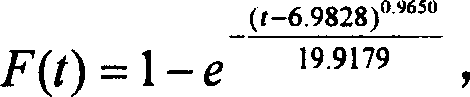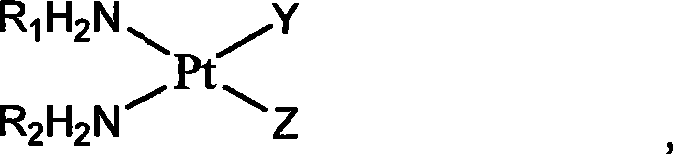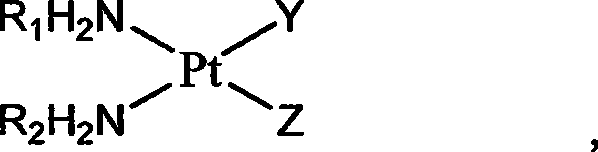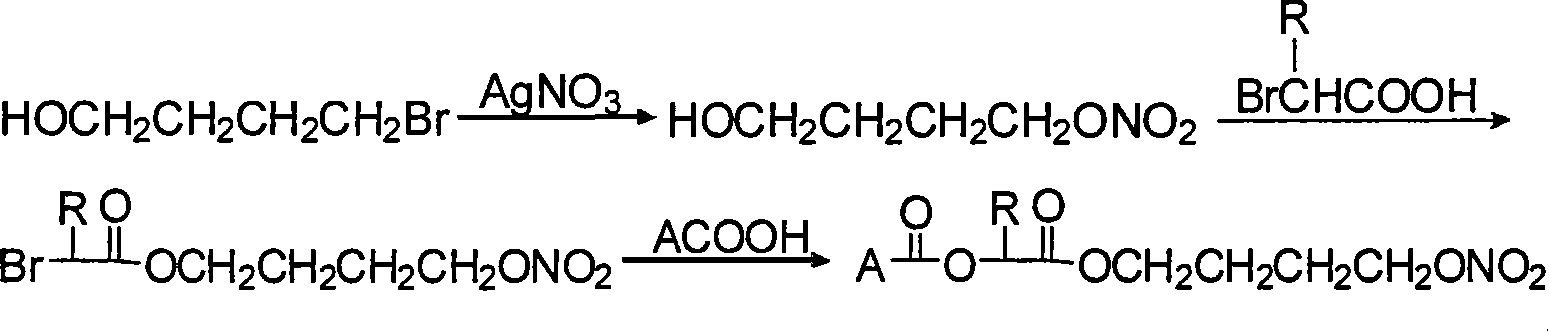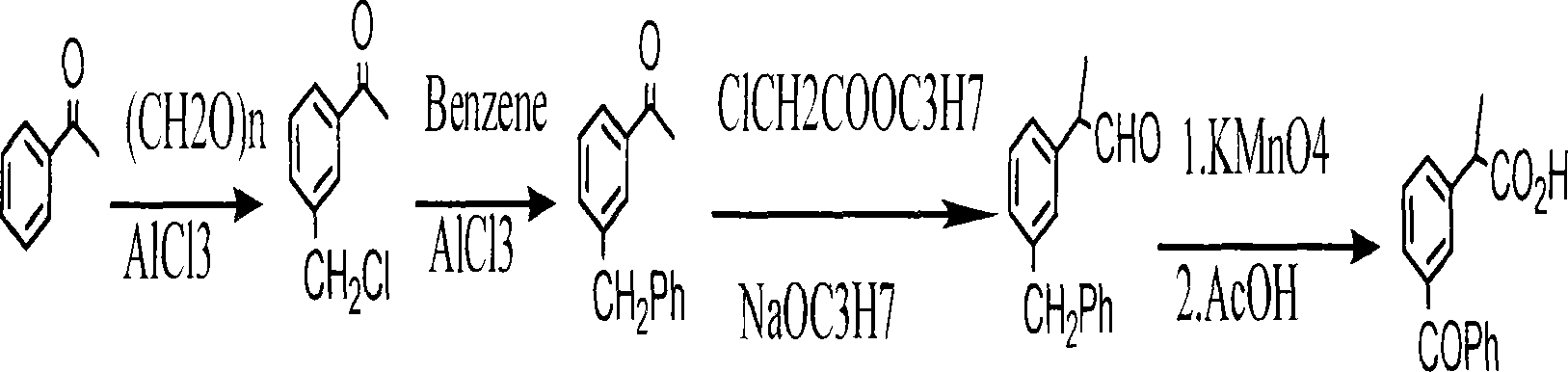Patents
Literature
239 results about "Ketoprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
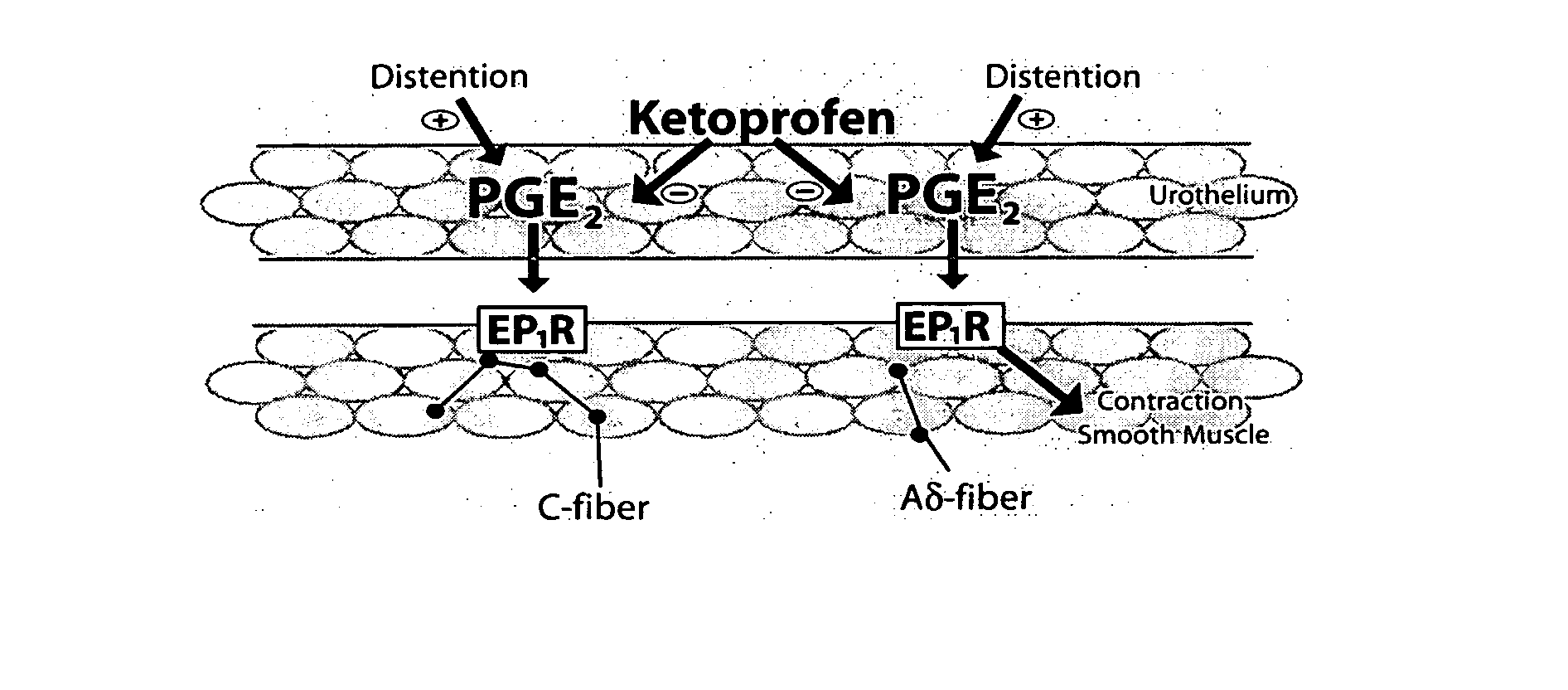
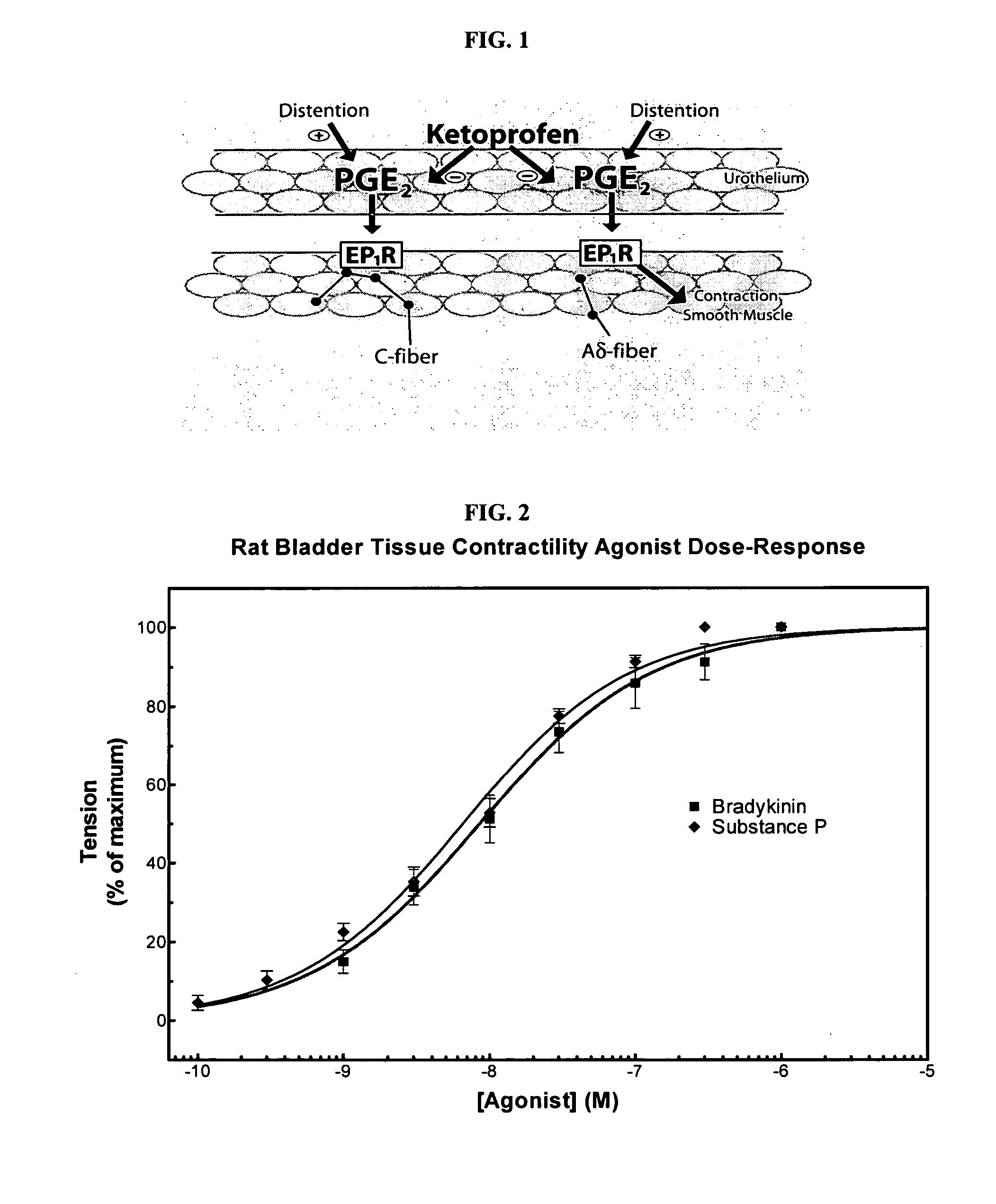
