Patents
Literature
46results about How to "Solve yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing-ester by using solid chlorine substitution of phosgene
ActiveCN1660868AHigh selectivityHigh activityEsterified saccharide compoundsSugar derivativesSucroseEthyl acetate
A process for preparing trichlorosucrose-6-ester by solid-state phosgene chlorinating includes dissolving solid phosgene in inertial solvent, slowly dropping it in the solution of sucrose-6-ester in dimethyl formamide, stirring, heating while reaction, alkali solution neutralizing, distilling to remove solvent, dissolving in water, decoloring by activated carbon, extracting in organic solvent and crystallizing.
Owner:JK SUCRALOSE
Preparation method of boron nitride nano-tube
The invention relates to a preparation method of a boron nitride nano-tube, comprising the steps of: leading inorganic porous ceramic material and ammonia to have ammoniation reaction for 5-24h at 800-1200 DEG C, and obtaining crude product of the boron nitride nano-tube, wherein the element mole ratio in the inorganic porous ceramic material is Mg: Fe: B: O= 1: (0.10-1.65): (0.33-1.95): (0.5-5); and separating and purifying the obtained crude product, and obtaining the boron nitride nano-tube. The preparation method has the advantages that (1) batch preparation of the boron nitride nano-tube product can be realized, the yield is more than 80%, and the purity is about 85%; the preparation technique is simple, the power consumption is lower, and the method is suitable for industrial batch production; and (2) the adopted inorganic porous ceramic material has good mechanical strength at higher temperature and can be taken as filler, and compared with other powder or block raw material, the inorganic porous ceramic material is more beneficial to preparing the boron nitride nano-tube by gas-solid phase reaction.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of parthenolide
The invention relates to an extraction and preparation method of parthenolide, and particulary relates to an extraction method of parthenolide from Magnolia grandiflora root bark. The extraction and preparation method comprises: crushing Magnolia grandiflora root bark as a raw material, extracting with an appropriate polar organic solvent to obtain total lactones, separating the total lactones by column chromatography, collecting the required ingredients, and then crystallizing the collected ingredients with an appropriate solvent to obtain parthenolide. The extraction method of parthenolide from Magnolia grandiflora root bark provided by the invention is suitable for industrial production.
Owner:新沂尚德药缘药业有限公司 +1
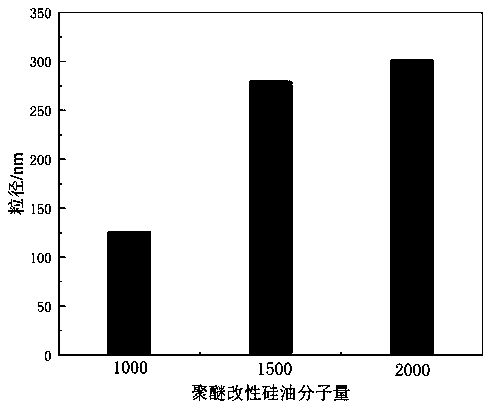
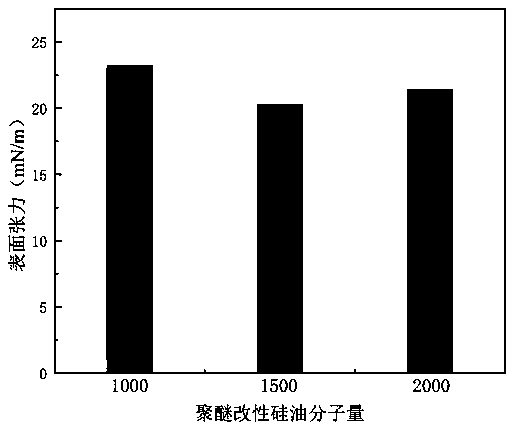
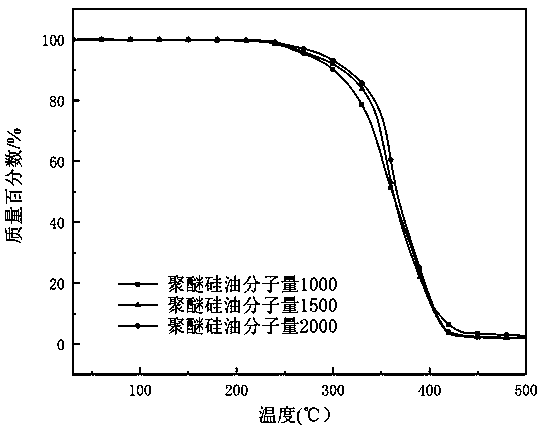
Preparation method of isocyanate modified polyether silicone oil nonionic emulsion and product and application thereof
ActiveCN110042667ASolve yieldSolve problems such as purification difficultiesCarbon fibresFiberCarbon fibers
The invention provides a preparation method of an isocyanate modified polyether silicone oil nonionic emulsion and a product and application thereof. The method comprises the steps of synthesizing double-end containing hydrogen silicone oil, synthesizing double-end hydroxyl modified silicone oil, synthesizing isocyanate modified polyether silicone oil, adding a proper amount of water for emulsifying, performing co-emulsifying with cationic amino modified epoxy silicone oil, and performing compounding to prepare a carbon fiber precursor oil agent. The technical problems met during an addition reaction of polyether modified silicone oil and high molecular weight polyethylene glycol monoallyl ether through containing hydrogen silicone oil that the reaction is difficult, the yield is low, theseparation purification is hard, and the color is dark are solved, and meanwhile the problems that the storage stability of amino modified silicone oil in the weak acid water phase is poor, compounding with cationic amino modified epoxy silicone oil is performed to prepare the carbon fiber precursor oil agent, after oiling, the carbon fiber precursor is stuck to rollers in the high-temperature drying and rolling process are solved.
Owner:吉林乾仁新材料有限公司
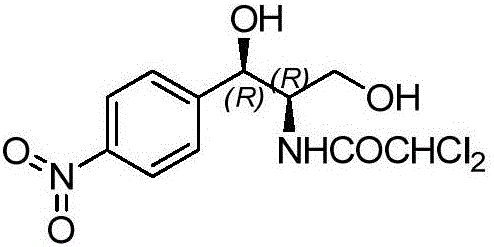
Preparation method of chloramphenicol compounds
The invention provides a preparation method of chloramphenicol compounds. The preparation method comprises the following steps: adding a chloramphenicol intermediate product to a buffer solution; then, adding ketoreductase to the solution so as to obtain a mixed solution; and conducting a reaction on the mixed solution at 25-35 DEG C, so that the chloramphenicol compounds are obtained. The preparation method of the chloramphenicol compounds provided by the invention not only reduces raw material cost but also improves economy of raw materials and improves yield; and the problems of the prior art that isomer by-products, obtained from the preparation of chloramphenicol, cannot be used, raw material cost is high and yield is low are solved.
Owner:SUZHOU LEAD BIOTECH CO LTD
Preparation method of gold nucleus-quantum dot satellite structure assembly
ActiveCN105036073ASolve assembly difficultiesSolve yieldNanostructure manufactureMicrobiological testing/measurementQuantum dotBiology
The invention discloses a preparation method of a gold nucleus-quantum dot satellite structure assembly, belonging to the technical field of analytical chemistry. A high-yield assembly method of a gold nucleus-quantum dot satellite structure comprises the following steps of synthesis of gold nano particles with the particle size of 35nm, preparation of quantum dots with the particle size of 7nm, DNA-1 modification on the gold nano particles, complementary sequence DNA-2 modification on the quantum dots, assembling of the gold nucleus-quantum dot satellite structure, and purification of the gold nucleus-quantum dot satellite structure. By adopting the preparation method, the problems that quantum dot assembling is difficult and the yield is low are solved, and a way is paved for developing a fluorescence sensor based on quantum dot assembling.
Owner:JIANGNAN UNIV
Method for extracting fulvic acid from lignite
The invention discloses a method for extracting fulvic acid from the lignite and belongs to the method for extracting the fulvic acid. The method comprises the following steps: 1, weathering the lignite crushed to 80-200 mesh in the sunlight; wherein the weathering illumination temperature is 15-30 DEG C, the weathering effective illumination time is 140-210 hours, producing the lignite sample; 2, adding the hydrogen peroxide with the mass concentration of 30% in the lignite sample processed in step 1 according to the mass ratio of the lignite to the hydrogen peroxide of (1:1.5)-(1:2.5) to obtain the mixtures of the lignite sample and the hydrogen peroxide, performing microwave oxidation degradation on the mixtures at 40-60 DEG C for 10-30 minutes; 3, adding distilled water in the oxidation-degraded mixtures, wherein the mass ratio of the distilled water to the lignite sample is 5:1; performing centrifugal separation, filtering the supernatant, evaporating by a rotary evaporator, performing vacuum drying to obtain the fulvic acid solid. The method has the advantages of green and environmentally friendly, economical and effective, is high in productivity and extraction efficiency of the fulvic acid, simple in process, low in cost and little in environment pollution.
Owner:CHINA UNIV OF MINING & TECH
Method for preparing (S)- or (R)-2-aminobutanamide through transaminase biocatalysis
ActiveCN106834372AHigh optical purityReduce manufacturing costFermentationHigh selectivityRaw material
The invention discloses a method for preparing (S)- or (R)-2-aminobutanamide through transaminase biocatalysis. The method comprises the following steps: taking transaminase as a biocatalyst and catalyzing carbonyl on 2-carbonylbutylamide into amino in the presence of an amino donor, thereby obtaining the (S)- or (R)-2-aminobutanamide. The used transaminase refers to any one of Cv-omega-TA WT, Cv-omega-TA W60C, Vf-omega-TA, ATA-117 and ATA S9. According to the method disclosed by the invention, the (S)- or (R)-2-aminobutanamide is prepared by taking the 2-carbonylbutylamide as a raw material through a biocatalysis method. The transaminase is used for catalysis of carbonyl amide substances first, the transaminase with high efficiency and high selectivity can be successfully screened, the reaction selectivity is high, the product yield is 85% or higher, the ee value of the product is more than 99.5% or higher, and the problems in the prior art that the product yield is low and the ee value is not high are completely solved.
