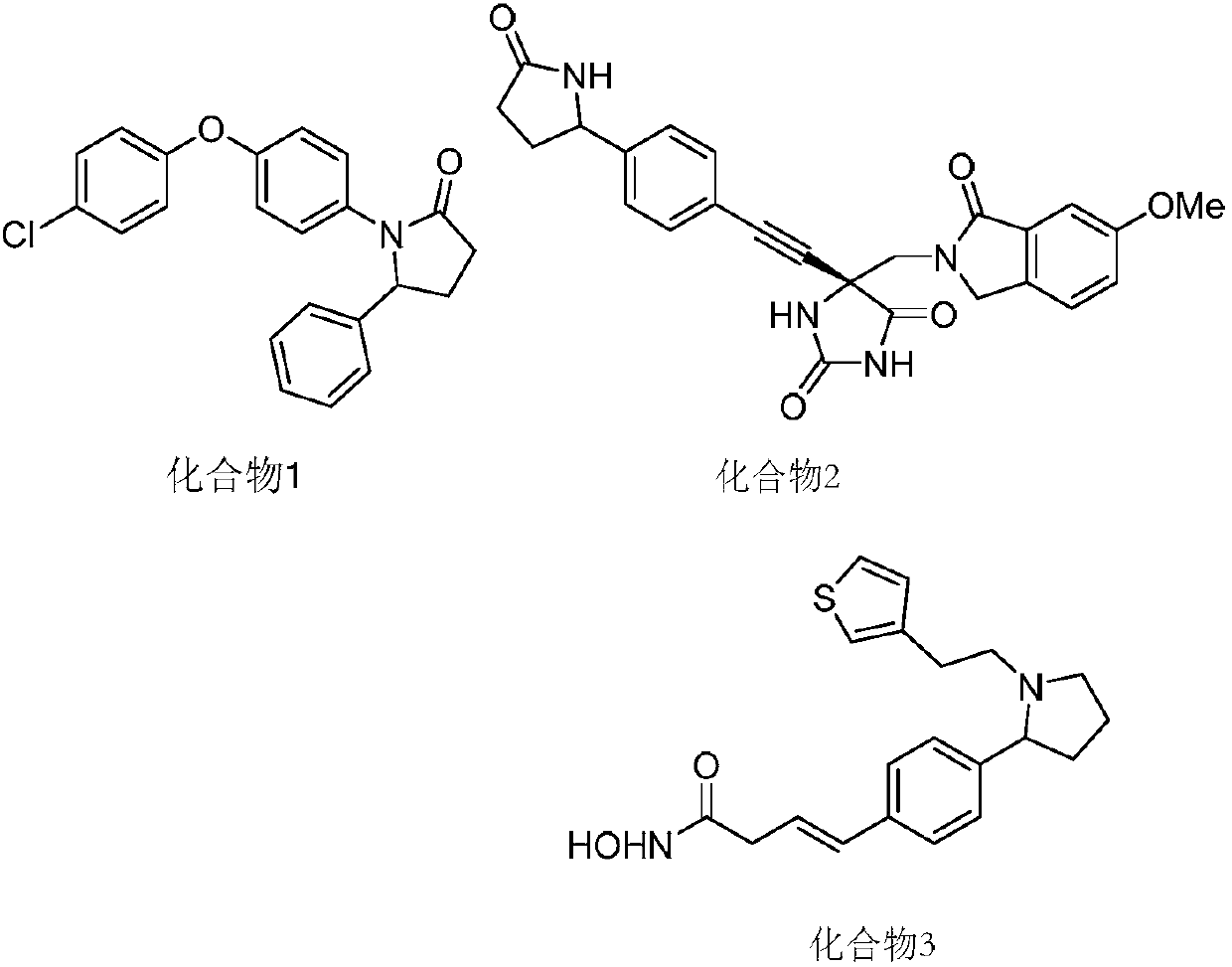
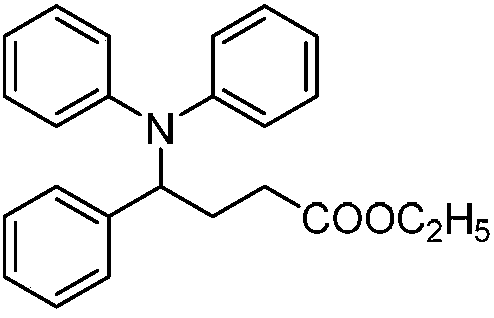
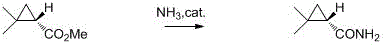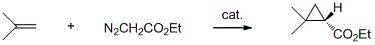Patents
Literature
54 results about "Ethyl diazoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
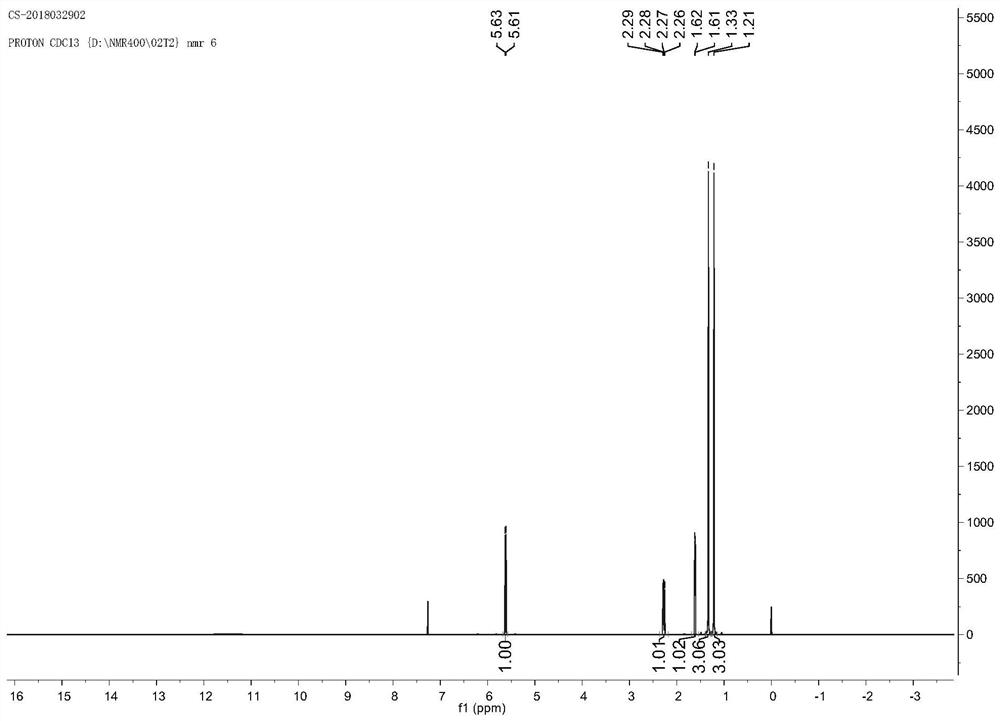
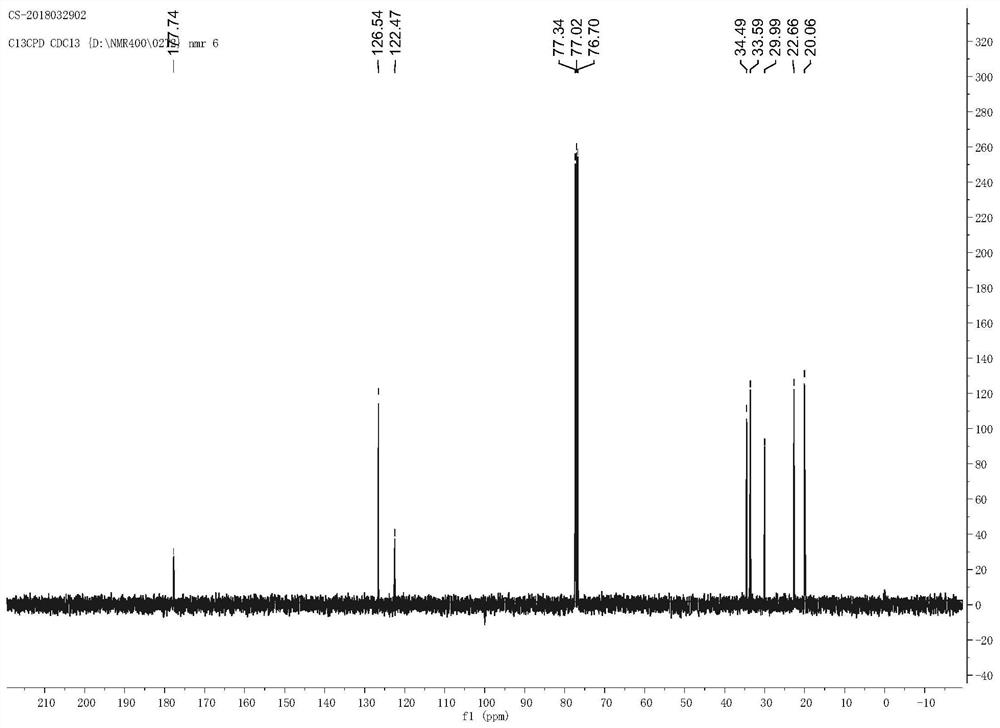
Ethyl diazoacetate (N=N=CHC(O)OC₂H₅) is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water.
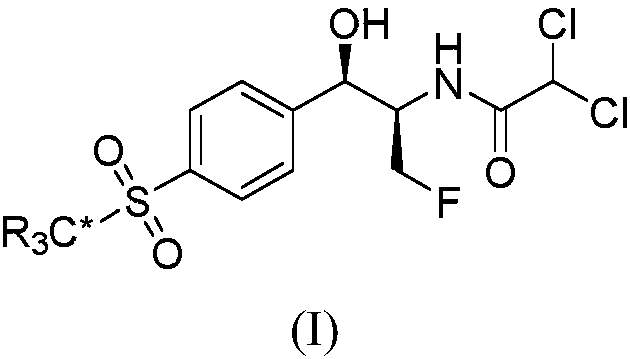
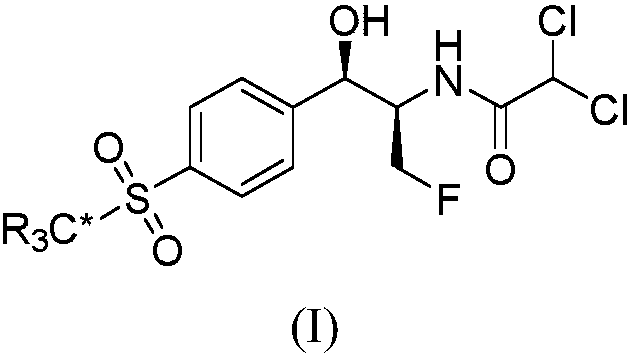
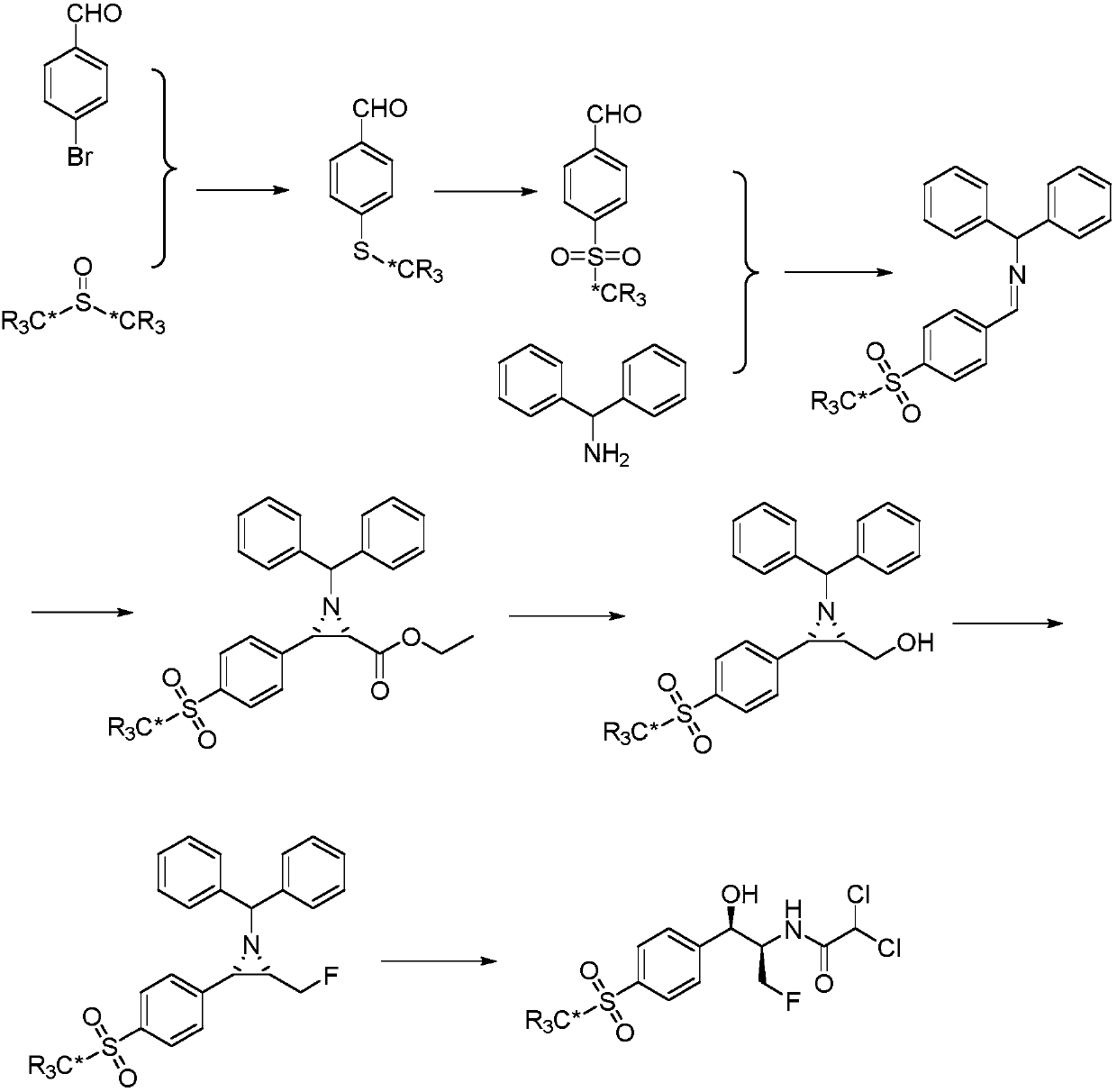
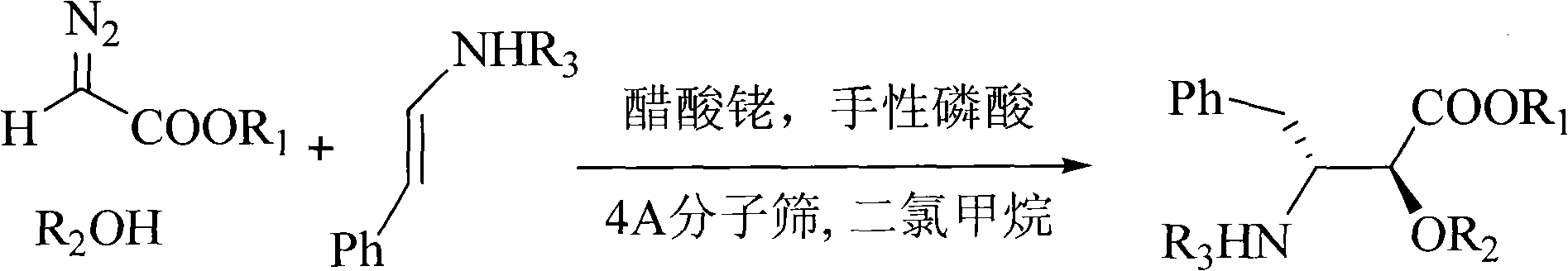
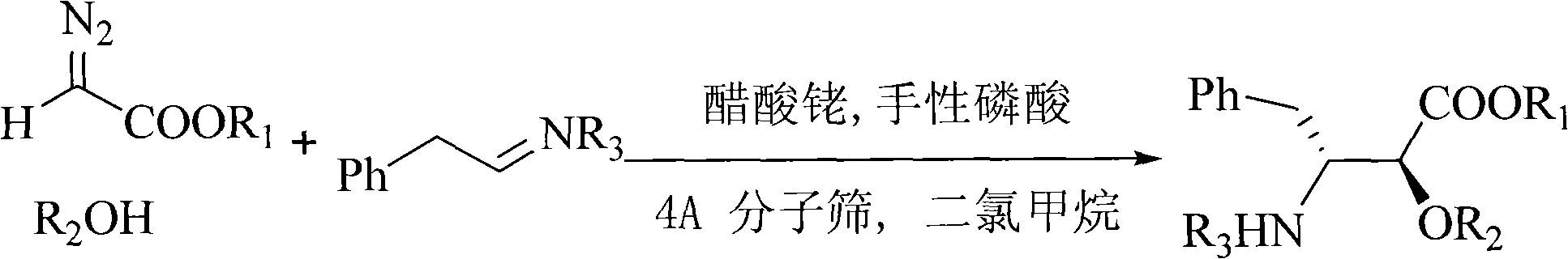
Method for synthesizing optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative
InactiveCN101538226AHigh atomic economyHigh selectivityCarbamic acid derivatives preparationOrganic compound preparationSolventCoordination complex
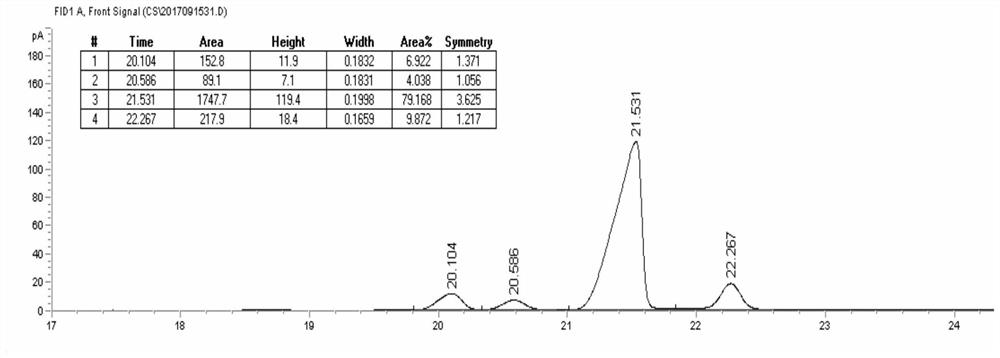
The invention relates to a method for synthesizing optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative, relating to a process for synthesizing the alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative. The method adopts ethyl diazoacetate, alcohol and styrylamine or benzyl imine as raw materials, chiral phosphoric acid and rhodium carboxylic acid or chiral phosphoric acid and copper (I) metal complex as catalyst, organic solvent as solvent, and 4molecular sieve, or 3 molecular sieve, or 5 molecular sieve as activating agent; and after one step of reaction, the dissolvent is removed to obtain a crude product. The crude product is processed by the operation of column chromatography by a solution in which the volume ratio of ethyl acetate to sherwood oil ranges from 1:50 to 1:20 to obtain the optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative. The mol ratio of the components is as follows: diazocompound to alcohol to styrylamine or benzyl imine to chiral phosphoric acid to rhodium carboxylic acid or copper(I) metal complex is equal to 1.1:1:1.2:0.02:0.02; and the proportion of the activating agent is 2 to 5g per mmol diazocompound. The method has the advantages of high atom economy, selectivity and yield, and easy and safe operation.
Owner:EAST CHINA NORMAL UNIV
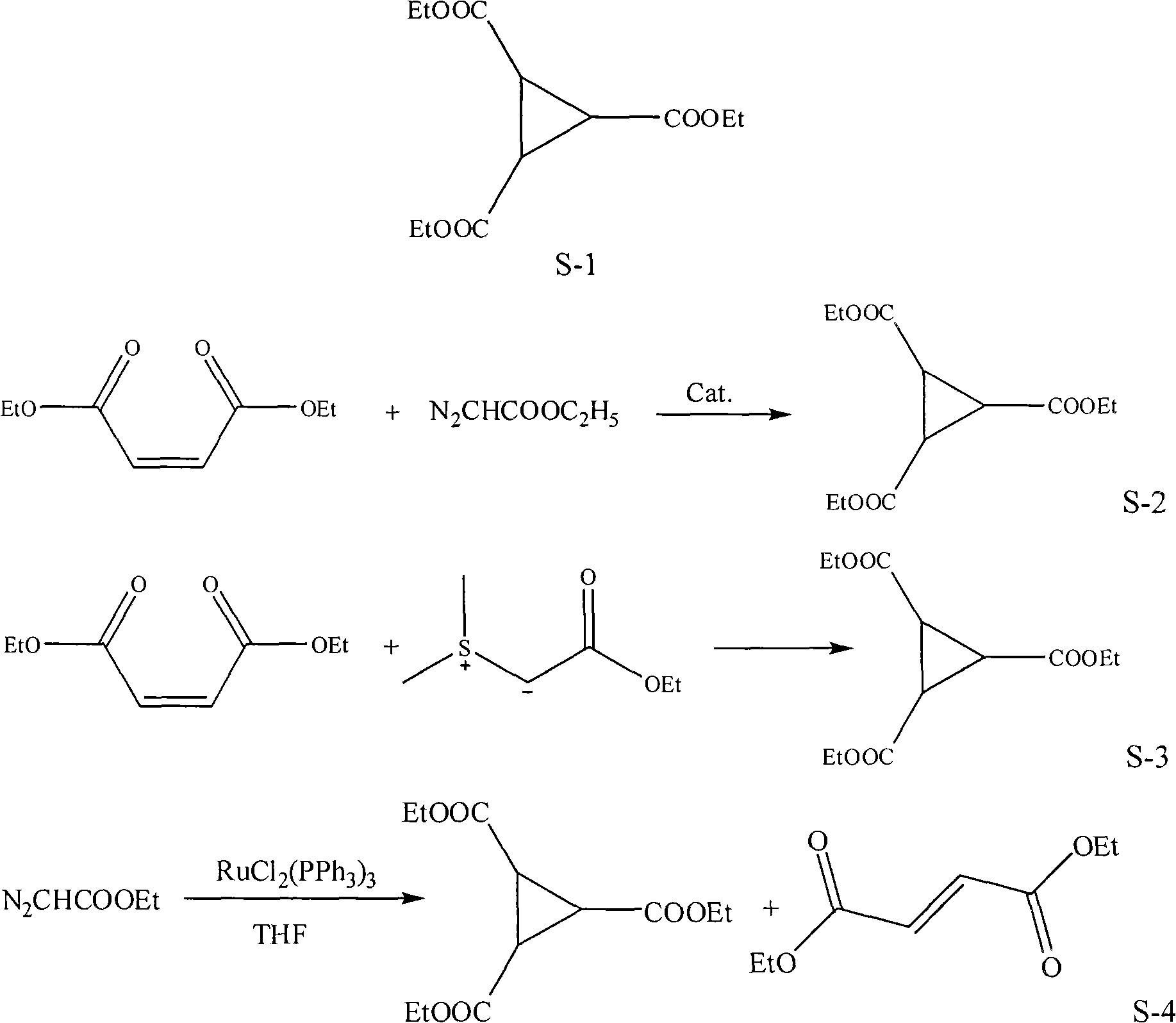
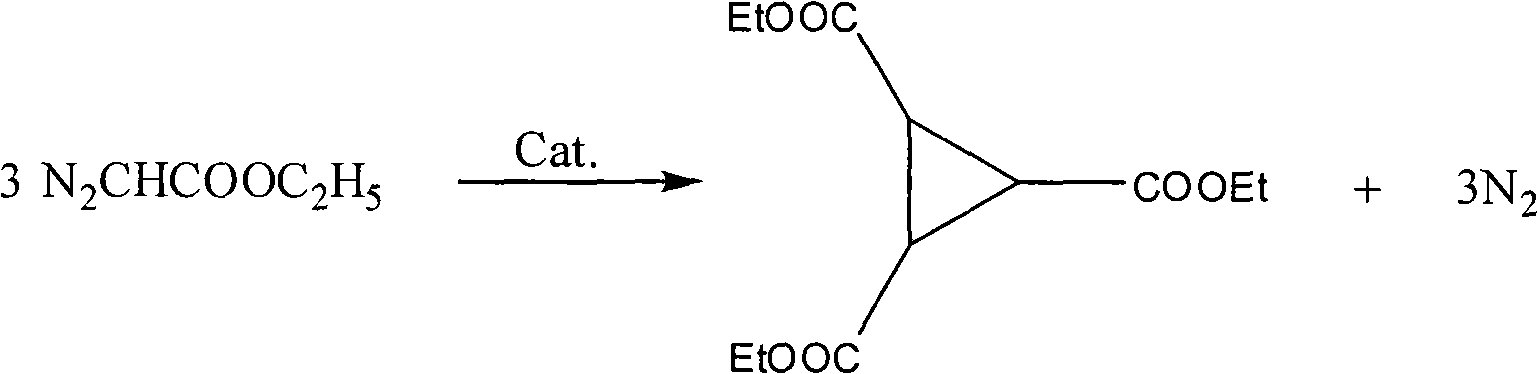
Preparation method of 1,2,3-cyclopropyl tricarboxylate
InactiveCN101844984ALow costHigh yieldOrganic compound preparationCarboxylic acid esters preparationAcetic acidOrganic solvent
