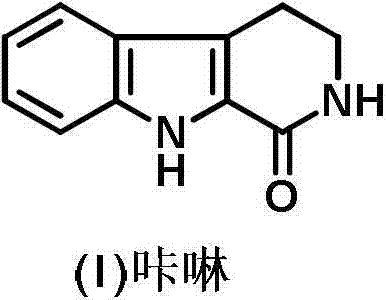
Preparation method of 2, 3, 4, 9-tetrahydro-beta-carboline-1-one
A carboline and equation technology, applied in the field of preparation of 2,3,4,9-tetrahydro-β-carboline-1-one, can solve the problem that the raw material carboxylic acid valerolactam is difficult to obtain, cannot realize industrial production, Harsh reaction conditions and other problems, to achieve the effect of ingenious process design, less pollution and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 20g of indolepropionic acid (0.106mol), 65g of toluene and 22g of thionyl chloride (0.185mol) into a 500ml four-neck flask, and start stirring. React at 70-75°C for 4 hours, distill the excess thionyl chloride under reduced pressure, evaporate to dryness, and add 100ml of fresh toluene. Add 10g of sodium azide (0.153mol), react at 10-15°C for 30 minutes, add 30ml of water, let stand to separate layers, and remove the lower water layer. The organic layer was heated to 85°C and stirred for 2.0 hours. Cool to room temperature, add 10g of zinc chloride, and stir overnight. After suction filtration, the filter cake was washed with water until neutral, and dried to obtain 15.7 g of a yellow solid with a yield of 80.1%. Melting point: 180.7-183.0°C;
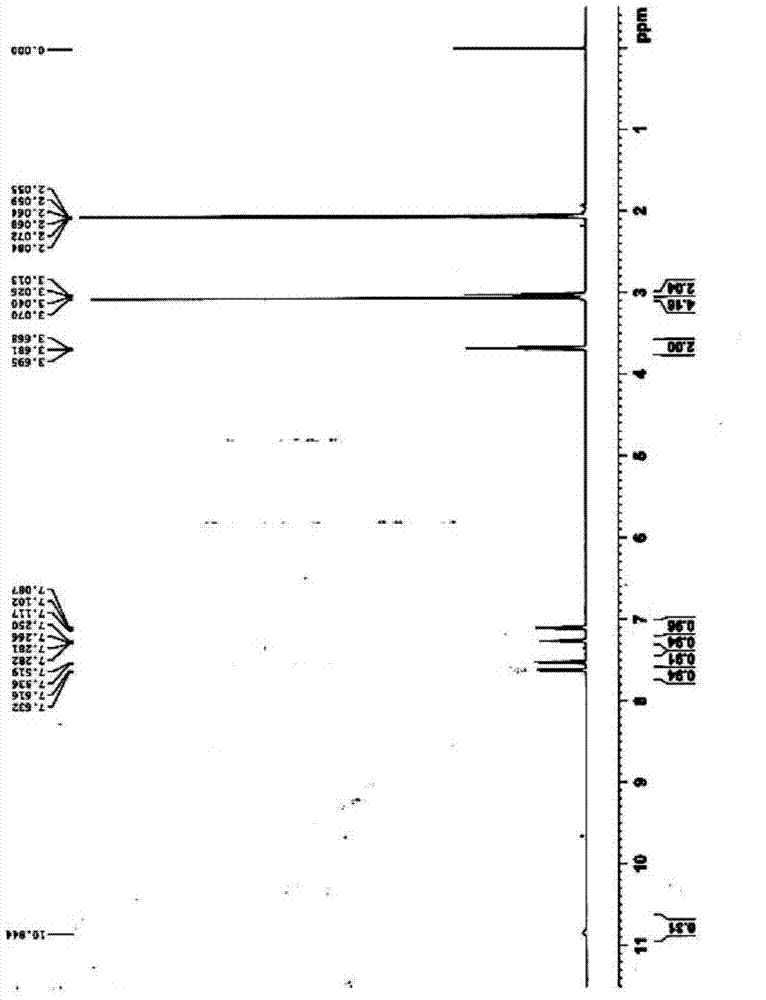
[0040] 1 HNMR(400MHZ,Acetone-d6):δ3.01(2H,m),3.04(1H,m),3.66(2H,m),7.08(1H,m),7.21(1H,m),7.51(1H, d),7.61(1H,d),10.84(1H,s);
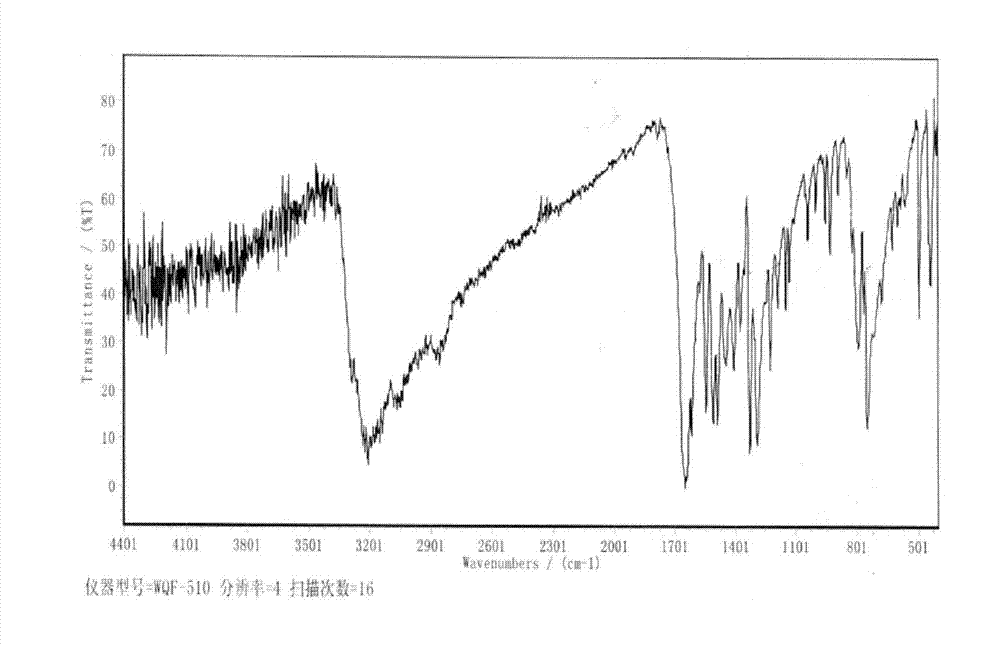
[0041] IR(KBr):3210(NH),2990,2870(CH),1665(C=O),1548,1512,1490,1455,1416(Ph and CH),1328,129...
Embodiment 2