Owner:HAINAN UNIVERSITY
Method for microwave-assisted fast and efficient preparation of phenethyl caffeate
ActiveCN104262156ASafe preparationEfficient preparationOrganic compound preparationCarboxylic acid esters preparationCaffeic acidPhenethyl alcohol
The invention relates to a preparation method of phenethyl caffeate. The preparation method is characterized by comprising the following steps: 1) putting reaction raw materials, namely adding caffeic acid, phenethyl alcohol, a reaction solvent and a molecular sieve into a reaction kettle, and uniformly stirring at normal temperatures to obtain a pre-mixture; introducing nitrogen to remove oxygen for 5 minutes to obtain the deoxidized pre-mixture, wherein the molar ratio of caffeic acid and phenethyl alcohol is (1:1)-(1:20), the mass ratio of caffeic acid and the reaction solvent is (1:1)-(1:20), and the addition amount of the molecular sieve is 50-80g / L; 2) carrying out microwave-assisted transesterification, namely adding 100-200ppm antioxidant into the deoxidized pre-mixture, controlling the temperature at 120-140 DEG C, stirring at an ordinary pressure for reaction for 5-20 minutes under a microwave-assisted condition, stopping microwave heating, and naturally cooling to 40 DEG C; and 3) carrying out post-treatment of a product to obtain phenethyl caffeate. The method has the characteristics of rapid reaction, simplicity in operation and mild reaction condition, the utilization ratio of raw materials can be effectively improved, and the prepared product phenethyl caffeate is high in purity and yield.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
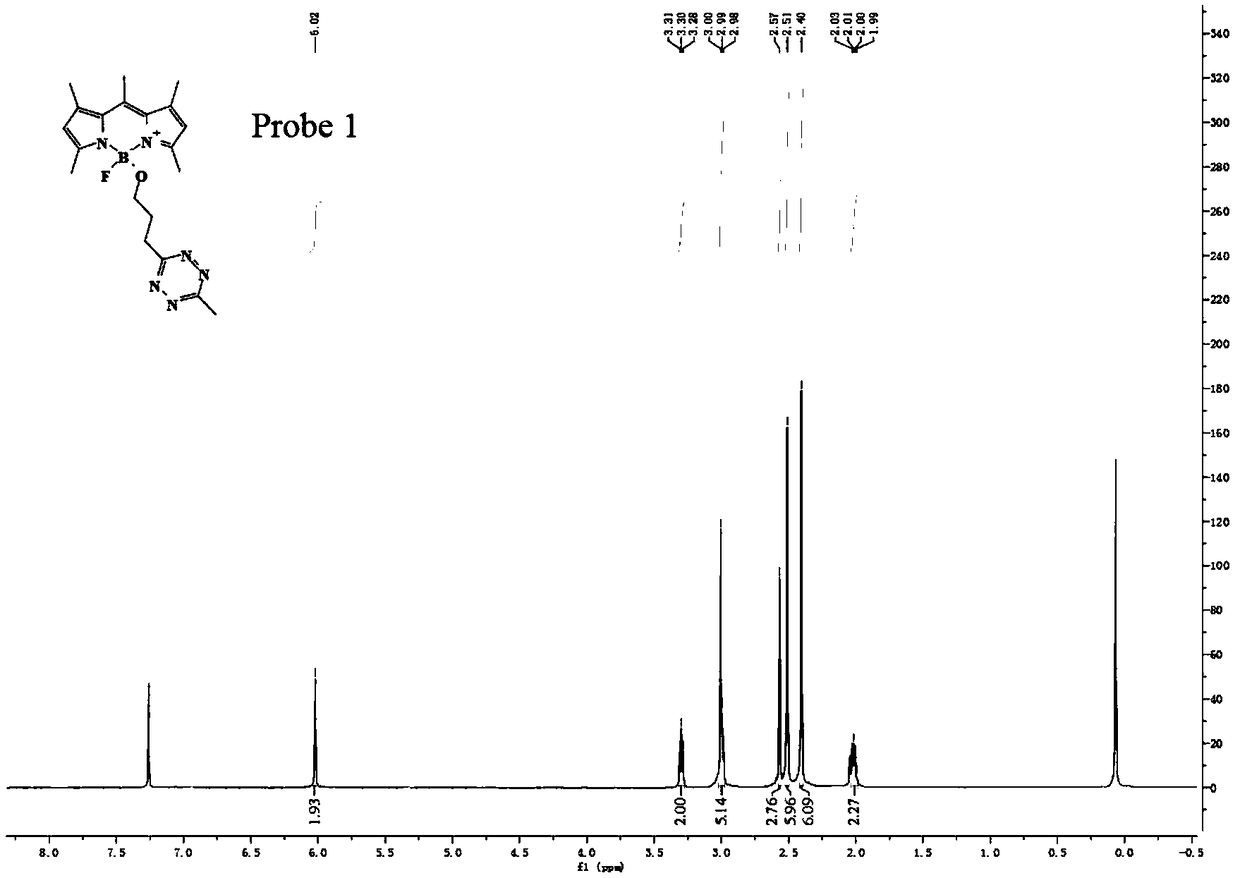
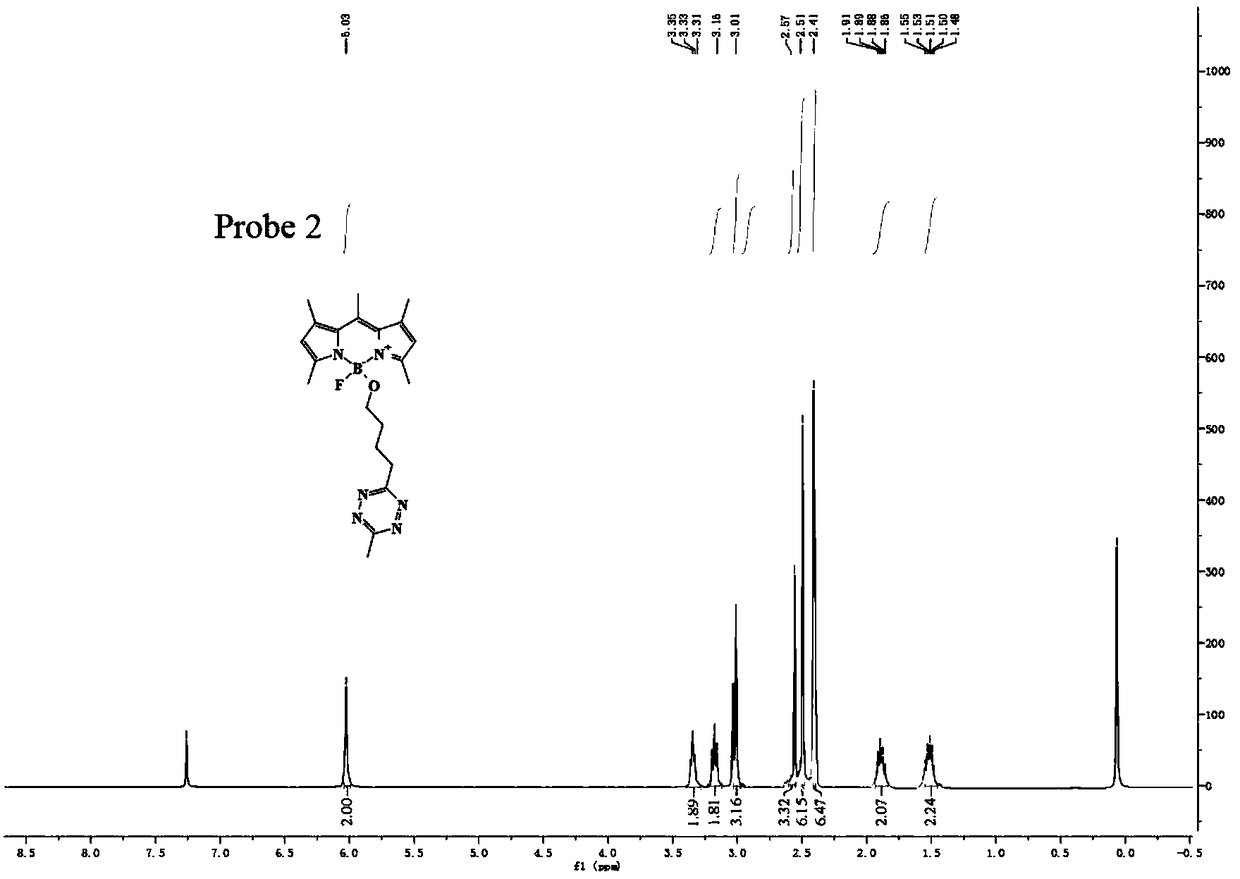
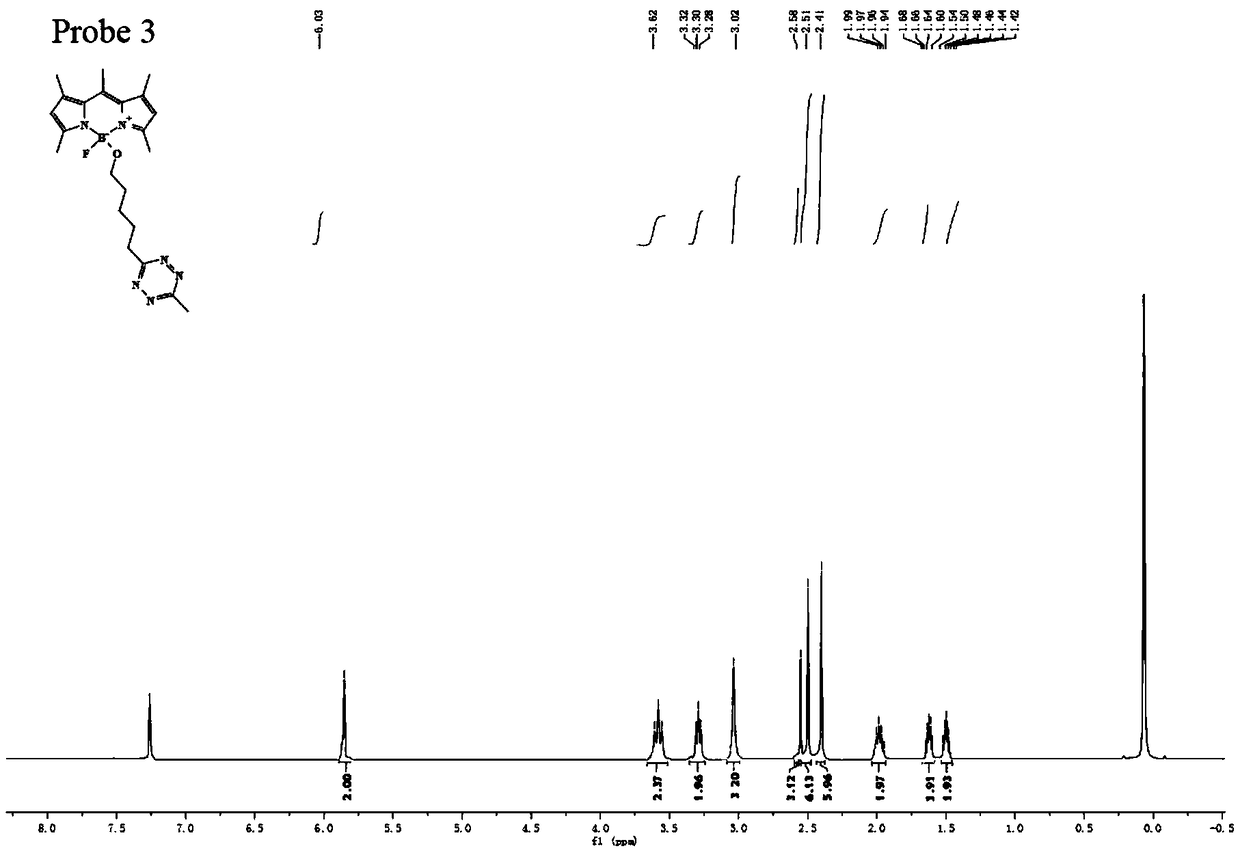
Preparation method of BODIPY-tetrazine bio-orthogonal probe
InactiveCN108997402AHigh priceSolve complexityGroup 3/13 element organic compoundsFluorescence/phosphorescenceSolubilityOrganic layer
The invention discloses a BODIPY-tetrazine bio-orthogonal probe and a preparation method thereof. The preparation method comprises the steps: 1) dissolving BODIPY1-2 and TMSOTf in an anhydrous acetonitrile-dichloromethane mixed solvent, mixing evenly, stirring the reaction solution for 30 min under a condition of the temperature of 0 DEG C, and activating BODIPY1-2; 2) adding tert-butanol quenchedTMSOTf to the reaction solution, then dropwise adding 2,6-dimethylpyridine and a tetrazine Tza-Tzg pre-mixed acetonitrile solution, and carrying out a stirring reaction of the mixed solution under acondition of the temperature of 0 DEG C for 10 min; and 3) quenching the reaction in the step 2 by water, then extracting by DCM, separating an organic layer, drying the organic phase by Na2SO4, concentrating in vacuum, carrying out separated purification of the reaction product by a silica gel column, and thus obtaining the BODIPY-tetrazine bio-orthogonal fluorescent probe. The BODIPY-tetrazine bio-orthogonal probe is synthesized by the reaction of the BODIPY compound with the tetrazines. The yield in the synthesis process is greatly increased, the water solubility of the probe is greatly improved, the probe has the fluorescent opening properties, and the improvements are conducive to an application of the probe in cell imaging.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Preparing method for ginsenoside Rh2
InactiveCN105603036ADifficult to extractReduce pollutionMicroorganism based processesFermentationGinsenoside Rh2Therapeutic effect
The invention relates to the technical field of medicine and discloses a preparing method for ginsenoside Rh2. The preparing method comprises the steps of strain culture, raw material selecting, biological fermentation and conversion, filter pressing, sterilization and drying, and thus a product is obtained. Ginsenoside Rh2 extract prepared through the preparing method is high in treatment effect and purity, good in absorption and stable in quality; meanwhile, the preparing method is good in separation effect, high in content, simple in process, convenient to implement, low in cost and suitable for industrial production.
Owner:杭州双马生物科技股份有限公司
Synthetic method of saccharide-containing copolymer with biological specific recognition performance
InactiveCN110283286ASolve protection problemsSolve yieldColor/spectral properties measurementsSynthesis methodsSolvent
The invention relates to a synthetic method of a saccharide-containing copolymer with biological specific recognition performance. The synthetic method comprises following steps: in an inert atmosphere, alpha-D-mannose monomer M1 and Grubbs third-generation catalyst are reacted in a solvent; beta-D-galactose monomer M2 is added for reaction; ethyl vinyl ether is added to stop polymerization, and post-treatment is carried out so as to obtain a segmented copolymer containing alpha-D-mannose and beta-D-galactose. Compared with the prior art, the polymer monomer synthetic method is simple and reliable; the obtained polymer structure is linear; the molecular weight is controllable; and the synthetic method can be applied in the fields such as high molecular material and biological medicine engineering.
