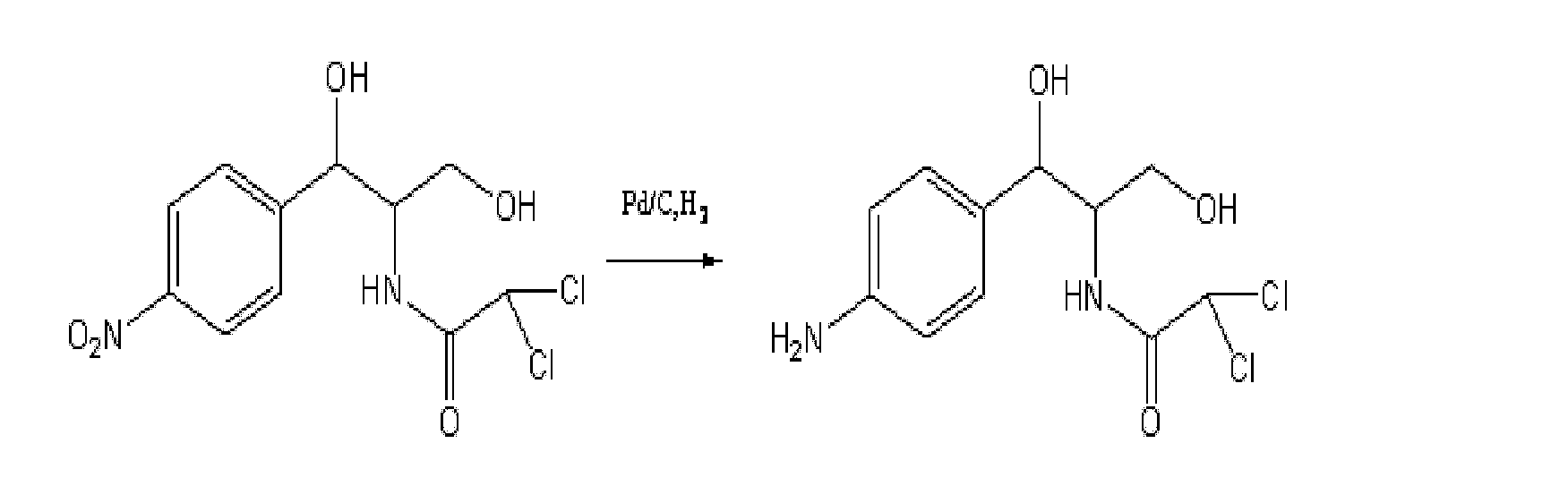
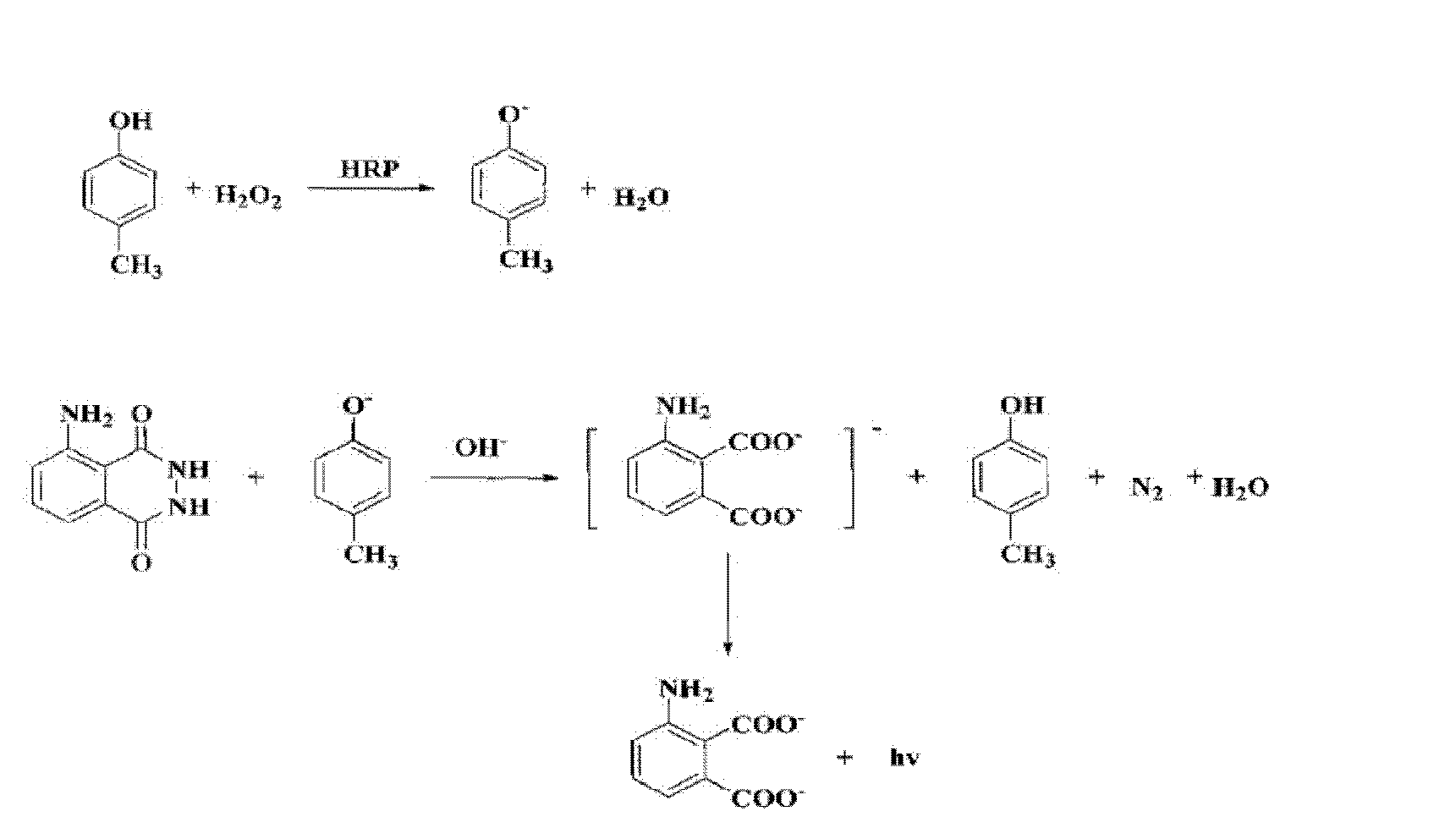
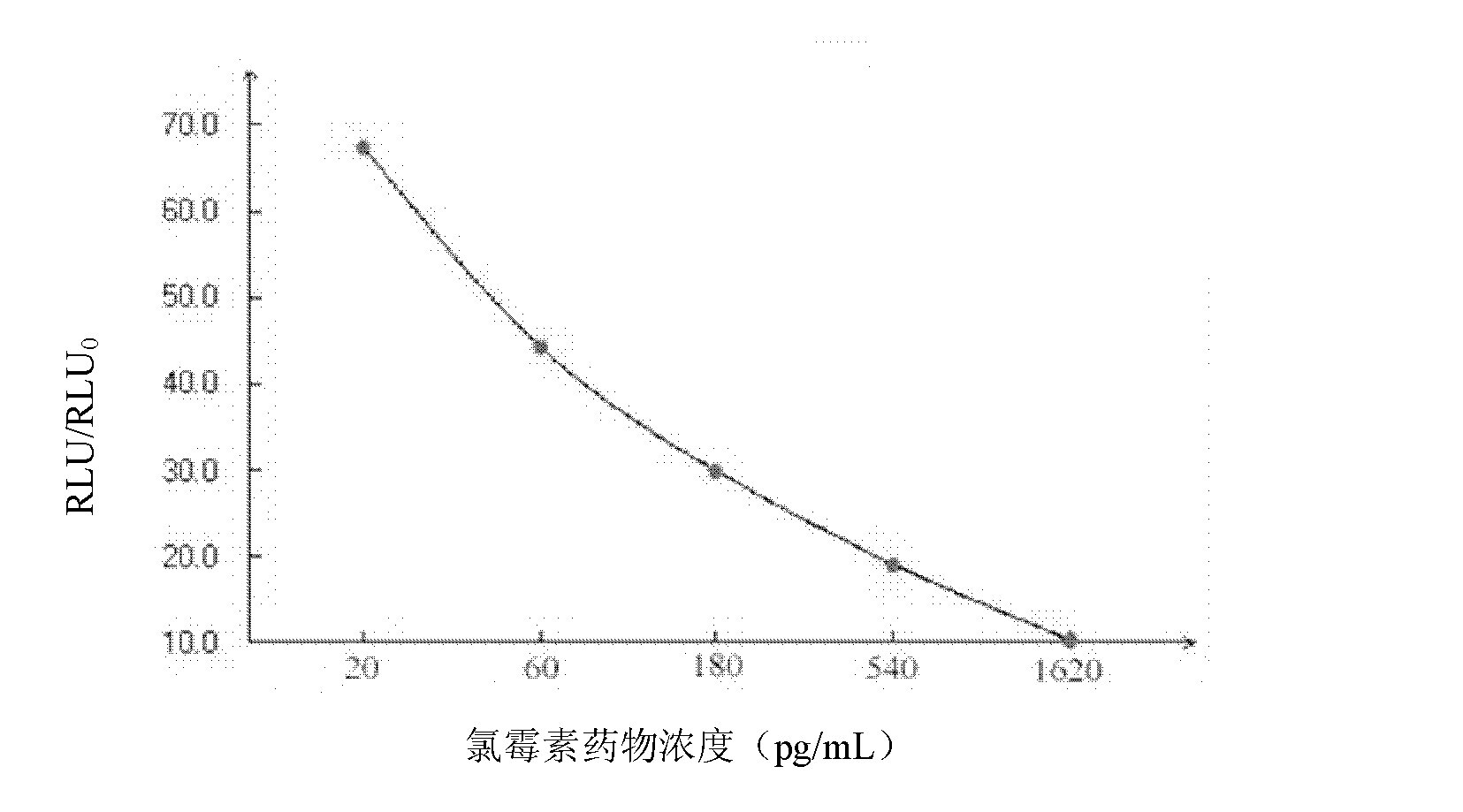
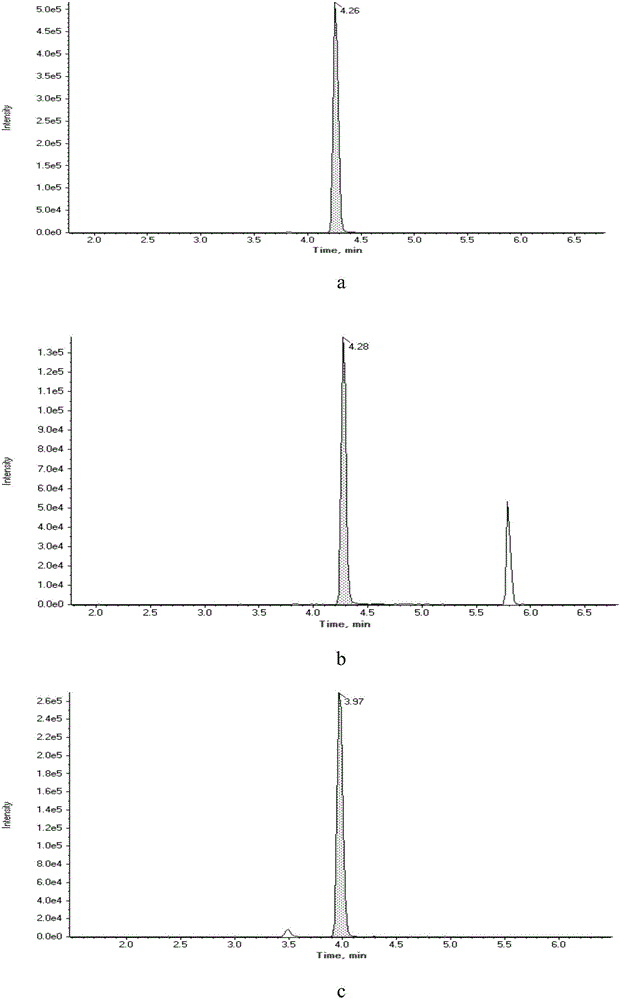
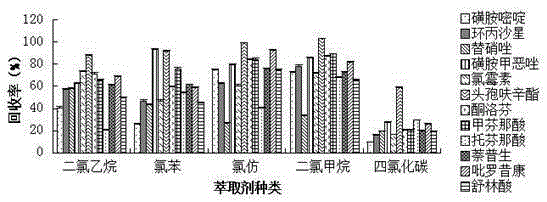
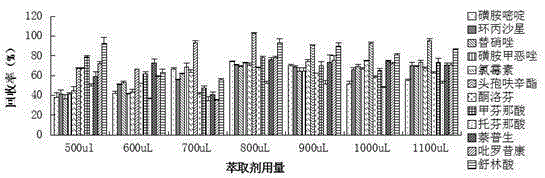
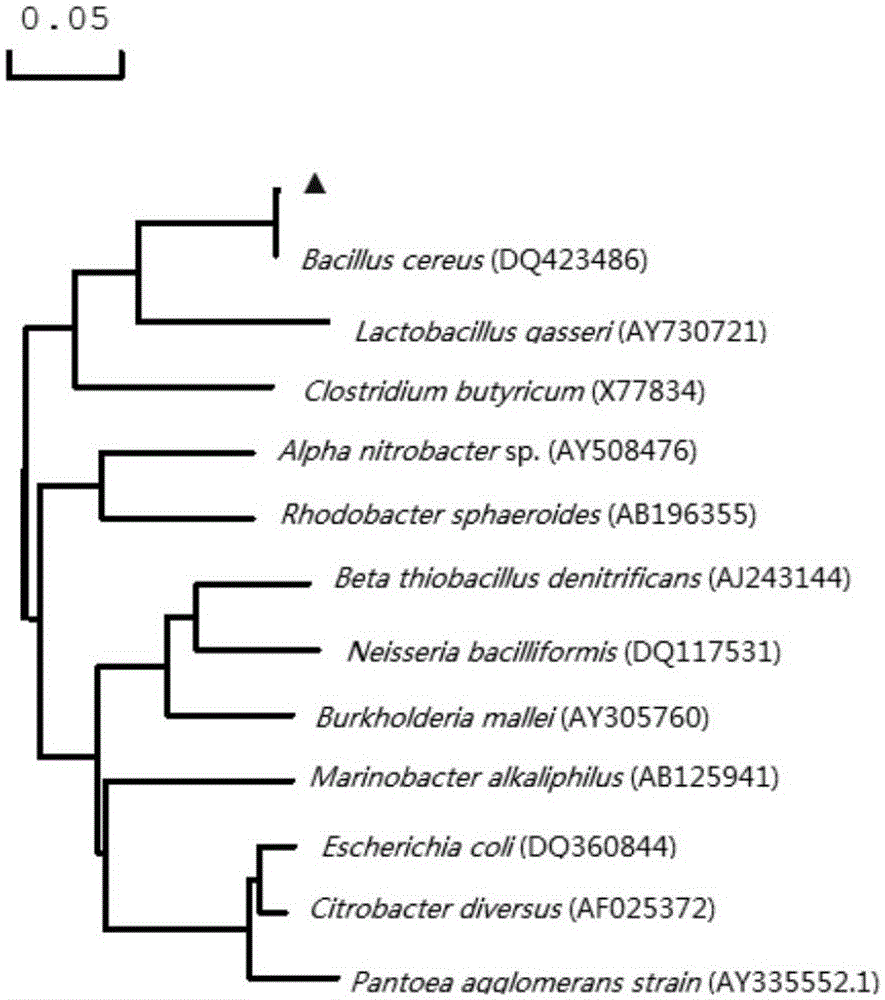
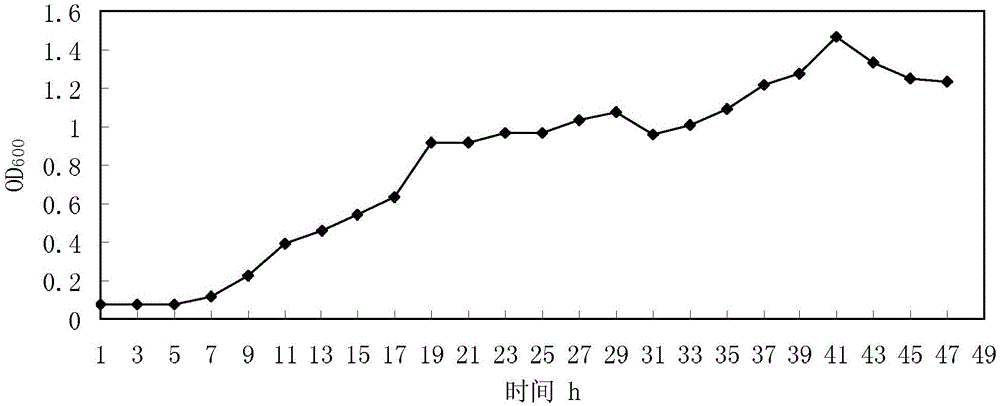
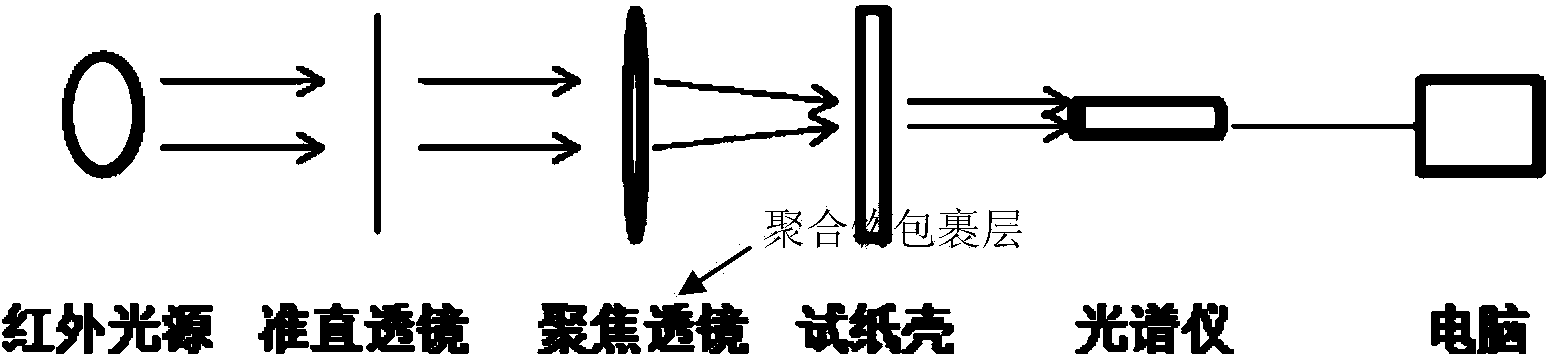
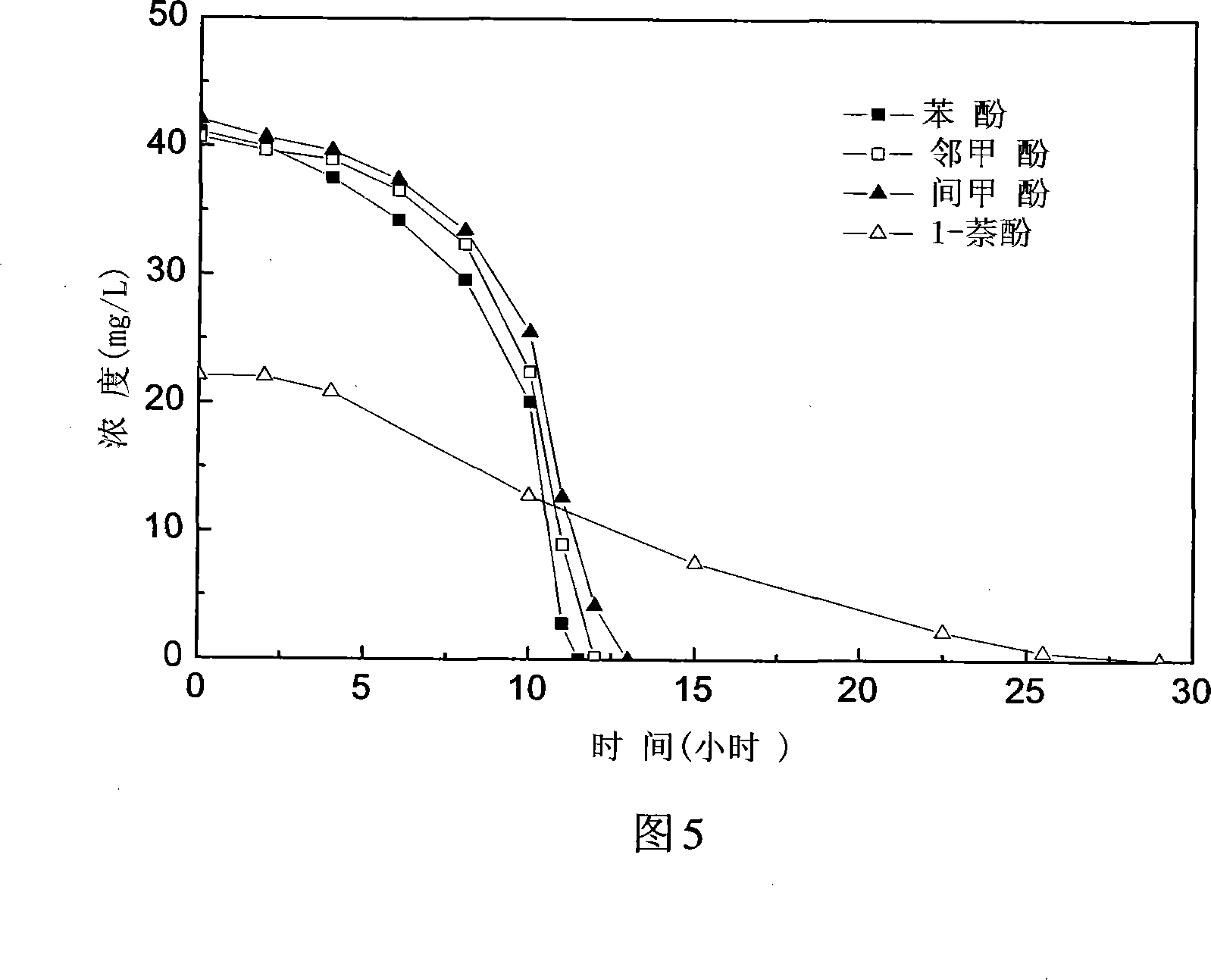Patents
Literature
510 results about "CHLORAMPHENICOL TOXICITY" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chloramphenicol may cause bone marrow suppression during treatment; this is a direct toxic effect of the drug on human mitochondria. This effect manifests first as a fall in hemoglobin levels, which occurs quite predictably once a cumulative dose of 20 g has been given.