The invention discloses a preparation method of 1,2,3-cyclopropyl tricarboxylate, comprising the following steps of: heating ethyl diazoacetate as a raw material for carrying out a cyclization reaction in the presence of organic solvents and catalysts at the reaction temperature of 35-110DEG C for 2-6h, wherein the mass ratio of the catalysts to the ethyl diazoacetate is 1:10-50; and rectifying after the reaction is finished to obtain the 1,2,3-cyclopropyl tricarboxylate. The method for preparing the 1,2,3-cyclopropyl tricarboxylate has the characteristics of concise process, high yield, low cost and less discharge of three wastes.
Owner:ZHEJIANG UNIV
Synthesis method of cyclopropane compounds
ActiveCN111732509AImprove reaction efficiencyPromote environmental protectionGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationCompound aPtru catalyst
The invention provides a synthesis method of cyclopropane compounds. The cyclopropane compounds have a structure as shown in a general formula I which is described in the specification. The synthesismethod comprises the following step: reacting olefin compounds A with ethyl diazoacetate under the catalytic action of a supported rhodium catalyst to obtain the cyclopropane compounds, wherein the structural formula of the olefin compounds A is shown in the specification. The synthesis method provided by the invention has the advantages of high reaction efficiency, short time, yield even reaching90% or above, and good repeatability. In addition, copper halide or an acetyl halide similar additive is not needed in the synthesis method, and the supported rhodium catalyst is adopted, so that theenvironmental protection property is relatively high.
Owner:ASYMCHEM LIFE SCI TIANJIN
Preparing method of cis, trans-ethyl 2, 2-dimethyl-3-(1-isobutenyl)cyclopropane-1-carboxylate
PendingCN106316845AEasy to operateHigh yieldOrganic compound preparationCarboxylic acid esters separation/purificationSolventSodium nitrite
The invention discloses a method of preparing cis, trans-ethyl 2,2-dimethyl-3-(1-isobutenyl)cyclopropane-1-carboxylate, comprising of making the ethyl glycinate hydro and sodium nitrite in the solvent diazo-react without additional addition of organic acid or mineral acid to get ethyl diazoacetate which is to react with the 2,5-Dimethyl-2,4-hexadiene under the catalyst effect and then be desolvated and rectificated to get ethyl chrysanthemumate.The invention is easy to use with high production yield and high contents.
