Preparation method of BODIPY-tetrazine bio-orthogonal probe
A bioorthogonal, fluoroboron fluorescence technology, applied in the field of biological probes, can solve the problems of complex synthesis steps, poor water solubility, low yield, etc., and achieve the effects of improving fluorescence intensity, enhancing fluorescence intensity and reducing fluorescence background signal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
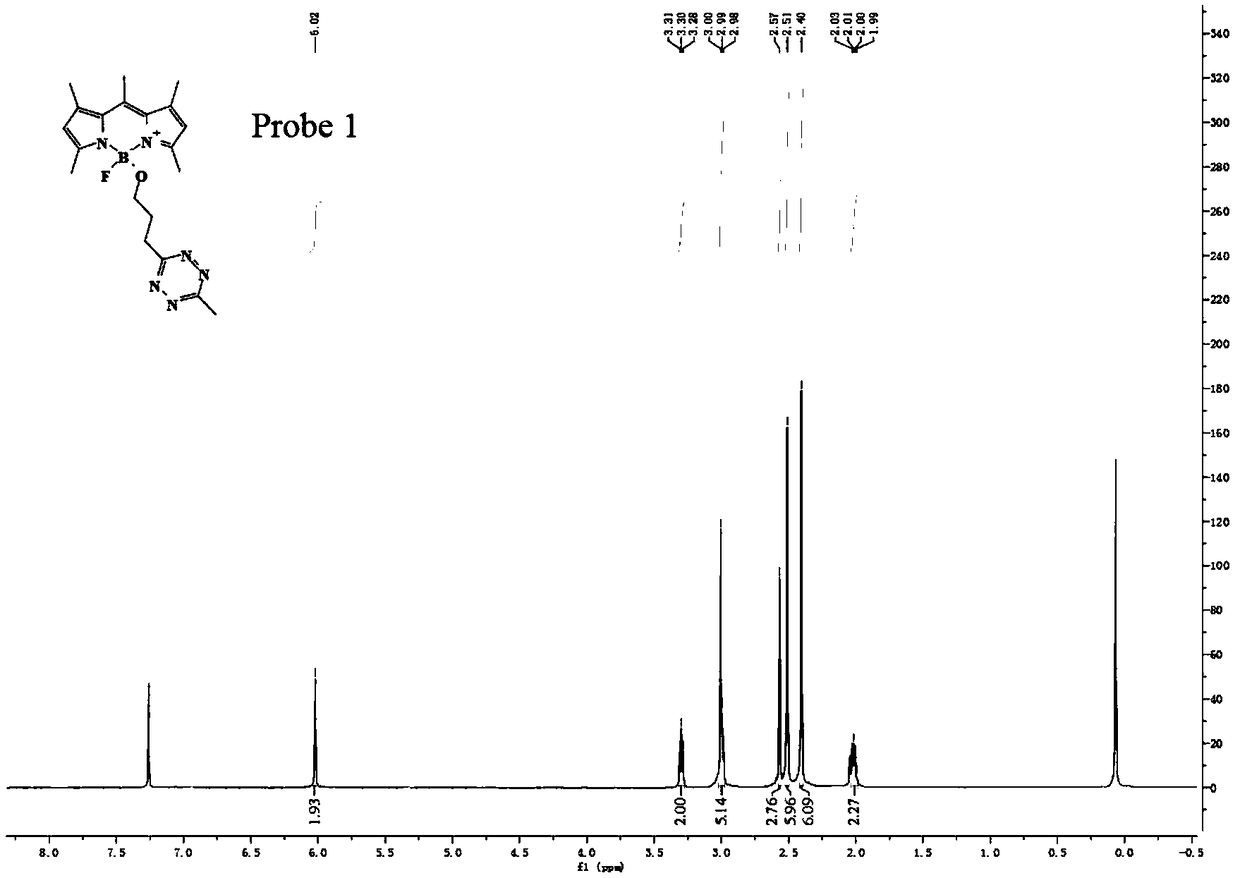
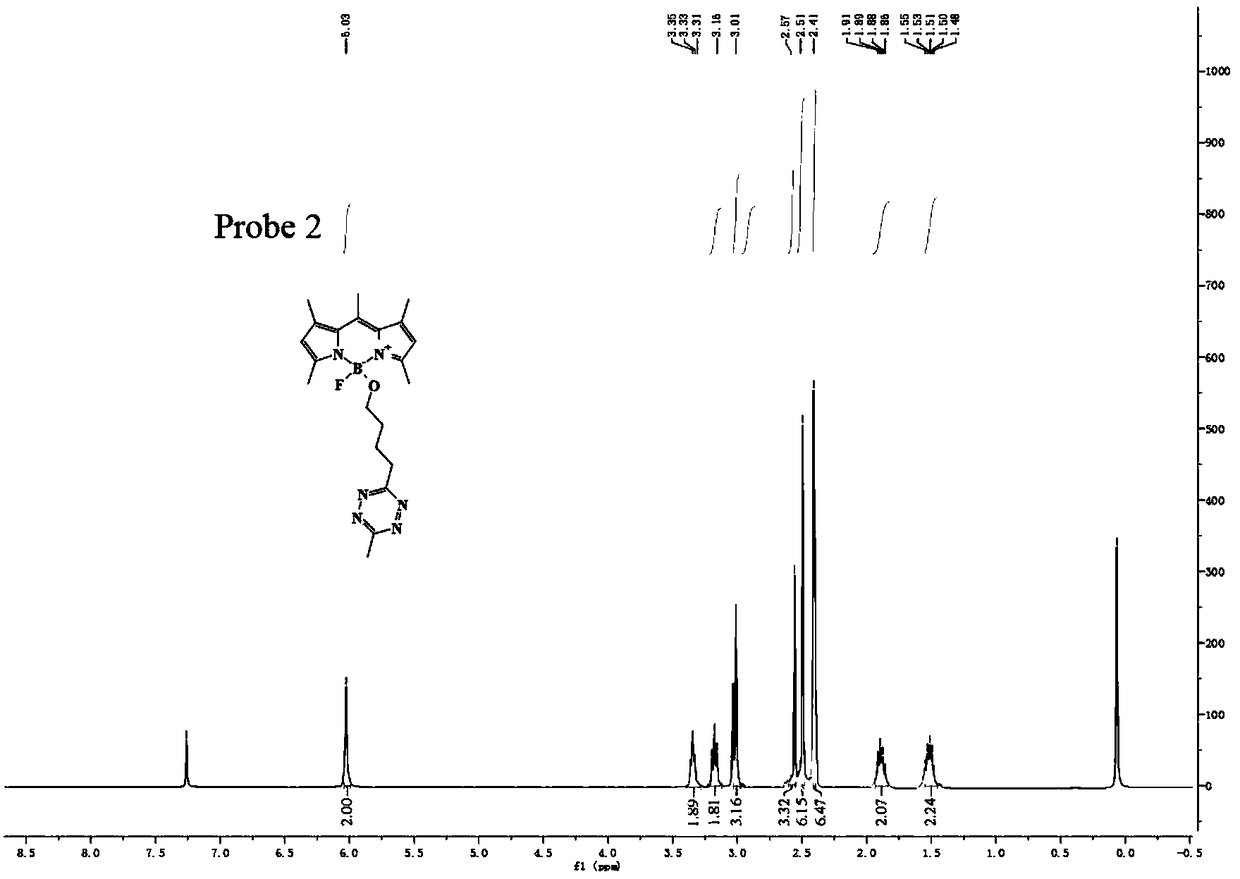
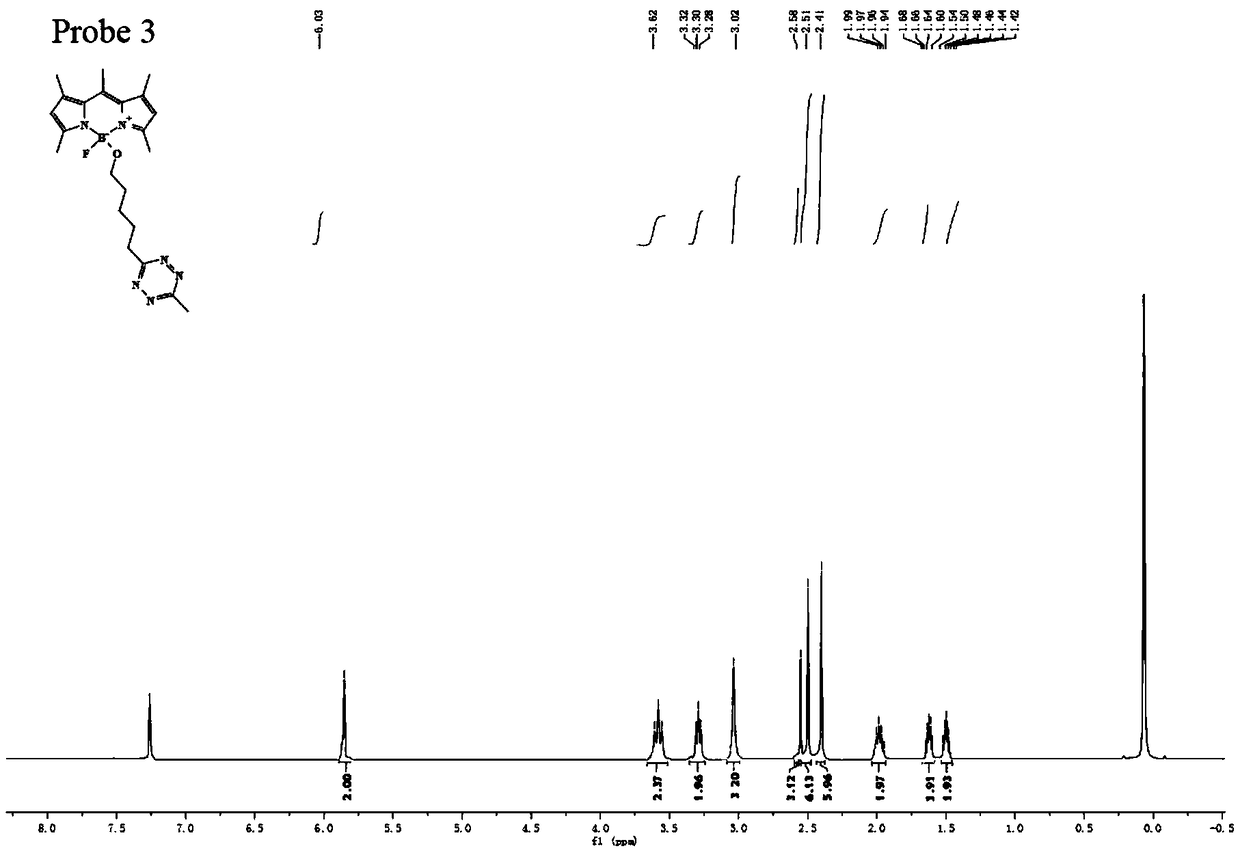
[0042] The preparation method of flubofluorine-tetrazine bio-orthogonal probe, the reaction structural formula is as follows, such as Figure 18 ,
[0043]
[0044] The specific operation method is: add 5 mmol of TMSOTf to 9 ml of anhydrous acetonitrile solution containing 1 mmol of BODIPY1-2, stir and react