Chloramphenicol chemiluminescence enzyme-linked immunodetection kit
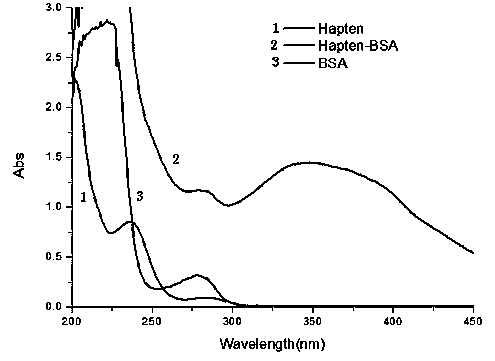
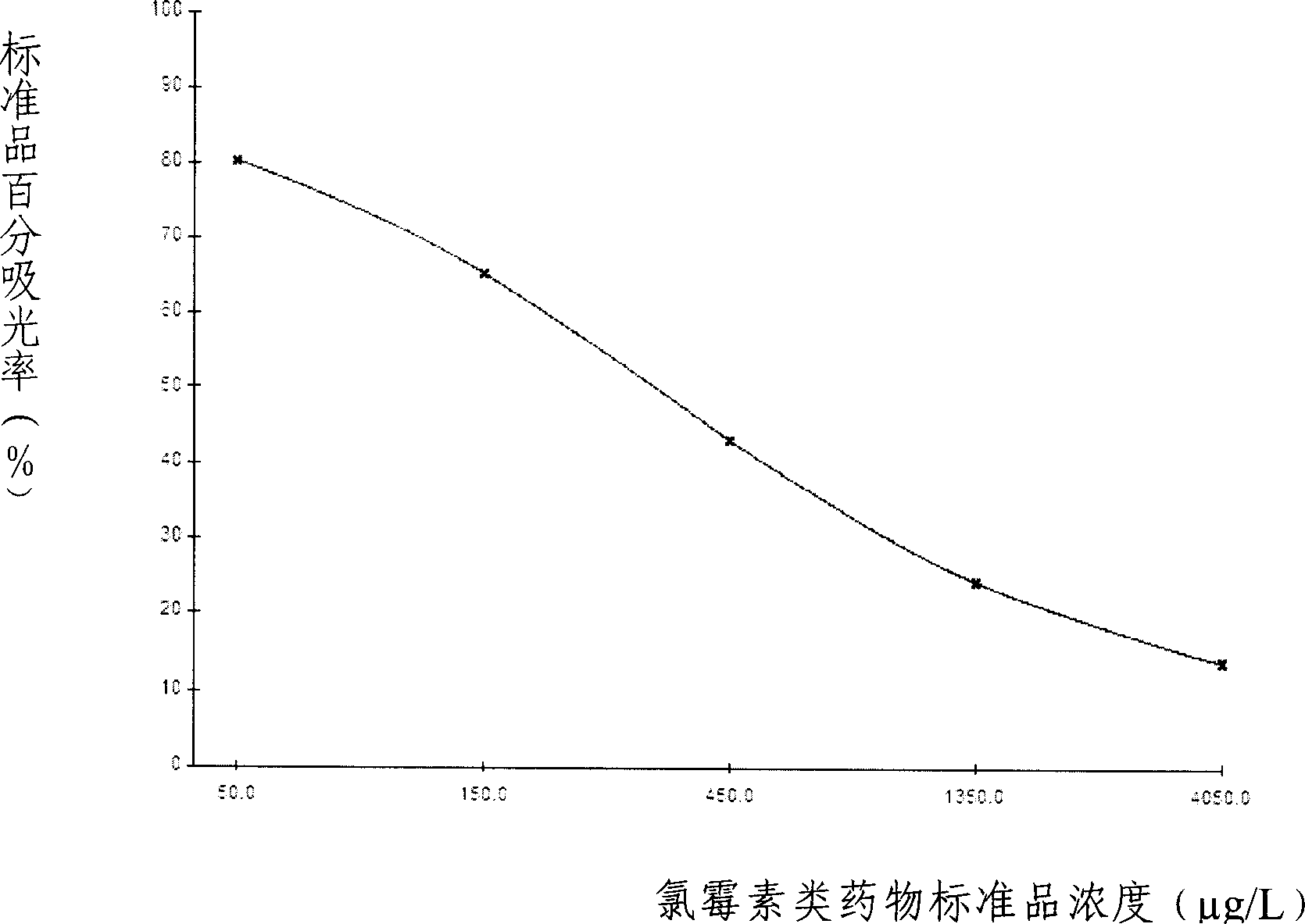
The present invention discloses a chloramphenicol chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a chloramphenicol and carrier protein conjugate, and the reagents comprise horseradish peroxidase-labeled chloramphenicol monoclonal antibody, a series of chloramphenicol standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The chloramphenicol chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, and high accuracy, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of chloramphenicol residues in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp) and milk.
Owner:BEIJING KWINBON BIOTECH
Detection method of multiresidue of 5 nitrofuran metabolites and chloramphenicol in shrimp
ActiveCN106124653AImprove extraction efficiencyOvercoming the limitations of separate assaysComponent separationMetaboliteQuantitative accuracy
A detection method of a nitrofuran metabolites and chloramphenicol multiresidue in shrimp belongs to the technical field of aquatic products detection. The method is as below: hydrolyzing a sample with hydrochloric acid, subjecting nitrofuran metabolites to derivatization by using 2-nitrobenzaldehyde, adjusting the pH value to 6.5-7.5, adding acetonitrile, adding an extraction salt package; conducting liquid-liquid extraction of a target compound by using acetonitrile; purifying and concentrating a supernatant; then determining by a liquid chromatography-tandem mass spectrometer, and quantifying by an internal standard method. The method uses a novel sample extraction and purification mode for simultaneous analysis and determination of two prohibited drugs with the highest detection rate in shrimp, overcomes the limitations of separate determination of two drugs in the detection method of the prior art, improves the extraction efficiency of chloramphenicol in actual positive samples, greatly improves the work efficiency, shortens the work time and saves reagent consumption and labor costs. The method adopts the isotope internal standard method for quantification, the determination result is more accurate and reliable, has high sensitivity, and good reproducibility and quantitative accuracy.
Owner:青岛菲优特检测有限公司
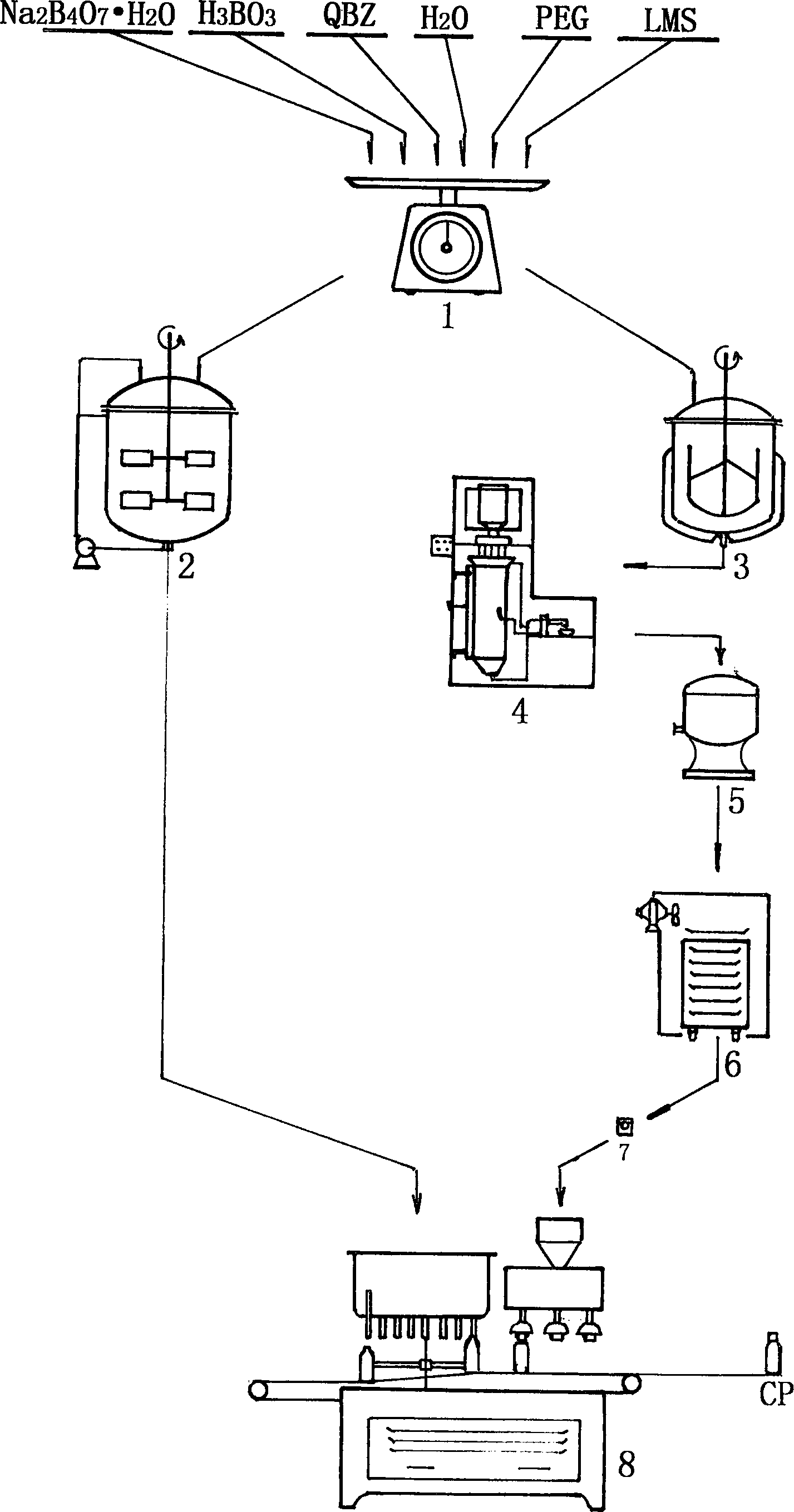
Drop pill type eye drip fluid of chloramphenicol, and preparation method
InactiveCN1872038AEnsure stable qualityImprove stabilityOrganic active ingredientsSenses disorderDiseasePolyethylene glycol
A dripping pill of Chloromycetin eye drops for treating trachnoma, keratitis, conjunctivitis, eyelid inflammation, etc is proportionally prepared from polyethanediol as matrix and Chloromycetin. It is applied in conjunction with the buffer liquid prepared from borax, boric acid, hydroxyphenyl ethylether and the water for injection. Its preparing process is also disclosed.
Owner:济宁光明制药有限公司
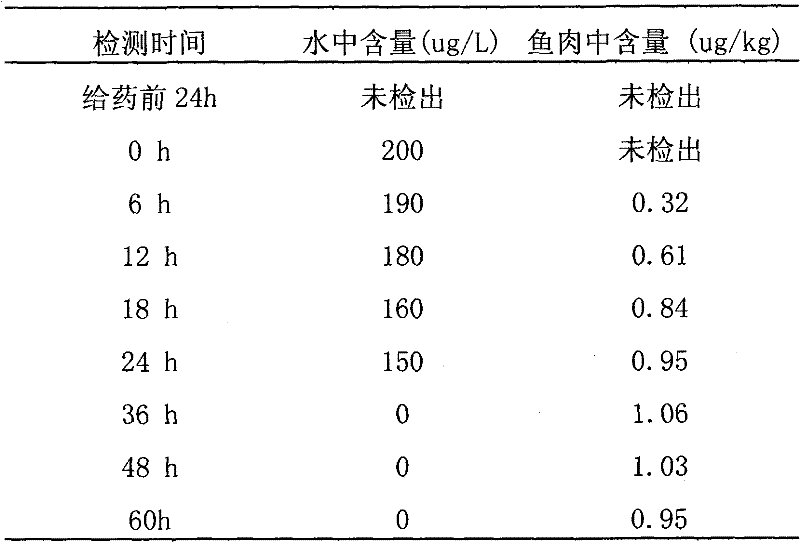
A kind of preparation method of chloramphenicol residual freeze-dried powder standard sample in carp muscle
InactiveCN102288464AGuaranteed uniformityGuaranteed stabilityPreparing sample for investigationFreeze-dryingCarp
The invention provides a preparation method for a standard sample of chloramphenicol residual matrix lyophiled powder in the muscle of a carp, which belongs to the technical field of animal food matrix standard substances in the residual detection of veterinary medicines. The problem that the bigger difference exists between the result of treatments such as extraction, purification and the like and a daily analytical sample as the condition of combining a target object with a matrix and a really detected sample are not consistent completely is solved. The method comprises the following steps of: carrying out cultivation and addition on a live carp by adopting chloramphenicol, so that the chloramphenicol is contained in carp in vivo, culturing the carp in water which does not contain a medicine until the medicine concentration in vivo reaches a stable metabolic state, fishing the carp, quickly freezing the carp to a temperature below -18 DEG C, unfreezing the frozen carp at a room temperature, taking a muscle part to homogenate to make into minced fillets, freezing, drying, screening and bottling the minced fillets, and encapsulating the minced fillets in a vacuum way, so as to prepare and obtain the standard sample of the chloramphenicol residual lyophiled powder in the muscle of the carp. The chloramphenicol concentration in the prepared standard sample is 1.0-10.0 [mu]g / kg.
Owner:时文春
Preparation method of lignin hierarchical porous carbon material with endellite as template
InactiveCN105664848ARich reservesLarge specific surface areaOther chemical processesWater contaminantsPotassium hydroxideBiological activation
The invention relates to a method for preparing a lignin-based hierarchical porous carbon material with halloysite as a template, which belongs to the technical field of environmental functional material preparation; the invention uses halloysite nanotubes, a natural mineral that is abundant in reserves and cheap and easy to obtain, as a template ; Sodium lignosulfonate and halloysite are mixed by liquid phase impregnation; then calcined, the template carbon material is initially obtained, which contains more mesopores and macropores; pores, and further obtain hierarchical porous carbon materials; the hierarchical porous carbon materials prepared by this method have been successfully applied to the efficient separation of tetracycline and chloramphenicol in aqueous environments, and have excellent regeneration performance.