Owner:江苏优普生物化学科技股份有限公司
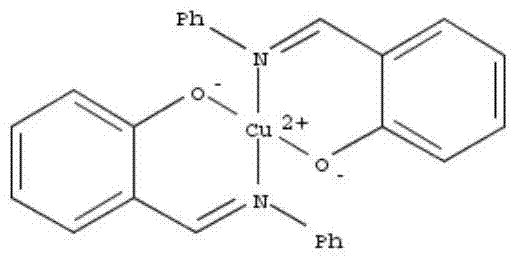
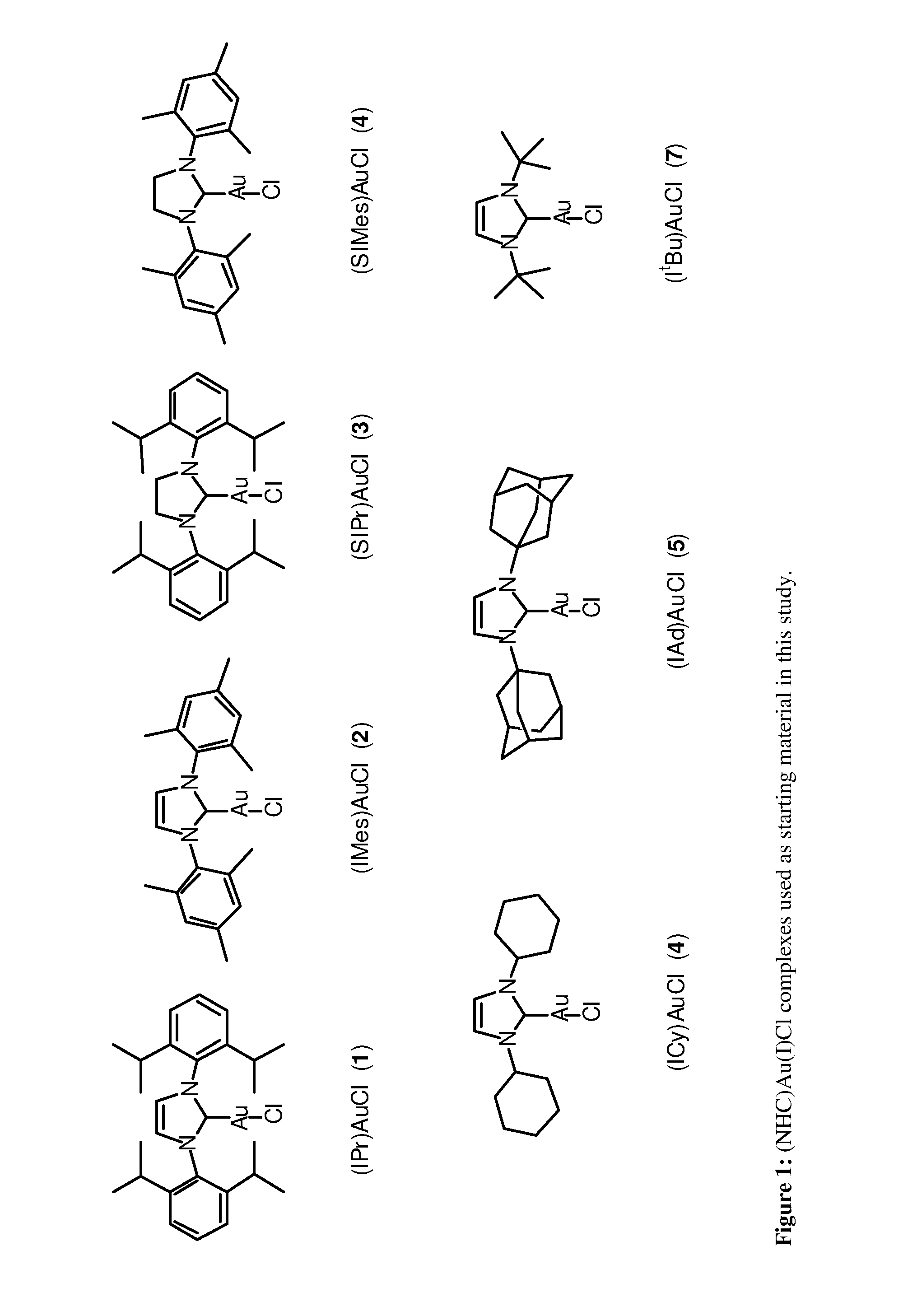
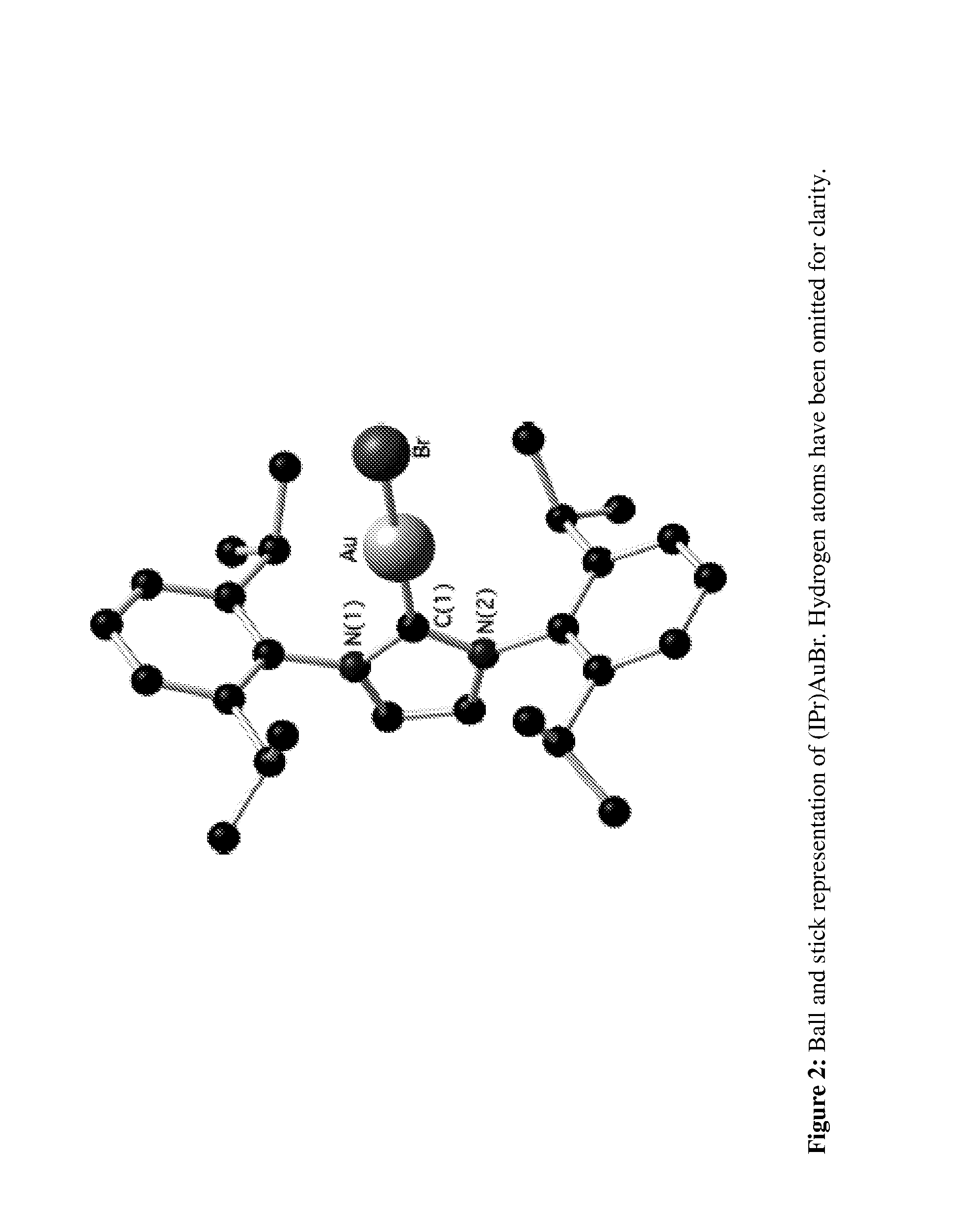
Gold complexes for catalysIS and preparation thereof
A number of cationic gold(I) and neutral gold(III) complexes have been synthesized and found to be stabilized by the use of N-heterocyclic carbene ligands. These species are often employed as in situ-generated reactive intermediates in gold catalyzed organic transformations. An isolated, well-defined cationic species was tested in gold mediated carbene transfer reactions from ethyl diazoacetate.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Synthetic method for stable isotope labeled chloramphenicol
InactiveCN107827687AIncrease usageThe synthesis steps are simpleIsotope introduction to heterocyclic compoundsOrganic compound preparationStable Isotope LabelingOrganic synthesis
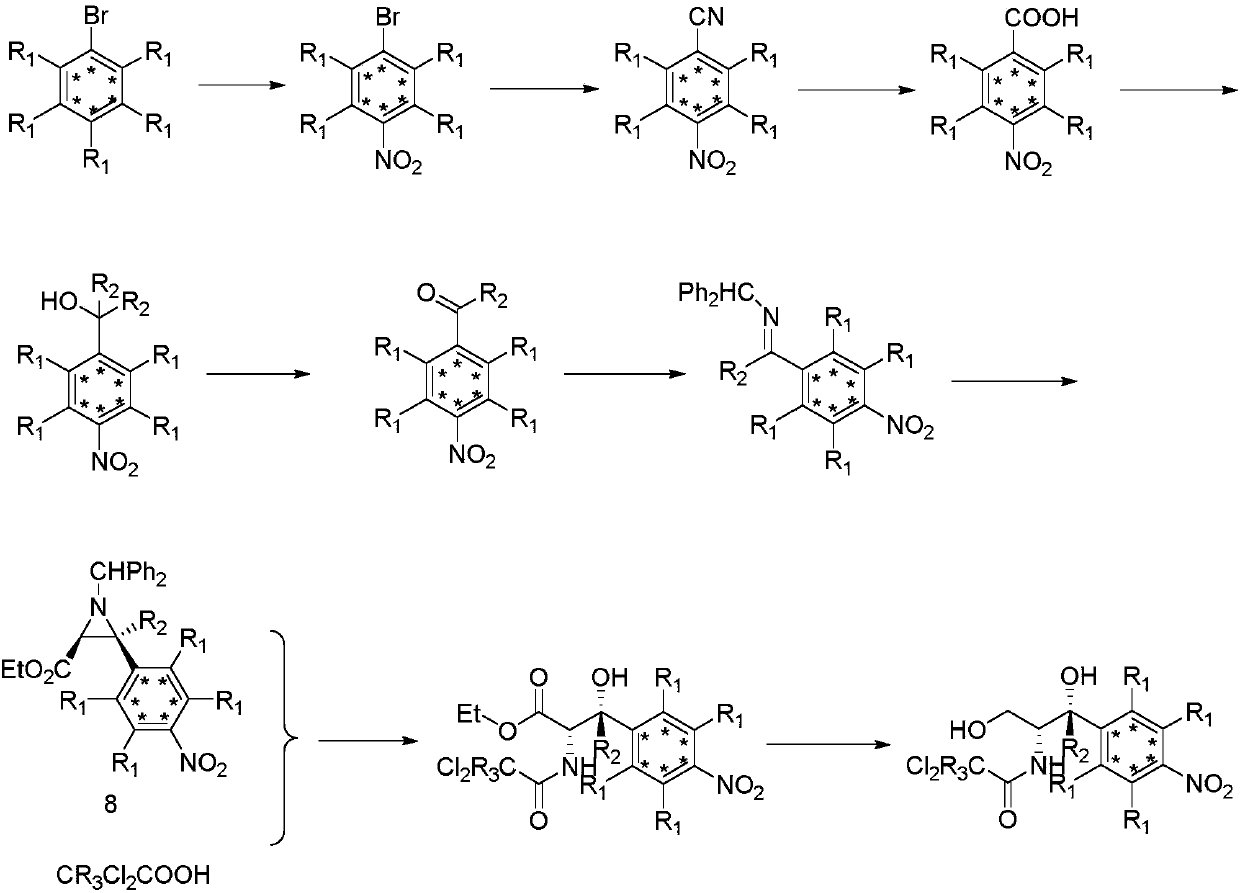
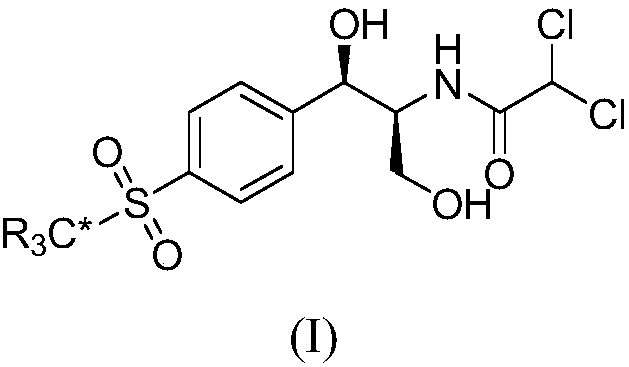
The invention relates to a synthetic method for stable isotope labeled chloramphenicol and belongs to the field of organic synthesis. The synthetic method for stable isotope labeled chloramphenicol ischaracterized in that a stable isotope labeled bromobenzene derivative is taken as a raw material, the raw material is subjected to para-position nitration, cyan substitution, cyan hydrolysis and reduction, and is further oxidized into carbonyl, a substitution reaction is performed on carbonyl and an aromatic ring to obtain amine which is condensed into imine, imine further reacts with ethyl diazoacetate under the action of (R)-VAPOL and triphenyl borate to build an ethylene imine structure, ring opening is performed on ethylene imine under an acid condition, and at last, an ester group is reduced to obtain stable isotope labeled chloramphenicol. The synthetic method is simple in step and simple and convenient to operate; and the product is high in chemical purity and isotope abundance and can be used for internal standard substances for veterinary drug residue test in the food safety field and study of the chloramphenicol metabolic mechanism.
Owner:山东辉璟生物医药科技有限公司
Synthetic method for stable isotope labeled thiamphenicol
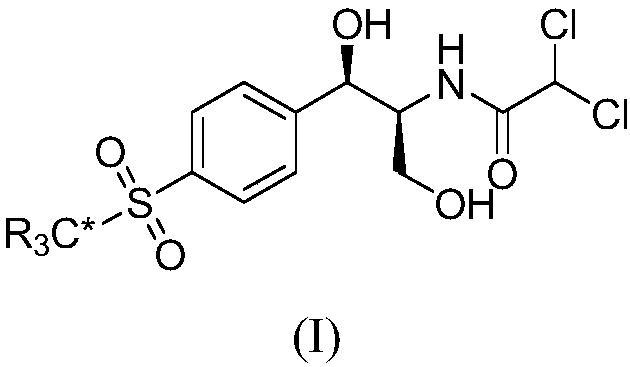
ActiveCN107827791AIncrease usageThe synthesis steps are simpleOrganic compound preparationOrganic chemistry methodsStable Isotope LabelingBenzaldehyde
The invention relates to a synthesis method for stable isotope labeled thiamphenicol and belongs to the field of organic synthesis. The synthesis method for stable isotope labeled thiamphenicol is characterized in that p-bromobenzaldehyde and stable isotope labeled dimethylsulfoxide are taken as raw materials, the raw materials are synthesized to obtain stable isotope labeled p-methylthiobenzaldehyde, oxidization is performed to obtain stable isotope labeled 4-methylsulfonyl benzaldehyde, next, condensation is performed on stable isotope labeled 4-methylsulfonyl benzaldehyde and benzhydrylamine to obtain imine, then imine further reacts with ethyl diazoacetate under the action of (R)-VAPOL and triphenyl borate to build an ethylene imine structure fragment, at last, ring opening is performed on ethylene imine under a dichloroacetic acid condition, an ester group is reduced to synthesize stable isotope labeled thiamphenicol. The raw materials required for synthesis and an intermediate are simple and easily accessible, and the target product (stable isotope labeled thiamphenicol) is high in purity and stable isotope abundance, can be used for internal standard substances for veterinary drug residue test in the food safety field and study of the thiamphenicol metabolic mechanism, and has an important practical application value.
