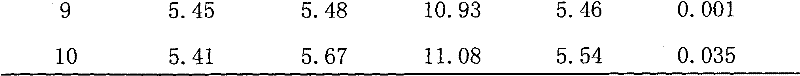A kind of preparation method of chloramphenicol residual freeze-dried powder standard sample in carp muscle
A standard sample, chloramphenicol technology, applied in the preparation of test samples, etc., can solve the problems of not representing the existence of the target analyte, affecting the reliability and validity of the results, and not representing the target analyte, etc. Economic value and market competitiveness, uniform and stable samples, and convenient transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
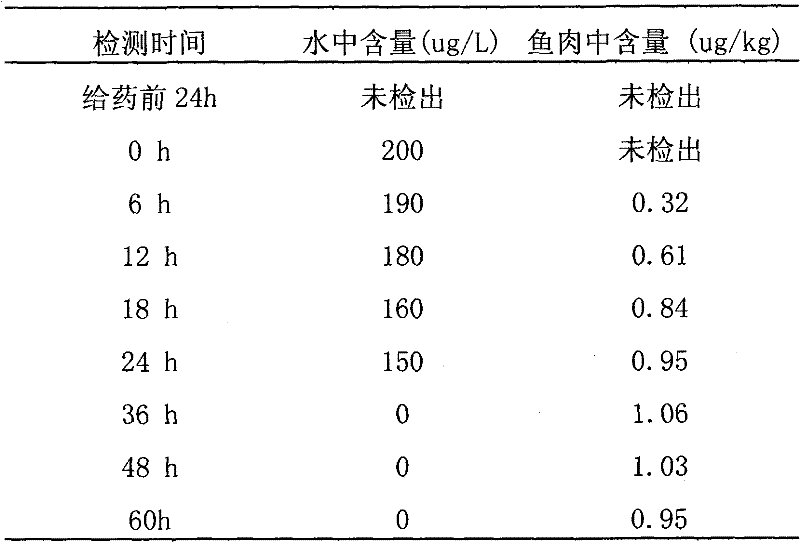
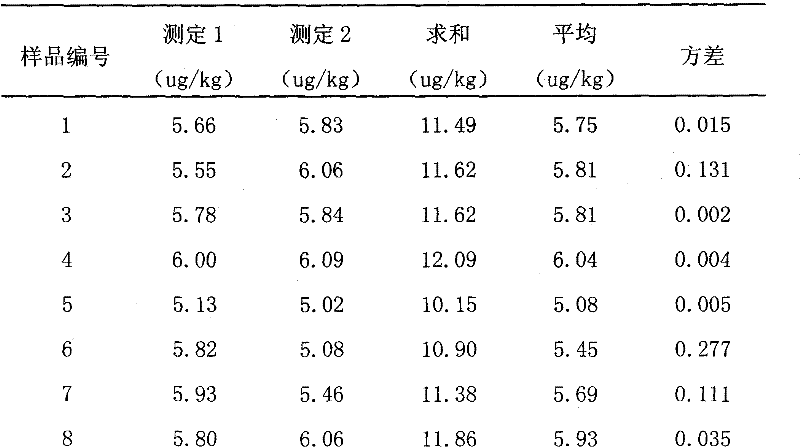
[0011] Embodiment 1, a kind of preparation method of chloramphenicol residual freeze-dried powder standard sample in carp muscle, present embodiment takes control target content level 5.0ug / kg as an example, and its specific steps are as follows:
[0012] (1) Selection of experimental animals: Live carps purchased from the market require uniform size and average weight of about 1.5kg. It should be confirmed that the sample does not contain chloramphenicol, and random sampling should be carried out. After the test confirms that the sample is negative, it should be fed. After the negative samples were fed for 2 days at 20°C-25°C, the injured and unhealthy carps were removed;
[0013] (2) Drug breeding addition: Add medical chloramphenicol tablets to fish ponds, add drugs to make the concentration of chloramphenicol in the water 200ug / L according to the amount of breeding water, and monitor and detect the drug concentration in the water body and in the fish body in real time. Unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com