Owner:SHANGHAI INST OF TECH
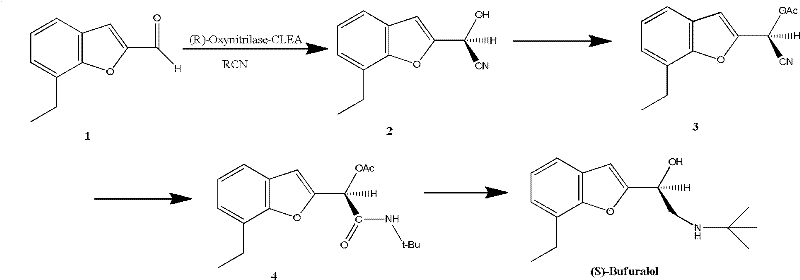
Method for preparing optically active (S)-bufuralol by enzyme catalysis
InactiveCN102649973AHigh optical purityHigh yieldFermentationCross-linked enzyme aggregateReaction step
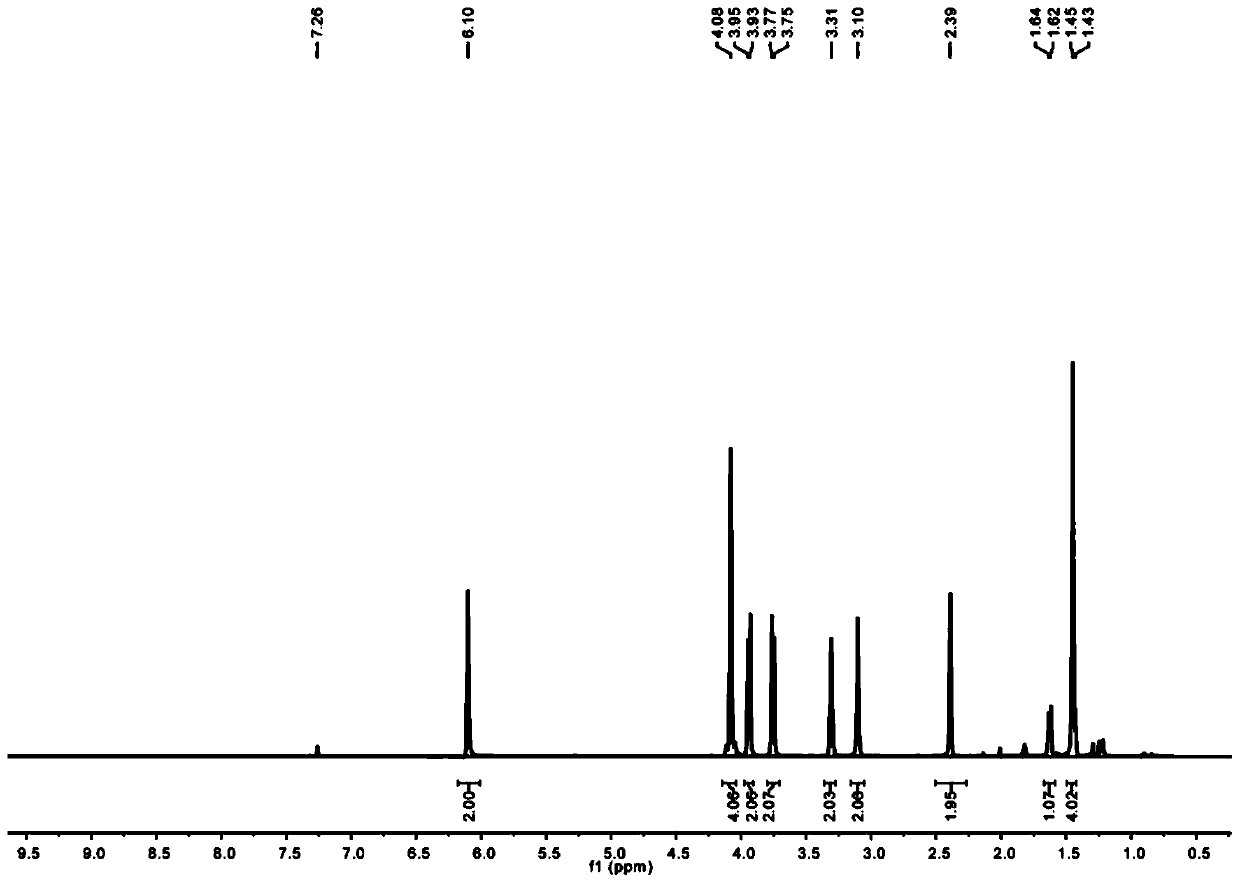
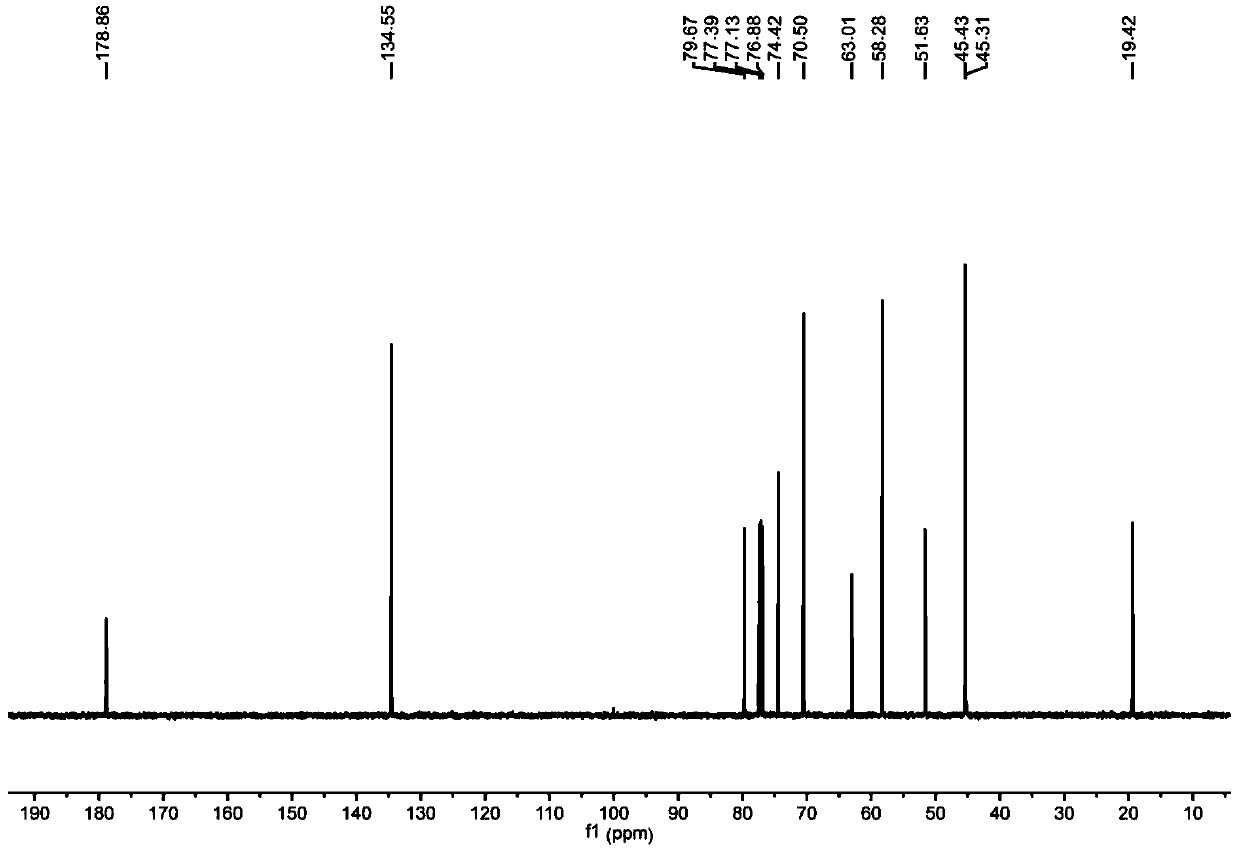
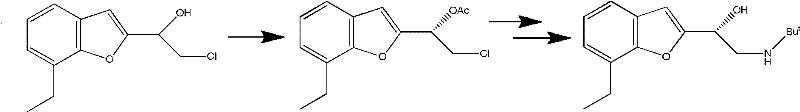
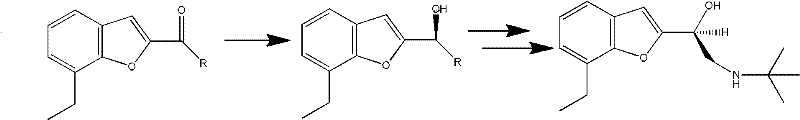
The invention provides a method for preparing optically active (S)-bufuralol by enzyme catalysis. By taking 7-ethylbenzofuran-2-formaldehyde as a raw material, the (S)-bufuralol is obtained through the catalysis asymmetric cyanation of cross-linked enzyme aggregates (CLEA for short) of (R)-hydroxynitrile lyase [(R)-Oxynitrilase], hydroxyl protection, Ritter reaction and amide reduction reaction. The preparation method for the (S)-bufuralol has the advantages that the reaction steps are simple, the reaction condition is mild, the yield is high and the optical purity of the obtained (S)-bufuralol is high.
Owner:NANJING UNIV OF TECH
Ultra-fine boron carbide polycrystalline powder prepared through organic boron-containing precursor self-propagating method
The invention relates to ultra-fine boron carbide polycrystalline powder prepared through an organic boron-containing precursor self-propagating method. In the method, the raw materials, namely, PVA- boric acid precursor, boron anhydrous and metal magnesium powder are mixed according to following mass ratio: PVA-boric acid precursor: B2O3: Mg = 100: (350 - 360): (380 - 420); through the self-propagating reaction, the obtained product is stirred and soaked in concentrated hydrochloric acid, and is leached, a filter cake is washed to neutrality with distilled water, and the washed filter cake is dried, the ultra-fine boron carbide polycrystalline powder is obtained. The invention has the advantages that firstly, the problem of small yield, low yield, and low purity of the ultra-fine boron carbide powder is solved , and the preparation process is simple; secondly, the prepared boron carbide is boron-rich type boron carbide B13C2, and has important application value; thirdly, the yield rate of the boron carbide is more than or equal to 95 percent, the free boron content is lower than or equal to 0.65 percent, and the free C content is lower than or equal to 2.65 percent.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
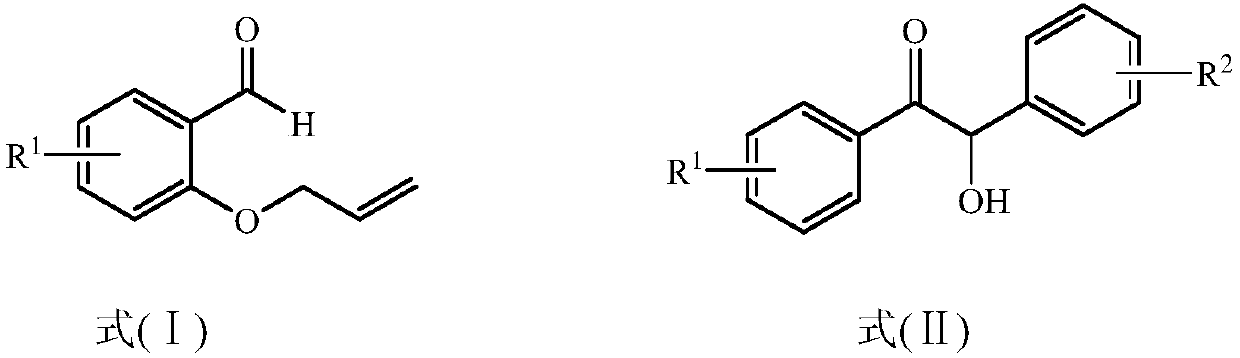
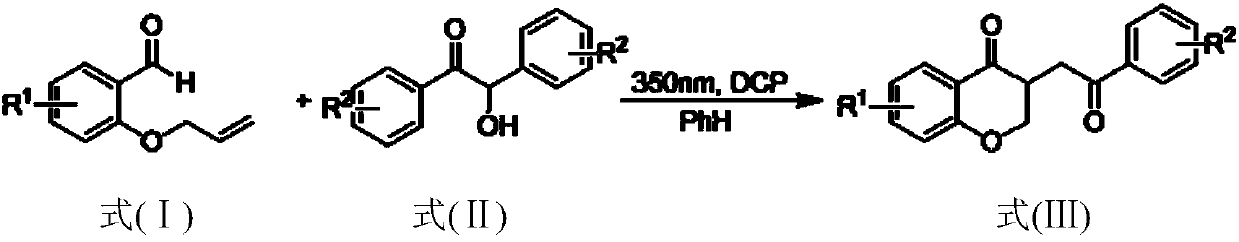
Method for preparing 2,3-dihydrobenzopyran-4-one derivative
ActiveCN107586285ASolve the cumbersome synthesis stepsSolve yieldOrganic chemistryEnvironmental resistanceSynthesis methods
The present invention provides a method for preparing a 2,3-dihydrobenzopyran-4-one derivative, and the method comprises the following steps: under nitrogen atmosphere at room temperature, dissolvinga 2-allyloxybenzaldehyde derivative represented by formula (I) and a benzoin derivative represented by formula (II) in an organic solvent, adding a photosensitizer, evenly mixing, placing under an ultraviolet lamp for light irradiation reaction, removing the solvent by rotary evaporation, and separating and purifying by silica gel column chromatography to obtain the resulting product 2,3-dihydrobenzopyran-4-one derivative. The preparation method solves the problems of cumbersome steps, low yield and poor environmental protection property of the existing synthesis method, can react under normaltemperature and normal pressure, and has the advantages of mild reaction conditions, no need of transition metal catalysis, simple operation, no pollution, safety, environmental protection, low costand the like. R1 and R2 are hydrogen, halogen or alkyl.