Owner:山东辉璟生物医药科技有限公司
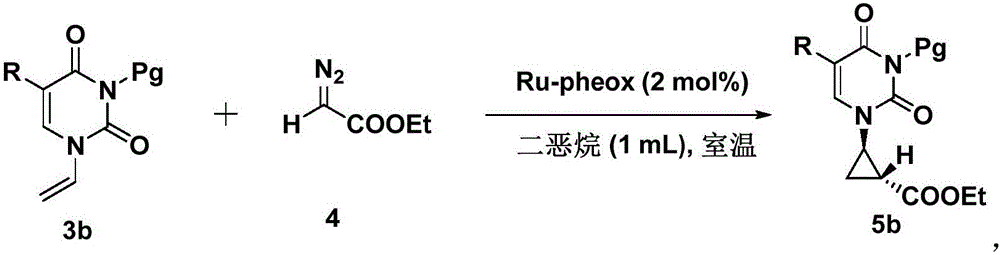
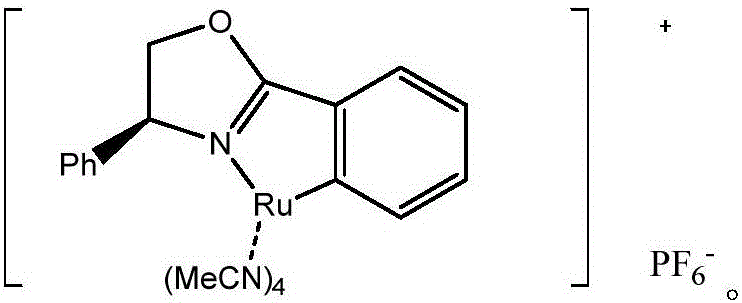
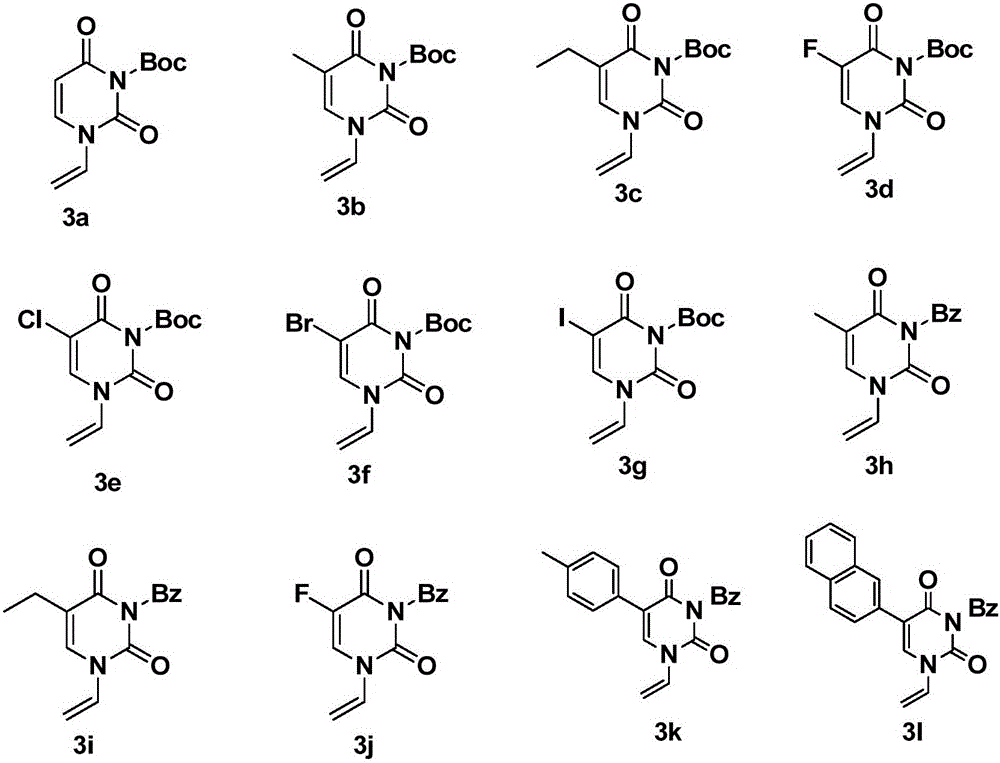
Chiral tri-carbocyclic pyrimidine nucleoside analogue and preparation method thereof
InactiveCN105693627ARaw materials are easy to getEasy to operateOrganic chemistryAntiviral drugRoom temperature
The invention discloses a chiral tri-carbocyclic pyrimidine nucleoside analogue and a preparation method of the chiral tri-carbocyclic pyrimidine nucleoside analogue. The preparation method comprises the following steps of taking dioxane as a solvent, adding Ru-pheox as a ligand and a catalyst, then adding an ethyl diazoacetate reactant, stirring at a room temperature for 4 minutes, and reacting at the room temperature. The preparation method disclosed by the invention is a simple, convenient, green and efficient method for synthesizing the chiral tri-carbocyclic pyrimidine nucleoside analogue, aims to solve the problems of expensive raw materials and complicated process in the process of synthesizing the kind of compound, and has the advantages that a reference value is provided for the synthesis and application of nucleoside drugs, and the raw material is provided for the research of new antiviral drugs and new antitumor drugs.
Owner:HENAN NORMAL UNIV
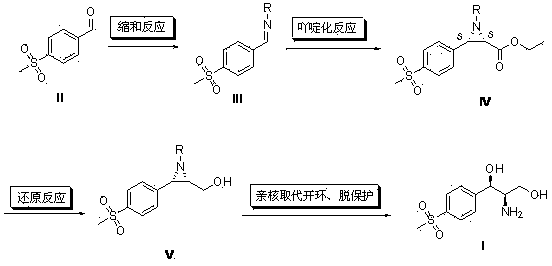
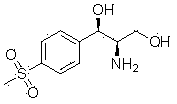
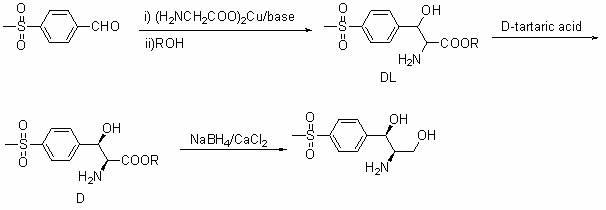
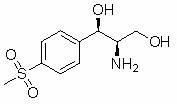
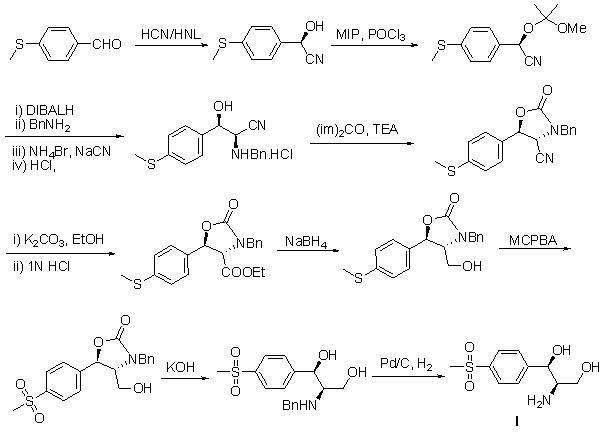
Method for synthesizing (1R, 2R)-1-p-methyl sulfone phenyl-2-amino-1,3-propanediol
InactiveCN102010355AShort routeMild conditionsOrganic chemistryOrganic compound preparationBenzaldehydeEthyl acetate
The invention discloses a method for synthesizing (1R, 2R)-1-p-methyl sulfone phenyl-2-amino-1,3-propanediol, which belongs to the technical field of chemical medicines. The method comprises the following steps: 1. carrying out condensation on p-methylsulphonyl benzaldehyde (II) and primary amine so as to obtain a compound (III); 2. in the presence of a catalyst, carrying out acridine reaction on the compound (III) and ethyl diazoacetate so as to obtain a compound (IV); 3. carrying out reduction on the compound (IV) so as to obtain a compound (V); and 4. in the presence of an acid, carrying out ring-opening reaction and de-protection reaction on the compound (V) by a one-pot method so as to obtain the (1R, 2R)-1-p-methyl sulfone phenyl-2-amino-1, 3-propanediol. The method provided by the invention has the advantages of novel design, simple and short route, mild conditions, good enantioselectivity, high yield, and good industrialized production prospect, and is simple and convenient in operation.
Owner:FUDAN UNIV
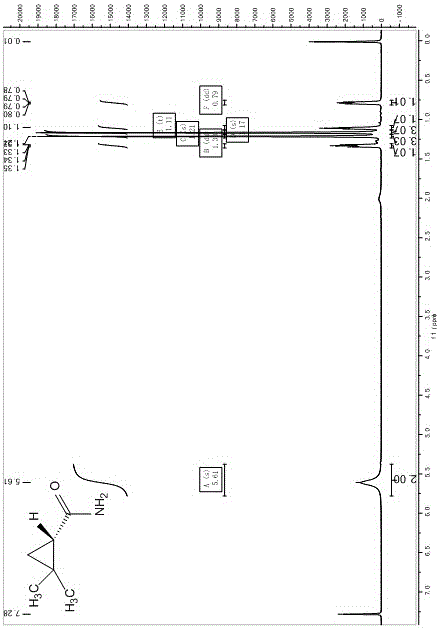
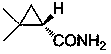
Preparation method of chiral dimethyl cyclopropyl carboxamide
InactiveCN104193645AOrganic compound preparationCarboxylic acid amide separation/purificationAlkyl transferDiazoacetic ester
The invention discloses a preparation method of chiral dimethyl cyclopropyl carboxamide. The method comprises a step of asymmetric cyclopropyl alkylation and a step of catalytic amidation of cyclopropyl formic ether, wherein in the step of asymmetric cyclopropyl alkylation, a cyclopropyl alkylation reaction is carried out on ethyl diazoacetate and isobutene under the catalysis of a chiral ligand complex of a cuprous salt so as to obtain (S)-dimethyl cyclopropyl formate; and in the step of catalytic amidation of cyclopropyl formic ether, an ammonolysis reaction is carried out on the (S)-dimethyl cyclopropyl formate by one step so as to directly obtain (S)-2,2-dimethyl cyclopropyl carboxamide, and refining the carboxamide with an alcohol so as to obtain the chiral dimethyl cyclopropyl carboxamide with chemical purity being greater than 99.5% and an e.e. value being greater than 99.5%. Thus, the method used for synthesizing the (S)-2,2-dimethyl cyclopropyl carboxamide is environment-friendly, simple, rapid and efficient.
Owner:SHANGHAI INST OF TECH
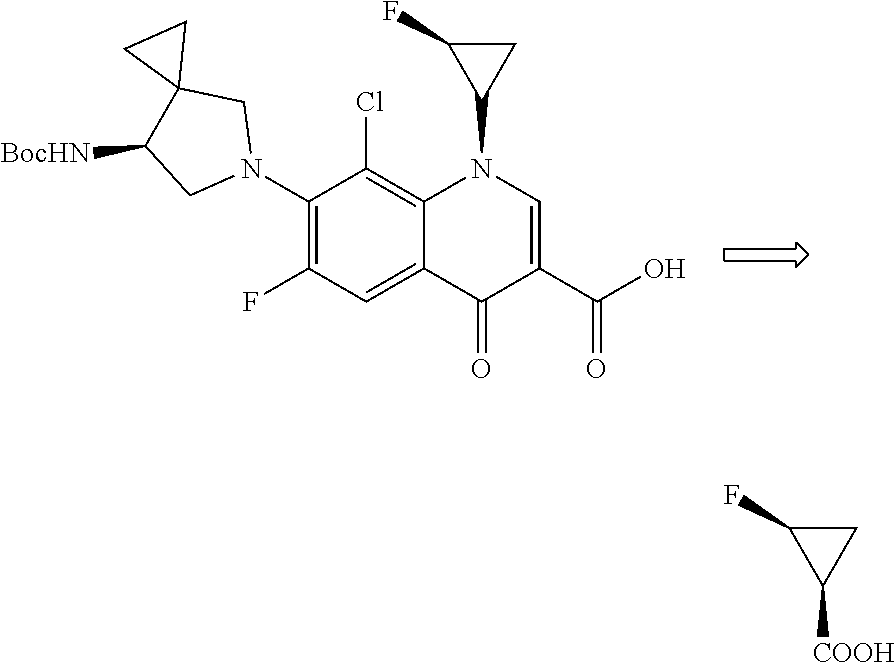
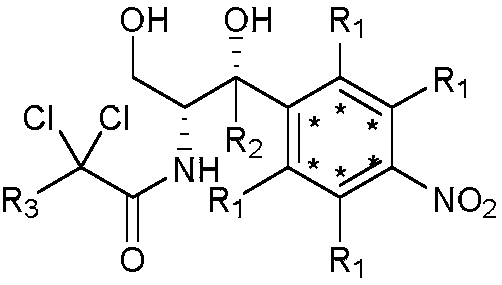
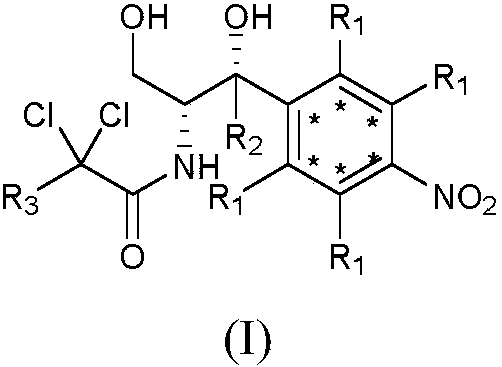
2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof
The invention belongs to the technical field of medicine and discloses a 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative of a formula (I) as well as a preparation method thereof. The preparation method comprises the following steps: performing Witting reaction on II to obtain III, catalyzing cyclopropanation reaction of ethyl diazoacetate and the III by rhodium acetate to obtain IVand V, performing hydrolysis, performing Curtius rearrangement to obtain VI and VII; (1) removing Boc from the VI or VII and performing reduction and ammoniation to obtain a compound VIII or IX; or (2) performing substitution on the VI or VII and 2-chlorine-1-morpholine ethane-1-ketone to obtain X or XI; or (3) performing reduction and ammoniation on the VI or VII and (4-oxycyclohexyl)tert-butylcarbamate, and removing Boc to obtain a compound XII or XIII; or (4) performing Suzuki coupling on the VI or VII and substituted arylboronic acid, removing Boc to obtain XIV or XV, and performing reduction and ammoniation to obtain VIII or IX. The derivative of the formula (I) has high inhibition activity, has high selectivity on homologous enzyme such as monoamine oxidase and LSD2, and is expected to be developed into a medicine for treating diseases such as acute myelogenous leukemia.