[0044]Add 20 g of indole propionic acid (0.106 mol), 35 g of tetrahydrofuran and 25 g of oxalyl chloride (0.197 mol) into a 500 ml four-neck flask, and start stirring. React at 50-60°C for 4 hours, distill the excess oxalyl chloride under reduced pressure, evaporate to dryness, and add 25 g of fresh tetrahydrofuran. Add 8g of sodium azide (0.123mol) and react at 0-5°C for 1 hour. Add 100ml of toluene and 30ml of water, stir to dissolve, and separate the lower aqueous layer. The organic layer was heated to 80°C and stirred for 2.5 hours. Cool to room temperature, add 40g hydrochloric acid / 20g water, and stir overnight. After suction filtration, the filter cake was washed with water until neutral, and dried to obtain 14.9 g of a yellow solid with a yield of 75.8%. Melting point: 180.1-182.5°C.
Embodiment 3
[0046] Add 20g of indole propionic acid (0.106mol), 90g of acetone and 20g of triethylamine into a 500ml four-necked flask, and start stirring. 20 g of methyl chloroformate (0.211 mol) were added dropwise at 0-8°C. After dropping, add 10.5g of potassium azide (0.129mol) and react at 5-10°C for 2 hours. Add 100ml of toluene and 30ml of water, stir to dissolve, and separate the lower aqueous layer. The organic layer was heated to 85°C and stirred for 1.5 hours. Cool to room temperature, pass through 0.5mol hydrogen chloride gas, and stir overnight. After suction filtration, the filter cake was washed with water until neutral, and dried to obtain 15.4 g of a yellow solid with a yield of 78.1%. Melting point: 178.4-181.6°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com