Berberine azole compound and preparation method and application thereof
An azole compound, berberine azole technology, applied in the field of preparation of the compound, can solve the problems of low solubility of berberine, low bioavailability, many times of medication, etc. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
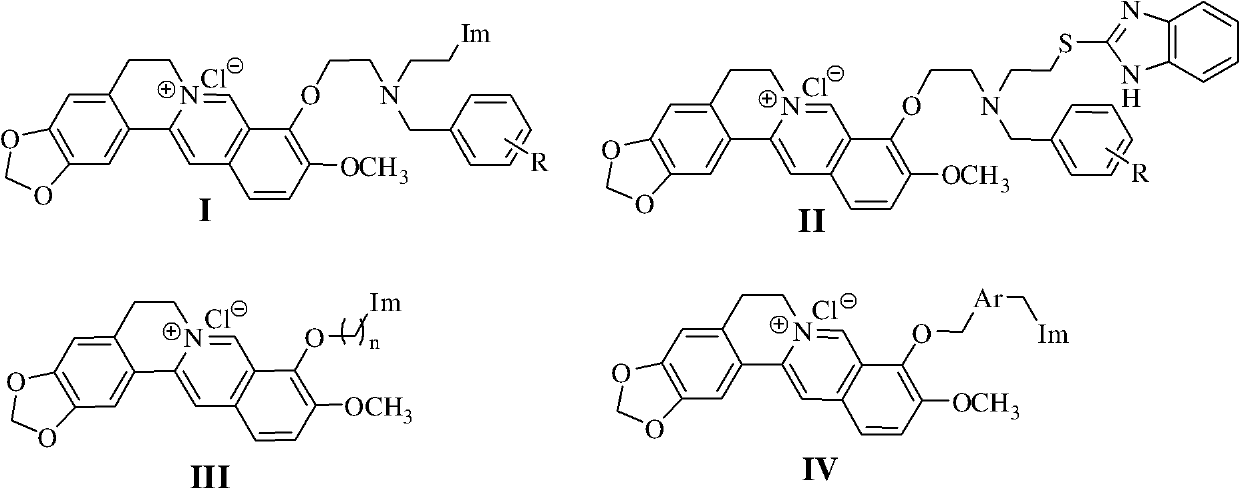
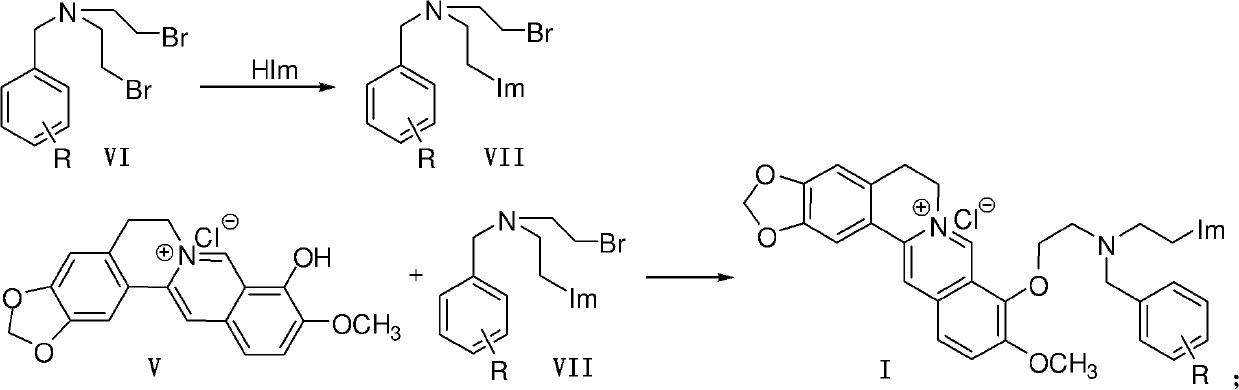
[0046] Embodiment 1, the preparation of berberine azole compound and hydrochloride thereof shown in general formula as I
[0047]
[0048] 1. Preparation of Intermediate VII
[0049] Method 1: In a 100mL round bottom flask, add azole compound (HIm), anhydrous potassium carbonate and acetonitrile (10mL), stir and heat up to 60°C for 1 hour, then add raw material VI under ice-water bath cooling condition, and react at room temperature , monitor the progress of the reaction with thin-layer chromatography (TLC); after the reaction is completed, extract with ethyl acetate, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, and purify by column chromatography (with a volume ratio of 1 to 3:5 Ethyl acetate-petroleum ether mixture is the eluent) to obtain intermediates VII-1~VII-20. The specific experimental conditions and results are shown in Table 1.