Owner:EAST CHINA NORMAL UNIV +1
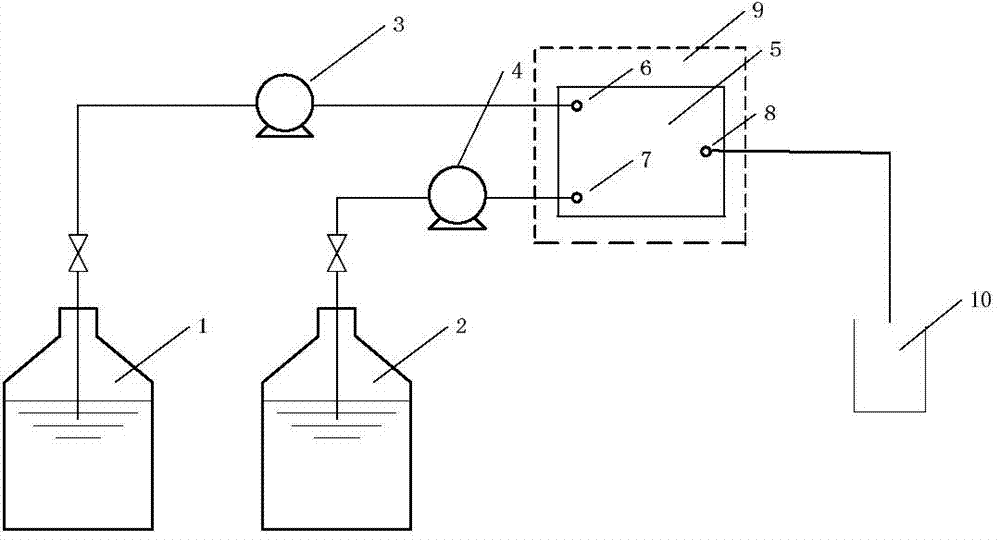
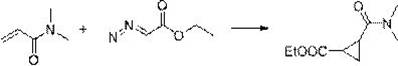
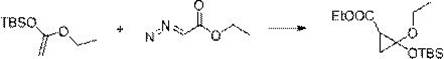
Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether
The invention relates to the preparation of cyclopropane carboxylate, in particular to a method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether under the mild condition of the catalysis of non-noble metal. The method comprises the following steps of: taking thiophene and ethyl diazoacetate as raw materials, adopting a compound of Cu or Co as a catalyst, adopting a compound which does not contain an alcoholic hydroxyl group, a carboxylic acid group, a primary amino radical and a secondary amino group as a solvent, dripping the ethyl diazoacetate or a solution thereof into the mixture of the catalyst and the thiophene, stirring, reacting for 5-24min at the temperature of 0-120 DEG C until a reaction mixture does not discharge gas any more, then distilling off the solvent andthe excessive thiophene and separating by the methods of column chromatography or reduced pressure distillation and the like to obtain the 4-thio-bicyclo[3.1.0]-2-hexene-6-formic ether, wherein calculated by molar weight, the use quantity of the thiophene in the reaction is 1-200 times that of the ethyl diazoacetate, the use quantity of the catalyst is 0.0001-100mol% of that of the ethyl diazoacetate, and the volume of the solvent is 0-50 times the volume of the thiophene. The invention has low cost, mild condition, better selectivity and higher economic value.
Owner:DALIAN UNIV
Synthetic method for stable isotope labeled florfenicol
InactiveCN107827688AThe synthesis steps are simpleHigh yieldIsotope introduction to heterocyclic compoundsOrganic compound preparationStable Isotope LabelingBenzaldehyde
The invention relates to a synthetic method for stable isotope labeled florfenicol and belongs to the field of organic synthesis. The synthetic method for stable isotope labeled florfenicol is characterized in that p-bromobenzaldehyde and stable isotope labeled dimethylsulfoxide are taken as raw materials, the raw materials are synthesized to obtain stable isotope labeled p-methylthiobenzaldehyde,oxidization is performed to obtain stable isotope labeled 4-methylsulfonyl benzaldehyde, next, condensation is performed on stable isotope labeled 4-methylsulfonyl benzaldehyde and benzhydrylamine toobtain imine, then imine further reacts with ethyl diazoacetate under the action of (R)-VAPOL and triphenyl borate to build an ethylene imine structure fragment, at last, ester group is reduced, a hydroxyl group is fluoridize, and ring opening is performed on ethylene imine under a dichloroacetic acid condition to synthesize stable isotope labeled florfenicol. The raw materials required for synthesis are simple and easily accessible, and the target product (stable isotope labeled florfenicol) is high in purity and stable isotope abundance, can be used for internal standard substances for veterinary drug residue test in the food safety field and study of the florfenicol metabolic mechanism, and has an important practical application value.
Owner:山东辉璟生物医药科技有限公司
Method for preparing 4-aminobutyrate derivatives
ActiveCN107892655ASolve the cumbersome synthesis stepsSolve yieldOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisEvaporation
The invention discloses a method for preparing 4-aminobutyrate derivatives, and relates to a method for preparing the 4-aminobutyrate derivatives. The purpose of the invention is to solve the problemsof complicated steps, a low yield, and environmental pollution in the synthesis of the 4-aminobutyrate derivatives by using conventional methods. The method comprises the following steps: a diarylamine derivative, an olefin derivative and ethyl diazoacetate are dissolved into an organic solvent under a nitrogen atmosphere and room temperature, then a photocatalyst is added, mixing is performed uniformly, the mixed material is placed below a blue LED lamp, an illumination reaction is performed, rotary evaporation is performed to remove the solvent, chromatography separation purification is performed by using a silica gel column, and therefore the product, one 4-aminobutyrate derivative, is obtained. The reaction in the method provided by the invention can be performed under a normal temperature and normal pressure, the reaction conditions are mild, the yield can reach 80%, and the method has the advantages of simple operation, no pollution, safety, environmental friendliness and low costs, and is applied to the field of organic synthesis.
Owner:HARBIN INST OF TECH
Carbene-diazo compound-olefine aldehyde terpolymer and application of carbene-diazo compound-olefine aldehyde terpolymer as bidirectional conversion fluorescent material and anti-cancer drug
InactiveCN104311822ANo damageDeep tissue penetrationOrganic active ingredientsAntineoplastic agentsPolymer scienceCarbene
The invention discloses a carbene-diazo compound-olefine aldehyde terpolymer and a preparation method thereof. The preparation method comprises the following steps: a diazo compound and olefine aldehyde are used as polymerization reaction monomers and after reaction, reprecipitation, centrifugation and vacuum drying of precipitates are carried out to obtain the product. The polymer is mainly prepared by copolymerization of C1 / C1N2 / C2 and a main chain of the polymer mainly comprises carbene, diazo compound and olefine aldehyde. The polymer not only has a down-conversion fluorescent property, but also has an up-conversion fluorescent property, meanwhile, has high optical stability, can be widely applied to the field of optics and optical detection; meanwhile, the polymer has anti-cancer activity and can be applied to the field of medicine.
Owner:WUHAN UNIV
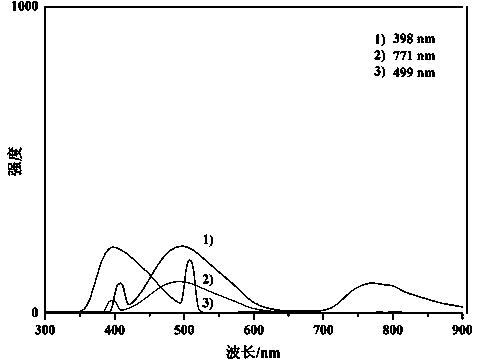
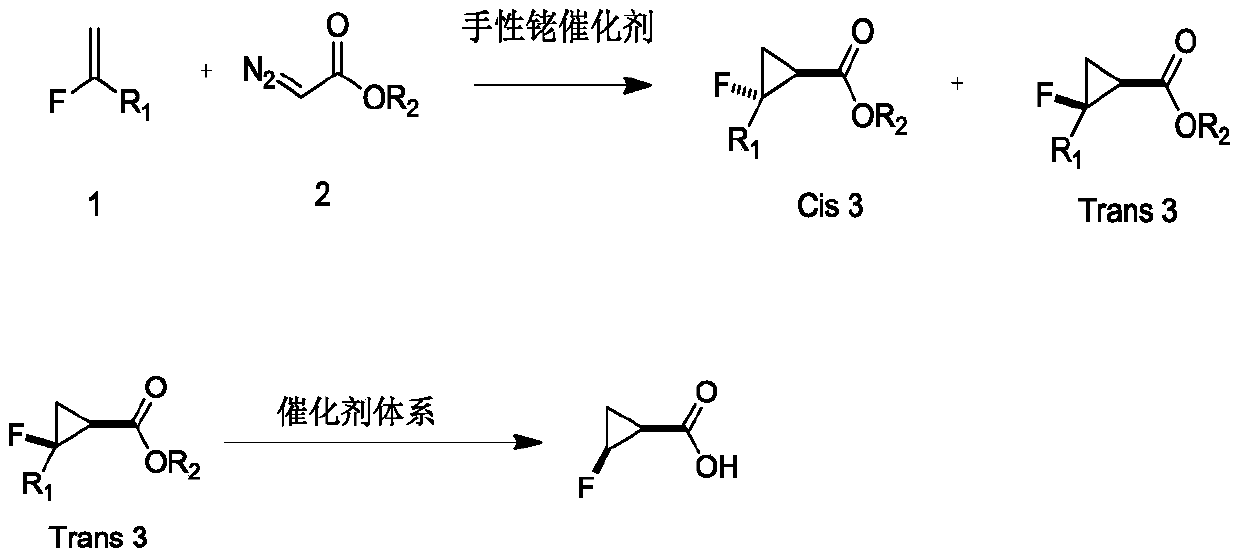
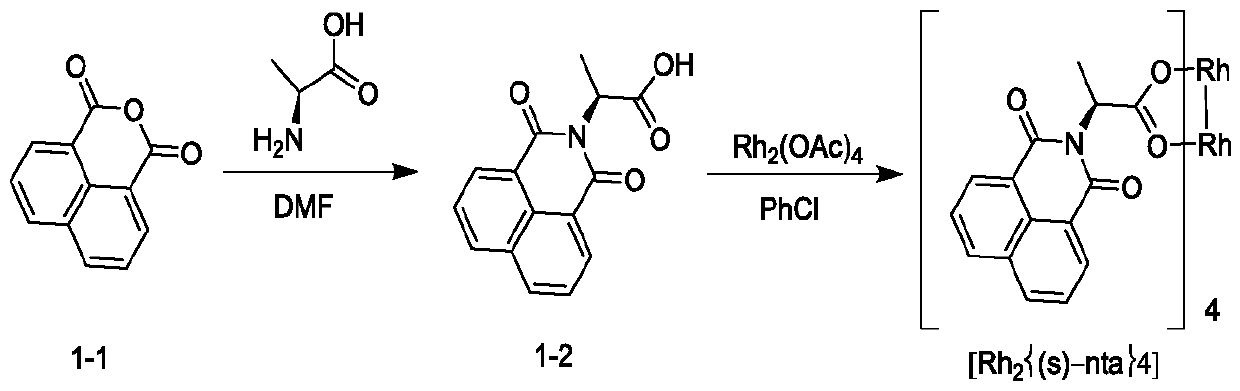
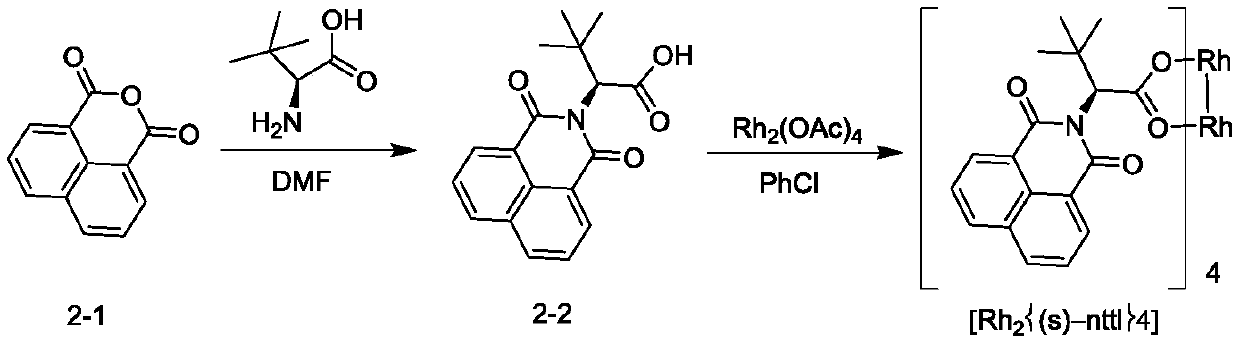
Novel method for asymmetric synthesis of (1S,2S)-2-fluorocyclopropanecarboxylic acid under catalysis of chiral rhodium catalyst
ActiveCN110483272AHigh SS/RR valueGood universalityOrganic compound preparationOrganic chemistry methodsEnantioselective synthesisEthyl ester
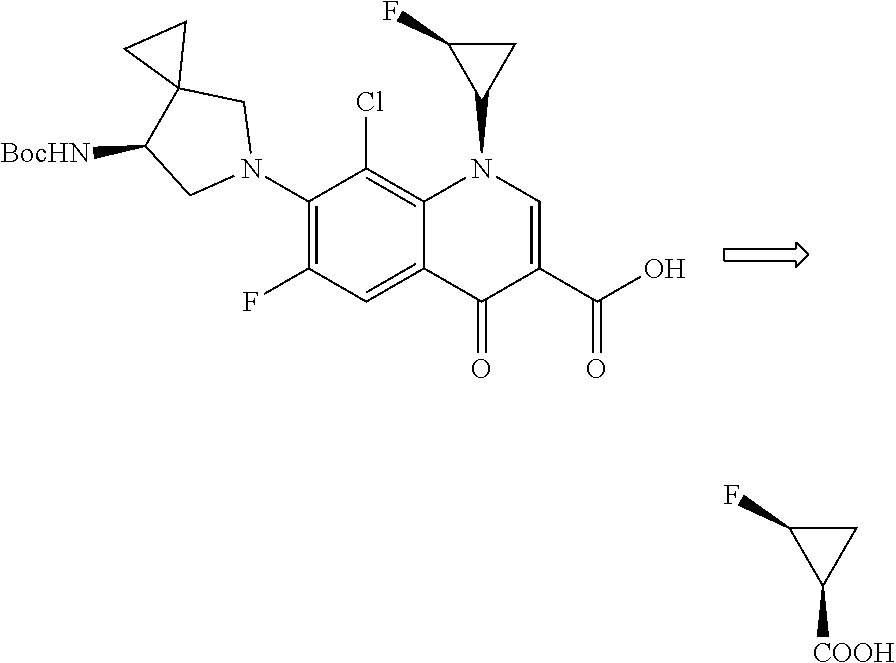
The invention provides a novel method for asymmetric synthesis of (1S, 2S)-2-fluorocyclopropanecarboxylic acid under catalysis of a chiral rhodium catalyst. The chiral rhodium catalyst not only can catalyze 1-fluoro-1-benzenesulfonyl ethylene and ethyl diazoacetate to carry out asymmetric cyclization reaction, but also can catalyze 1-fluoro-1-chloroethylene and ethyl diazoacetate to carry out asymmetric cyclization reaction. The method provided by the invention has the advantages that the universality is good, and the reaction yield is high, wherein the SS / RR value of (1S, 2S)-2-fluorocyclopropanecarboxylic acid is as high as 98%, so that the preparation cost is greatly reduced, and the method is suitable for industrial production.
