Copper-zinc catalyst for preparing N-methylaniline and N,N-dimethylaniline as well as preparation method and application of copper-zinc catalyst
A technology of dimethylaniline and methylaniline, which is applied in the field of copper-zinc catalyst and its preparation, can solve the problems of simplification, strong pollution, complex reaction process, etc., and achieve cost reduction, simple reaction process, and few types of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
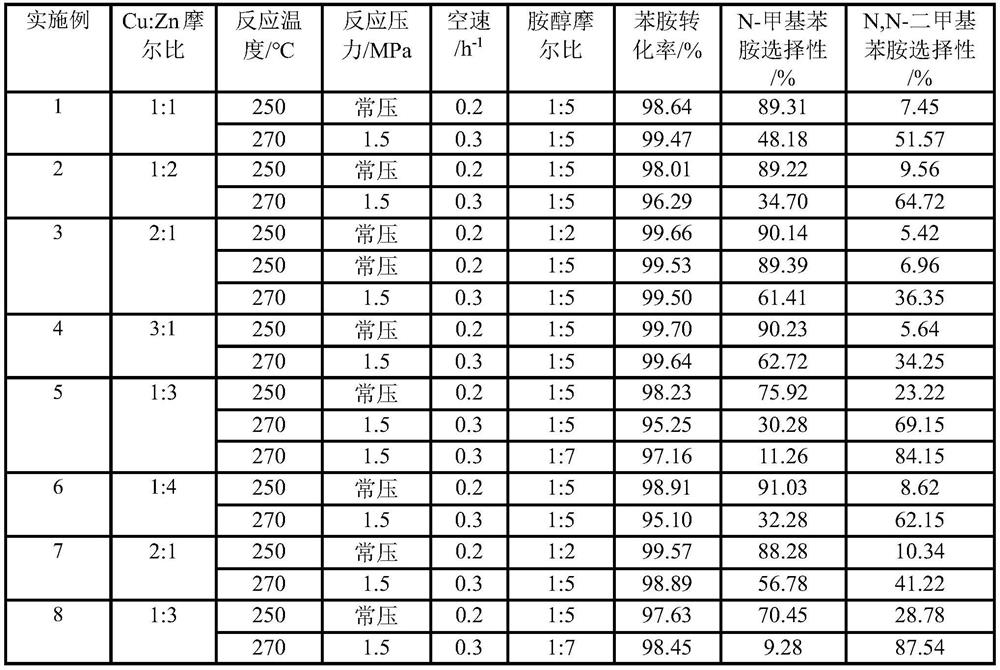
[0023] Example 1: A catalyst for preparing N-methylaniline and N,N-dimethylaniline and a method for preparing N-methylaniline and N,N-dimethylaniline
[0024] (1) The preparation method of copper-zinc catalyst:
[0025] S1. First weigh 53g of sodium carbonate and dissolve it in 530g of water and stir at 70°C and 300r / min;
[0026] S2. Weigh again 74.4g zinc nitrate hexahydrate and 60.4g copper nitrate trihydrate and dissolve them in 200g water, drop them into the sodium carbonate solution through a constant pressure dropping funnel, the pH is 7.5 after the addition is complete, continue to react for 1h, and naturally Aged for 2 hours under cooling, then washed twice and then suction filtered;
[0027] S3. The precipitate after suction filtration was dried at 90° C. for 8 hours, and then calcined at 400° C. for 4 hours. Characterized by BET, the specific surface area of the catalyst is 17.1m 2 / g, pore size 16.6nm.
[0028] (2) Utilize above-mentioned copper-zinc catalyst...
Embodiment 2
[0033] Example 2: A catalyst for preparing N-methylaniline and N,N-dimethylaniline and a method for preparing N-methylaniline and N,N-dimethylaniline
[0034] (1) The preparation method of copper-zinc catalyst:
[0035] S1. First weigh 53g of sodium carbonate and dissolve it in 530g of water and stir at 70°C and 300r / min;
[0036] S2. Take again 99.2g zinc nitrate hexahydrate and 40.3g copper nitrate trihydrate and dissolve them in 200g water, drop them into the sodium carbonate solution through a constant pressure dropping funnel, after the addition is complete, the pH is 7.5, continue to react for 1h, naturally Aged for 2 hours under cooling, then washed twice and then suction filtered;
[0037] S3. The precipitate after suction filtration was dried at 90° C. for 8 hours, and then calcined at 400° C. for 4 hours. Characterized by BET, the specific surface area of the catalyst is 21.8m 2 / g, pore size 19.7nm.
[0038] (2) Utilize above-mentioned copper-zinc catalyst to ...
Embodiment 3
[0043] Example 3: A catalyst for preparing N-methylaniline and N,N-dimethylaniline and a method for preparing N-methylaniline and N,N-dimethylaniline
[0044] (1) The preparation method of copper-zinc catalyst:
[0045] S1. First weigh 127.2g of sodium carbonate and dissolve it in 1272g of water and stir at 70°C and 300r / min;
[0046] S2. Weigh again 119g of zinc nitrate hexahydrate and 193.2g of copper nitrate trihydrate and dissolve them in 500g of water, drop them into the sodium carbonate solution through a constant pressure dropping funnel, after the addition is complete, the pH is 7.5, continue to react for 1h, and cool naturally Aged for 2 hours, then washed twice and filtered with suction;
[0047] S3. The precipitate after suction filtration was dried at 90° C. for 8 hours, and then calcined at 400° C. for 4 hours. Characterized by BET, the specific surface area of the catalyst is 17.2m 2 / g, pore size 17.2nm.
[0048] (2) Utilize above-mentioned copper-zinc cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com