Owner:ZHEJIANG SHENGXIAO CHEM
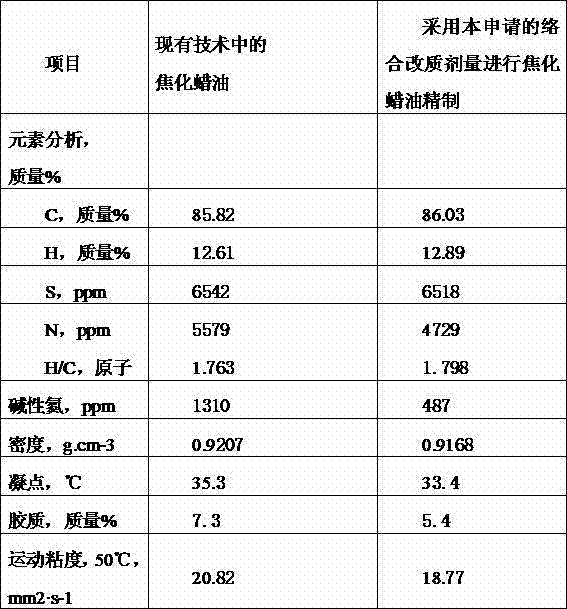
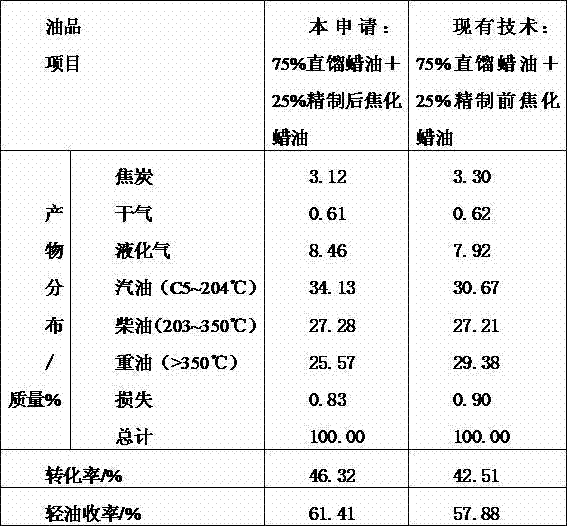
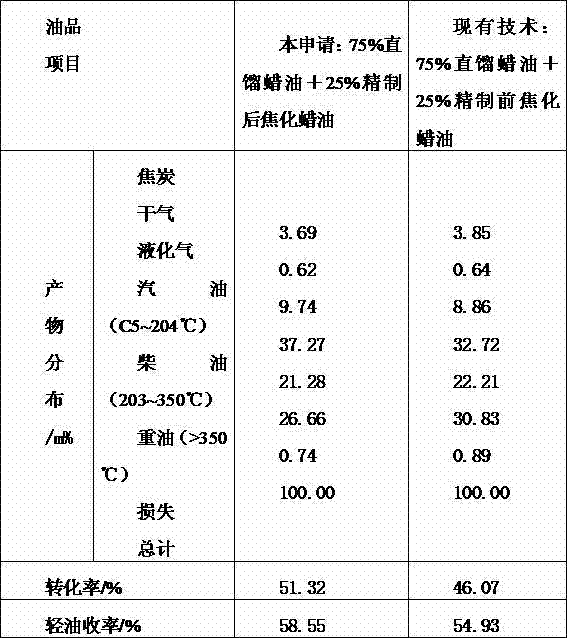
Coking wax oil complexing denitrification refining method
The invention relates to an additive added in the coking wax oil refining process, in particular to a coking wax oil complexing denitrification refining method. A coking wax oil complexing modifier comprises the following ingredients in parts by mass: 50 percent to 85 percent of complexing agents, 2 percent to 10 percent of precipitating agents, 0.5 percent to 10 percent of corrosion inhibitors and 2 percent to 30 percent of cosolvents. A combination has higher denitrification reaction activity, meanwhile, reaction products can also be fast separated through settling from oil products, efficiency and reliability of the complexing denitrification refining operation are ensured, various nonideal ingredients in the coking wax oil can be effectively removed, the corrosion on equipment in the process of the complexing process can also be avoided, meanwhile, the reaction products can be fast separated through settling from the oil products, the influence on production devices and environment is not caused, and the environment pollution is reduced.
Owner:湖北本心环保科技股份有限公司
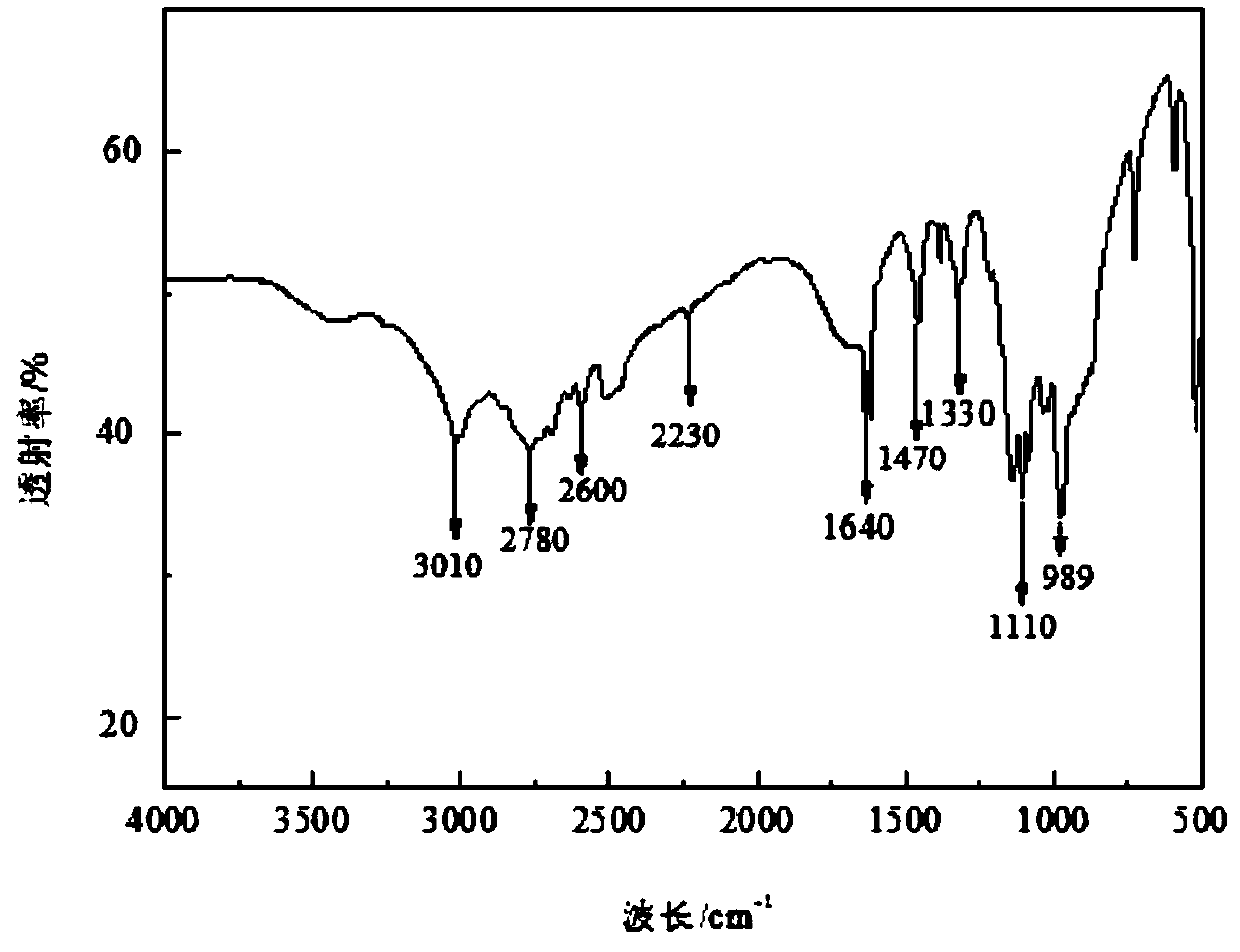
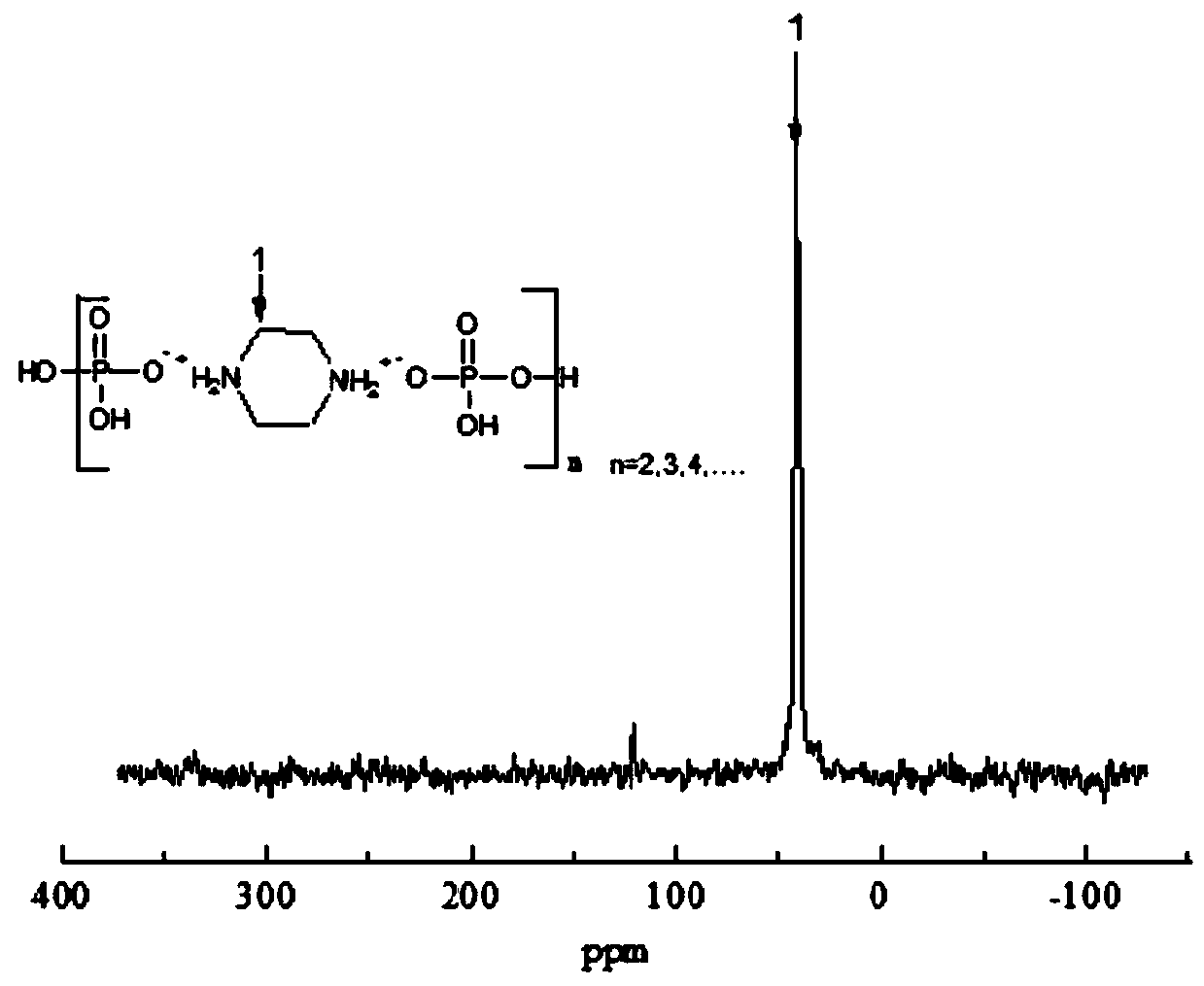
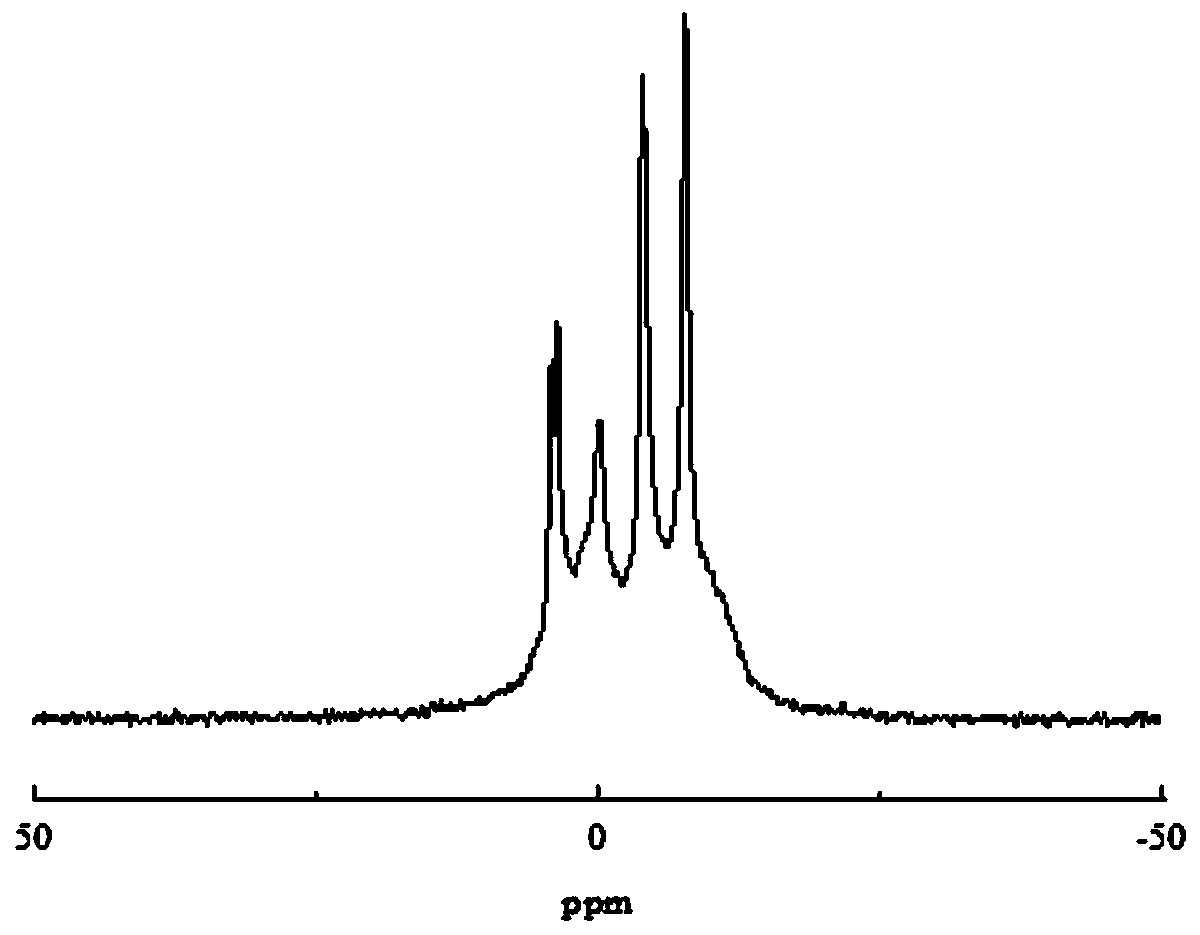
Halogen-free phosphorus-nitrogen additive-type flame retardant and preparation method thereof
InactiveCN110818948ASolve yieldSolve poor charcoal performanceOrganic chemistryO-Phosphoric AcidPhosphate
The invention relates to a halogen-free phosphorus-nitrogen additive-type flame retardant and a preparation method thereof. The preparation method comprises the following steps: dissolving anhydrous piperazine with deionized water; adding a phosphoric acid solution, reacting under inert conditions to obtain intermediate piperazine diphosphate, and carrying out dehydration polycondensation reactionon the intermediate piperazine diphosphate to obtain polypiperazine phosphate which is the halogen-free phosphorus-nitrogen additive-type flame retardant. The halogen-free phosphorus-nitrogen additive-type flame retardant is not easily hydrolyzed, has favorable charring performance and heat stability; reaction conditions are mild, operation is safer, synthesis efficiency is higher. According to the preparation method of the halogen-free phosphorus-nitrogen additive-type flame retardant, a phosphoric acid structure and a piperazine structure are introduced into a same flame retardant moleculestructure, so that better intermolecular synergistic effect is achieved; it is shown by thermogravimetric analysis results that at 800 DEG C, the polypiperazine phosphate carbon yield is 16.4wt%, thepiperazine diphosphate carbon yield is 14.1wt%, and the polypiperazine phosphate carbon yield is 16.3% higher than that of the piperazine diphosphate, it indicates that the flame retardant polypiperazine phosphate has good char forming performance and thermal stability.
Owner:珠海格力绿色再生资源有限公司
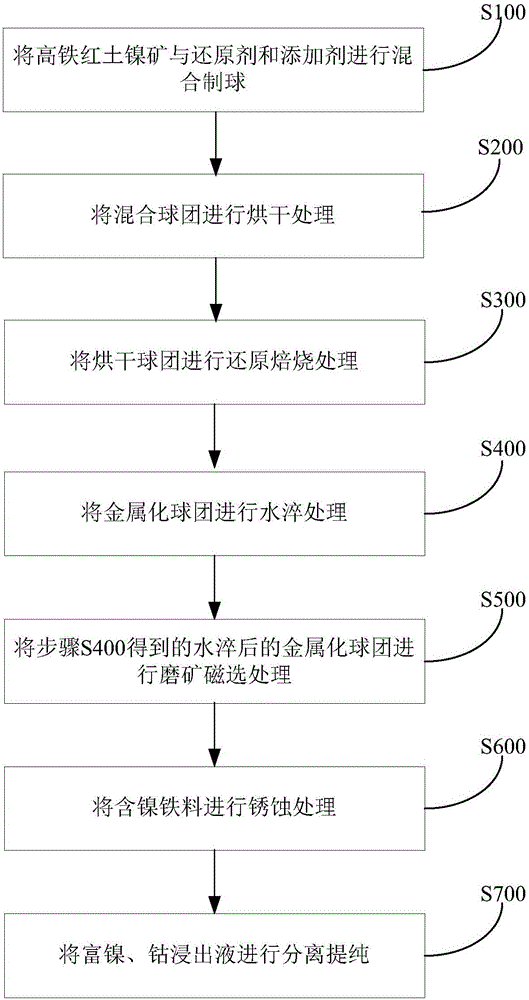
Method and system for processing high-iron laterite nickel ore
ActiveCN105925818AEfficient use ofEfficient removalProcess efficiency improvementProduction rateFerric oxide hydrate