Owner:CHEN STONE GUANGZHOU CO LTD
A kind of preparation method of chiral dimethyl cyclopropanamide
InactiveCN104193645BOrganic compound preparationCarboxylic acid amide separation/purificationAlkyl transferAlcohol
The invention discloses a preparation method of chiral dimethyl cyclopropyl carboxamide. The method comprises a step of asymmetric cyclopropyl alkylation and a step of catalytic amidation of cyclopropyl formic ether, wherein in the step of asymmetric cyclopropyl alkylation, a cyclopropyl alkylation reaction is carried out on ethyl diazoacetate and isobutene under the catalysis of a chiral ligand complex of a cuprous salt so as to obtain (S)-dimethyl cyclopropyl formate; and in the step of catalytic amidation of cyclopropyl formic ether, an ammonolysis reaction is carried out on the (S)-dimethyl cyclopropyl formate by one step so as to directly obtain (S)-2,2-dimethyl cyclopropyl carboxamide, and refining the carboxamide with an alcohol so as to obtain the chiral dimethyl cyclopropyl carboxamide with chemical purity being greater than 99.5% and an e.e. value being greater than 99.5%. Thus, the method used for synthesizing the (S)-2,2-dimethyl cyclopropyl carboxamide is environment-friendly, simple, rapid and efficient.
Owner:SHANGHAI INST OF TECH
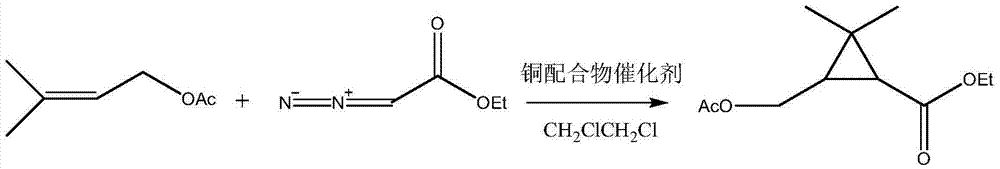
3-acetoxy methyl-ethyl-2,2-dimethylcyclopropanecarboxylate synthesis method
InactiveCN104710313AHigh yieldImprove securityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidSynthesis methods
The invention relates to a 3-acetoxy methyl-ethyl-2,2-dimethylcyclopropanecarboxylate synthesis method, which comprises that an ethyl diazoacetate solution and an isopentenyl acetate solution are continuously pumped into a micro-reactor and react at a temperature of 80-110 DEG C, wherein the ethyl diazoacetate solution comprises (a) ethyl diazoacetate solution and (b) 1,2-dichloroethane, and the isopentenyl acetate solution comprises (c) isopentenyl acetate and (d) a copper coordination compound catalyst. According to the present invention, the copper coordination compound is innovatively adopted as the catalyst so as to accelerate the reaction, and the 1,2-dichloroethane is adopted as the solvent so as to make the reaction be performed under the homogeneous condition, such that the reaction characteristics and the micro-reactor advantages are combined through the selection of the two reagent, the synthesis method has characteristics of short reaction time, continuous process, high safety and high efficiency, and the product yield under the optimal condition can achieve 80%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
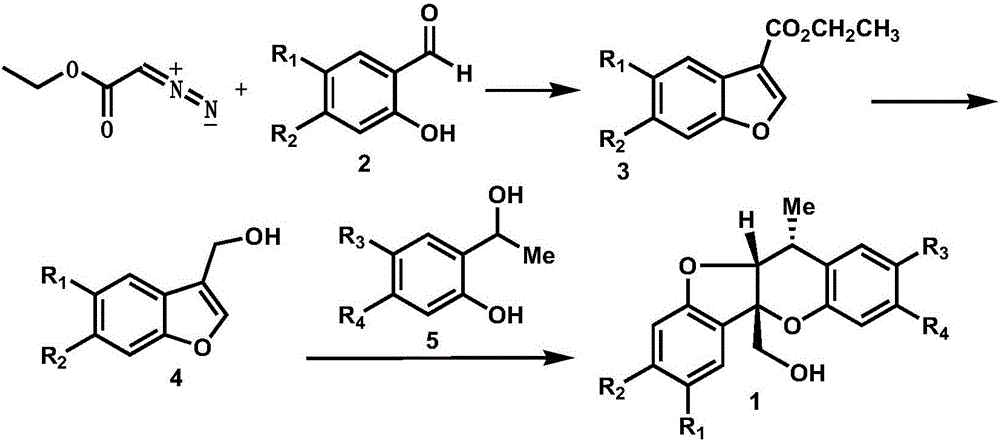
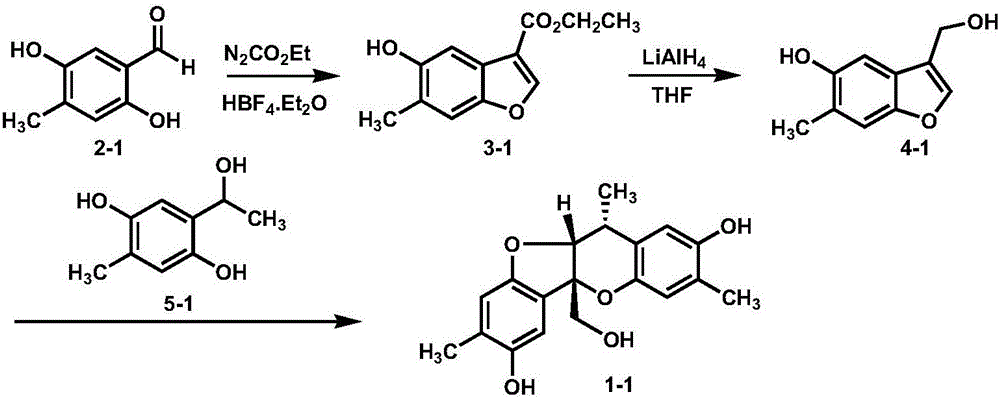
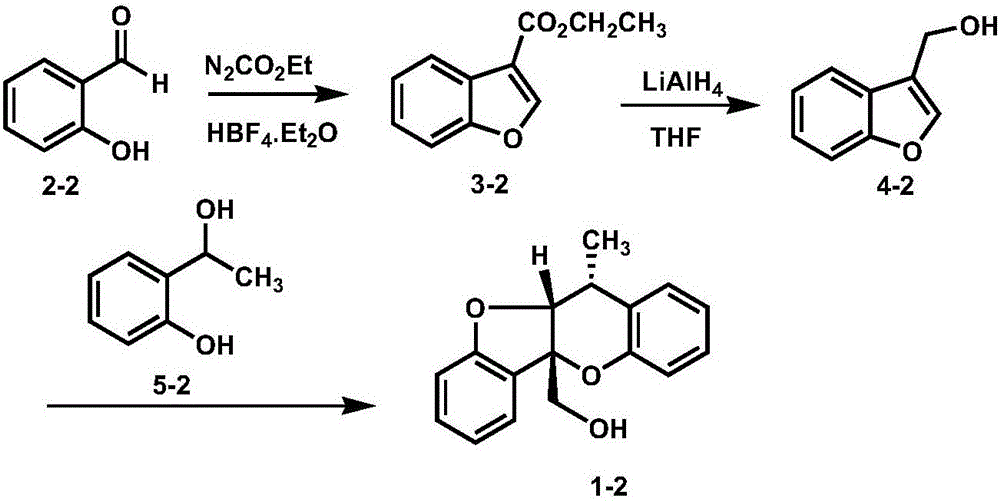
Synthetic method of paeonia veitchii lynch alcohol and structural analogue thereof
InactiveCN105801594ARealized the first chemical synthesisSimple and fast operationOrganic chemistryFuranChemical synthesis
The invention discloses a synthetic method of paeonia veitchii lynch alcohol and a structural analogue thereof. The method comprises the following steps: forming a benzofuran derivative under the catalysis of lewis acid by adopting commercially available or known salicylaldehyde or a derivative thereof and ethyl diazoacetate as raw materials, and performing intermolecular cycloaddition reaction between a reduction product of the benzofuran derivative and a salicyl alcohol compound to obtain a paeonia veichii lynch alcohol natural product or a structural analogue thereof, so that the first chemical synthesis of the paeonia veitchii lynch alcohol and the structural analogue thereof is realized. The gram-scale preparation of the natural product paeonia veitchii lynch alcohol and the structural analogue thereof can be realized only by virtue of three steps of chemical transformation in a whole synthetic route, and the synthetic route has the advantages of simplicity, high efficiency, simplicity in operation, low cost and the like and is suitable for the mass synthesis of the paeonia veitchii lynch alcohol and the structural analogue thereof, so that an important substance foundation is provided for evaluating the biological activity of the natural product paeonia veitchii lynch alcohol and the structural analogue thereof.
