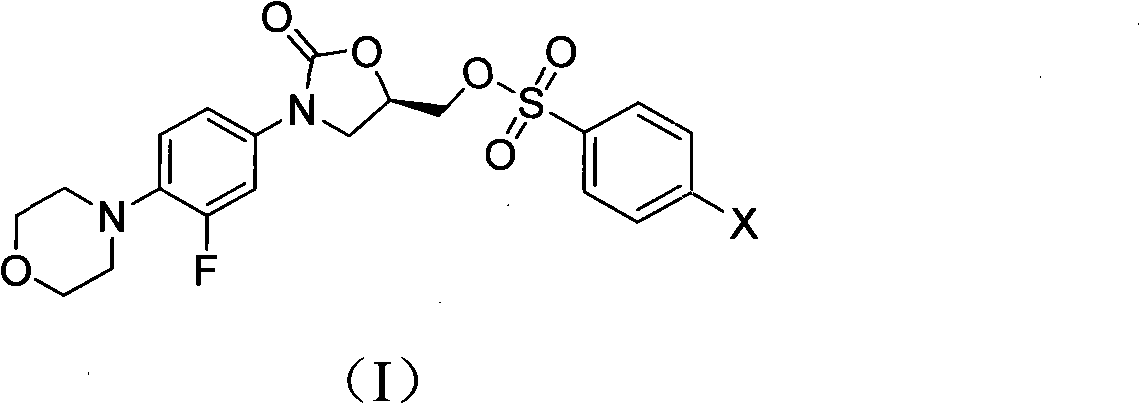
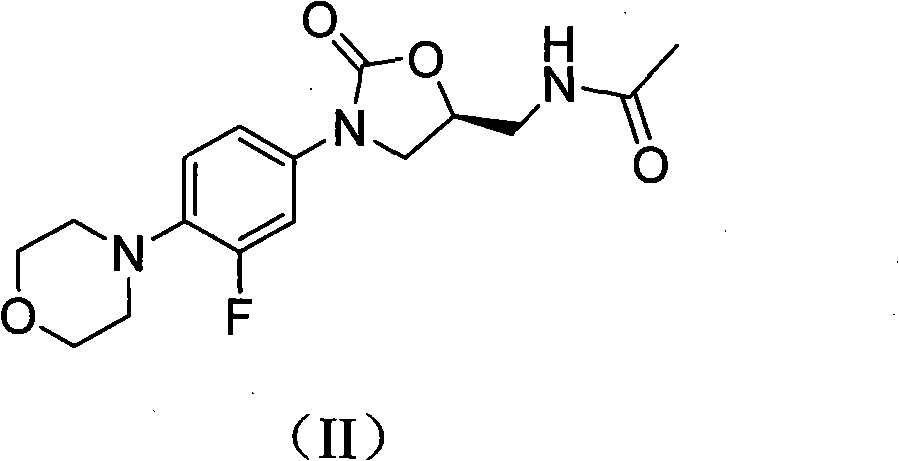
Novel Linezolid intermediate, its preparation method and novel preparation method of Linezolid
A technology of linezolid and intermediates, applied in the direction of organic chemistry, can solve the problems of high cost, low yield, harsh reaction conditions, etc., and achieve the effects of low cost, reduction of hydrolyzed products, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0036] Preparation of embodiment 13-fluoro-4-morpholinyl nitrobenzene
[0037] Add morpholine (3.50 g, 40.1 mmol), triethylamine (4.05 g, 40.1 mmol) and ethyl acetate (30 mL) into the three-neck flask and stir. Add 3,4-difluoronitrobenzene (5.80 g, 36.5 mmol) dropwise, and react at room temperature for 17 hours. TLC monitoring showed that the reaction was completed, filtered, and the filter cake was washed with water to obtain 7.39 g of the product 3-fluoro-4-morpholinylnitrobenzene in the form of yellow crystals, with a yield of 89.7%. 1 H NMR (CDCl 3 ): δ3.27 (4H, m), 3.87 (4H, m), 6.92 (1H, t, 7.0), 7.90 (1H, m), 7.98 (1H, m).
Embodiment 23
[0038] Preparation of embodiment 23-fluoro-4-morpholinylaniline
[0039] Add 3-fluoro-4-morpholinylnitrobenzene (5.14g, 22.7mmol), NH 4 Cl (6.22g, 116.0mmol) aqueous solution, ethanol and reduced iron powder (4.46g, 79.5mmol), stirred, heated to reflux reaction, TLC monitoring, the reaction was completed, filtered, the filtrate was concentrated, the residue was cooled and filtered, filtered The cake was washed with water and dried to obtain 4.12 g of brown powdery solid product 3-fluoro-4-morpholinoaniline, yield: 92.6%. 1 H NMR (CDCl 3 ): δ3.05 (4H, m), 3.61 (4H, m), 3.89 (4H, m), 6.43 (2H, m), 6.83 (1H, m).
Embodiment 3
[0040] The preparation of embodiment 3N-benzyloxycarbonyl-3-fluoro-4-morpholinylaniline
[0041] Add 3-fluoro-4-morpholinylaniline (4.42g, 22.4mmol), NaHCO 3 (3.53g, 42.0mmol), acetone and water, stirred, and added benzyl chloroformate (CBZ-Cl) (4.42g, 25.8mmol) dropwise in an ice-salt bath, and the dropwise addition was completed. The system was heated to room temperature and reacted. TLC monitoring, the reaction is complete. The reaction solution was poured into water and stirred, filtered, the filter cake was washed with water, and dried to obtain a crude product, which was recrystallized to obtain 6.69 g of light orange product N-benzyloxycarbonyl-3-fluoro-4-morpholinoaniline, yield: 89.9%, Chemical purity: 99.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com