Owner:JIANGSU UNIV
Method for determining 10 kinds of antibiotics in water environment through combination of sample pre-treatment technology and HPLC-MS
The present invention relates to a method for determining 10 kinds of antibiotics in a water environment through combination of a sample pre-treatment technology and HPLC-MS, and belongs to the field of detection of safety of trace organic contaminant residue in the water environment. The method is characterized in that a water sample is separated and enriched through combination of solid phase extraction and dispersive liquid-liquid microextraction (SPE-DLLME), and then an ultra-high performance liquid chromatography-mass spectrometry instrument (UPLC-MS / MS) is adopted as a detection tool to directly determine the contents of 10 kinds of common antibiotics in the water environment (drinking water, tap water, river water, sewage treatment plant influent and effluent), wherein the 10 kinds of the common antibiotics respectively are sulfadiazine, sulfamethoxazole, oxytetracycline, tetracycline, doxycycline, ciprofloxacin, levofloxacin, chloramphenicol, cefuroxime axetil and tinidazole. According to the present invention, the water sample pre-treatment method and the instrument detection conditions are investigated and optimized, and the optimal SPE-DLLME-UPLC-MS / MS method is established and is successfully applied for the real sample determination; and compared with the traditional method, the method of the present invention has advantages of high sensitivity, high extraction recovery rate, wide application objects, environmental protection, and the like.
Owner:SHENYANG PHARMA UNIVERSITY
Simultaneous detection method of fluoroquinolones medicines and chloramphenicols medicines in food
InactiveCN101957348ARapid Quantitative and Qualitative AnalysisEasy to handleComponent separationMaterial analysis by electric/magnetic meansFluoro quinolonesMedicine
The invention builds a liquid chromatograph-mass spectrometer database of 13 types of floxacins medicines and 3 types of chloramphenicols medicines, and an LC-MS / MS detection method. The method has the advantages of simple preprocessing and good repeatability. Combining with the chromatograph-mass spectrometer database built in the invention, the method can realize the rapid quantitative and qualitative analysis of 13 types of the fluoroquinolones medicines and 3 types of the chloramphenicols medicines, the analysis time is shortened by a half, and the method is extremely suitable for the import and export rapid confirmation work.
Owner:中华人民共和国珠海出入境检验检疫局
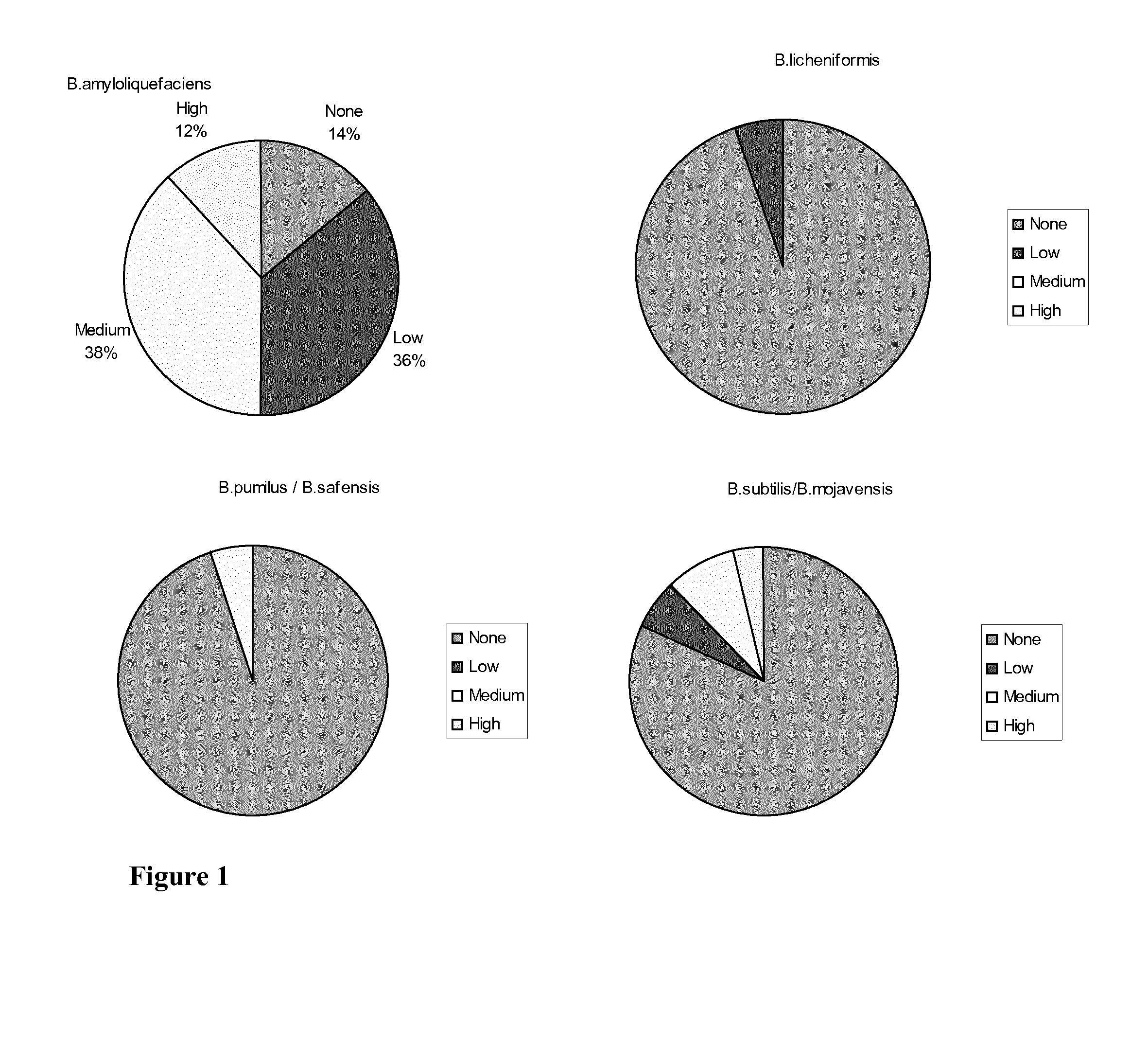
Antibiotic sensitive bacillus strains having antimicrobial effect against e. coli and clostridium perfringens and having high sporulation capacity
A Bacillus strain characterized by (i): sensitivity for ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol; (ii) antimicrobial activity against E. coli and Clostridium perfringens; and (iii) a sporulation percentage of at least 80 when measured after 2 days of incubation. The invention further relates to a method for selecting such strains. Many of the identified strains according to the invention are of the species Bacillus amyloliquefaciens. Some of the Bacillus amyloliquefaciens were further identified as Bacillus amyloliquefaciens subsp. amyloliquefaciens whereas others were identified as amyloliquefaciens subsp. plantarum. A Bacillus strain of the invention may be used as a feed additive to animal feed where it has a probiotic effect.
Owner:CHR HANSEN AS
Chinese and western compound medicine for treating stomach disease
InactiveCN1698813AAdjust the spleen and stomachShort treatment timeDigestive systemBird material medical ingredientsAbdomen diseasesStomach cancer
The invention discloses a Chinese and western compound medicine for treating stomach diseases, which comprises seventeen traditional Chinese medicinal materials including pilose asiabell root, white atractylodes rhizome, Poria cocos, chicken's gizzard-skin, astragalus root, licorice root, haw, medicated leaven and malt, and a small number of Western medicinal materials including chloramphenicol, terramicin and furazolidone.
Owner:张丽君
Bacillus subtilis strain for excreting nattokinase at high efficiency and preparation technology of high-purity nattokinase
InactiveCN107058204AIncrease enzyme activityBacteriaMicroorganism based processesRestriction enzyme digestionDigestion
The invention relates to a bacillus subtilis strain for excreting nattokinase at high efficiency. An aprN gene of bacillus subtilis NK is amplified by a PCR (polymerase chain reaction) technique; codons of initial thirty amino acids are optimized according to the codon preference of the bacillus subtilis, a recombination expression plasmid pHT01-aprN is established, and the correctness is verified through the restriction enzymes digestion, PCR amplification and sequencing. The recombination expression plasmid pHT01-aprN is imported into the bacillus subtilis 168 by an electric shocking method, and is performed with resistance screening by adopting chloramphenicol, so as to obtain engineering bacteria. After the fermenting condition is optimized, and the expression is induced by IPTG, the highest enzyme activity in a shake culture fermenting liquid is 289U / ml; the highest enzyme activity in a high-density fermenting liquid is 7778U / ml; the highest enzyme activity of a nattokinase preparation prepared by affinity column chromatography is 981731U / g.
Owner:上海诺金科生物科技有限公司
Method for measuring 12 types of remaining medicine in water environment through separation and enrichment
ActiveCN105424825ASimplified processing stepsImprove extraction efficiencyComponent separationWater dischargePretreatment method
The invention relates to a method for measuring 12 types of remaining medicine in a water environment through separation and enrichment at the same time, and belongs to the field of safety detection of a trace of organic pollutant residue in the water environment. The content of 12 types of frequently-used medicine in the water environment (drinking water, faucet water, river water and water discharged into and out of sewage treatment plants) is directly measured with an ultra performance liquid-chromatography-mass spectrometer (UPLC-MS / MS) as a detection tool after a water sample is subjected to solid phase extraction combined with ultrasonic-assisted dispersion liquid-liquid micro-extraction (UA-DLLME) separation and enrichment. The 12 types of antibiotic include ketoprofen, ciprofloxacin, tinidazole, tolfenamic acid, sulfadiazine, sulindac, naproxen, sulfamethoxazole, chloramphenicol, cefuroxime axetil, piroxicam and mefenamic acid. Inspection and optimization are conducted on a sample pretreatment method and instrument detection conditions of the water sample, and the optimal UA-DLLME method is established and is successfully applied to practical sample detection. Compared with a traditional method, the method has the advantages of being high in sensitivity, high in extraction and recycle rate, wide in suitable object, friendly to the environment, and the like.
Owner:SHENYANG PHARMA UNIVERSITY +1
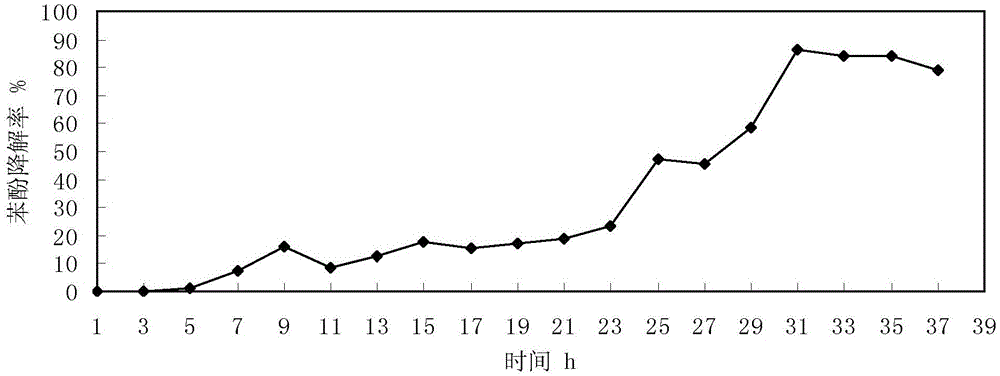
Bacillus cereus capable of degrading phenol and having electrogenesis characteristic and application thereof
The invention discloses bacillus cereus capable of degrading phenol and having an electrogenesis characteristic and application thereof and relates to bacillus cereus and application thereof. The bacillus cereus can degrade phenol and has the electrogenesis characteristic, and a foundation is laid for microorganism-utilized phenol treatment. The bacillus cereus capable of degrading the phenol is bacillus cereus WL027 and is preserved at the China Center for Type Culture Collection on July 10, 2015, and the preservation number is CCTCC No. M 2015439. The bacillus cereus WL027 can degrade phenol, has the electrogenesis characteristic, has good penicillin, chloramphenicol and tetracycline tolerance, has salt tolerance and can use aniline as a substrate. The bacillus cereus can be applied to microbial fuel cells.