at 0°C for 30 minutes to activate BODIPY1-2. Then add 4 mmol t-BuOH to quench TMSOTf, quickly add 5 mmol 2,6-lutidine and 5 mmol tetrazine Tza-Tzg mixed anhydrous acetonitrile solution, and continue stirring for 10 min at 0°C. The reaction was quenched with water, and the reaction product was extracted with DCM. The organic phase was dried with anhydrous sodium sulfate, spin-dried, and separated on a silica gel column to obtain a fluboron-tetrazine bioorthogonal probe. The reaction equation and yield are as follows.
[0045] In this example, in the reaction structure formula, the synthesis method using Tza is obtained by using the prior art method.
[0046] In the pre...
Embodiment 2
[0126] Fluorescence performance test of the flubofluorine-tetrazine bio-orthogonal fluorescent probe prepared in Example 1:
[0127] The fluborine-tetrazine bio-orthogonal fluorescent probe prepared above was prepared into a fluborine-tetrazine bio-orthogonal fluorescent probe solution (concentration is 5 μm / ml, solvent is 20% DMF and 80% mixed solvent ), under the conditions of 495nm excitation light irradiation at room temperature, continuously irradiated for 10min, the maximum fluorescence intensity of the flubororine-tetrazine bioorthogonal fluorescent probe still remained above 90% of the initial value, as Figure 15 As shown, it shows that the boron-tetrazine bioorthogonal fluorescent probe can be applied to long-term cell imaging.
[0128] The live cell imaging of the boron-tetrazine bio-orthogonal fluorescent probe, the specific operation method is: the above-prepared boron-tetrazine bio-orthogonal fluorescent probe 7 (0.5% cross) is dissolved in In the PBS solution, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com