The invention discloses a method and system for processing high-iron laterite nickel ore. The content of iron in the high-iron laterite nickel ore is not lower than 30 wt%. The method comprises the steps that 1, the high-iron laterite nickel ore, reducing agents and additives are mixed to prepare a ball, and a mixed pellet is obtained; 2, the mixed pellet is dried, and a dried pellet is obtained; 3, the dried pellet is subjected to reducing roasting processing, and a metalized pellet is obtained; 4, the metalized pellet is subjected to water quenching processing; 5, the metalized pellet which is subjected to water quenching processing and obtained through the step 4 is subjected to ore grinding magnetic separation processing, and an iron material containing nickel and tailings are obtained; 6, the iron material containing the nickel is subjected to rusting processing, and hydrated ferric oxide and a leaching agent rich in the nickel and cobalt are obtained; and 7, the leaching agent rich in the nickel and the cobalt is subjected to separation and purification, and metal nickel and metal cobalt are obtained. By means of the method for processing the high-iron laterite nickel ore, the problems that obtained product nickel is low in grade and production rate when the high-iron laterite nickel ore is processed through a traditional smelting technology can be solved, and the high-iron laterite nickel ore can be effectively used.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
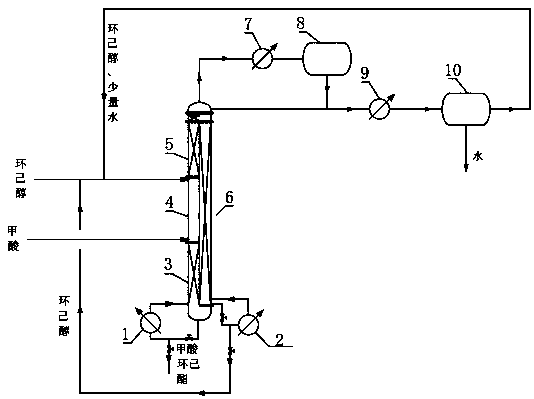
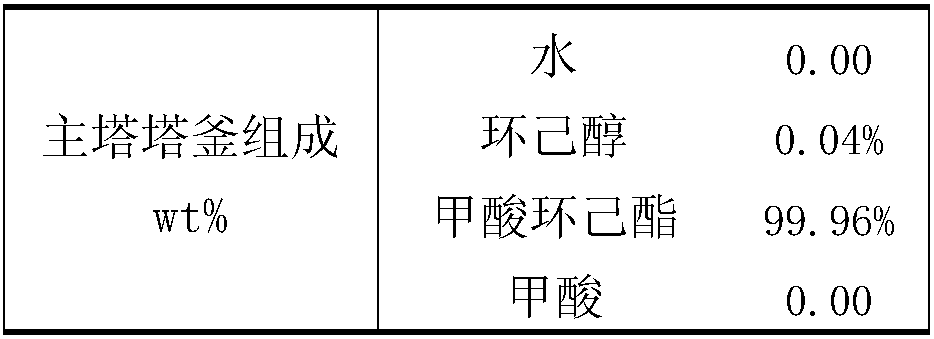
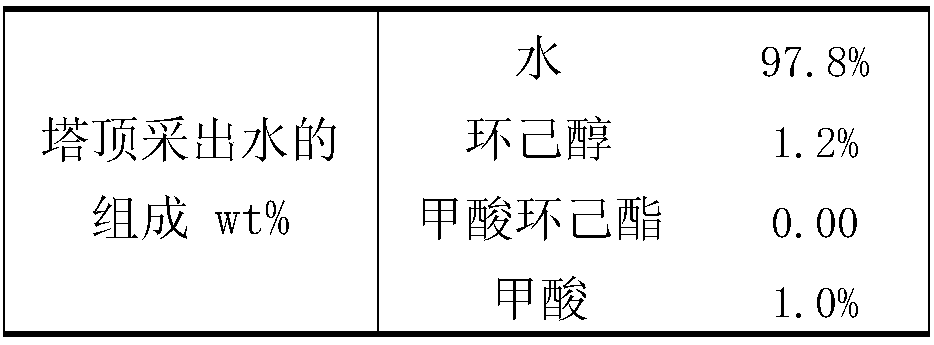
Production technology for producing cyclohexyl formate by dividing wall reaction rectification
ActiveCN108516934ASolve the problem of energy consumptionSolve yieldOrganic compound preparationChemical industryPolymer scienceFormate
The invention discloses a production technology for producing cyclohexyl formate by dividing wall reaction rectification. The technology comprises the following steps: (1) adding 50 wt% of cyclohexylformate, 25 wt% of cyclohexanol and 25 wt% of formic acid into a main rectification tower bottom, adding cyclohexanol into an auxiliary rectification tower bottom, starting a reboiler I and a reboilerII when the height of the liquid level in every tower bottom reaches 70% of the height of the corresponding tower bottom, and controlling the pressure in every tower to be 10-15 kPa; and (2) adding cyclohexanol and formic acid into a main rectification tower when the temperature of the main rectification tower bottom reaches 90-100 DEG C, performing a reaction under the action of a solid acid catalyst, removing water and recovering cyclohexanol from the tower top of the main rectification tower, extracting the cyclohexyl formate from the main rectification tower bottom, extracting the cyclohexanol from the auxiliary rectification tower bottom, and recycling the extracted cyclohexanol. The technology has the advantages of simplicity, reduction of production devices, production cost saving,solving of the problems of high complex energy consumption and low yield of original technologies for producing the cyclohexyl formate through directly reacting formic acid with cyclohexanol, and realization of high purity of the obtained cyclohexyl formate.