Owner:SHAANXI NORMAL UNIV
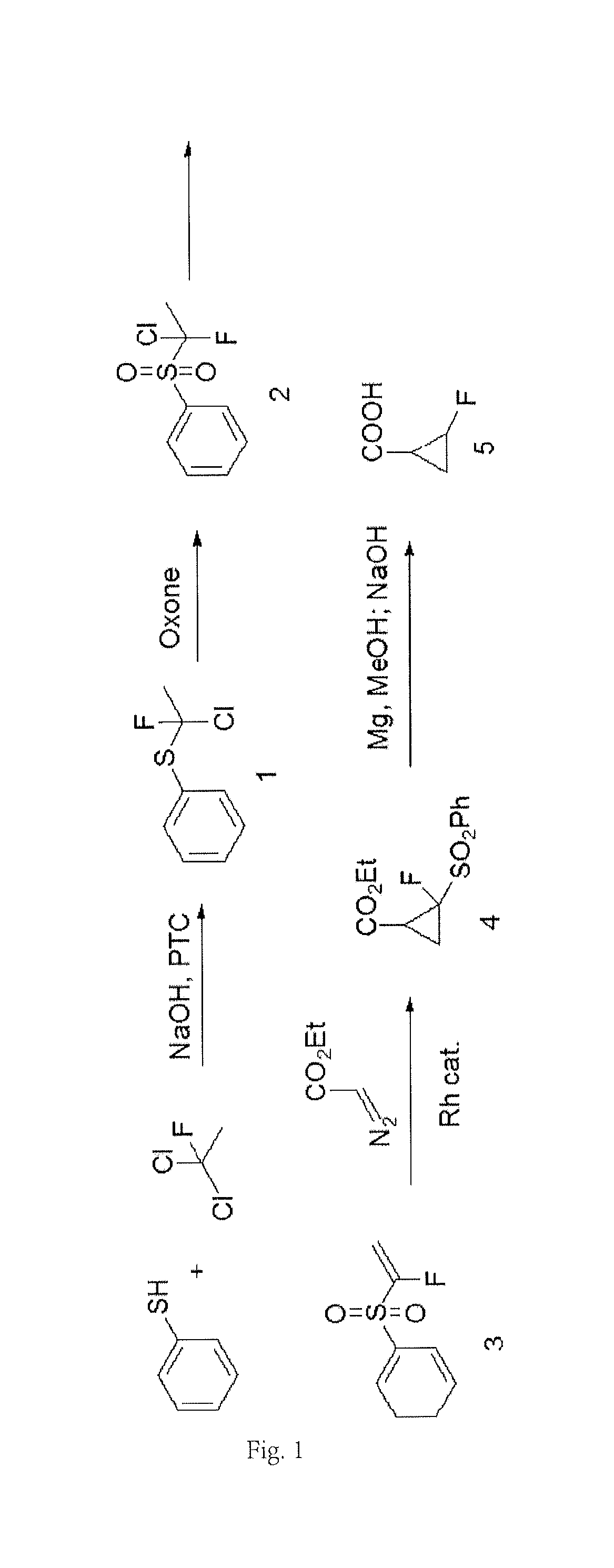
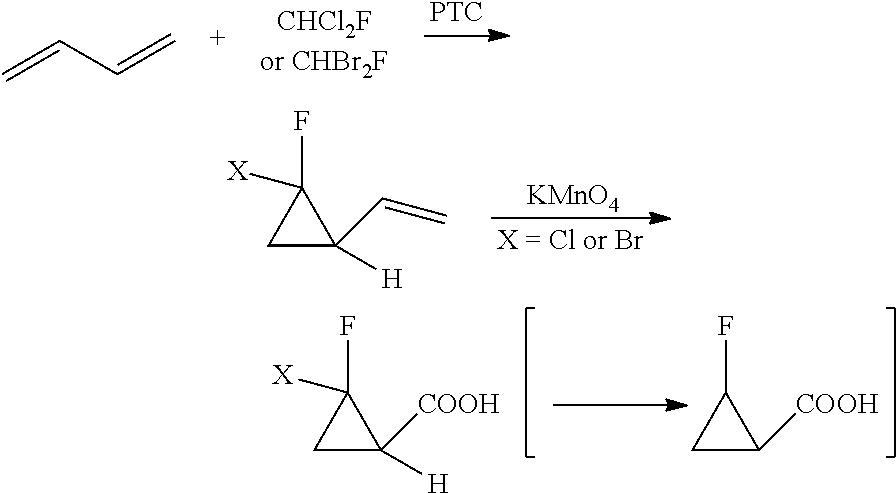
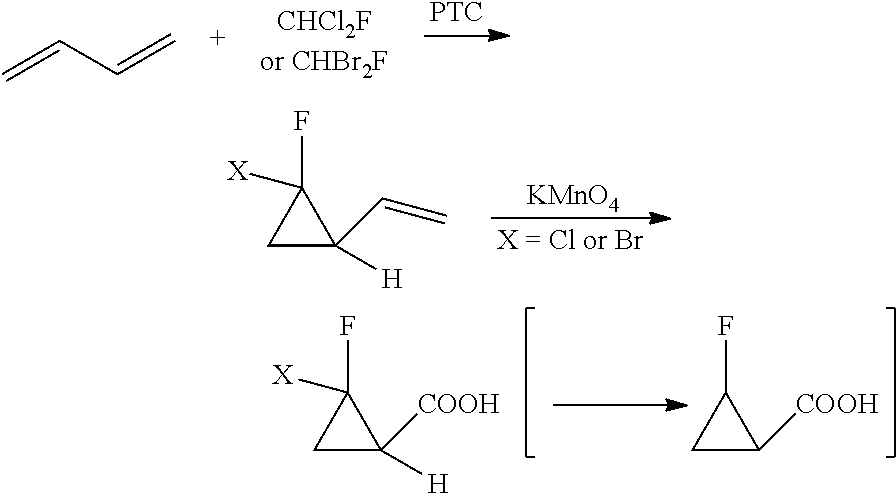
New method for synthesizing 2-fluorocyclopropane carboxylic acid
ActiveUS20180370893A1Safely scaledHigh reaction yieldPreparation from carboxylic acid saltsOrganic compound preparationCarboxylic acidEthyl fumarate
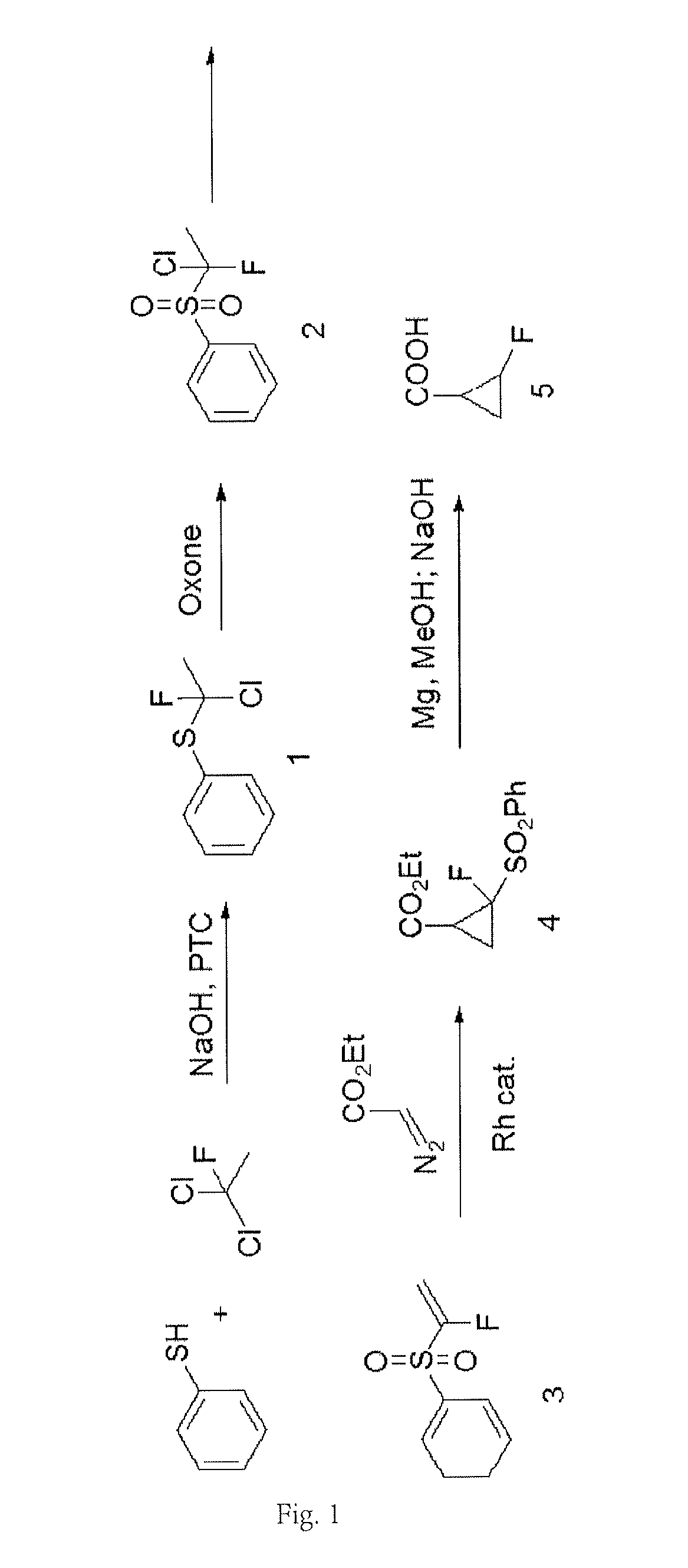
Disclosed is a new method for synthesizing 2-fluorocyclopropanecarboxylic acid comprising: 1) performing reaction of 1,1-dichloro-1-fluoroethane with thiophenol in the presence of an alkali, to produce a phenyl sulfide intermediate; 2) performing oxidation reaction of the phenyl sulfide intermediate with Oxone; 3) performing elimination reaction of the product of Step 2) in the presence of an alkali, to obtain 1-fluoro-1-benzenesulfonyl ethylene; 4) performing addition reaction of the 1-fluoro-benzenesulfonyl ethylene with ethyl diazoacetate in the presence of a catalyst, to obtain a cyclopropane intermediate; 5) performing elimination reaction of the cyclopropane intermediate in the presence of an alkali before acidification, to obtain 2-fluorocyclopropanecarboxylic acid. Herein, the synthetic route is short, used materials are bulk commodities, and raw materials are inexpensive and readily available. The process can be safely scaled up by replacing commonly used mCPBA reagents with Oxone. Further, reaction yield is improved, production cost is greatly reduced, and operation is simplified.
Owner:CHEN STONE GUANGZHOU CO LTD
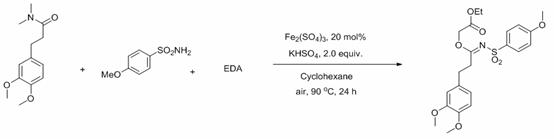
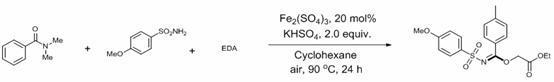
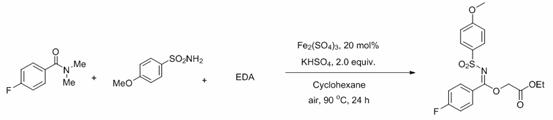
A kind of method for preparing n-sulfonimide
ActiveCN113527154BHigh yieldResponse to the Green EconomySulfonic acid amide preparationIron sulfateHydrogen Sulfate
The invention discloses a method for preparing N-sulfonimide: using ferric sulfate as a catalyst or no catalyst, and potassium hydrogen sulfate as an auxiliary agent to realize the three-component reaction of amide, sulfonamide and ethyl diazoacetate to prepare N ‑Sulfonimide has the following advantages: cheap and green catalyst, more economical reaction, wide substrate versatility, readily available raw materials, can be carried out in the air, and simple post-processing, which is conducive to the synthesis of drug molecules and large-scale industrialization applications in . At the same time, the reactants, catalysts, assistants and the like used in the present invention are cheap and easy to obtain, the reaction composition is reasonable, no ligands and toxic metal catalysts are required, the atom economy is high, the reaction steps are few, and a higher reaction can be obtained only in one step. Yield, in line with the requirements and directions of contemporary green chemistry and medicinal chemistry.
Owner:SUZHOU UNIV
Production method of high-content cyclogalbanate synthetic perfume
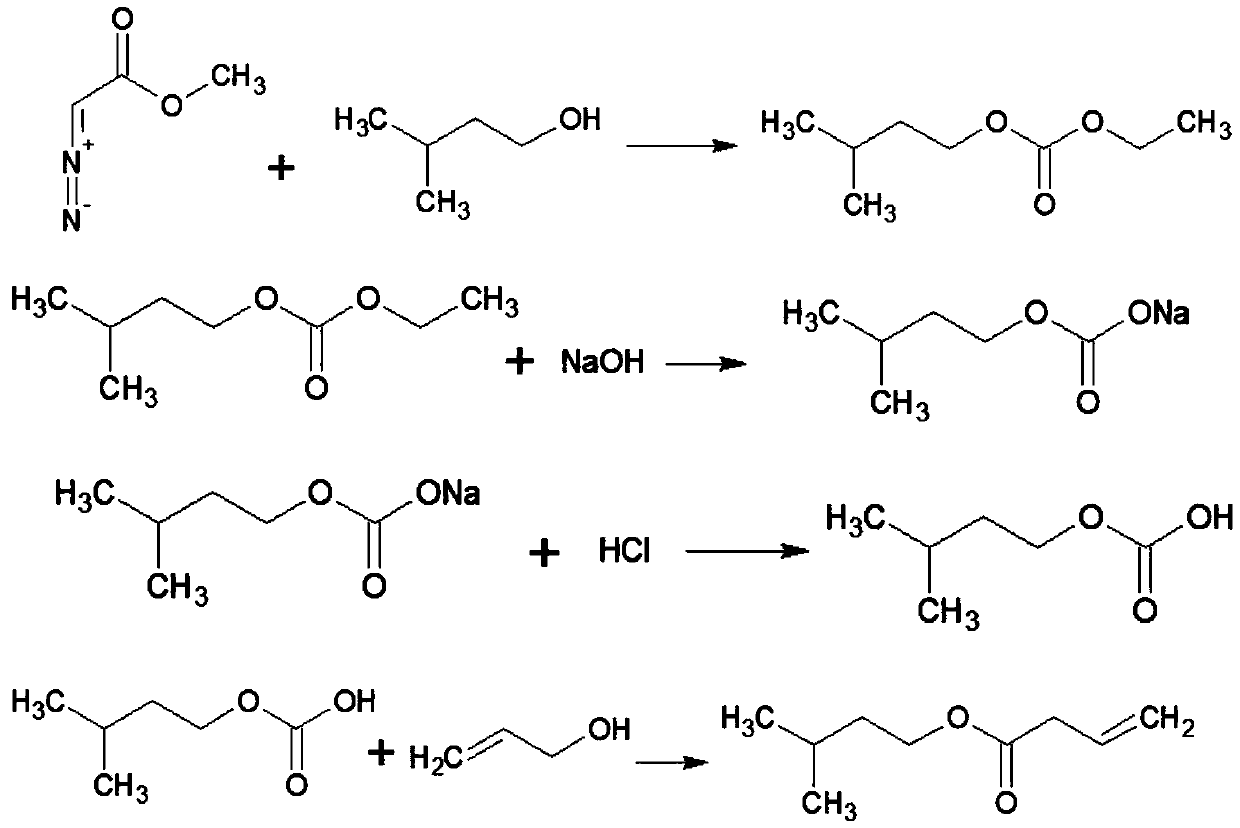
InactiveCN110483287AAvoid slow-onset irritating problemsShort reaction timeOxygen-containing compound preparationOrganic compound preparationSolid acidEthyl acetate
The invention discloses a production method of a high-content cyclogalbanate synthetic perfume, which comprises the following steps: (1) with dichloroethane as a solvent, and ethyl diazoacetate and cyclohexanol as starting raw materials, carrying out O-H insertion reaction to obtain ethyl cyclohexyloxyacetate under protection by nitrogen gas with a solid catalyst, and saponifying and acidifying the reaction product to obtain cyclohexyloxyacetic acid; (2) carrying out esterification reaction on cyclohexyloxyacetic acid and allyl alcohol by taking solid acid as a catalyst, so as to obtain a cyclogalbanate perfume product. According to the method, cyclohexanol and ethyl diazoacetate are taken as initial raw materials, and O-H insertion reaction, saponification, acidification and esterification reaction are performed to produce the high-content cyclogalbanate perfume, so that the reaction time is greatly shortened, the technological process is relatively short, the raw materials are easy to obtain, and the total yield is increased.