Owner:HARBIN UNIV OF SCI & TECH
Compsns-and methods for trapping and inactivating pathogenic microbes and spermatozoa
Antimicrobial and contraceptive compositions and methods which prevent and / or reduce the risk of transmission of sexually transmitted diseases through sexual activity as well as prevent and / or reduce the risk of pregnancy are provided. The compositions contain (1) a matrix-forming agent, (2) a bio-adhesive agent, (3) a buffering agent, (4) optionally a humectant, (5) optionally a preservative, and (6) water; wherein the composition is suitable for application within the vagina; wherein the compositions form a semisolid matrix on contact with ejaculate (thereby trapping ejaculated microbes and spermatozoa); wherein the composition causes hardening of cervical mucus (thereby decreasing the probability of sperm entry); wherein the composition forms a bio-adhesive layer over vaginal surfaces (thereby preventing or reducing the risk of contact of STD-causing microbes with the vaginal surfaces); wherein the composition maintains an acidic vaginal pH of less than about 5 in the presence of semen ejaculated from the male; and wherein the composition does not significantly impair the natural microbiological balance within the vagina. The antimicrobial and contraceptive compositions may also contain additional antimicrobial and / or contraceptive agents (e.g., nonoxynol-9, octoxynol-9, benzalkonium chloride, phosphorylated hesperidins, sulfonated hesperidins, polystyrene sulfonates, substituted benzenesulfonic acid formaldehyde co-polymers, H2SO4-modified mandelic acids, povidone iodine, itraconazole, ketoconazole, metronidazole, clotrimazole, fluconazole, teraconazole, miconazole, tinidazole, iconazole, chloramphenicol, nystatin, cyclopiroxolamine, and the like).
Owner:RUSH UNIV MEDICAL CENT
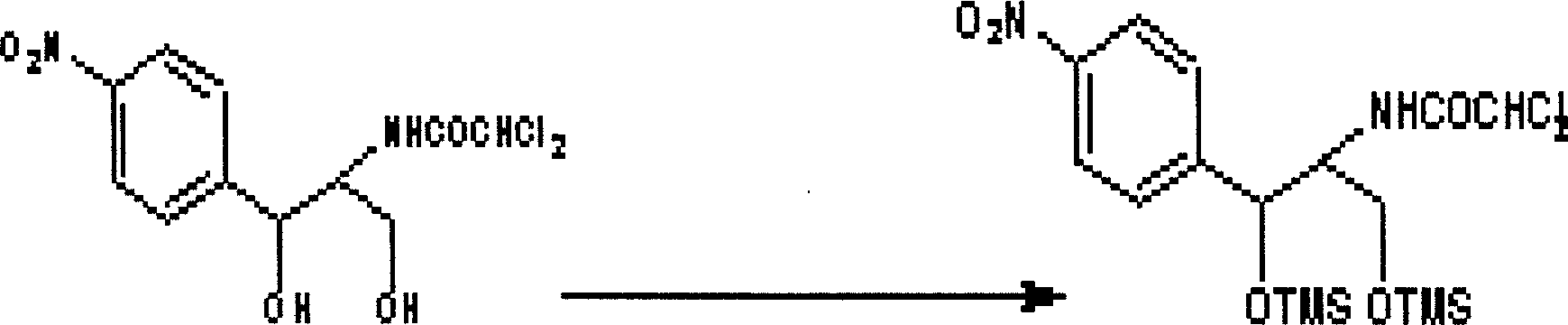
Chloramphenicol affinity column and preparation method and use thereof
InactiveCN1731182AHigh enrichment efficiencyImprove purification efficiencyMaterial analysisSepharoseSolvent
The invention relates to a method for preparing chloramphenicol affinant column and its usage, which adopts protein A -Sepharose 4 fast flow as phase carrier, the protein A of the carrier is coupled with the chloramphenicol antibody; the coupled chloramphenicol antibody is bonded with the fixed phase chemistry; the affinant column can suit for many stroma; it uses protein A so that IgG can be connected with it with high desirability and large load capacity of the chloramphenicol column.
Owner:王家红
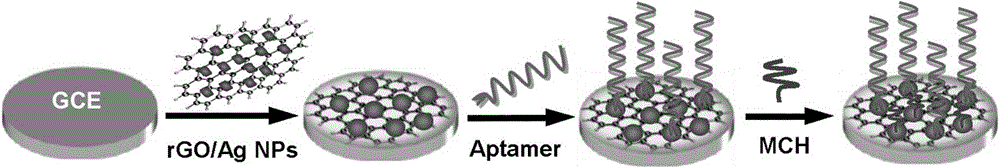
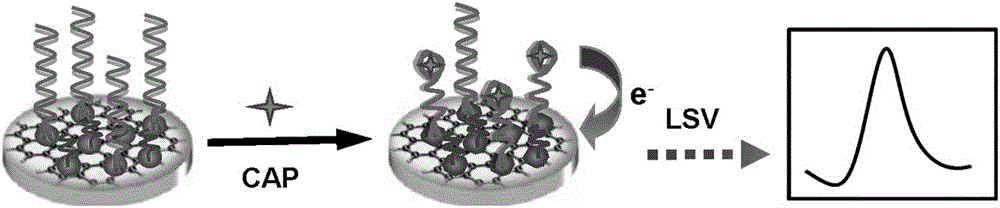
Electrochemical aptamer sensor for rapid detection of chloramphenicol
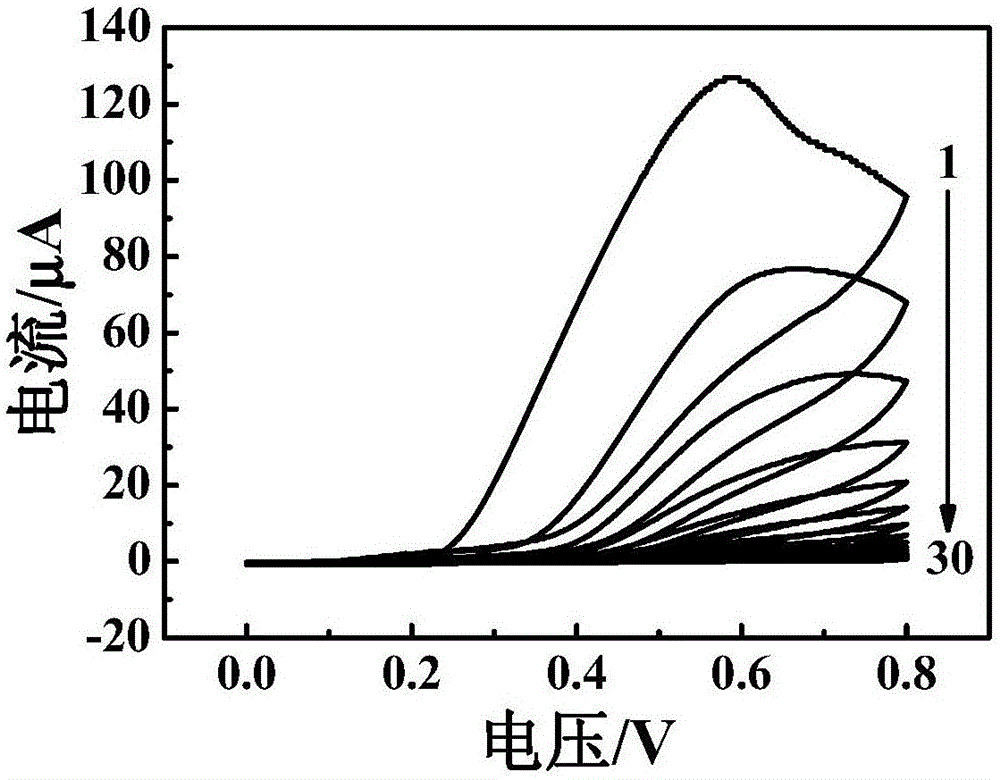
ActiveCN106198695AWide range of target moleculesHigh affinityMaterial electrochemical variablesSulfurCHLORAMPHENICOL TOXICITY
The invention discloses an electrochemical aptamer sensor for rapid detection of chloramphenicol. The electrochemical aptamer sensor is assembled by fixing an aptamer and silver nanoparticles on a nano-composite onto a glassy carbon electrode through a silver-sulfur bond. Chloramphenicol in a sample is quantitatively captured onto the surface of the sensor, and under the catalytic action of the nano-composite, an electrical signal is generated. The electrochemical aptamer sensor has advantages of high sensitivity, fast response, simple operation, low cost, and good selectivity. The invention is of great significance for widespread practical application of the aptamer sensor in fields of medical analysis and food safety, etc.
Owner:HUBEI NORMAL UNIV
Erythrocyte preservative solution
ActiveCN105028389APrevent invasionProtect normal metabolismDead animal preservationBiological testingAntigenInosine
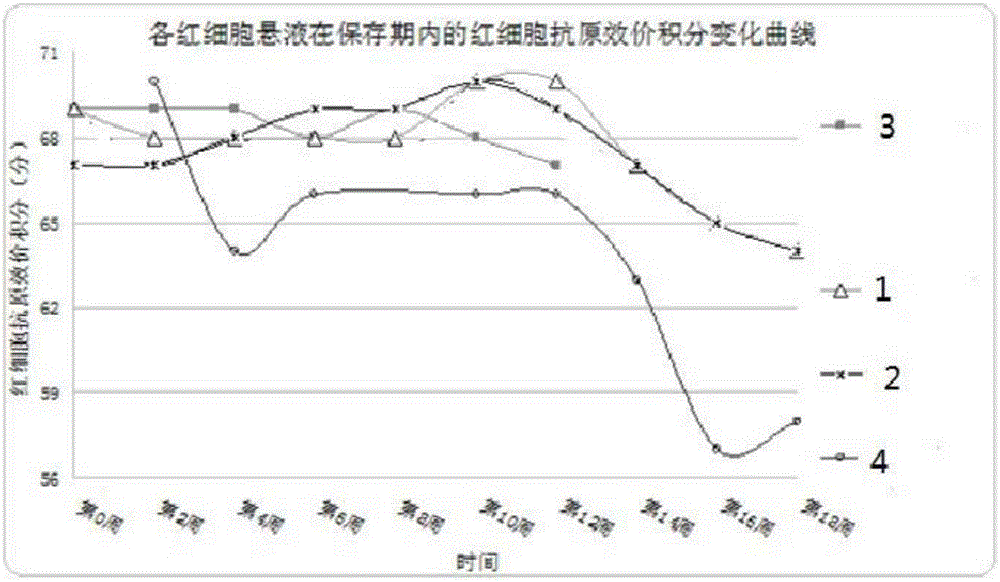
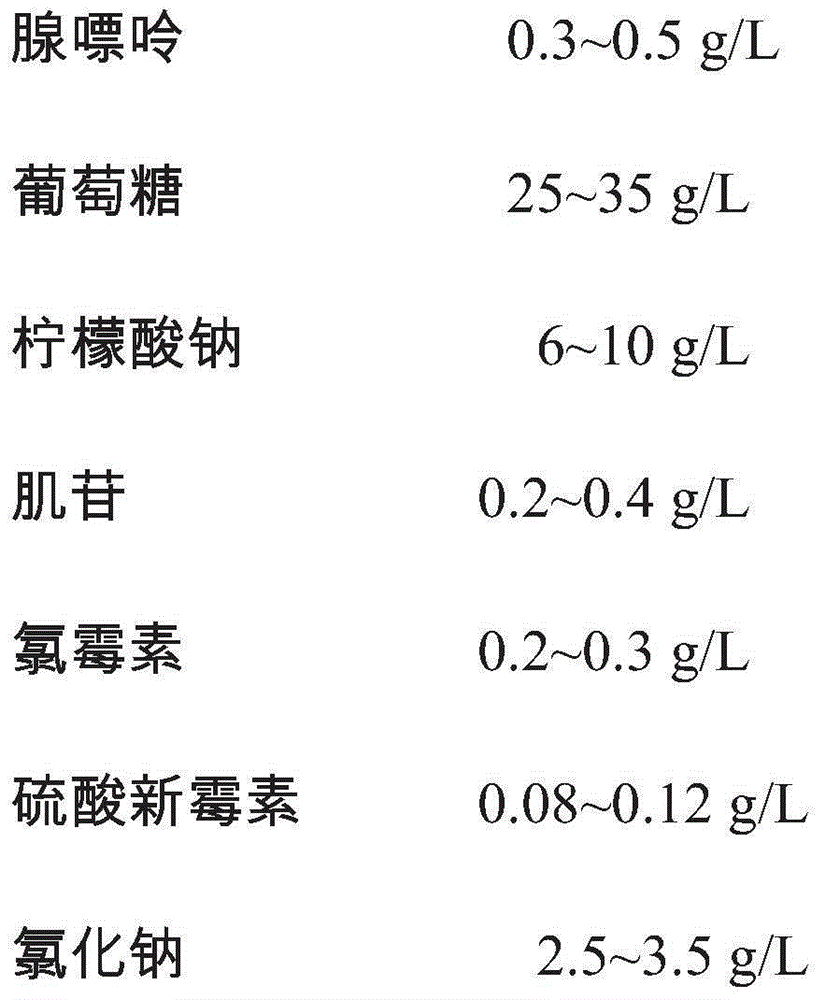
The invention provides an erythrocyte preservative solution. The solution comprises the following components by content: 0.3-0.5g / L of adenine, 25-35g / L of glucose, 6-10g / L of sodium citrate, 0.2-0.4g / L of inosine, 0.2-0.3g / L of chloramphenicol, 0.08-0.12g / L of neomycin sulfate, 2.5-3.5g / L of sodium chloride and 0.04-0.06g / L of sodium dihydrogen phosphate. According to the preservative solution, the preservation period of erythrocytes can be 14 weeks, and the erythrocyte suspension preserved in the solution has a relatively high antigen titer in the preservation period.