Owner:YANTAI UNIV
Novel Linezolid intermediate, its preparation method and novel preparation method of Linezolid
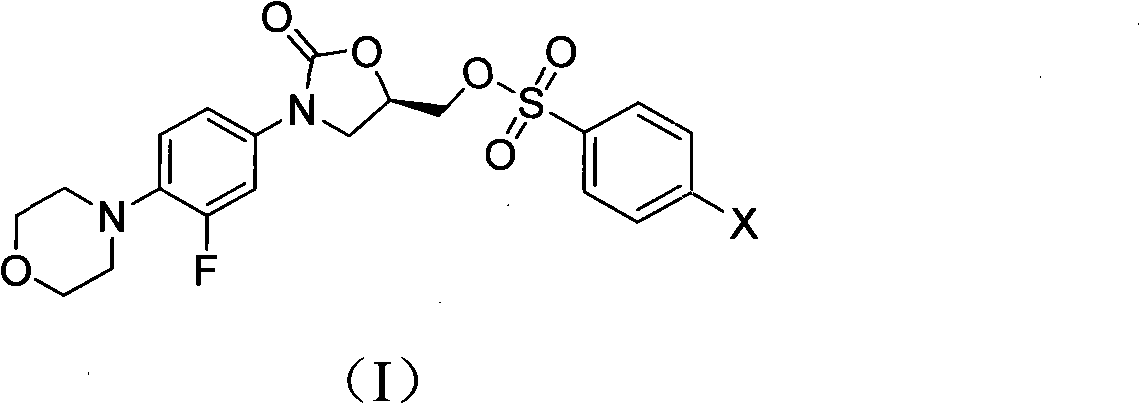
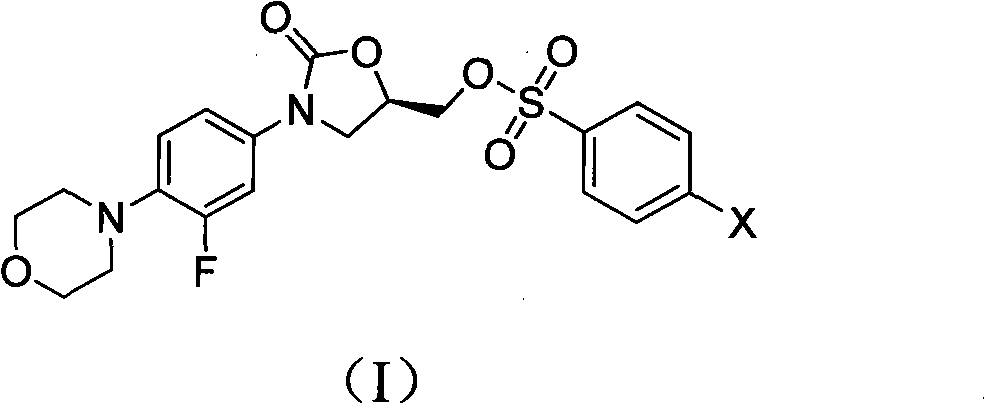
The invention discloses a novel Linezolid intermediate, its preparation method and a novel preparation method of Linezolid, a structure of the Linezolid key intermediate is shown as a formula (I), in the formula (I), X is fluorine, chlorine, bromine or iodine. According to the invention, the Linezolid intermediate solves the problems of poor solubility of the Linezolid intermediate, and low yield and purity of the synthesized Linezolid in the prior art. The preparation methods of the invention have the advantages of easy preparation process, easy raw material acquisition, low cost, easy purification of intermediate product and final product, high yield and purity, and are suitable for large-scale industrial production.
Owner:SHANDONG SALUBRIS PHARMA
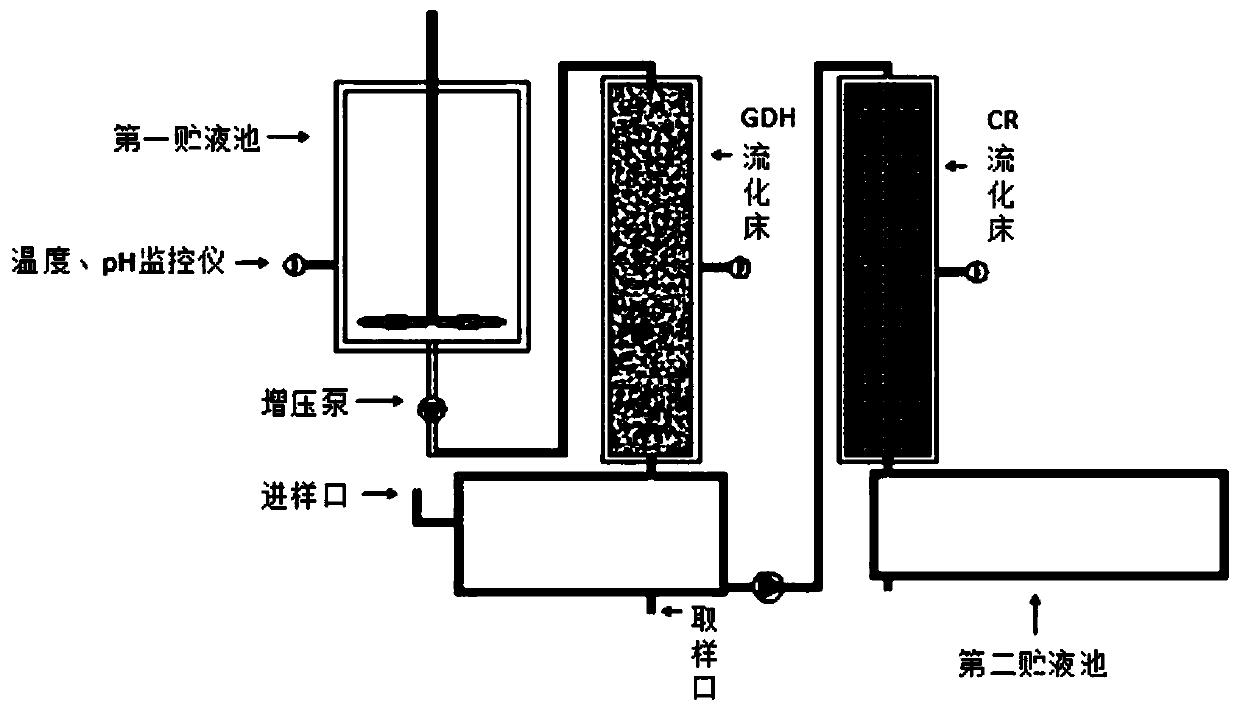
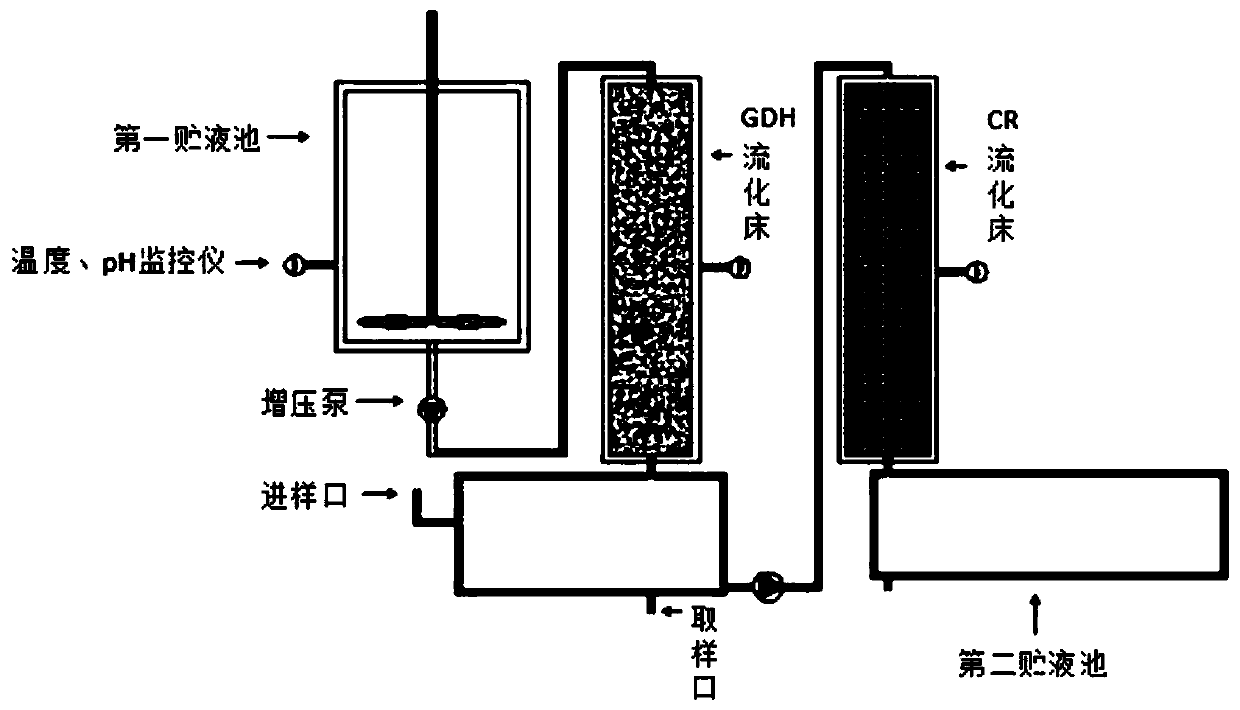
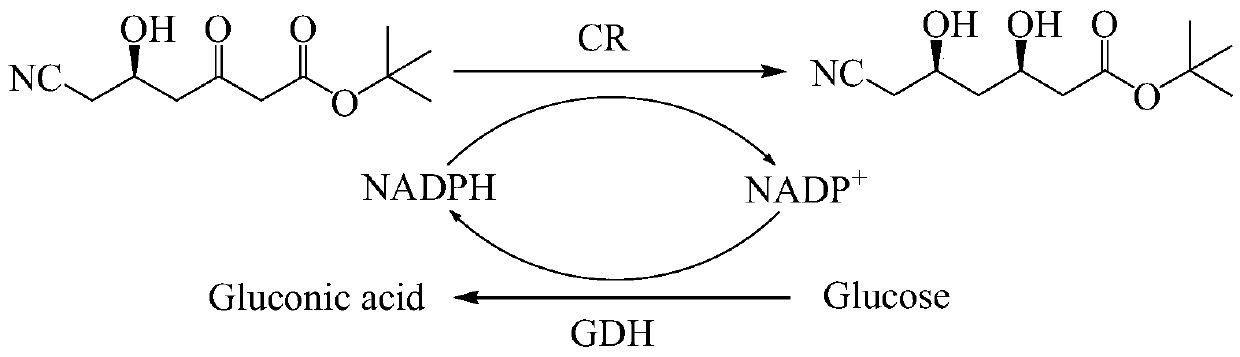
Method for continuously catalyzing and synthesizing ATS-7 by immobilized enzyme fluidization bed
PendingCN109913514AHigh catalytic efficiencyIncrease profitFermentationChemical industryEnzyme catalyzed
The invention belongs to the field of pharmaceutical and chemical industry, and particularly relates to a method for continuously catalyzing and synthesizing ATS-7 by an immobilized enzyme fluidization bed. Mixed liquid of glucose and a nicotinamide adenine dinucleotide phosphate (NADP+) generates reduced nicotinamide adenine dinucleotide phosphate (NADPH) through a GDH (glutamate dehydrogenase) bed, and mixed solution of ATS-6 and generated NADPH is implemented through a CR fluidized bed, so that the ATS-7 is continuously catalyzed and synthesized by the immobilized enzyme fluidization bed. By the continuous reaction catalysis method of the immobilized enzyme fluidization bed, enzyme catalyzing efficiency is improved; enzyme utilization rate is increased, the catalytic efficiency of the immobilized enzyme is improved by 3.1-4.2 times as compared with that of traditional free enzyme, reaction processes can be controlled by independently adjusting different sample feeding flow speed, the immobilized enzyme cannot easily fall off, continuously catalyzing reaction can be achieved, the immobilized enzyme and a product are conveniently separated, the yield of the product is improved, and purification steps are simplified, and industrialization is more facilitated.