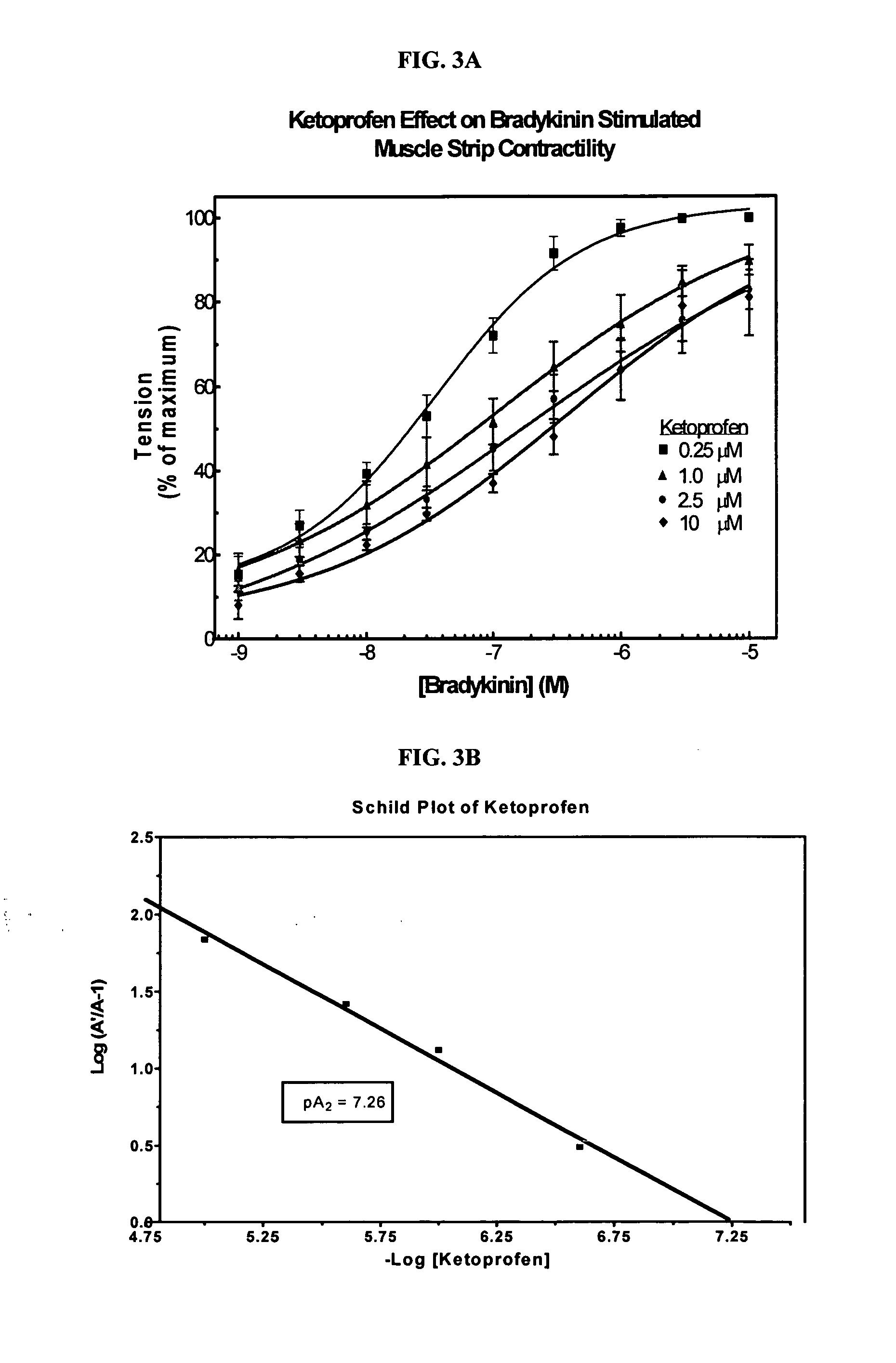
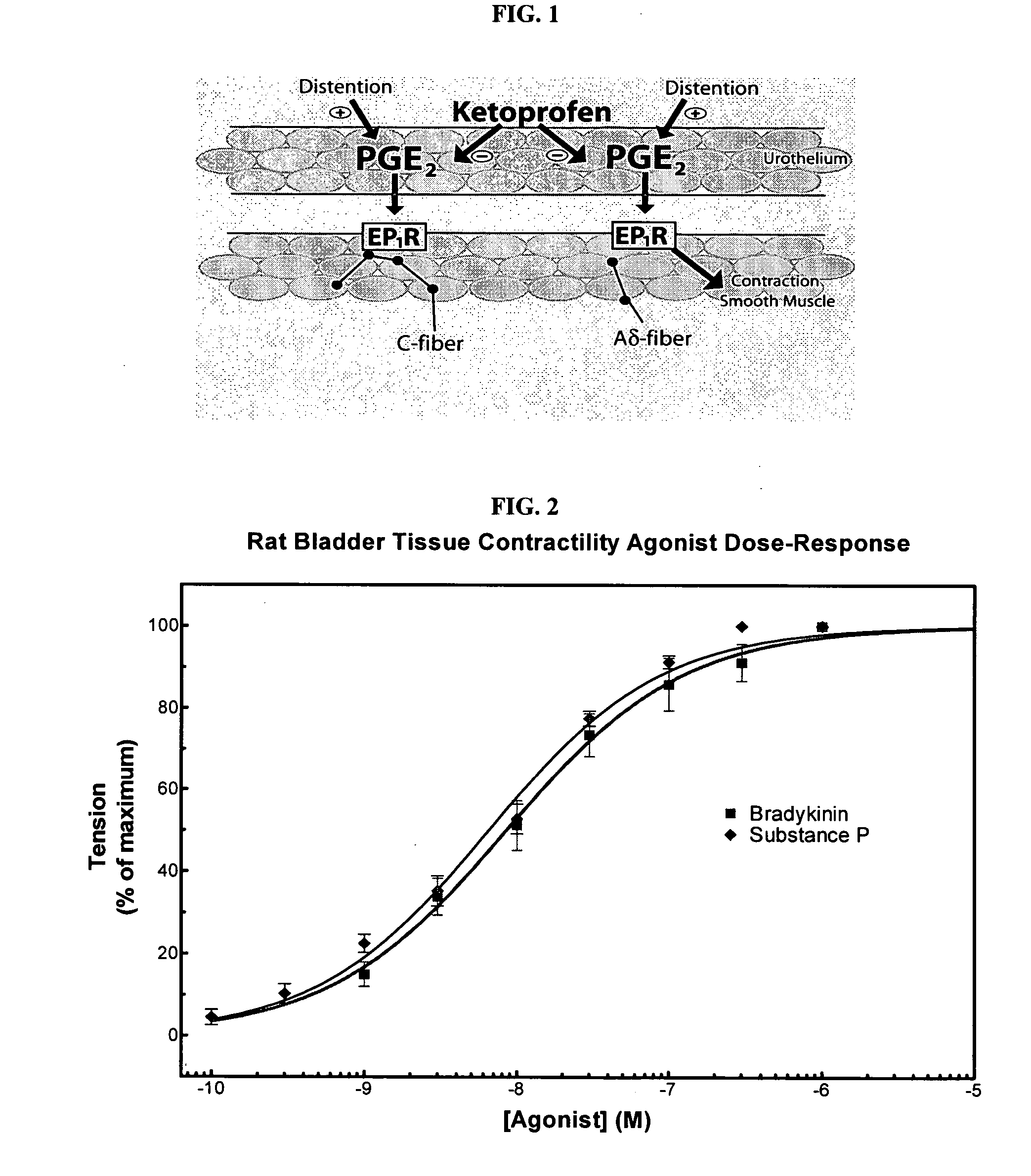
Ketoprofen is used to relieve pain from various conditions.