Owner:苏州苏大赛尔免疫生物技术有限公司
Chloramphenicol universal monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104263701AHigh affinityHigh detection sensitivityMicroorganism based processesTissue cultureBALB/c1,3-Propanediol
Owner:JIANGNAN UNIV
ELISA kit for detecting chloramphenicols in animal derived food
ActiveCN1766629AQuick checkLow pre-processing requirementsBiological testingSite monitoringElisa kit
The invention provides an enzyme immune agent box for detecting chloromycetin drugs of animal foodstuff which comprises: enzyme mark plate which coats chloromycetin drugs antigen or antibody, chloromycetin drugs mouse monoclonal antibody compression solution, chloromycetin drugs standard solution, enzyme mark material, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Compositions and method for treating infection in cattle and swine
Owner:INTERVET INC
Single-resistance escherichia coli-bacillus subtilis shuttle expression vector and application thereof
InactiveCN104232675AEasy genetic manipulationEase of plasmid transformationBacteriaHydrolasesEscherichia coliAgricultural science
The invention discloses a single-resistance escherichia coli-bacillus subtilis shuttle expression vector and application thereof. The bacillus subtilis shuttle expression vector provided by the invention comprises the following elements: a bacillus subtilis formed strong promoter P43, a sequence which encodes signal peptide, multiple cloning sites, an antibiotics resistance gene (kalamycin or chloramphenicol), a bifunctional promoter sequence which can start resistance gene expression of kalamycin or chloramphenicol in escherichia coli and bacillus subtilis, and copy homing sequences of bacillus subtilis and escherichia coli. The expression vector disclosed by the invention not only can be stably copied in escherichia coli, but also can be stably copied in bacillus subtilis, and expresses homologous and heterologous proteins. All vectors just contain one resistance gene, the plasmid of which is less than 400bp, so that gene operation and plasmid conversion are easy to carry out, and the conversion ratio is high. Besides the expression vector of an exogenous gene, the vector can be further used for screening promoters and constructing a gene knock-out vector.
Owner:WUHAN RUIHENGDA BIOLOGICAL ENG
Citrobacter farmeri Citrobacter farmeriSC01 and application thereof
InactiveCN101240256AHigh average degradation rateBacteriaMicroorganism based processesSludgeCitrobacter farmeri
The invention discloses a fashi citric acid rod Citrobacter farmeri SC01 and the application. The bacterium is obtained by artificial accumulation culture and separation cleansing from phenols cyanogen waste water treatment station one level aerobic pool sludge. The bacterium is preserved in china typical culture entreasure center of Wuhan University on January 7th 2008 year whose short form is CCTCC and preserving identification serial number is CCTCC NO: M208004. The fashi citric acid rod Citrobacter farmeri SC01 incorporating the invention can degrade phenyl hydrate, 2-methylphenol, m-cresol, 1-naphthyl hydroxide, 696mg / L m-cresol in 114 hours on aerobic condition, and the average degrading rate of speed ran up to 8.94mg / (L h) and the bacterium is sensitive to acheomycin, chloramphenicol, rifampicin and streptomycin, and the bacterium can be used safe and wide in harnessing and repairing wasted water and soil phenols pollution because the bacterium can not carryover drug-resistance factor spreading in aborigines bacterium when the bacterium is released into environment.
Owner:SOUTH CHINA UNIV OF TECH
Light-induced chemiluminescent immunoassay kit and test method for chloramphenicol
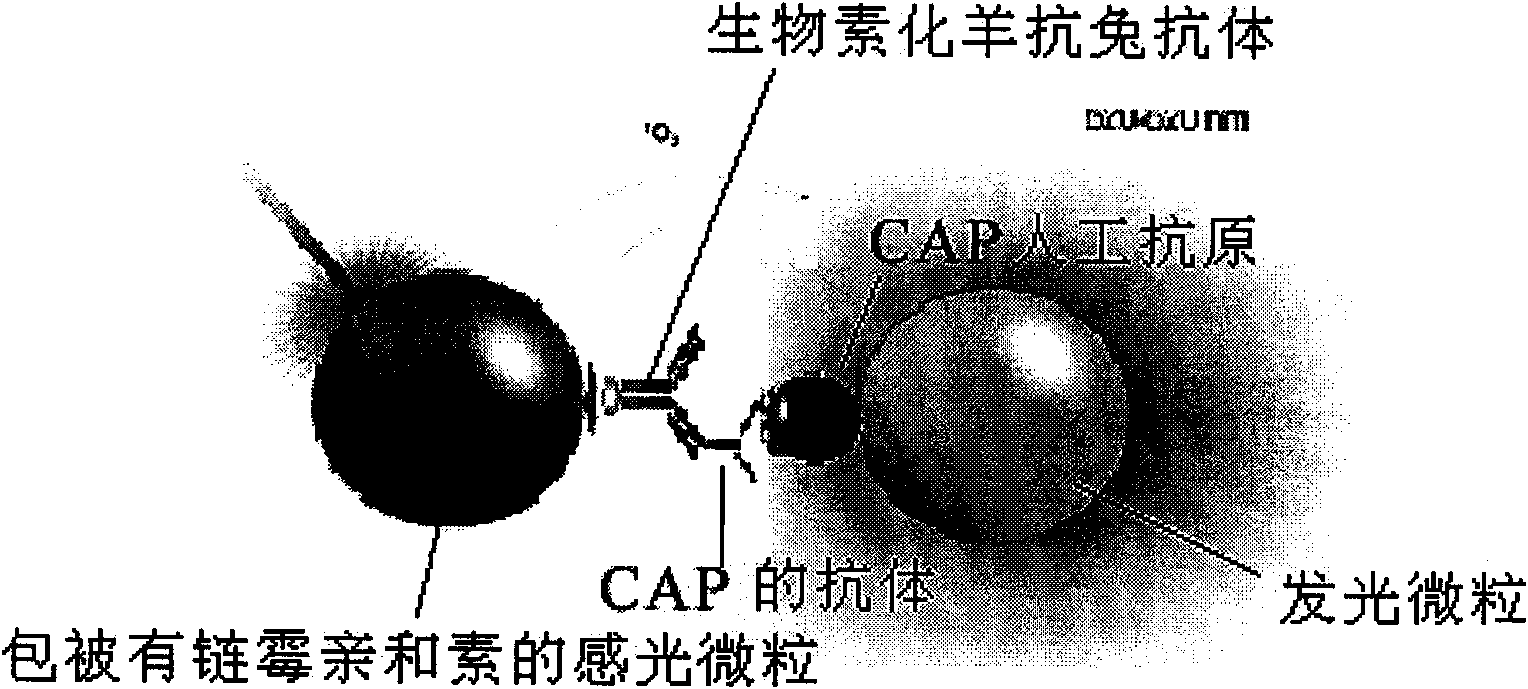
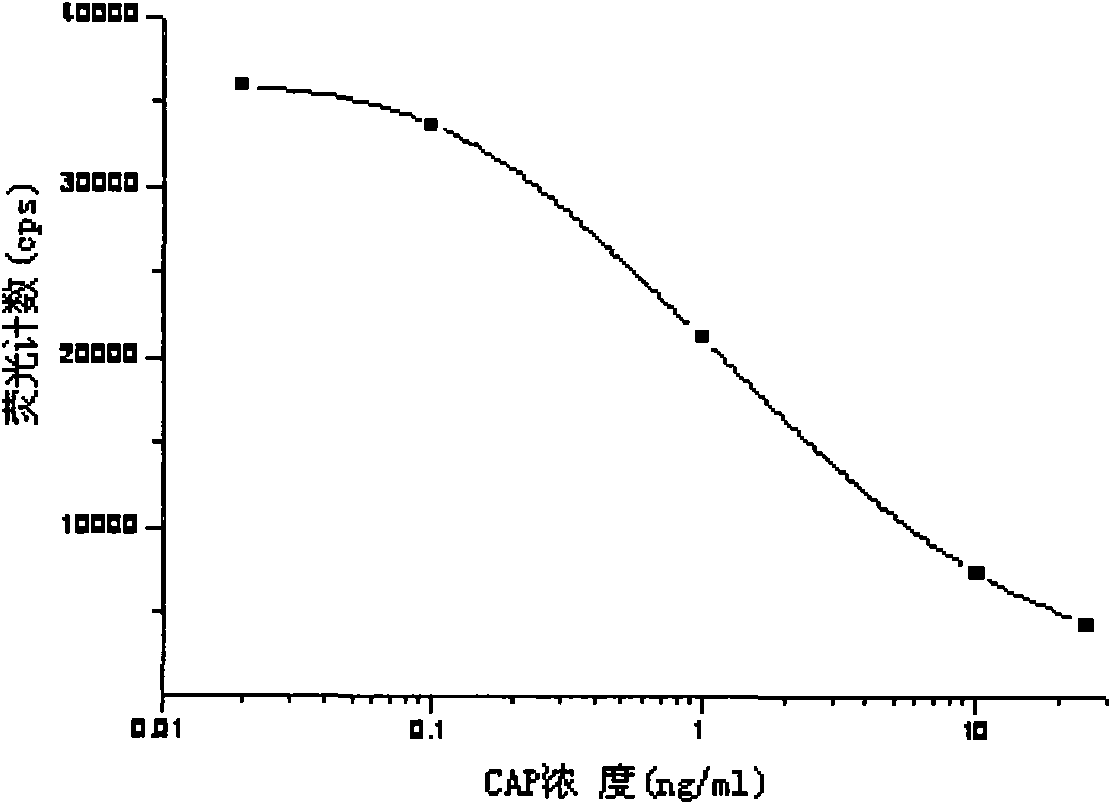
InactiveCN101603961ASimple structureSimple and fast operationPreparing sample for investigationBiological testingBiotin-streptavidin complexFluorescence
The invention discloses a light-induced chemiluminescent immunoassay kit and a test method for chloramphenicol (CAP) and belongs to the technical field of light-induced chemiluminescent immunoassay technology. A nontransparent white microporous plate is sequentially added with CAP-OVA coated luminous particles, a CAP standard substance or a sample to be tested, a rabbit anti-CAP antibody and a biotin goat anti-rabbit antibody for reaction without light and then is added with streptavidin-coated light sensitive particles for after-incubation test. The CAP-OVA on the luminous particles and free CAP compete to be connected to the CAP antibody to form a compound body with the biotin goat anti-rabbit antibody and the streptavidin-coated light sensitive particles. Under the excitation of red light, the compound body transfers energy to the luminous particles through the generation and transmission of singlet ionized oxygen for generating fluorescence. A light induced chemiluminescent detector is used to detect the intensity of an optical signal, and the CAP content of the sample can be determined by referring to a standard curve according on the basis that the intensity of the optical signal is in inverse proportion to the CAP concentration of the sample. The method is used for determining the CAP content of foods such as honey, milk and eggs. The kit is simple in structure, short in determination time, high in sensitivity and simple and convenient in operation.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Method for detecting antibiotic in environment sample
InactiveCN108318613AEfficient enrichmentRealize detectionComponent separationNorfloxacinSurface water
The invention relates to the technical field of environment detection and is suitable for measuring erythrocin, chloramphenicol, sulfamethoxazole, sulfadiazine, roxithromycin, cefotiam, pyridazol, norfloxacin, ofloxacin, tetracycline and doxycycline in sewage, surface water and bottom mud. After being sampled, a water sample and a mud sample are pretreated and detected through ultra-high performance liquid chromatography-triple quadrupole mass spectrometry, and qualitative diagnosis is conducted through the characteristic ion pair (m / z) of a target compound, a standard curve is drawn accordingto the response value and the corresponding concentration, and the external standard method is used in quantification, so that the contents of various antibiotics in the sample are obtained. The method is accurate, sensitive and simple, and is suitable for measuring the contents of 11 typical antibiotics in the sewage, the surface water and the mud at the same time.