Owner:ANHUI HYEA AROMAS
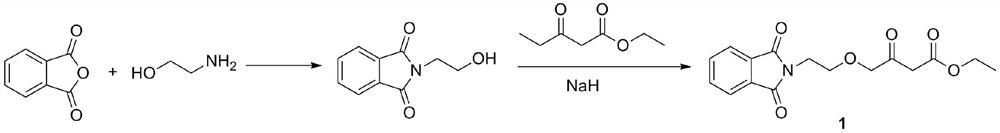
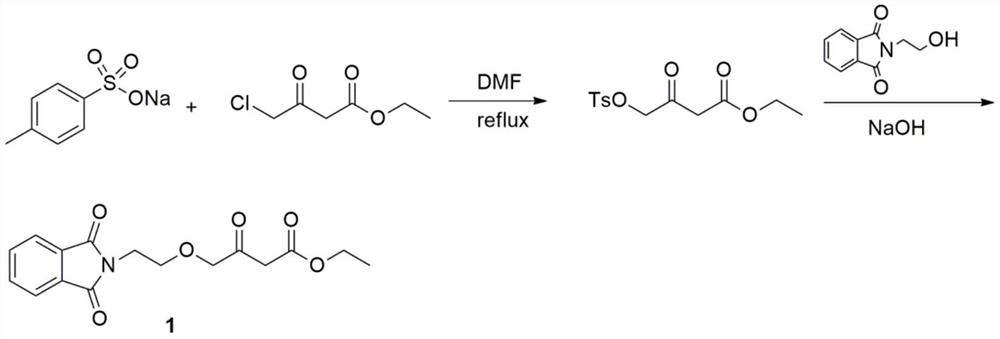
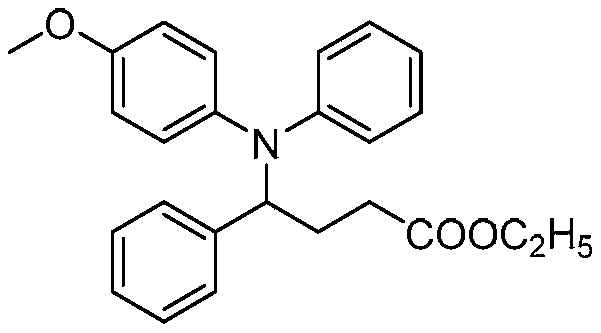
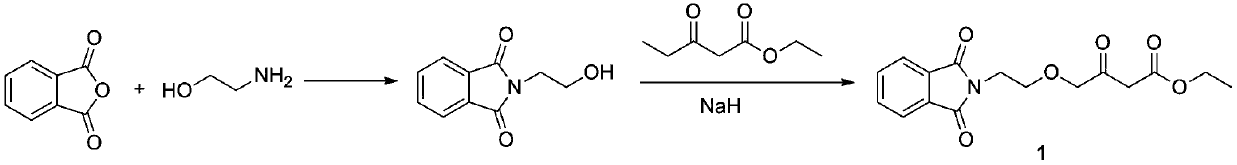
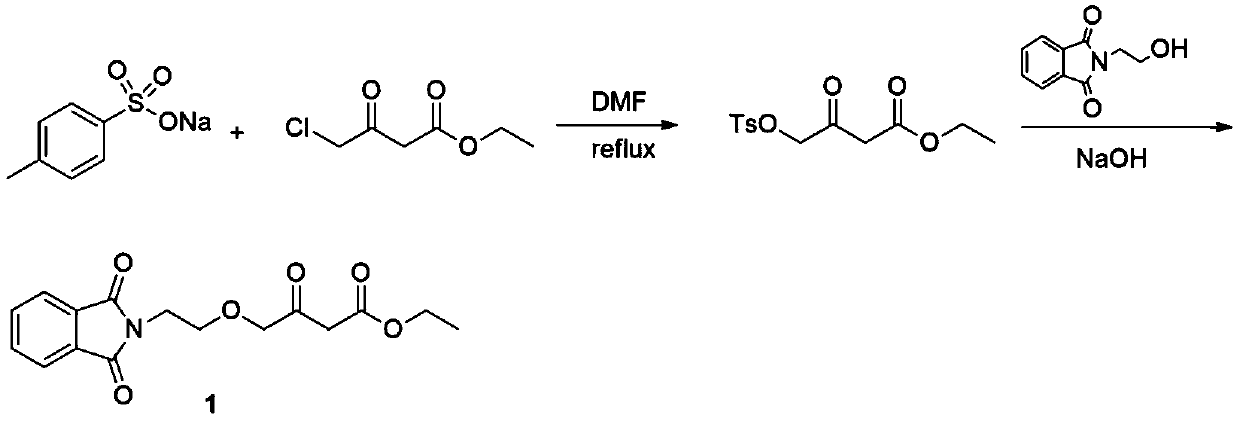
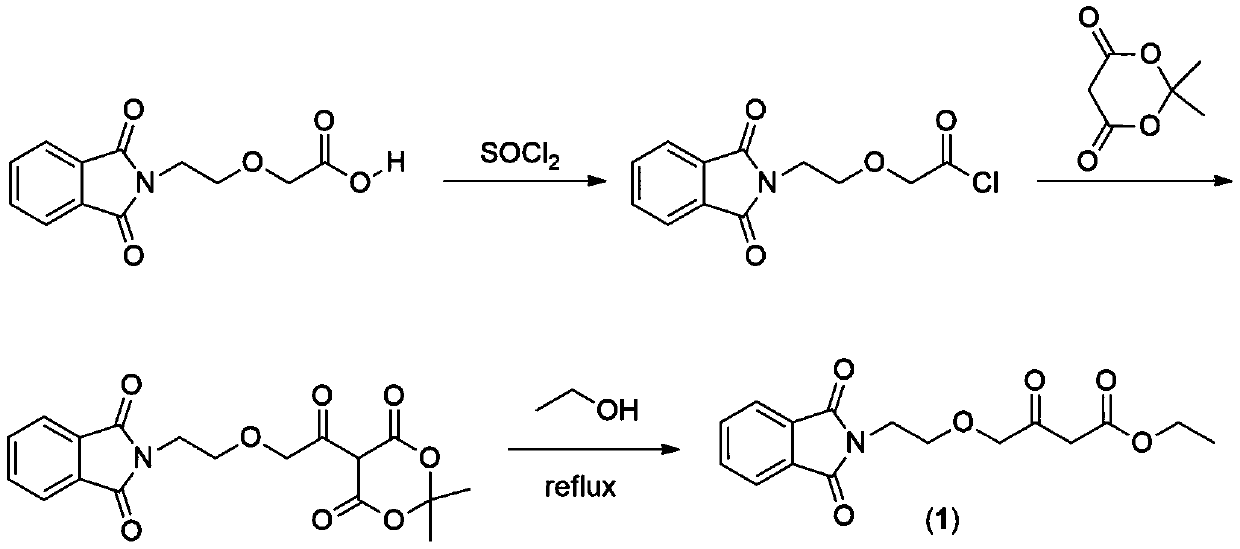
A kind of preparation method of amlodipine key intermediate
The invention discloses a preparation method of a key intermediate of amlodipine, and relates to the field of pharmaceutical synthesis. The specific steps are as follows: compound 3 (2-(2-(2-hydroxyethoxy)ethyl)isoindoline-1,3-dione) is obtained by reacting with DMSO (dimethyl sulfoxide) and oxalyl chloride Compound 2 (2-(2-(1,3-dioxaisoindolin-2-yl)ethoxy)acetaldehyde); then compound 2 and ethyl diazoacetate, catalyzed by Lewis (Lewis) acid A C-H bond insertion reaction occurs under the following conditions to obtain compound 1 ((2-(1,3-dioxaisoindolin-2-yl)ethoxy)-3-oxabutyric acid ethyl ester). The preparation method of the present invention avoids the NaH route commonly used in current production, the total yield is equivalent to the NaH route, and the raw materials are cheaper and easier to obtain, thereby greatly improving the safety and production efficiency, and reducing the total cost by about 30%. Suitable for industrial production.
Owner:CHANGZHOU UNIV
A method for preparing 4-aminobutyrate derivatives
ActiveCN107892655BMild reaction conditionsEasy to operateOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisEvaporation
The invention discloses a method for preparing 4-aminobutyrate derivatives, and relates to a method for preparing the 4-aminobutyrate derivatives. The purpose of the invention is to solve the problemsof complicated steps, a low yield, and environmental pollution in the synthesis of the 4-aminobutyrate derivatives by using conventional methods. The method comprises the following steps: a diarylamine derivative, an olefin derivative and ethyl diazoacetate are dissolved into an organic solvent under a nitrogen atmosphere and room temperature, then a photocatalyst is added, mixing is performed uniformly, the mixed material is placed below a blue LED lamp, an illumination reaction is performed, rotary evaporation is performed to remove the solvent, chromatography separation purification is performed by using a silica gel column, and therefore the product, one 4-aminobutyrate derivative, is obtained. The reaction in the method provided by the invention can be performed under a normal temperature and normal pressure, the reaction conditions are mild, the yield can reach 80%, and the method has the advantages of simple operation, no pollution, safety, environmental friendliness and low costs, and is applied to the field of organic synthesis.
Owner:HARBIN INST OF TECH
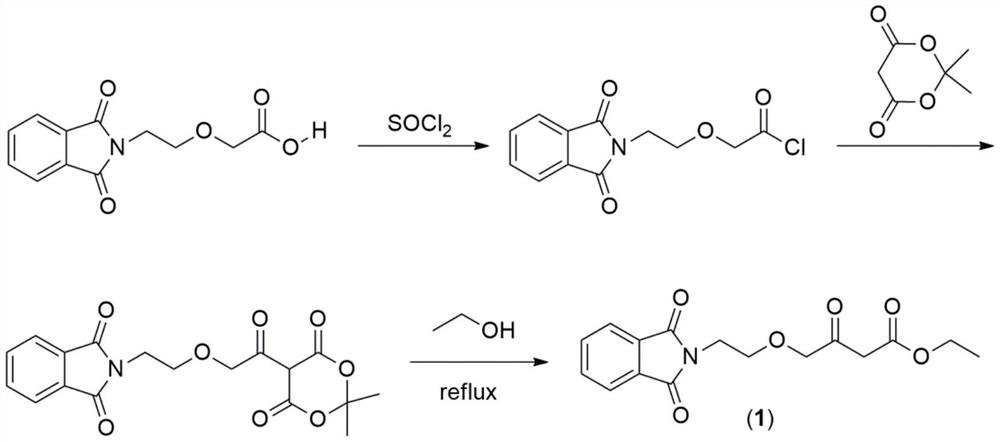
Preparation method of amlodipine key intermediate
ActiveCN111303006AShort synthetic routeImprove securityOrganic chemistryEthyl groupLewis acid catalysis
The invention discloses a preparation method of an amlodipine key intermediate, which relates to the field of drug synthesis. The preparation method comprises the following specific steps of reactinga compound 3 (2-(2-(2-hydroxyethoxy) ethyl) isoindoline-1, 3-diketone) with DMSO (dimethyl sulfoxide) and oxalyl chloride to obtain a compound 2 (2-(2-(1, 3-dioxaisoindoline-2-yl) ethoxy) acetaldehyde), then, enabling the compound 2 and ethyl diazoacetate to be subjected to a C-H bond insertion reaction under the catalysis of Lewis (Lewis) acid, and acquiring a compound 1 ((2-(1, 3-dioxaisoindoline-2-yl) ethoxy)-3-oxabutyrate). According to the preparation method, a NaH route commonly adopted in current production is avoided, the total yield is equivalent to that of the NaH route, and the rawmaterials are cheaper and easier to obtain, so that the safety and the production efficiency are greatly improved, the total cost is reduced by about 30%, and the preparation method is suitable for industrial production.
Owner:CHANGZHOU UNIV
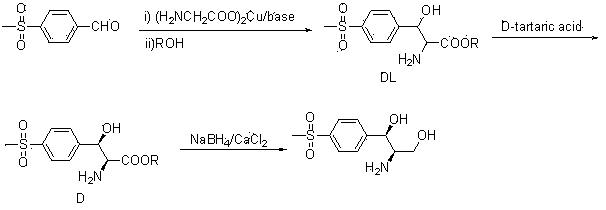
Method for synthesizing (1R, 2R)-1-p-methyl sulfone phenyl-2-amino-1,3-propanediol
InactiveCN102010355BEase of industrial productionOrganic chemistryOrganic compound preparationPtru catalystEthyl acetate
The invention belongs to the technical field of chemical medicines, and specifically relates to a synthesis method of (1R,2R)-1-p-thiamphenylphenyl-2-amino-1,3-propanediol (I). The method comprises: 1. Compound (III) is prepared by condensing p-thymphenylbenzaldehyde (II) with primary amine. 2. Under the action of a catalyst, compound (III) undergoes acridinization reaction with ethyl diazoacetate to generate compound (IV). 3. Compound (IV) is reduced to obtain compound (V). 4. Under the action of acid, compound (V) undergoes a one-pot ring-opening reaction and deprotection reaction to obtain (1R,2R)-1-p-thiamphenylphenyl-2-amino-1,3-propanediol ( I). The method of the invention has the advantages of novel design, short route, mild conditions, simple operation, good enantioselectivity, high yield and good industrialized production prospect.
Owner:FUDAN UNIV
Production method for catalytically synthesizing galbanate spice by using solid acid
InactiveCN110483288AAvoid slow-onset irritating problemsAvoid irritationOxygen-containing compound preparationOrganic compound preparationSolid acidNitrogen gas
The invention discloses a production method catalytically synthesizing galbanate spice by using solid acid. The production method comprises steps of: (1) with dichloroethane as a solvent and ethyl diazoacetate and isoamyl alcohol as initial raw materials, carrying out O-H insertion reaction under a solid catalyst under protection by nitrogen gas to obtain ethyl isopentoxyacetate, saponifying and acidifying the reaction product to obtain isopentoxyacetic acid; (2) carrying out condensation reaction on isopentoxyl acetic acid and allyl alcohol by taking solid acid as a catalyst to obtain a galbanate spice product. According to the method, isoamyl alcohol and ethyl diazoacetate are used as initial raw materials, and O-H insertion reaction and condensation reaction are performed to produce thegalbanate spice, so that the reaction time is greatly shortened, the technological process is relatively short, the raw materials are easy to obtain, and the total yield is increased; solid-liquid separation of the solid acid catalyst is realized by adopting an automatic backwashing filtering technology, the method has the advantages of no corrosion to equipment and simple post-treatment, and theproblems that the equipment is corroded by the existing liquid acid and the acid-containing wastewater pollutes the environment are solved.
Owner:ANHUI HYEA AROMAS
Production device and method for catalytically synthesizing allyl amyl glycolate perfume with solid acid
InactiveCN110563577ASimple post-processingReduce generationPreparation from carboxylic acid saltsOrganic compound preparationFiltrationSolid acid
The invention discloses a production device and method for catalytically synthesizing an allyl amyl glycolate perfume with solid acid. The production method comprises the following steps of by takingdichloroethane as a solvent, under the nitrogen protection condition, performing O-H insertion reaction to obtain isopentyloxy ethyl acetate by adopting a solid rhodium catalyst, ethyl diazoacetate and isoamyl alcohol as starting raw materials, and then performing saponification and acidification to obtain isoamylacetic acid; and then by taking the solid acid as a catalyst, performing condensationreaction on the isoamylacetic acid and allyl alcohol to obtain an allyl amyl glycolate perfume product. By adopting the production device and method, the reaction time is greatly shortened, the process flow is relatively short, the raw materials are available, and the total yield is increased; and for a solid catalyst, solid-liquid separation of the solid acid catalyst is realized by adopting anautomatic backwashing filtration technology, the solid acid catalyst has the advantages of easiness in separation with a liquid phase reaction system, non corrosion to equipment and simpleness in post-treatment, overcomes the problems that existing liquid acid corrodes the equipment, and acid-contained wastewater pollutes environment and is high in selectivity, and solid-liquid separation can be performed at a lower temperature.
Owner:ANHUI HYEA AROMAS
Method for synthesizing 2-fluorocyclopropane carboxylic acid
ActiveUS10385000B2Safely scaledPreparation from carboxylic acid saltsOrganic compound preparationCarboxylic acidElimination reaction
Owner:CHEN STONE GUANGZHOU CO LTD
A method for preparing trans-D-chrysanthemic acid
ActiveCN110467527BStarting materials are cheap and readily availableEasy to synthesizeOrganic compound preparationOrganic chemistry methodsPtru catalystOrganic synthesis
The invention provides a method for preparing trans-D-chrysanthemic acid, which belongs to the field of organic synthesis. The method of the present invention is that 2-methyl-5,5,5-trichloro-2-pentene and ethyl diazoacetate are synthesized by an asymmetric cyclopropanation reaction to trans-derothrin, and then hydrolyzed to generate trans- D-V-chrysanthemic acid (DV-chrysanthemic acid). The chiral ruthenium catalyst used is generated in situ by ruthenium salts and chiral tridentate P,N,N-ligands in various polar and non-polar solvents. The present invention can conveniently synthesize trans D-chrysanthemic acid (DV-chrysanthemic acid), and its enantiomeric excess percentage is as high as 90%. The invention has the characteristics of simple operation, readily available raw materials, wide application range of substrates, high enantioselectivity and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
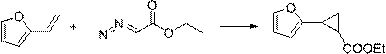
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000021.png)
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000031.png)
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000041.png)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka.patsnap.com/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00011.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka.patsnap.com/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00021.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka.patsnap.com/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00022.PNG)