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
InactiveUS20070248639A1Inhibiting pain/inflammationPrevent spasmsBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
ActiveUS20060263393A1Inhibits pain/inflammation and spasmInhibiting pain/inflammationBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
Medicinal fibre used for treating cutaneous inflammation and pain, preparation and application thereof
InactiveCN101724934AEasy to prepareLow costOrganic active ingredientsAntipyreticFiberOrganic solvent
The invention relates to medicinal fibre used for treating cutaneous inflammation and pain, which comprises the components of cellulose acetate nano fibre, a ketoprofen ester precursor medicine and a medicinal percutaneous sorbefacient, wherein the ketoprofen ester precursor medicine accounts for 0.1-30% of the total weight of the fibre, the medicinal percutaneous sorbefacient accounts for 0.1-10% of the total weight of the fibre, and the cellulose acetate nano fibre accounts for 1-99% of the total weight of the fibre; the preparation of the medicinal fibre comprises the following steps: dissolving the ketoprofen ester precursor medicine, the medicinal percutaneous sorbefacient and the cellulose acetate into an organic solvent to prepare spinning liquid and then spinning to prepare the required medicinal fibre. The medicinal fibre has the effect of treating cutaneous inflammation and pain, has simple preparation method, convenient source of the raw materials and low cost, is friendly to the environment and is suitable for industrialized production; the fibre can be prepared into medicine-carrying clothings or other fabrics which directly contact with the skin of a human body, and the percutaneous administration is safe and comfortable.
Owner:DONGHUA UNIV +1
Ketoprofen patch delivery system
InactiveUS20050152958A1Increase chanceMaximizes ketoprofenOrganic active ingredientsSheet deliveryControlled releasePain disorder
A controlled release ketoprofen patch for the topical application of ketoprofen is described, in addition to methods of treating inflammatory disorders and pain disorders by the administration of the controlled release ketoprofen patch.
