Synthesis method for compound and application thereof in field of medicines used for improving insulin resistance
A synthesis method and compound technology, applied in the field of improving insulin resistance drugs, in the field of compound synthesis, can solve the problems of low synthesis yield and complicated purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example is one of the specific examples of synthesizing SN158.
[0037] 1.1 Step i—preparation of intermediate product 1 (5-bromo-2,4-hydroxybenzaldehyde)
[0038] 2,4-Dihydroxybenzaldehyde (10 g, 72.4 mmol) was dissolved in acetic acid, and the reaction was placed in an ice bath. Under stirring condition, liquid bromine (3.78ml, 72.4mmol) was slowly added dropwise, and the reaction temperature was slowly raised to room temperature. After reacting for 3 hours, the reaction solution was poured into 100 mL of cold water, filtered, washed with 100 mL of cold water, dried, and recrystallized with 1:1 acetonitrile / toluene to obtain 13.09 g of intermediate product 1 crystals (yield: 83%).
[0039] Structural characterization of intermediate product 1:
[0040] Melting point: 165-168°C.
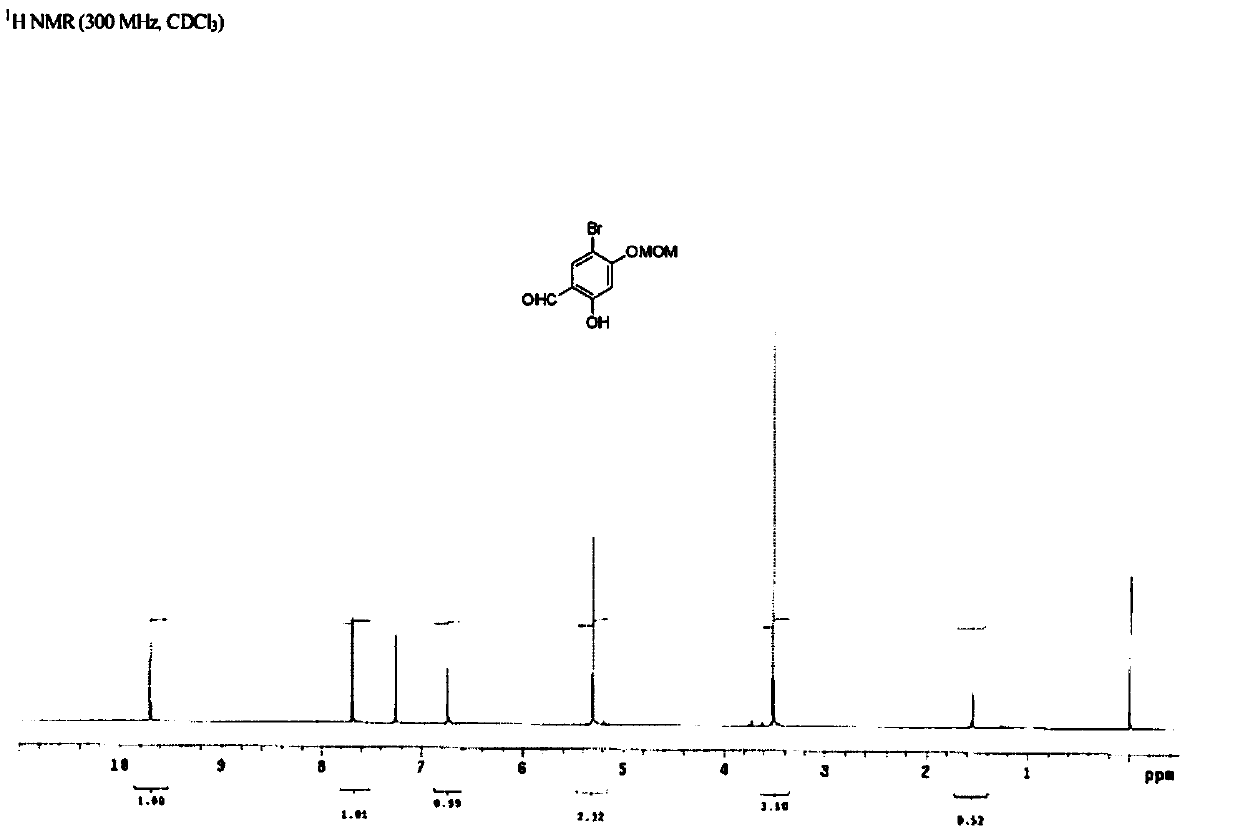
[0041] 1.2 Step ii—Preparation of intermediate product 2 (5-bromo-2-hydroxyl-4-(methoxymethyl)benzaldehyde)
[0042] Intermediate 1 (10.9 g, 50 mmol) and potassium carbonate (20.8 g,...