Owner:JIANGSU UNIV OF TECH
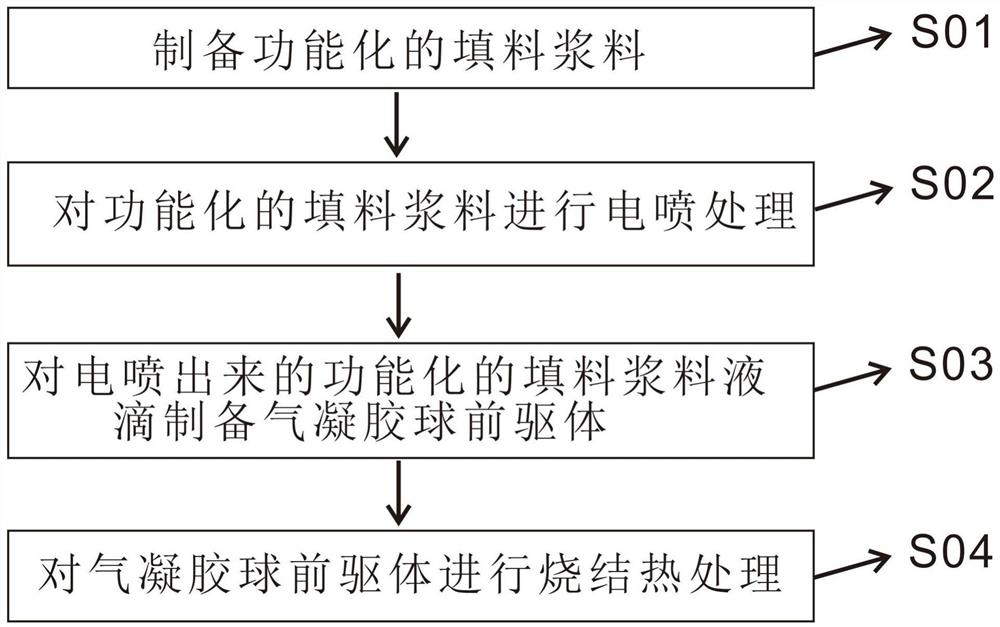
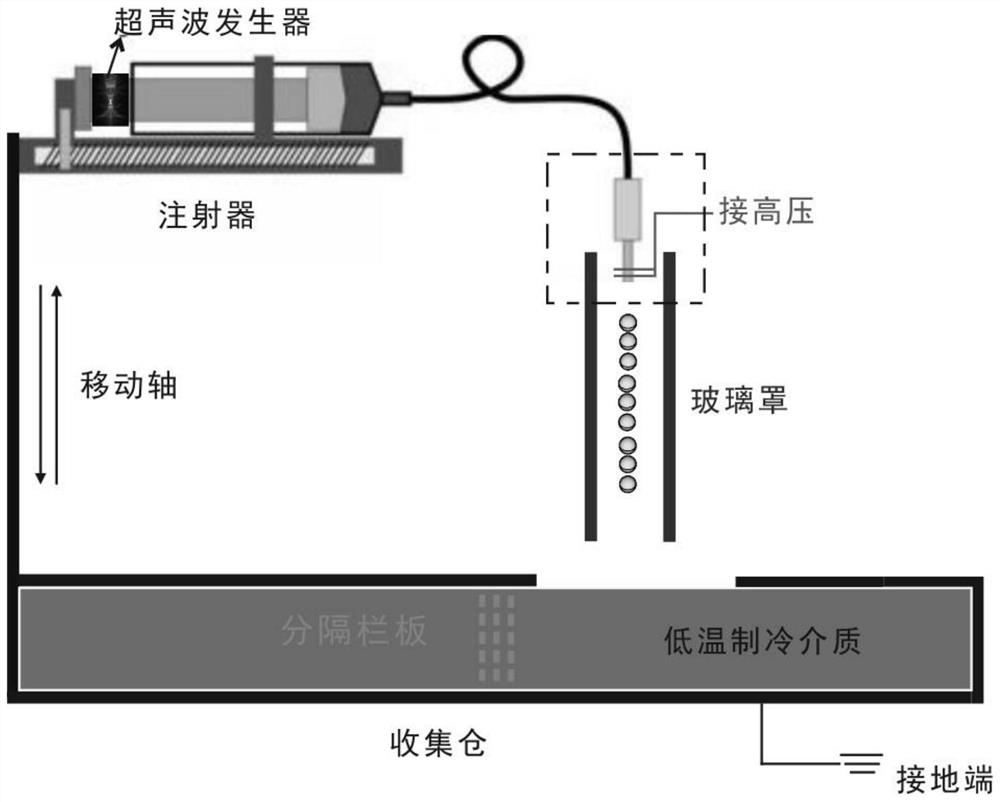
Size-controllable aerogel ball as well as preparation method and application thereof
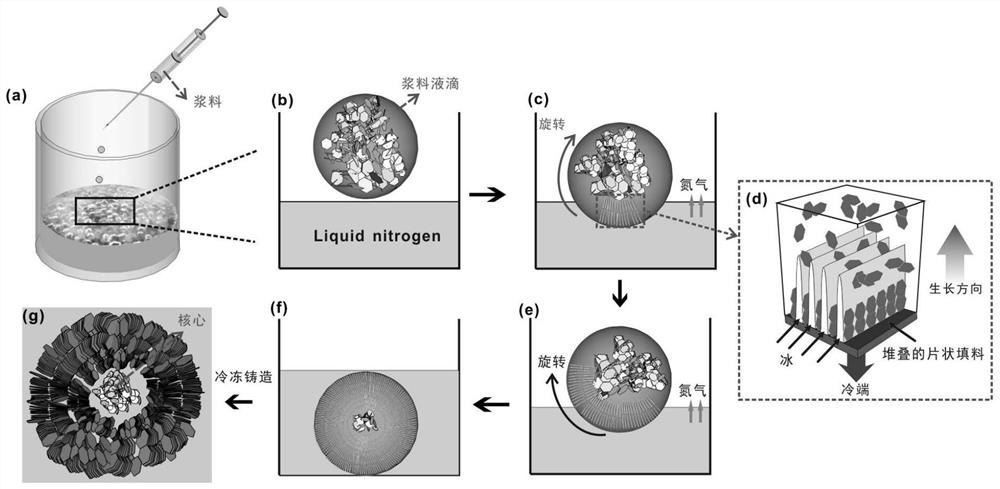
PendingCN112551494AGood size controlEasy to makeNitrogen compoundsZinc oxides/hydroxidesFreeze-dryingSlurry
The invention belongs to the technical field of nano porous materials, and discloses a size-controllable aerogel ball as well as a preparation method and application thereof. The preparation method ofthe aerogel ball comprises the following steps: mixing a filler, a viscosity regulator, salt and water according to a mass ratio of 1:(0.05-0.2):(0.025-0.05):(5-50) to obtain functionalized filler slurry; putting the functionalized filler slurry into an electronic injection treatment device to obtain filler slurry droplets with controllable diameters; dropwise adding a low-temperature refrigerating medium, carrying out freezing treatment to form a frozen mixture, and carrying out freeze drying treatment to obtain an aerogel ball precursor; and carrying out first and second sintering heat treatment on the aerogel ball precursor to obtain the aerogel ball. The aerogel ball is wide in applicable material range, controllable in aerogel size, simple in preparation process, relatively mild in reaction condition, high in yield, controllable in aerogel ball diameter and high in porosity, and has a good application prospect in various fields.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Preparation method for disubstituted styrene derivative
ActiveCN110305054AHas catalytic propertiesHas biological propertiesOrganic chemistryOrganic synthesisEvaporation
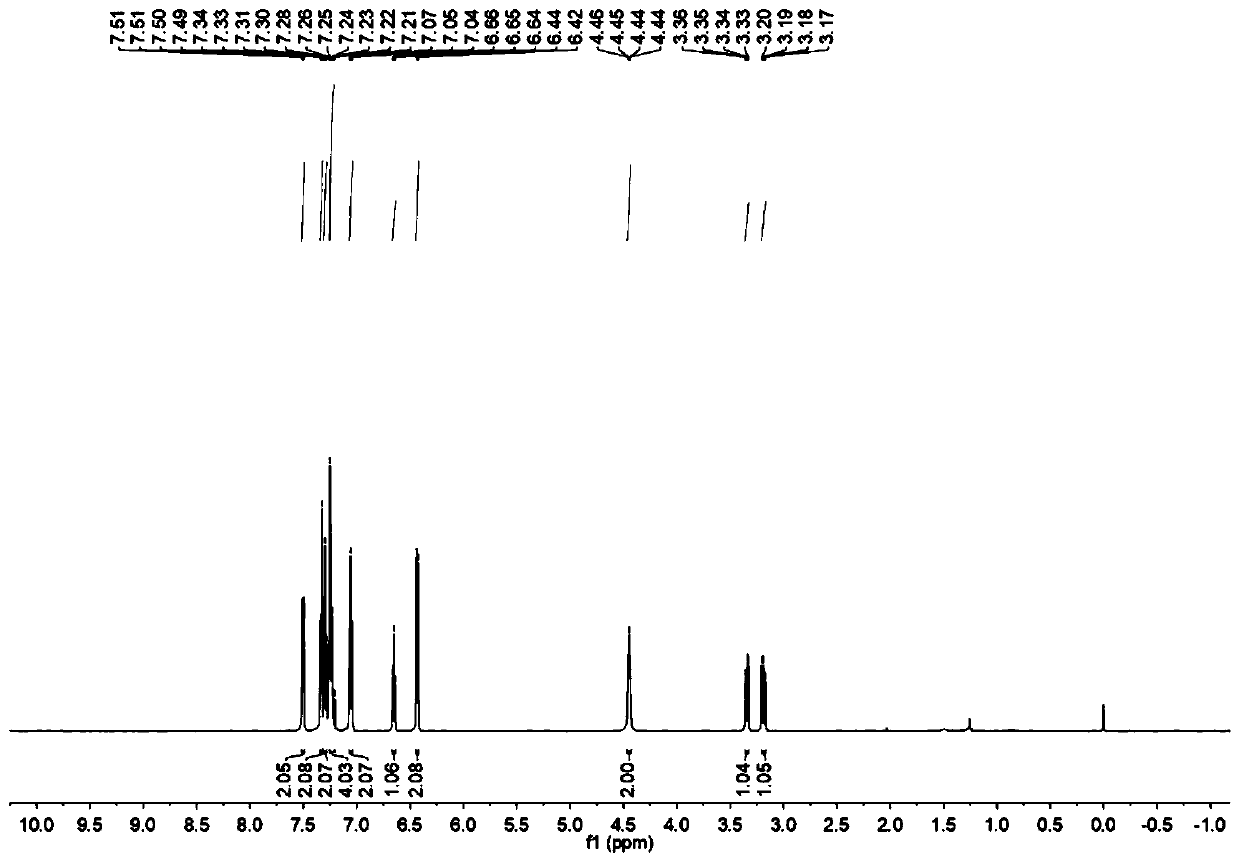
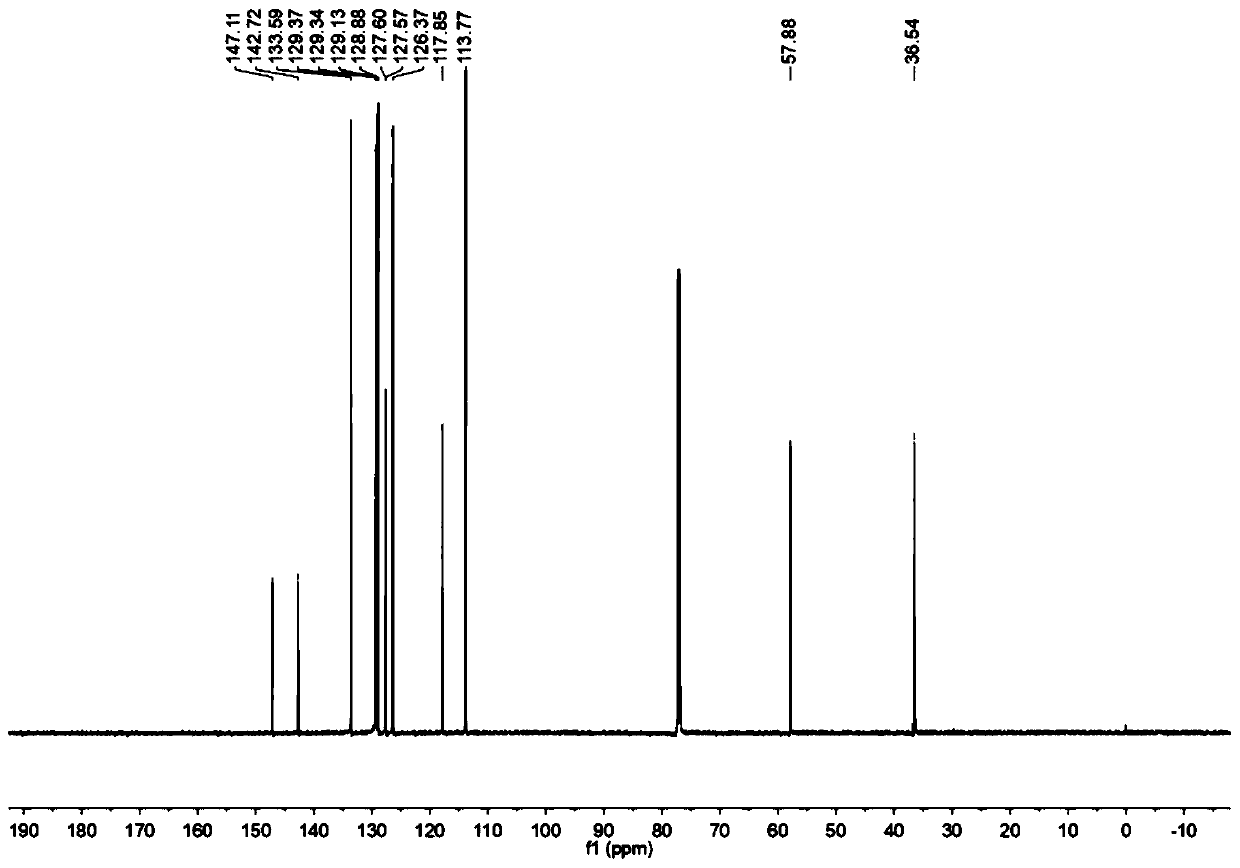
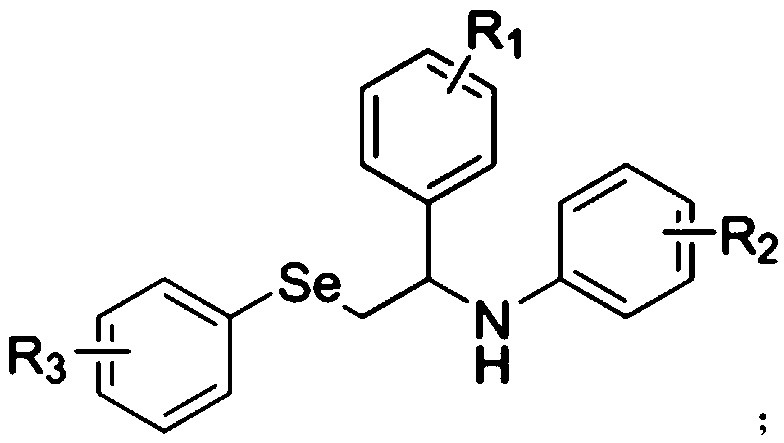
The invention relates to a preparation method for a disubstituted styrene derivative. The objective of the invention is to solve the problems of complicated steps, low yield and pollution to the environment for a conventional method for synthesizing the disubstituted styrene derivative. The preparation method provided by the invention comprises the following steps: dissolving a styrene derivative,an aniline compound, a diphenyl diselenide compound and a photocatalyst into an organic solvent at room temperature under a nitrogen or oxygen atmosphere, carrying out uniform mixing, placing an obtained mixture under blue LEDs lamps for a reaction, after the reaction is completed, carrying out rotary evaporation to remove a solvent, and carrying out separation and purification so as to obtain the disubstituted styrene derivative. The invention provides a simple one-step synthetic method. The method provided by the invention solves the problems of the need of heating for conventional synthesis, low yield caused by a transition metal catalyst system and poor environmental friendliness of a method, and seeks a synthetic route with greenness, high efficiency, mild conditions, simple method and convenient operation. The preparation method provided by the invention is applied to the field of organic synthesis.