Owner:APR APPLIED PHARMA RES
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Topical ketoprofen composition
ActiveUS8822537B2Relieve painGood skin permeabilityBiocideNervous disorderChemical compositionHeadache severe
A topical composition, specifically an oil-in-water emulsion, comprised of ketoprofen and oxybenzone in a physiologically acceptable topical carrier. The composition is applied topically to alleviate pain, especially pain associated with migraine headache. The composition has good photostability as well as freeze / thaw stability.
Owner:ACHELIOS THERAPEUTICS
Preparation method and application of typical acidic drug multi-template molecularly imprinted polymer
InactiveCN102702428AUniquely identifiableIncreased sensitivityOther chemical processesAlkali metal oxides/hydroxidesPharmaceutical drugClofibric acid
The invention relates to a preparation method and application of a typical acidic drug multi-template molecularly imprinted polymer. The adopted template molecules are clofibric acid, diclofenac sodium, ibuprofen, naproxen and ketoprofen. The preparation method and the application of the molecularly imprinted polymer belong to the technical field of analysis, determination and removal of five typical acidic drugs in environmental samples. The preparation method is simple and reliable, and the prepared multi-template molecularly imprinted polymer has a uniform size, a large specific surface area and higher specific selectivity for acidic drugs. The five acidic drugs in the environmental samples can be separated and gathered rapidly and efficiently. The multi-template molecularly imprinted polymer can be directly applied in selective removal of the five acidic drugs in surface water and has better effects than other adsorption materials. The method is an effective method for specific separation of a series of substances with the same attributes and has broad application prospects.
Owner:TONGJI UNIV
Painless injectable compositions containing salts of 2-arylpropionic acids
