Method for preparing (S)- or (R)-2-aminobutanamide through transaminase biocatalysis
A technology of aminobutanamide and biocatalysis, which is applied in the direction of fermentation, etc., can solve the problems of large environmental pollution, low S-2-aminobutanamide ee value, high cost, etc., and achieve low product yield and high reaction selectivity , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In a 25ml three-neck round bottom flask, add 1.5ml of water, then add 0.2ml of DMSO, then measure 0.5mmol of methyl ethyl ketone into the round bottom flask, stir well, then add 2mmol of ammonium chloride, 2mmol of NaHCO 3 , and then added 0.01 mmol of tetrabutylammonium iodide, condensed and refluxed at 50°C for half an hour. Then, a hydrogen peroxide solution (containing 2.5 mmol of H 2 o 2 , 1ml of water), the dropwise addition was completed in 2 hours. After dripping, reflux reaction was carried out at 50°C for 16 hours, during which time the progress of the reaction was monitored by TLC silica gel plate or high performance liquid chromatography. After the reaction was completed, 20 mL of ethyl acetate was added for extraction, and then the ethyl acetate was removed under reduced pressure to obtain a yellow solid. The obtained yellow solid was purified, and then its structure was identified, and its NMR data are as follows:
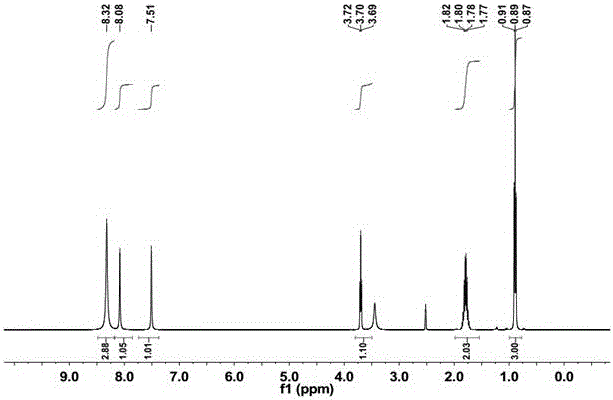
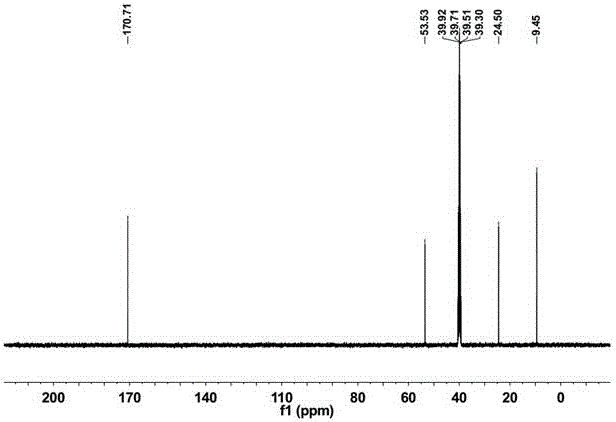
[0047] 1 H NMR (400 MHz, CDCl 3 )...
Embodiment 2
[0050] In a 25ml three-neck round bottom flask, add 1.5ml of water, then add 0.2ml of DMSO, then measure 0.5mmol of methyl ethyl ketone into the round bottom flask, stir well, then add 2mmol of ammonium chloride, 2mmol of NaHCO 3 , and then added 0.01 mmol of tetrabutylammonium iodide, condensed and refluxed at 70°C for half an hour. Then, a hydrogen peroxide solution (containing 2.5 mmol of H 2 o 2 , 1ml of water), the dropwise addition was completed in 2 hours. After dripping, the reaction was condensed and refluxed at 70° C. for 16 hours, during which time the progress of the reaction was monitored by TLC silica gel plate or high performance liquid chromatography. After the reaction was completed, 20 mL of ethyl acetate was added for extraction, and then the ethyl acetate was removed under reduced pressure to obtain a yellow solid, which was 2-carbonylbutyramide. In terms of butanone, the yield of 2-carbonylbutyramide is 80%, and the purity of the product tested by HPLC...
Embodiment 3
[0052] In a 25ml three-neck round bottom flask, add 1.5ml of water, then add 0.2ml of DMSO, then measure 0.5mmol of methyl ethyl ketone into the round bottom flask, stir well, then add 2mmol of ammonium chloride, 2mmol of K 2 CO 3 , and then added 0.01 mmol of CuI, condensed and refluxed at 70°C for half an hour. Then, a hydrogen peroxide solution (containing 2.5 mmol of H 2 o 2 , 1ml of water), the dropwise addition was completed in 2 hours. After dripping, it was condensed and refluxed at 70°C for 14 hours. During the period, the progress of the reaction was monitored by TLC silica gel plate or high performance liquid chromatography. After the reaction was completed, 20 mL of ethyl acetate was added for extraction, and then the ethyl acetate was removed under reduced pressure to obtain a yellow solid, which was 2-carbonylbutyramide. In terms of butanone, the yield of 2-carbonylbutyramide is 73%, and the purity of the product tested by HPLC is 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com