Compounds and preparation methods thereof, and uses of compounds in synthesis of brivaracetam
A compound and reaction technology, applied in the field of drug synthesis, to achieve high quality and avoid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
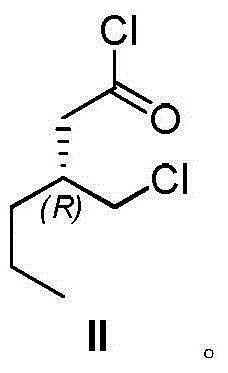
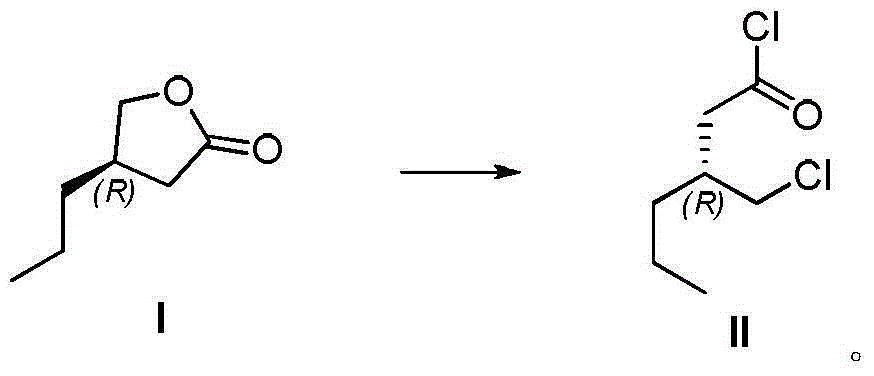
[0048] Embodiment 1 preparation formula II compound
[0049] Anhydrous zinc chloride (2.5 g, 18.3 mmol) was added to the reaction flask containing 20 mL of thionyl chloride, and the compound of formula I (12.0 g, 93.7 mmol) was added. Put the reaction bottle in an oil bath at 55°C for reaction, and stop the reaction when there is no raw material left after detection by gas chromatography (detection by gas chromatography), then stop the reaction, cool the system to room temperature, spin out thionyl chloride, and then distill under reduced pressure to obtain a yellowish liquid. Formula II compound, yield 68%. The nuclear magnetic data of formula II compound is as follows: 1 H NMR (400MHz, CDCl 3 ): δ3.67(1H,dd), 3.59(1H,dd), 2.58(1H,dd), 2.40(1H,dd), 2.20-2.31(1H,m), 1.25-1.53(4H,m), 0.93(3H,t).
[0050] The specific rotation of compound II is: [α] 23 D =+2.9 (C=10, CHCl 3 )
Embodiment 2
[0051] Embodiment 2 preparation formula II compound
[0052] Anhydrous zinc chloride (40 g, 0.29 mol) was added to the reaction flask containing 400 mL of thionyl chloride, and the compound of formula I (188 g, 1.47 mol) was added. Put the reaction bottle in an oil bath at 85°C for reaction. After GC detects that there are no remaining raw materials, stop the reaction, cool the system to room temperature, spin out thionyl chloride, and then distill under reduced pressure to obtain the compound of formula II as a light yellow liquid. The yield is 63.5%.
[0053] The nuclear magnetic data of formula II compound is as follows: 1 H NMR (400MHz, CDCl 3 ): 1 HNMR (400MHz, CDCl 3 ): δ3.67(1H,dd), 3.59(1H,dd), 2.58(1H,dd), 2.40(1H,dd), 2.20-2.31(1H,m), 1.25-1.53(4H,m), 0.93(3H,t).
[0054] The specific rotation of compound II is: [α] 23 D =+2.9 (C=10, CHCl 3 )
Embodiment 3
[0055] Embodiment 3 preparation formula IV compound
[0056] Add the compound of formula III (1.67g, 12mol) (purchased from Beijing Coupling Technology Co., Ltd.) into 40mL of dry dichloromethane, add triethylamine (2.43g, 24mmol), stir at room temperature for 30 minutes, then add formula II dropwise Compound (2.0 g, 10.8 mmol), after the dropwise addition was completed, stirred at room temperature until no raw material was detected by TLC. Add 30mL water, 4mL ethanol, extract and separate the organic phase, extract twice with 40mL dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, after drying, filter, and concentrate the filtrate to obtain the crude product of the compound of formula IV, the yield is 96.7% .
[0057] Purification by column chromatography (developing solvent polarity: ethyl acetate 100%) obtains the NMR data of the purified formula IV compound as follows: 1 H NMR (400MHz, CDCl 3 )δ6.20-6.45 (2H, m), 5.69 (1H, brs), 4.46 (1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com