InactiveUS7442832B2Not cause any painOrganic active ingredientsOrganic chemistryTiaprofenic acidMedicine
The invention relates to pharmaceutical compositions of alkylammonium salts of 2-aryipropionic acids, including ketoprofen, ibuprofen, naproxen and tiaprofenic acid. The compositions are suitable for parenteral administration arid, due to buffering at pH 8 to 9, cause less pain upon injection than previously known composition.
Owner:DOMPE FARM SPA
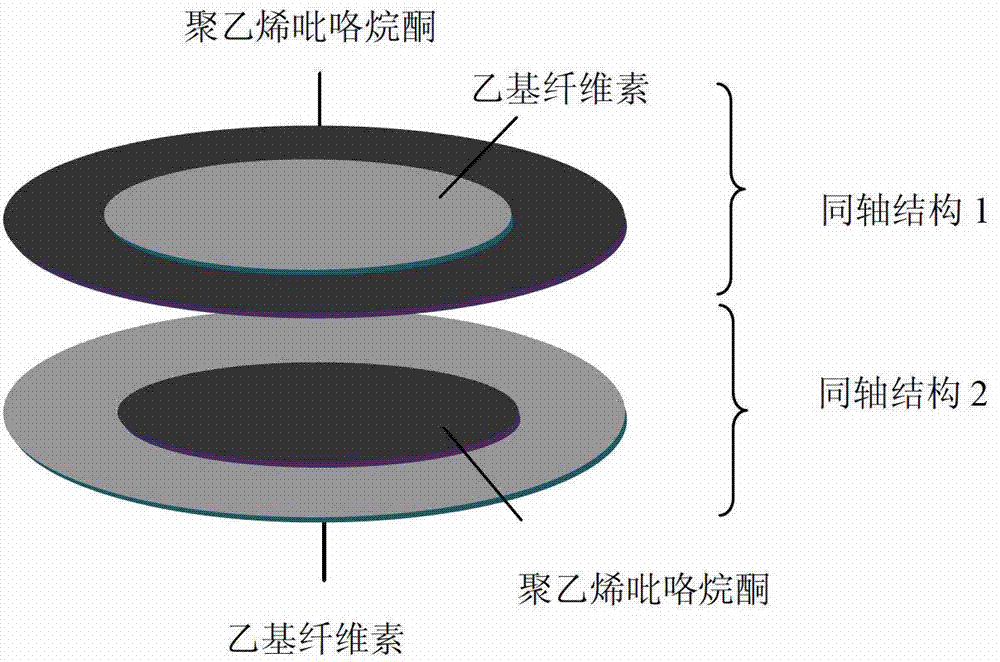
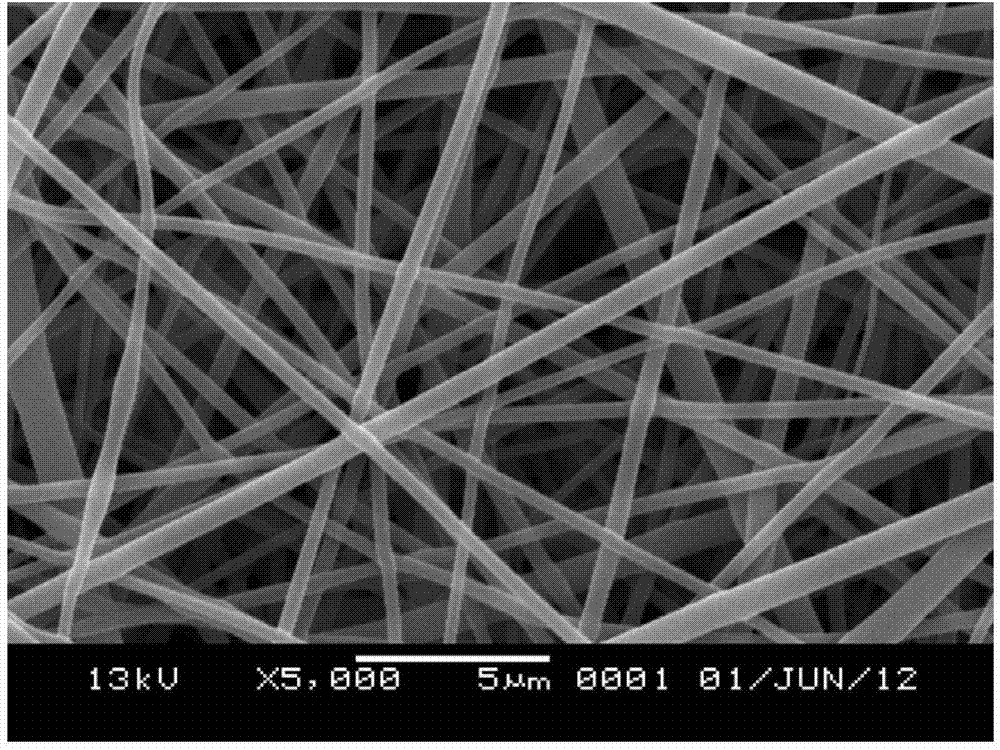
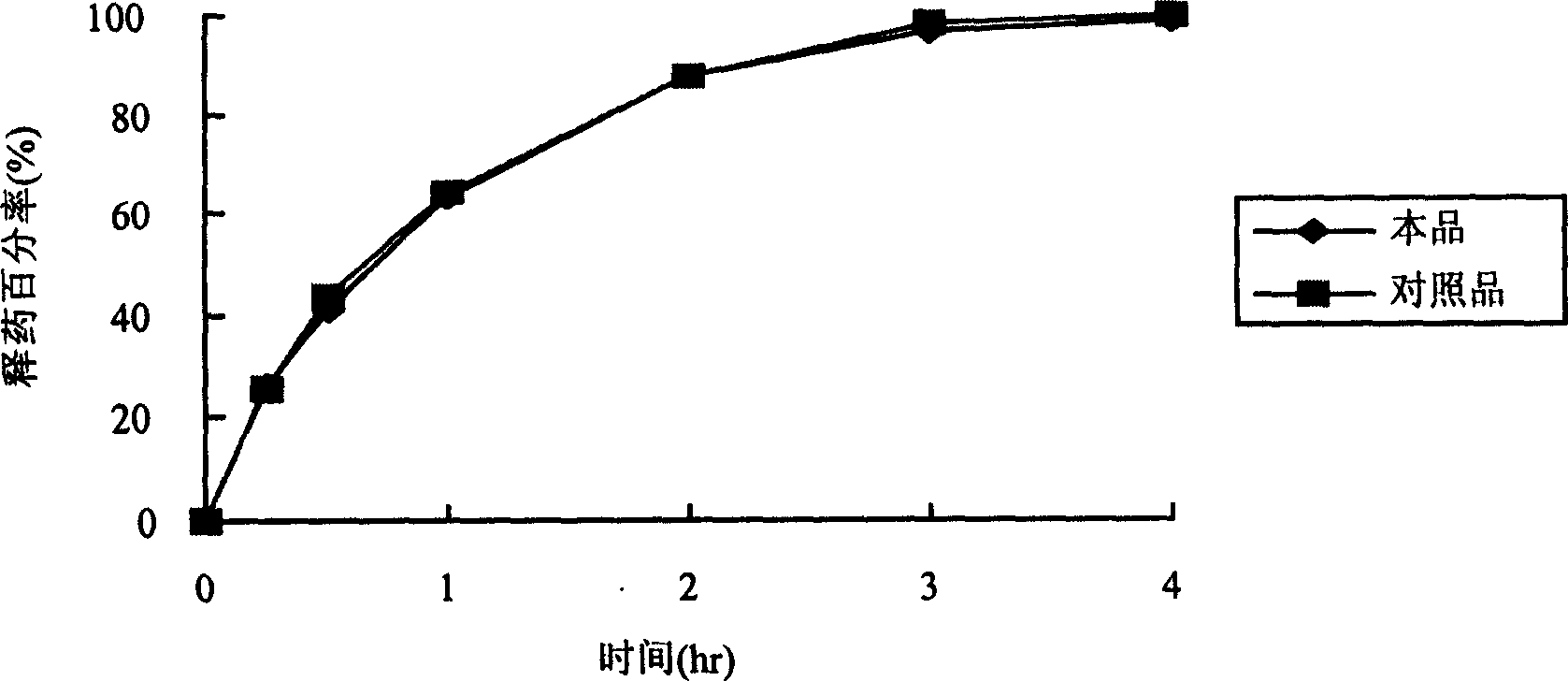
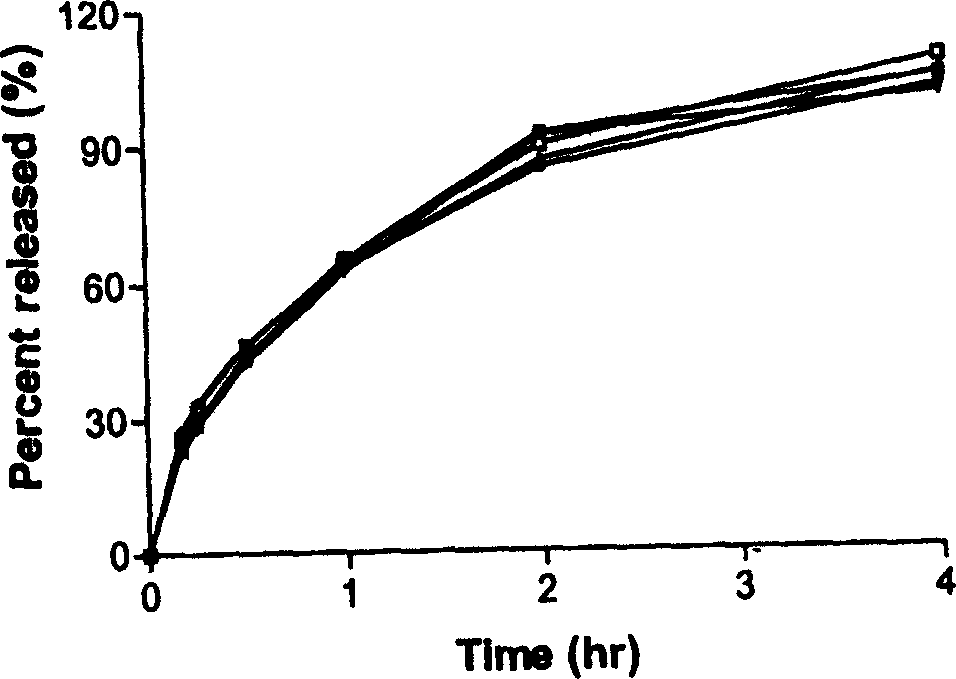
Two-phase drug-release multilayer drug-loaded nanofiber mat and preparation method thereof
InactiveCN102824641AImprove solubilityProlong the action timeAntipyreticAnalgesicsFiberElectrospinning
The invention relates to a two-phase drug-release multilayer drug-loaded nanofiber mat and a preparation method thereof. The fiber mat is a multilayer drug-loaded nanofiber membrane which consists of a water-soluble polymer, a water-insoluble polymer and a drug. The nanofiber membrane has the following two structures: a shell layer is the drug-contained water-soluble polymer, and a core layer is the drug-contained water-insoluble polymer; the shell layer is the drug-contained water-insoluble polymer, and the core layer is the drug-contained water-insoluble polymer. A preparation method for a nano analgesic comprises the following steps of: (1) preparing spinning solution containing analgesic ketoprofen; (2) preparing a multilayer nanofiber membrane through performing coaxial electrostatic spinning on the prepared spinning solution, and drying to obtain the drug-loaded nano analgesic. The nano analgesic can be used for rapidly and durably easing pains, can be easily carried, is convenient in administration and can be used for administrating drugs into intestinal tracts in the manner of targeting. The invention has the advantages that the preparation method is simple and is low in cost, and has no special requirements on equipment; and the two-phase drug-release multilayer drug-loaded nanofiber mat can be produced on a large scale.