Owner:四川国测检测技术有限公司
Chloramphenicol molecular imprinting electrochemical luminescence sensor and chloramphenicol detection method thereof
InactiveCN105067598AHigh degreeImprove stabilityChemiluminescene/bioluminescenceMaterial electrochemical variablesPolymer thin filmsElectrochemiluminescence
Embodiments of the present invention disclose a chloramphenicol molecular imprinting electrochemical luminescence sensor, which comprises an inert electrode and a chloramphenicol molecular imprinting polymer film on the inert electrode surface. The chloramphenicol molecular imprinting polymer film preparation steps comprise: adopting an acetic acid buffer solution containing chloramphenicol and o-phenylene diamine as an electrolyte, and adopting an inert electrode as a working electrode to construct a three-electrode system; using a cyclic voltammetry method to obtain a poly o-phenylene diamine-chloramphenicol film, wherein the polymerization potential is 0-0.8 V; and carrying out elution with an eluant to obtain the chloramphenicol molecular imprinting polymer film, wherein the pH value of the acetic acid buffer solution is 5.2, the molar concentration of the acetic acid buffer solution is 0.05-0.2 mol / L, and the molar concentrations of the chloramphenicol and the o-phenylene diamine respectively are 1-50 mmol / L. According to the present invention, the chloramphenicol molecular imprinting electrochemical luminescence sensor can be used for chloramphenicol detection, and has characteristics of high detection sensitivity and good selectivity.
Owner:BEIJING NORMAL UNIVERSITY +1
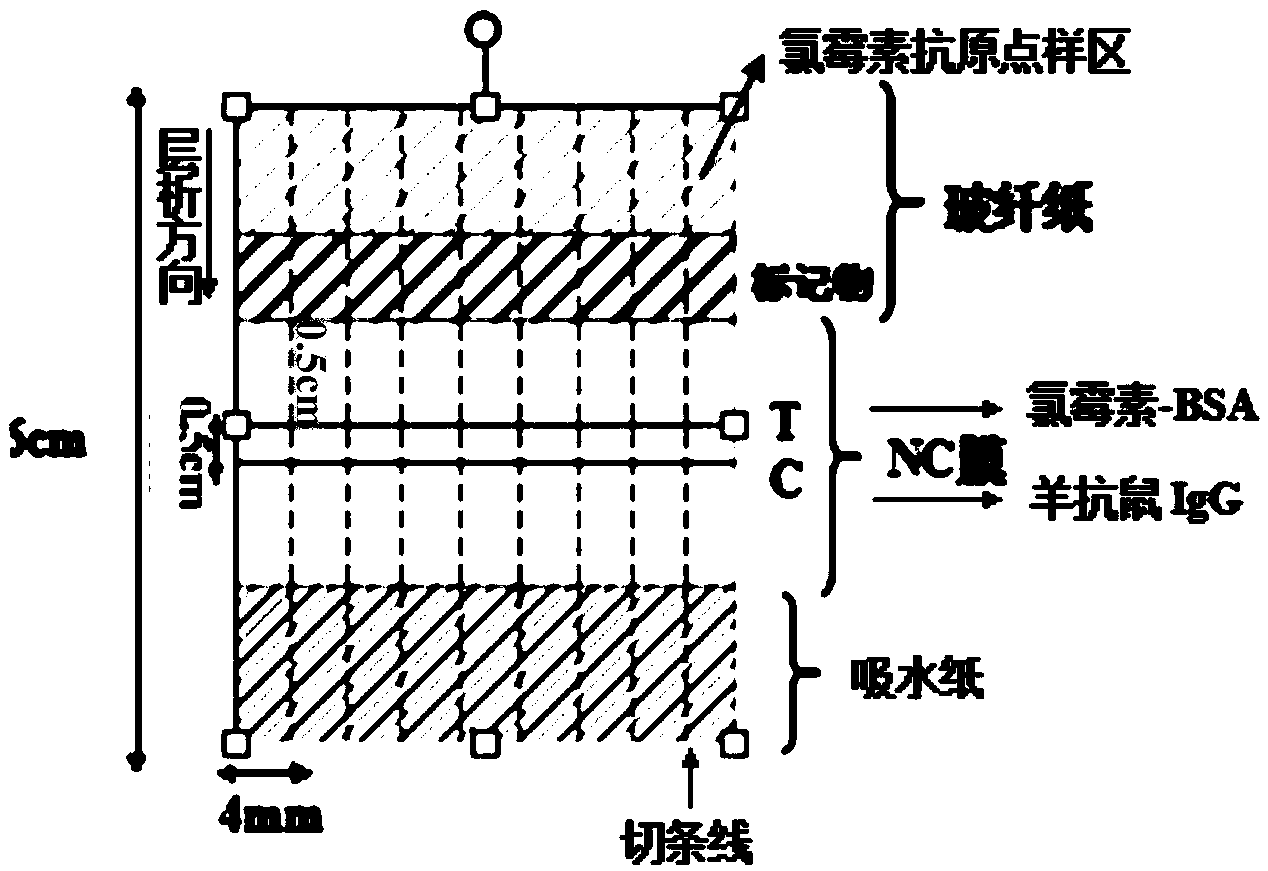
Chloramphenicol quantitative detection method based on up-conversion phosphor technology and immunochromatography technology
InactiveCN104374909AGood water solubilityGood dispersionFluorescence/phosphorescenceSolubilityAntigen
The invention relates to a chloramphenicol quantitative detection method based on an up-conversion phosphor technology and an immunochromatography technology, belonging to the fields of nano biomarkers, immunology and optical detection. The detection method mainly comprises the following steps that carboxylation modification is carried out on 200-300nm up-conversion fluorescent nanoparticles so that the up-conversion fluorescent nanoparticles become markers which are good in water solubility and high in dispersibility and can be easily coupled with biomolecules; a sample pad, a conjugate pad treated by the up-conversion markers, a nitrocellulose membrane (NC membrane) treated by antigen-antibody sample application and absorbent paper are combined together by a sticky bottom lining; antigen takes chloramphenicol-bull serum albumin (BSA) as a detection line (T), antibody takes goat-anti-mouse antibody as a quality control line (C), and the distance between the detection line and the quality control line is 0.5cm; the assembled test paper is cut into test paper strips which are 6cm long and 4mm wide by a strip cutting machine, the test paper strips are assembled into a shell to establish the immune chromatography test paper; chloramphenicol standard antigens with different concentration gradients are loaded to the sample pad in the test paper shell; after standing still for 10-15 minutes, the sample pad is put into an up-conversion detector for detection. The chloramphenicol quantitative detection method is simple in preparation technology and operation, can be completed without a complicated instrument or equipment, is rapid and sensitive, and has an important significance for accurately and quantitatively testing the content of chloramphenicol in food.
Owner:BEIJING UNIV OF TECH
Production of functional antibacterial fibre
InactiveCN1940171ARealize the shielding effectControl releaseVegetal fibresPolymer scienceCyclodextrin
It is a method of preparing functional antibacterial fiber. Its character lies in that CD grafted to cellulose fiber to Form cyclodextrin-cellulose fiber polymer including chloramphenicol serving to be antibacterial. Compared with the existing technology, the invention of the advantages are : a) the CD received cellulose fiber, CD Modified fiber formation, and then use the fiber of cyclodextrin inclusion drugs, achieving CD - cellulose polymer drug screening, controlled drug release, the protection function of drug activity; 2) Antibiotics can be functional fiber weaving, tailoring, made of various kinds of medicinal materials functional textiles, Surgical dressing for antibacterial materials, wartime emergency bandaging materials, and better meet the medical needs of a different.
Owner:ZHEJIANG SCI-TECH UNIV
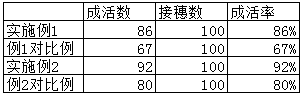
Strapping band for fruit tree grafting
The invention discloses a strapping band for fruit tree grafting, and the strapping band is used for strapping grafting scions onto rootstocks. The strapping band comprises a strip-shaped band body made of gauze. The band body is subjected to infiltration processing. The components of infiltrating liquid include, by weight, 1000-2000 parts of distilled water, 0.8-2 parts of vitamin C, 0-3 parts of tannic acid, 2-5 parts of phenylacetic acid, 0.03-0.05 part of potassium chloride, 0.2-0.5 part of ammonium nitrate and 0.01-0.02 part of chloramphenicol. The PH value of the infiltrating liquid is 5.2-7.2. By the strapping band for fruit tree grafting, coalescence of joints can be accelerated after grafting and survival rate of fruit grafting is increased.
