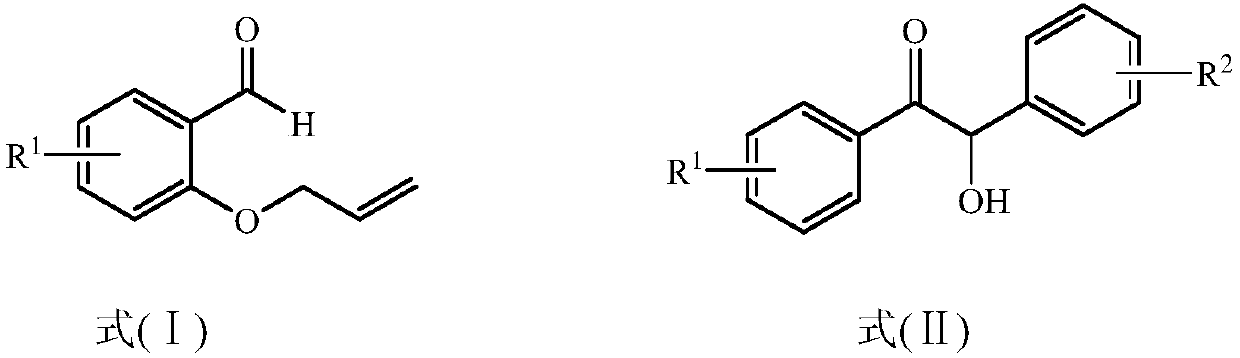
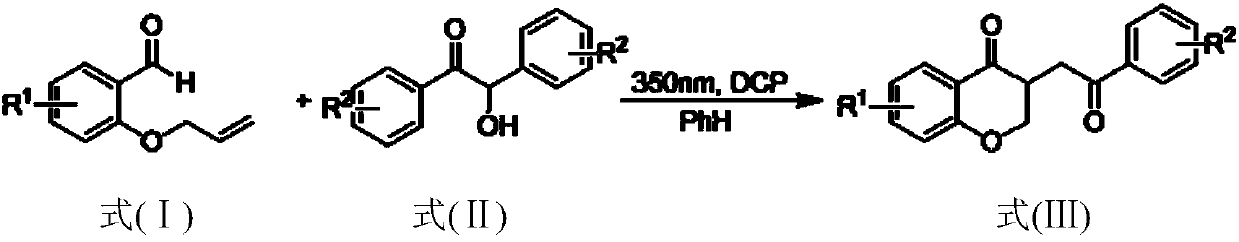
Method for preparing 2,3-dihydrobenzopyran-4-one derivative
A technology of dihydrobenzopyran and its derivatives, which is applied in the direction of organic chemistry, can solve problems such as damage, environmental pollution, and cumbersome steps, and achieve the effects of mild reaction conditions, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Into a 50mL photoreaction tube, add o-propenyloxybenzaldehyde (0.1mmol), benzoin (0.2mmol), dicumyl peroxide (DCP) (0.4mmol), dry benzene (15mL) sequentially, and then Ball bubbling to deoxygenate for 0.5h, then seal the reaction system and irradiate it with 350nm ultraviolet light for 24h. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether with a volume ratio of 50:1: ethyl acetate was used as an eluent for purification and separation by silica gel column chromatography to obtain the corresponding 3 -Benzoylmethyl-2,3-dihydrochromen-4-one, whose structural formula is:
[0022]
[0023] Purity 99%, productive rate 77%, its NMR data analysis is: 1 H NMR (400MHz, CDCl3) δ8.03 (d, J = 7.9Hz, 2H), 7.94 (d, J = 7.8Hz, 1H), 7.61 (t, J = 7.0Hz, 1H), 7.50 (dd, J =10.4,4.8Hz,3H),7.09–6.99(m,2H),4.67(dd,J=11.1,5.3Hz,1H),4.34(t,J=11.4Hz,1H),3.77–3.71(m, 1H), 3.62(ddd, J=1...
Embodiment 2
[0026] Into a 50 mL photoreaction tube, 5-methyl-2-propenyloxybenzaldehyde (0.1 mmol), benzoin (0.3 mmol), dicumyl peroxide (DCP) (0.5 mmol), dry acetonitrile ( 5 mL), deoxygenated by bubbling with a nitrogen balloon for 0.5 h, and then, the reaction system was sealed and irradiated with 350 nm ultraviolet light for 36 h. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether with a volume ratio of 30:1: ethyl acetate was used as an eluent for purification and separation by silica gel column chromatography to obtain the corresponding 3 -Benzoylmethyl-6-methyl-2,3-dihydrochromen-4-one, whose structural formula is:
[0027]
[0028] The purity is 98%, and the yield is 71%. Its NMR data are: 1 H NMR (400MHz, CDCl3) δ8.00(d, J=7.3Hz, 2H), 7.70(s, 1H), 7.59(t, J=7.4Hz, 1H), 7.48(t, J=7.6Hz, 2H ),7.30 (dd, J=8.4,1.9Hz,1H),6.89(d,J=8.4Hz,1H),4.61(dd,J=11.1,5.2Hz,1H),4.28(t,J=11....
Embodiment 3
[0031] Into a 50mL photoreaction tube, add 5-chloro-2-propenyloxybenzaldehyde (0.1mmol), benzoin (0.4mmol), dicumyl peroxide (DCP) (0.6mmol), dry dimethyl After sulfoxide (15 mL), deoxygenation was performed by bubbling a nitrogen balloon for 0.5 h, and then the reaction system was sealed and irradiated with 350 nm ultraviolet light for 30 h. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether with a volume ratio of 10:1: ethyl acetate was used as an eluent for purification and separation by silica gel column chromatography to obtain the corresponding 3 -Benzoylmethyl-6-chloro-2,3-dihydrochromen-4-one, whose structural formula is:
[0032]
[0033] The purity is 99%, and the yield is 84%. Its NMR data are: 1 H NMR (400MHz, CDCl3) δ8.00(d, J=7.4Hz, 2H), 7.87(d, J=2.6Hz, 1H), 7.60(t, J=7.4Hz, 1H), 7.49(t, J =7.6Hz, 2H), 7.43(dd, J=8.8, 2.6Hz, 1H), 6.96(d, J=8.8Hz, 1H), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com