Embodiment 2
[0069] This example is another specific example of synthesizing SN158.
[0070] 2.1 Step i—preparation of intermediate product 1 (5-bromo-2,4-hydroxybenzaldehyde)
[0071] 2,4-Dihydroxybenzaldehyde (10 g, 72.4 mmol) was dissolved in acetic acid, and the reaction was placed in an ice bath. Under stirring condition, liquid bromine (3.78ml, 72.4mmol) was slowly added dropwise, and the reaction temperature was slowly raised to room temperature. After reacting for 3 hours, the reaction solution was poured into 100 mL of cold water, filtered, washed with 100 mL of cold water, dried, and recrystallized with 1:1 acetonitrile / toluene to obtain 13.09 g of intermediate product 1 crystals (yield: 83%).
[0072] Structural characterization of intermediate product 1:
[0073] Melting point: 165-168°C.
[0074] 2.2 Step ii—Preparation of intermediate product 2 (5-bromo-2-hydroxyl-4-(methoxymethyl)benzaldehyde)
[0075] Intermediate 1 (10.9 g, 50 mmol) and potassium carbonate (20.8 g, 150...
Embodiment 3
[0115] This example is a specific example for verifying that the prepared SN159 improves insulin resistance.
[0116] C2C12 myoblasts were differentiated into myotubes over 4 days in DMEM medium with 2% horse serum. They were then cultured in DMEM containing 2% BSA and 10% FBS for 16 hours, and controlled for normal or insulin-resistant conditions by adding or not adding 0.75 mM palmitate.
[0117] After being stimulated by insulin for 1 hour, the myotubes were incubated with 50 μM 2-NBDG (purchased from Invitrogen) for 15 minutes, and washed 3 times with PBS to remove free 2-NBDG. In the Infinite M1000 microplate reader (TECKAN, Switzerland) to measure the fluorescence intensity of 2-NBDG entering the cell, the excitation wavelength is 485nm, and the emission wavelength is 535nm.
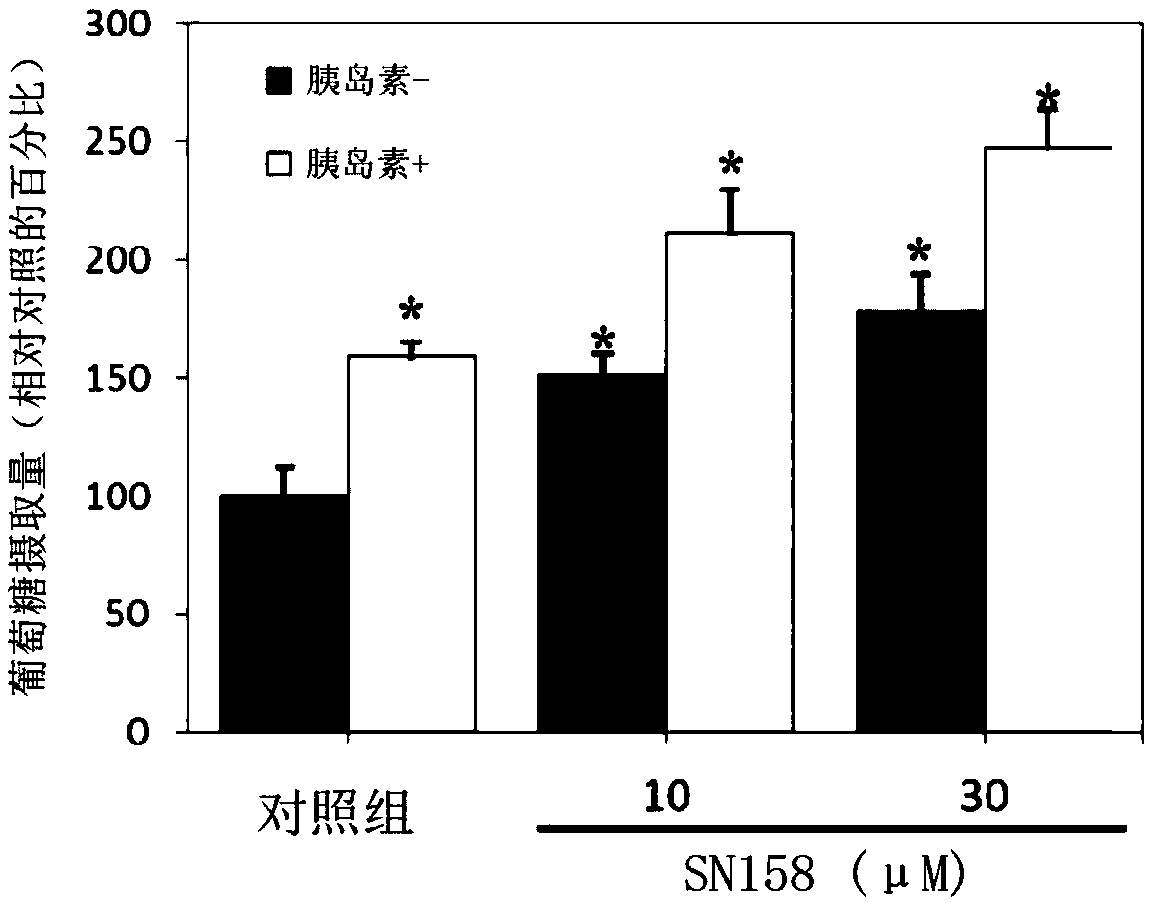
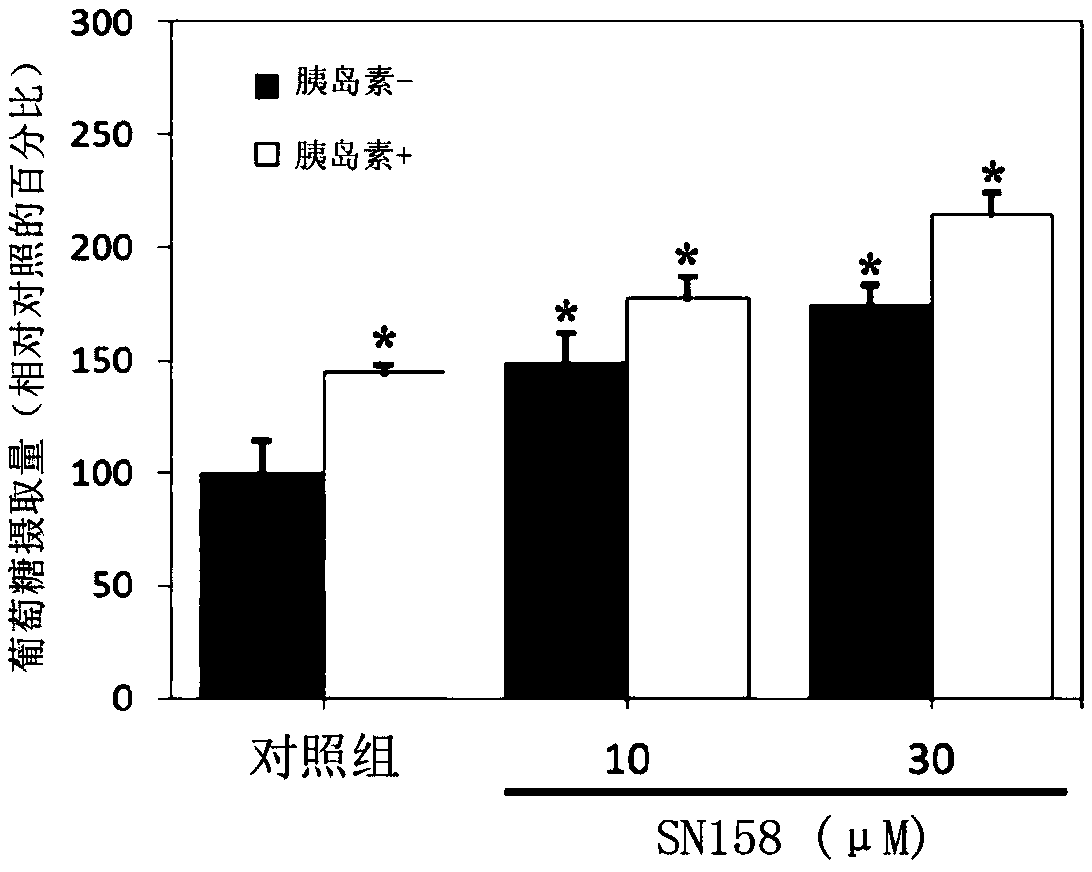
[0118] For the experimental results obtained, please refer to figure 1 and figure 2 ,in, figure 1 is the effect of compound SN159 on the glucose uptake of C2C12 myoblasts under normal condit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com