Owner:JIANGSU POLYTECHNIC COLLEGE OF AGRI & FORESTRY
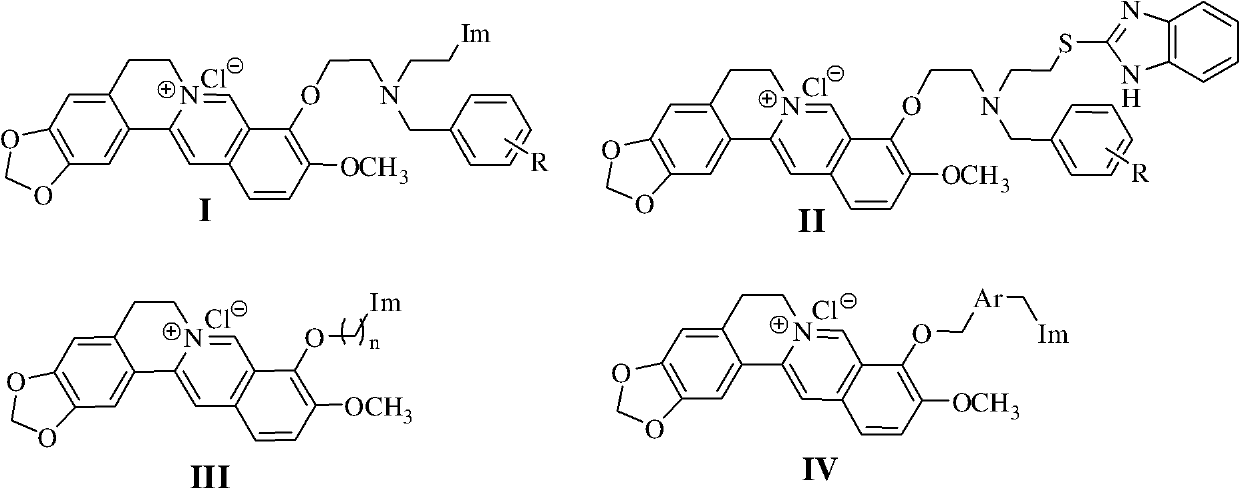
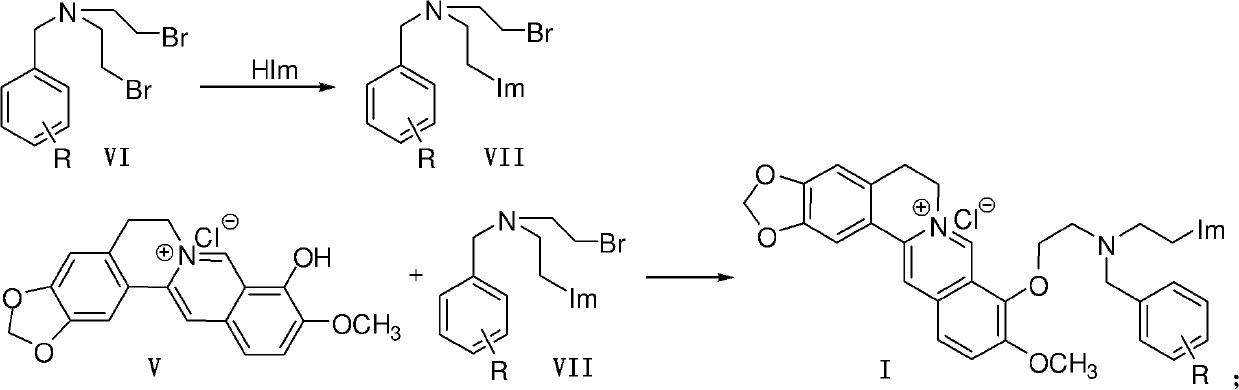
Berberine azole compound and preparation method and application thereof
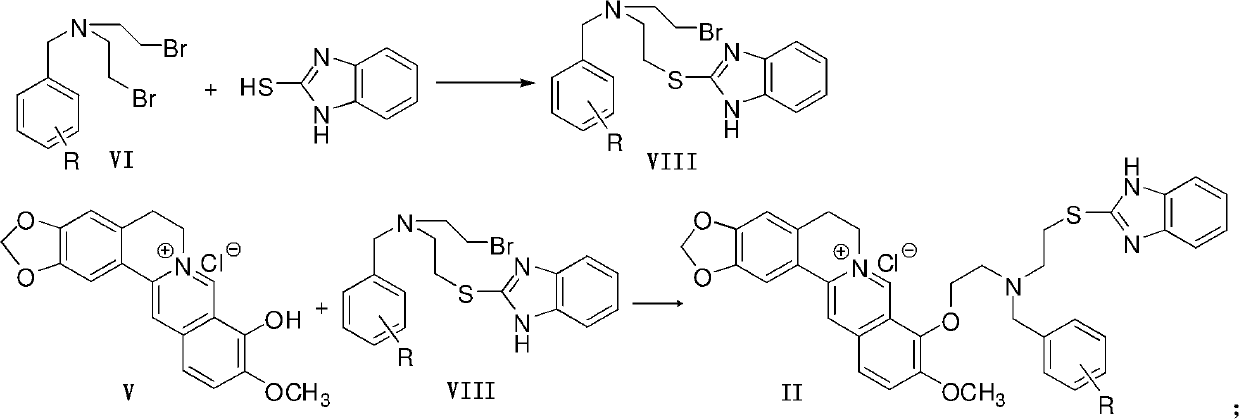
ActiveCN102516242AEasy to prepareRaw materials are easy to getAntibacterial agentsOrganic active ingredientsBerberineFluconazole
The invention discloses a berberine azole compound shown in general formulas such as I-IV, and a pharmaceutically acceptable salt thereof, and also discloses a preparation method of the compound, which comprises the following steps that: a compound VI and an azole compound HIm or 1H-2-mercaptobenzimidazole are reacted to obtain an intermediate VII or VIII, and then the obtained intermediate is reacted with a compound V to obtain the berberine azole compound shown in general formulas such as I or II; and the compound V and a compound IX or XI are reacted to obtain an intermediate X or XII, and then the obtained intermediate is reacted with the azole compound Him to obtain the berberine azole compound shown in general formulas such as III or IV. The berberine azole compound has a certain inhibitory activity on Gram-positive bacteria, Gram-negative bacteria and fungi, the anti-bacterial activity of part of the compounds is equal to or even stronger than that of chloramphenicol or norfloxacin, the antifungal activity of part of the compounds is equal to or even stronger than that of fluconazole, and the berberine azole compound can be used for preparing anti-microbial drugs.
Owner:SOUTHWEST UNIVERSITY
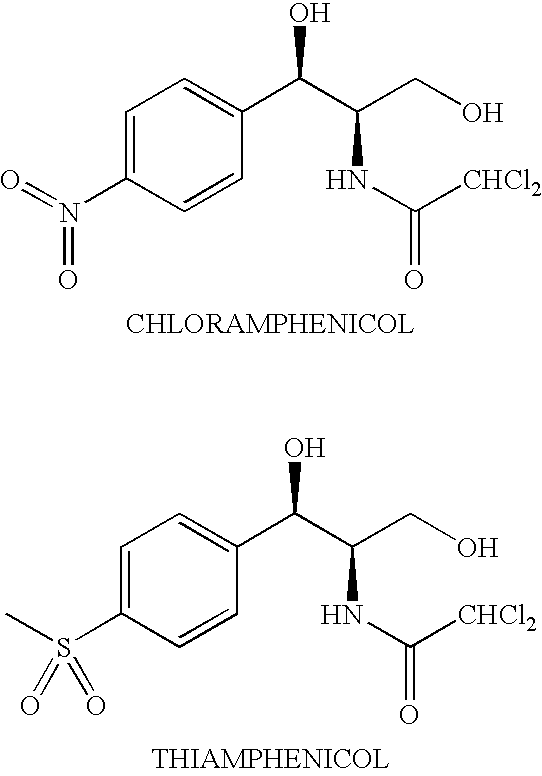
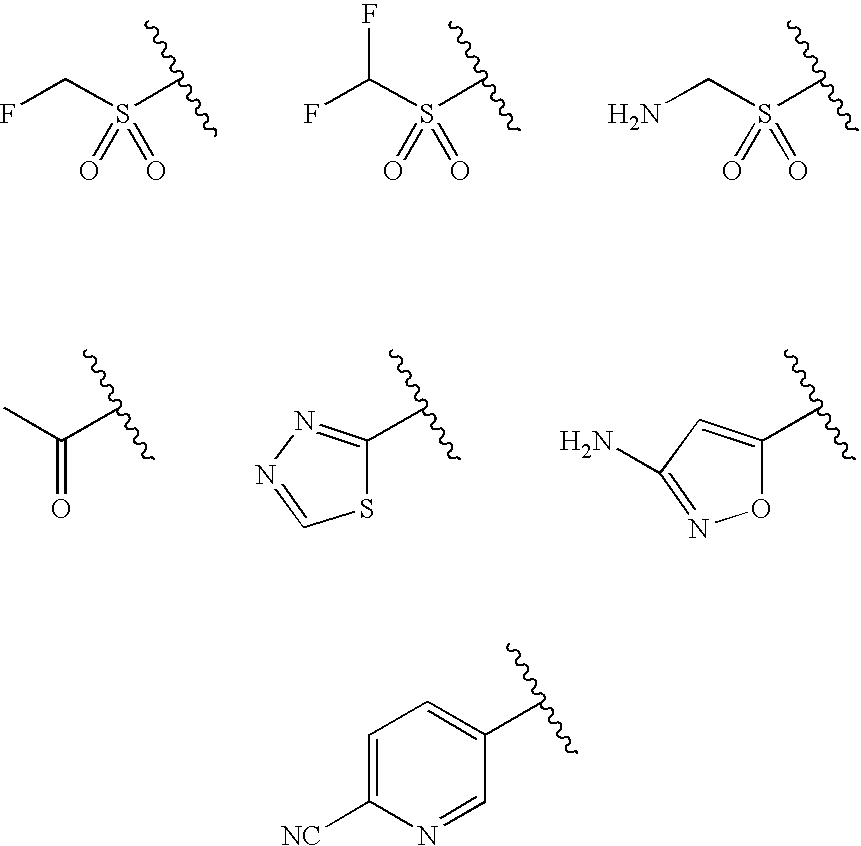
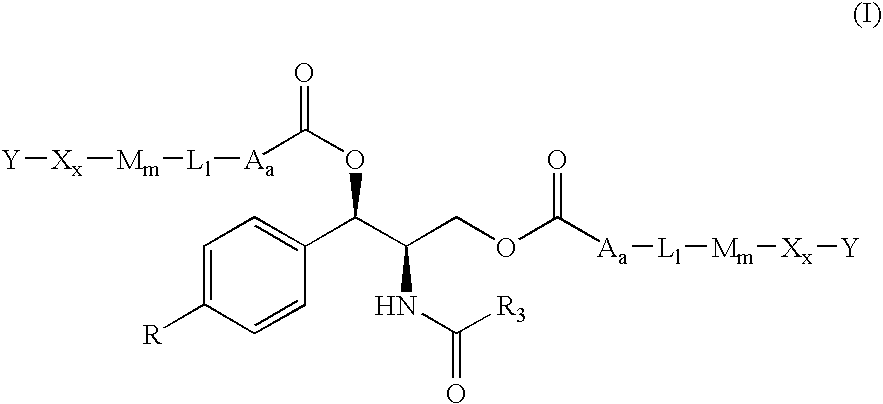
Water-Soluble Prodrugs of Chloramphenicol, Thiamphenicol, and Analogs Thereof
The present invention discloses certain novel prodrugs of chloramphenicol or thiamphenicol, or of an analog of either, including prodrugs of pharmaceutically acceptable salts of chloramphenicol or thiamphenicol or of their analogs, including nitrogen-containing esters of both alcohol groups of such compounds. In certain embodiments these novel prodrugs are sufficiently water-soluble to serve the functions needed of a prodrug of chloramphenicol or thiamphenicol or of an analog of either. In one embodiment, a certain subclass of the compounds also possesses the hydrolytic stability needed to maintain the prodrug in solution in the subject's system until appropriate conditions exist when the prodrug can hydrolyze, releasing the active compound in question.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
Spore suspension for improving yield of white muscardine silkworms and application of spore suspension
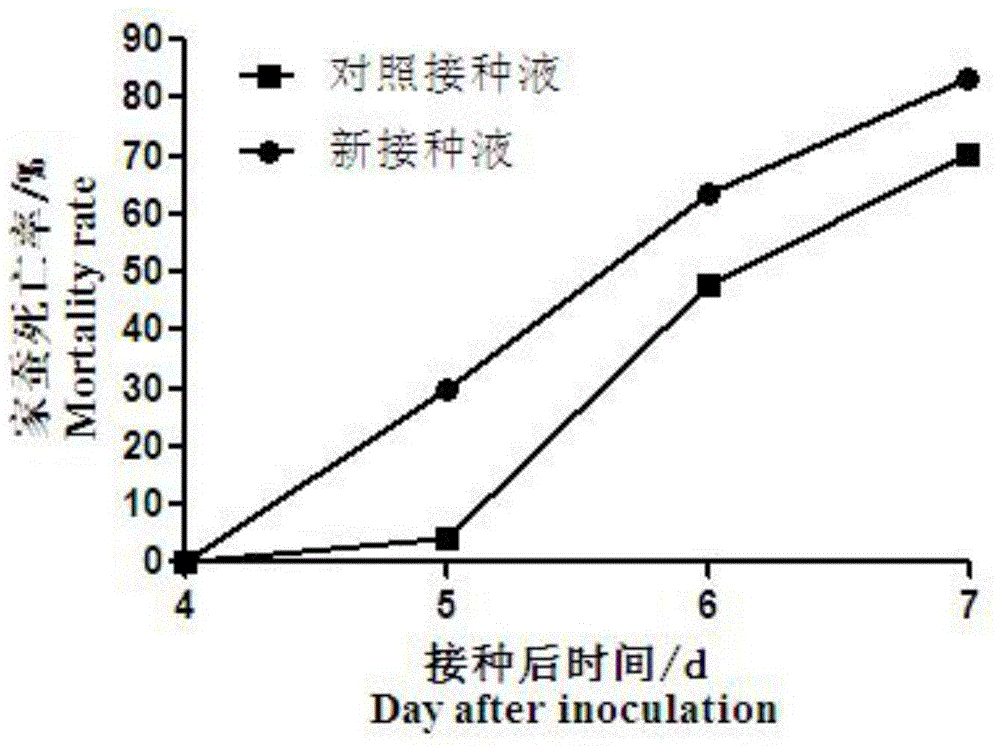
The invention discloses spore suspension for improving the yield of white muscardine silkworms and application of the spore suspension. The spore suspension for improving the yield of the white muscardine silkworms comprises the following raw materials: muscardine spore powder with 1x106-5x106 muscardine spores per mL, 0.2 part by mass of polysorbate-80, 1-3 parts by mass of cane sugar, 0.4-0.6 part by mass of peptone, 0.05 part of auxiliary materials and the balanced sterile water. The auxiliary materials can be magnesium sulfate, chloramphenicol and streptomycin sulfate. By the spore suspension for improving the yield of the white muscardine silkworms, the concentration of the optimized muscardine suspension is increased, the stickiness and the activity of the muscardine suspension are improved, the germination rate of the muscardine suspension is increased, and the yield and the quality of the white muscardine silkworms are improved obviously.
Owner:SERICULTURE & AGRI FOOD RES INST GUANGDONG ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com