Owner:HARBIN INST OF TECH
Method for synthesizing piperidine and homolog thereof from N-pyridine oxide and homolog thereof
The invention discloses a method for synthesizing piperidine and homolog thereof from N-pyridine oxide and homolog thereof. The method comprises the following steps: firstly weighing 95-110g of N-pyridine oxide and homolog thereof, 63g of formic acid and 500mL of a solvent, putting the N-pyridine oxide and homolog thereof and ammonium formate into a reaction kettle, adding the solvent, controllingthe temperature to 25-35 DEG C, uniformly stirring to carry out a reaction, washing with water after stirring is completed, and finally carrying out vacuum distillation to remove the solvent, therebyobtaining a product. By adopting the method disclosed by the invention, the problems that piperidine and homolog thereof are serious in environment pollution, low in yield, high in temperature and pressure, high in energy consumption and the like in a synthesis process since acid radical anion is generated can be solved, the method has the advantages of good environment protection, energy conservation, security and high yields, the yield of the product is up to 95% or greater, which is far greater than that of a conventional cycloaddition method, in addition, when reactions are carried out under normal temperature and normal pressure conditions, energy consumption in the reaction process can be reduced, the purposes of energy conservation and environment protection are achieved, and wideapplication prospects can be met.
Owner:ANHUI COSTAR BIOCHEM CO LTD
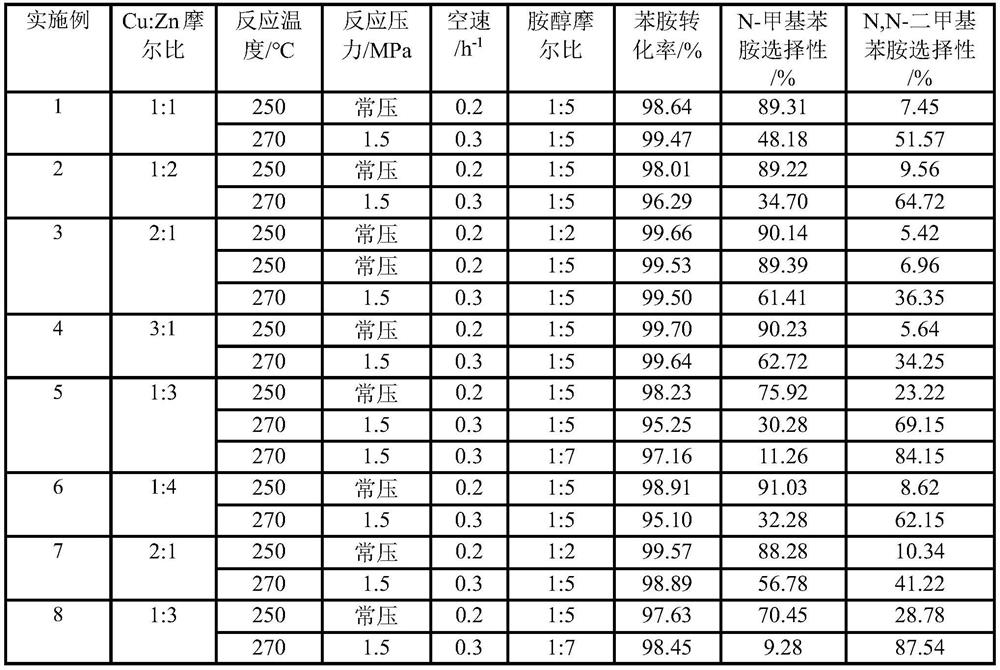
Copper-zinc catalyst for preparing N-methylaniline and N,N-dimethylaniline as well as preparation method and application of copper-zinc catalyst
PendingCN113976128AThe reaction process is simpleReduce typesOrganic compound preparationAmino compound preparationMethylanilineDimethylaniline N-oxide
The invention belongs to the technical field of catalyst N-methylation, and particularly relates to a copper-zinc catalyst for preparing N-methylaniline and N,N-dimethylaniline as well as a preparation method and application of the copper-zinc catalyst. The molar ratio of copper to zinc in the copper-zinc catalyst is (3:1)-(1:4), the specific surface area of the catalyst is controlled to be 15-30 m<2> / g, and the pore diameter is 15-25nm. The preparation method comprises the following steps: carrying out compression molding on a copper-zinc catalyst and graphite together, loading the molded material into a fixed bed reactor, carrying out catalytic reduction, and adjusting specific process conditions by taking aniline and methanol as raw materials in the fixed bed reactor to obtain N-methylaniline and / or N,N-dimethylaniline. According to the method, the problems of low yield, simplification and the like of the traditional preparation of the N-methylaniline and the N,N-dimethylaniline are solved, and meanwhile, the catalyst disclosed by the invention is few in raw material variety and simple in reaction process, so that the cost of preparing the N-methylaniline and the N,N-dimethylaniline is also greatly reduced.
Owner:CHANGZHOU UNIV
Method for synthesizing methylaminopyridine compound by one-step method
ActiveCN110606827AEasy to separate and purifyHigh purityOrganic chemistryChemical recyclingPalladium on carbonDistillation
The invention discloses a method for synthesizing a methylaminopyridine compound by a one-step method. The method comprises the following steps: with cyanopyridine as a raw material, methanol as a reaction solvent and a palladium-carbon catalyst as a catalyst, performing nitrogen replacement, and carrying out a reaction for 4-10 hours in an ammonia atmosphere under the conditions that the hydrogenpressure is 0.5-2.0 MPa and the temperature is 15-30 DEG C; cooling the reaction product to room temperature, filtering the reaction product, recovering filter residues for later use, and performingreduced pressure distillation on the filtrate, thereby obtaining the product methylaminopyridine compound. The method provided by the invention has the advantages of mild reaction conditions, simple steps, convenient product separation and purification, few side reactions, high yield and the like, the purity of the final product is higher than 99.9%, and the catalyst filtered and recovered after the reaction and the distilled reaction solvent can be recycled, so that the problems of low yield, high cost and the like in the prior art are solved.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Recombinant yeast strain for fermenting erythritol under high nitrogen condition, construction method and application thereof
ActiveCN110129381AImprove filtration efficiencyAddressing Fermentation DelaysMicroorganism based processesFermentationBiotechnologyFermentation
The invention discloses a recombinant yeast strain for fermenting erythritol under high nitrogen condition, a construction method and an application thereof. The recombinant strain is constructed by:obtaining the SNF1 gene in the whole genome of Yarrowia lipolytica, using the Yarrowia lipolytica genome as a template to separately amplify the upstream and downstream fragments of the SNF1 gene andthe URA3 fragment by PCR reaction, ligating the upstream and downstream fragments of the SNF1 gene to the upstream and downstream of the URA3 fragments by an overlap extension PCR method to obtain aSNF1 gene knockout component, transferring the SNF1 gene knockout module to the selected uracil-deficient Yarrowia lipolytica strain, and screening to obtain the Yarrowia lipolytica recombinant strainin which the SNF1 gene was knocked out. The strain eliminates the coupling between nitrogen hunger stress and erythritol synthesis by knocking out the SNF1 gene of Snf1 protein. The recombinant yeaststrain of the invention can synthesize erythritol under high nitrogen conditions, and the fermentation efficiency is obviously improved, which solves the problem of delayed fermentation and low yieldin industrial production of erythritol.
Owner:HUAIYIN TEACHERS COLLEGE
Synthesis method for compound and application thereof in field of medicines used for improving insulin resistance
ActiveCN110937986ASolve yieldSolve complexityOrganic active ingredientsOrganic compound preparationBenzaldehydePancreatic hormone
The invention belongs to the technical field of drug synthesis, and particularly relates to a synthesis method for a compound and application thereof in field of medicines used for improving insulin resistance. The invention provides the synthesis method of SN159. The synthesis method comprises the following steps: brominating a raw material, namely 2,4-dihydroxy benzaldehyde, protecting para-hydroxyl groups by using methoxymethyl groups, performing methylating by using dimethyl sulfate or methyl iodide, and carrying out a Claisen-Schmidt condensation reaction under an alkaline or acidic condition, thereby enhancing the yield of the finally produced compound from 18.9% in the prior art to 68% or above. According to the invention, column chromatographic purification is not needed in the preparation process, and the finally produced compound with a purity of 99% or above can be obtained only through recrystallization; and the method is suitable for large-scale / industrial production. According to the synthesis method of the compound and the application of the compound in the field of preparation of medicines used for improving insulin resistance, the technical defects that SN159 is low in synthesis yield and complex in purification in the prior art are overcome.
Owner:SHENZHEN LINGLAN BIO PHARMA TECH CO LTD
System and method for treating maize straws
PendingCN109504405ALow ashRealize resource utilizationBiofuelsOven incrustations prevention/removalPyrolytic carbonBriquette
The invention discloses a system and a method for treating maize straws. The system comprises a crusher, a mixing pool, a dryer, a granulator and a pyrolysis device, wherein the crusher is provided with a maize straw inlet and a maize straw particle outlet; the mixing pool is provided with a maize straw particle inlet, a vinegar inlet, an acid-leached maize straw outlet and a waste acid outlet; the maize straw particle inlet is connected with the maize straw particle outlet; the dryer is provided with an acid-leached maize straw inlet and a dry maize straw outlet; the acid-leached maize strawinlet is connected with the acid-leached maize straw outlet; the granulator is provided with a dry maize straw inlet and a straw briquette outlet; the dry maize straw inlet is connected with the dry maize straw outlet; the pyrolysis device is provided with a straw briquette inlet, a pyrolytic oil gas outlet and a pyrolytic carbon outlet; the straw briquette inlet is connected with the straw briquette outlet. By using the system to treat the maize straws, the pyrolysis efficiency is remarkably improved; the oil yield of obtained biomass is greatly improved; the obtained biomass and pyrolytic gas have higher quality.
Owner:WUHAN RUNDO BIOTECHNOLOGY CO LTD
Method of extracting fish oil from squid skin
The invention relates to a method of extracting fish oil from squid skin. The method comprises extracting and separating fish oil from squid skin through extraction solvents such as an ether, an esteror an alkane, and evaporating the extraction solvents through rotary evaporation so that the extraction solvents are successfully separated from the fish oil and the fish oil product is obtained. Themethod can extract fish oil from fish skin wastes and retain the nutrients such as oleic acid, DHA and EPA. The method changes the fish skin into valuables, has simple and easy processes and utilizesrenewable extraction solvents.
Owner:HARBIN INST OF TECH AT WEIHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com