Electrochemical aptamer sensor for rapid detection of chloramphenicol
An aptamer sensor, chloramphenicol technology, applied in the direction of material electrochemical variables, scientific instruments, instruments, etc., can solve the problems of long time, poor stability and high cost, and achieve simple operation, good stability and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
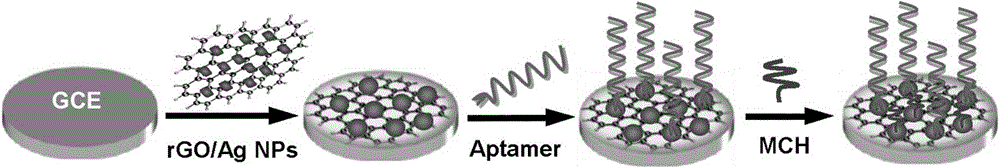
[0031] Example 1 A preparation method of an electrochemical aptasensor for rapid detection of chloramphenicol, comprising the following steps:
[0032] ① Preparation of reduced graphene (rGO) from polydiene dimethyl ammonium chloride (PDDA)
[0033] (1) Put 25mg of graphene oxide (GO) in 25mL of water, ultrasonically disperse until there are no particles in the solution, and obtain a 1mg / mL graphene oxide (GO) dispersion;
[0034] (2) Add 0.5mL of 20% polydiene dimethyl ammonium chloride (PDDA) electrolyte to the above dispersion, stir for 30min, add 0.875mL of 35% hydrazine hydrate solution, stir in an oil bath at 100°C for 24h and centrifuge Separation, washing the precipitate with water to neutrality and dispersing in 25mL of water to obtain 1mg / mL reduced graphene (rGO) of polydiene dimethyl ammonium chloride (PDDA);
[0035] ②Preparation of reduced graphene silver nanoparticles (rGO / AgNPs) composite
[0036] (1) Add 0.25mL of 100mM silver nitrate solution and 0.25mL of ...
Embodiment 2
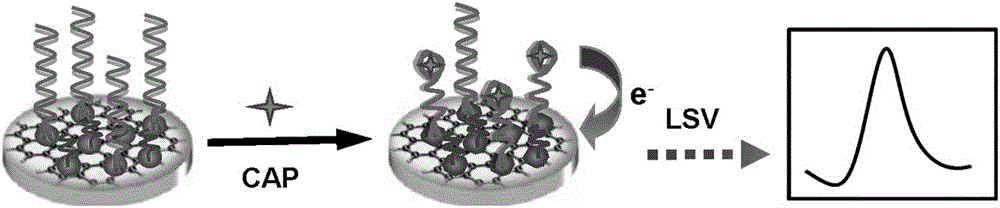
[0041] The detection of embodiment 2 chloramphenicol standard solution
[0042] (1) On the electrochemical aptamer sensor (rGO / AgNPs / aptamer) prepared in Example 1, different concentrations of chloramphenicol solutions were added dropwise, 10 μL each time, and incubated at 37°C for 40 min Wash with 10mM tris buffer (Tris-HCl);
[0043] (2) The above-mentioned electrochemical aptamer sensor (rGO / AgNPs / aptamer) was used as the working electrode, Ag / AgCl was used as the reference electrode, and the platinum column electrode was used as the counter electrode, and connected to the electrochemical workstation;
[0044] (3) Place the above-mentioned electrochemical aptasensor in 50mM pH 7.4 Tris-HCl buffer (Tris-HCl) containing 0.1 M potassium chloride detection solution, at a potential of -0.80 V to -0.10 V Perform linear sweep voltammetry in the range with a scan rate of 50mV / s. According to the recorded electrochemical signals, the working curves of chloramphenicol standard sol...
Embodiment 3
[0046] Example 3 Detection of Chloramphenicol in Milk
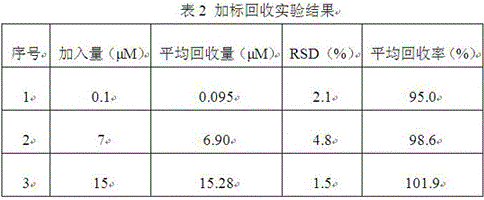
[0047] Obtain working curve according to embodiment 2, detect the content of chloramphenicol in the milk sample. Dissolve 1.0 g of milk sample in 5 mL of 50 mM Tris-HCl, adjust to pH = 4.6 with 20% acetic acid, incubate for 20 min and then centrifuge, take the supernatant and filter it through a 0.22 μm filter membrane, and adjust the filtrate to pH = 7.4 to test. The results showed that no chloramphenicol residues were detected in the milk samples. Then, chloramphenicol standard solution was added to the above-mentioned milk sample, and the standard addition recovery experiment was carried out. The experimental results are shown in Table 2 below.
[0048]
[0049] As can be seen from Table 2 above, the relative standard deviation (RSD) of the test results is 1.5-4.8%, and the average recovery rate is 95.0-101.9%, indicating that the electrochemical aptamer sensor of the present invention has higher accuracy and pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com