[0050] Table 1 Preparation conditions and results of intermediate VII
[0051]
[0052]
...
Embodiment 2
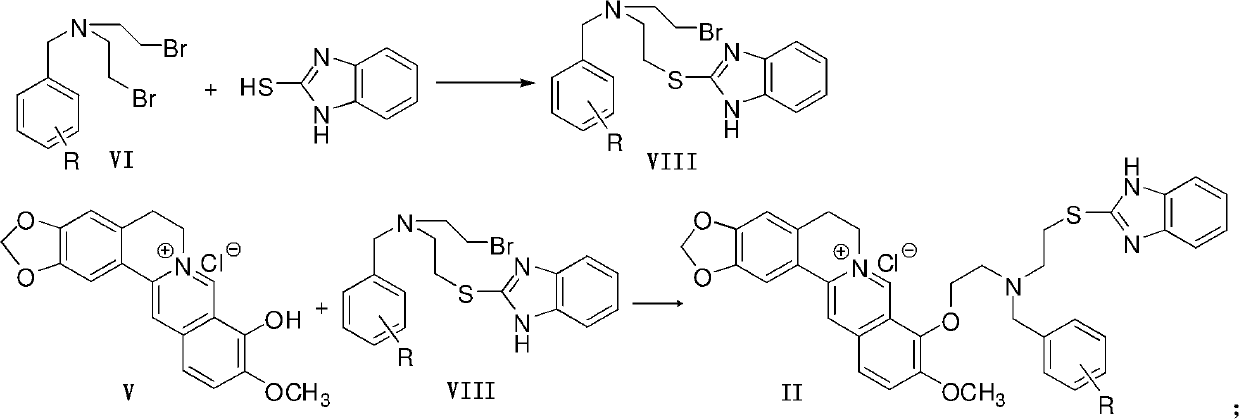
[0120] Embodiment 2, the preparation of berberine azole compound II-1
[0121]
[0122] 1. Preparation of Intermediate VIII-1
[0123] Consistent with the preparation method 1 of intermediate VII, in a 100mL round bottom flask, add 1H-2-mercaptobenzimidazole (1.58g, 10mmol), anhydrous potassium carbonate (2.76g, 20mmol) and acetonitrile (10mL), stir Raise the temperature to 60°C and react for 1 hour, then add raw material VI (4.37g, 12mmol) under ice-water bath cooling condition, react at room temperature, and monitor the reaction progress with TLC; after the reaction is completed, extract with ethyl acetate and wash with saturated sodium chloride solution , dried over anhydrous sodium sulfate, and purified by column chromatography (using ethyl acetate-petroleum ether mixture with a volume ratio of 1 to 3:5 as the eluent) to obtain Intermediate VIII-12.73g with a yield of 61%; White solid; m.p.: 138-140°C; 1 H NMR (300MHz, CDCl 3 ): δ7.59 (m, J=9.0Hz, 2H, Benim-Ph 3, 6-H...
Embodiment 3
[0126] Embodiment 3, the preparation of berberine azole compound III-1
[0127]
[0128] 1. Preparation of Intermediate X-1
[0129] According to the literature (Yan Ma, et al. Synthesis and evaluation of 9-O-substituted berberine derivatives containing aza-aromatic terminal group as highly selective telomeric G-quadruplex stabilizing ligands. Bioorganic & Medicinal Chemistry Letters, 2009, 19: 34714-3 ) method, using acetonitrile as a solvent, reacting raw material V with 1,6-dibromohexane at 75°C.
[0130] 2. Preparation of compound III-1
[0131] In a 100mL round bottom flask, add 1H-1,2,4-triazole (45mg, 0.65mmol), anhydrous potassium carbonate (150mg, 1.09mmol) and acetonitrile (10mL), stir and heat up to 60°C for 1 hour, Then cool to room temperature with an ice-water bath, add intermediate X-1 (240 mg, 0.46 mmol), react at 50 ° C, and monitor the progress of the reaction with TLC; after the reaction is completed, extract with chloroform, wash with saturated sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com