Owner:DONGHUA UNIV
Injection containing ketoprofen and preparation method thereof
The present invention provides one kind of injection containing ketoproten and its preparation process, and features that the injection contains ketoproten, alkaline assistant and stuffing in the molar ratio between ketoproten and alkaline assistant of 1 to 1, and the weight ratio between ketoproten and stuffing of 1 to 0-10. The injection containing ketoproten is superior to traditional orally taken ketoproten preparation, and has new administration way, high product quality, high bioavailability and fast acting.
Owner:汪洪湖
Percutaneous plaster and its preparation method
InactiveCN1565523ALess pollutionFast coating speedMedical devicesUnknown materialsTransdermal patchMedicine
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Transdermal plaster of aryl propionic non-steroid antiphlogistic
InactiveCN1387842AImprove adhesionImprove stabilityOrganic active ingredientsAntipyreticTransdermal patchWhole body
The present invention relates to medicine technology and is especially one kind of new preparation form. The transdermal plaster is noe kind of antiphlogistic containing Flubiprofen, Ketoprofen, Ibuprofen, Rosorolfen, Naproxan and other aryl propionic non-steroid. It has three parts including non-sticking layer, medicine layer and lining layer. The medicine layer incldues medicine dispersed in matrix, and the matrix consists of non-polar polymer and plasticizer and may contains tackifier, transdermal promoter and oxidant. It has accurate admistration amount, no stimulation to gastrointestinaltract, relatively higher local medicine density in the affected part and controllable medicine release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for measuring 12 types of remaining medicine in water environment through separation and enrichment
ActiveCN105424825ASimplified processing stepsImprove extraction efficiencyComponent separationWater dischargePretreatment method
The invention relates to a method for measuring 12 types of remaining medicine in a water environment through separation and enrichment at the same time, and belongs to the field of safety detection of a trace of organic pollutant residue in the water environment. The content of 12 types of frequently-used medicine in the water environment (drinking water, faucet water, river water and water discharged into and out of sewage treatment plants) is directly measured with an ultra performance liquid-chromatography-mass spectrometer (UPLC-MS / MS) as a detection tool after a water sample is subjected to solid phase extraction combined with ultrasonic-assisted dispersion liquid-liquid micro-extraction (UA-DLLME) separation and enrichment. The 12 types of antibiotic include ketoprofen, ciprofloxacin, tinidazole, tolfenamic acid, sulfadiazine, sulindac, naproxen, sulfamethoxazole, chloramphenicol, cefuroxime axetil, piroxicam and mefenamic acid. Inspection and optimization are conducted on a sample pretreatment method and instrument detection conditions of the water sample, and the optimal UA-DLLME method is established and is successfully applied to practical sample detection. Compared with a traditional method, the method has the advantages of being high in sensitivity, high in extraction and recycle rate, wide in suitable object, friendly to the environment, and the like.
Owner:SHENYANG PHARMA UNIVERSITY +1
Medicament microsphere and preparation method thereof
InactiveCN103610649AReduce solubilityHigh encapsulation efficiencyOrganic active ingredientsAntipyreticDipyridamoleMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a medicament microsphere and a preparation method thereof. The medicament microsphere comprises medicinal materials, a high polymer material and a surfactant, wherein the ratio of the medicament to the high polymer material is 1:(1-3); the medicament preferably is celecoxib, ketoprofen, dipyridamole and nimodipine. The medicament microsphere is prepared by adopting an O / W emulsified solvent diffusion-volatilization method. The O / W emulsified solvent diffusion-volatilization method comprises the following steps: respectively weighing the medicinal materials, the high polymer material and a release regulator according to the prescription amount, and then adding the weighted materials to an organic solvent; ultrasonically or mechanically stirring and dissolving as a disperse phase; taking a surfactant solution as a continuous phase; warming and stirring after low-temperature emulsification under an agitation state, so as to remove an organic solvent; and then carrying out solid separation, washing by distilled water, and drying, so as to obtain the medicament microsphere. Thus, the prepared microsphere is high in encapsulation efficiency and high in yield.
Owner:SHENYANG PHARMA UNIVERSITY
Edible Oral Strip or Wafer Dosage Form Containing Ion Exchange Resin for Taste Masking
ActiveUS20140155483A1Facilitate ionic bindingGood dispersionBiocideAntipyreticThroat irritationAdditive ingredient
An edible oral film strip dosage form containing an unpalatable acidic active pharmaceutical ingredient, particularly ketoprofen, and an ion exchange resin as a primary taste masking agent, along with an optional alkaline agent and further optionally containing one or more secondary taste masking agents is provided. The edible oral film strip dosage matrix is formed from at least one water soluble or miscible polymer(s). The optional secondary taste masking ingredients include one or more of flavoring agent(s), sweetener(s), cooling sensation agent(s), and taste receptor blocker(s). The inventive dosages minimize or completely mask the bitterness, burning sensation and throat irritation associated with many acidic active pharmaceutical ingredients. Methods for preparing the inventive edible oral film strip dosage forms are disclosed, as well as their method of administration.
Owner:LTS LOHMANN THERAPIE-SYST AG
Methods and compositions for treating and preventing trigeminal autonomic cephalgias, migraine, and vascular conditions
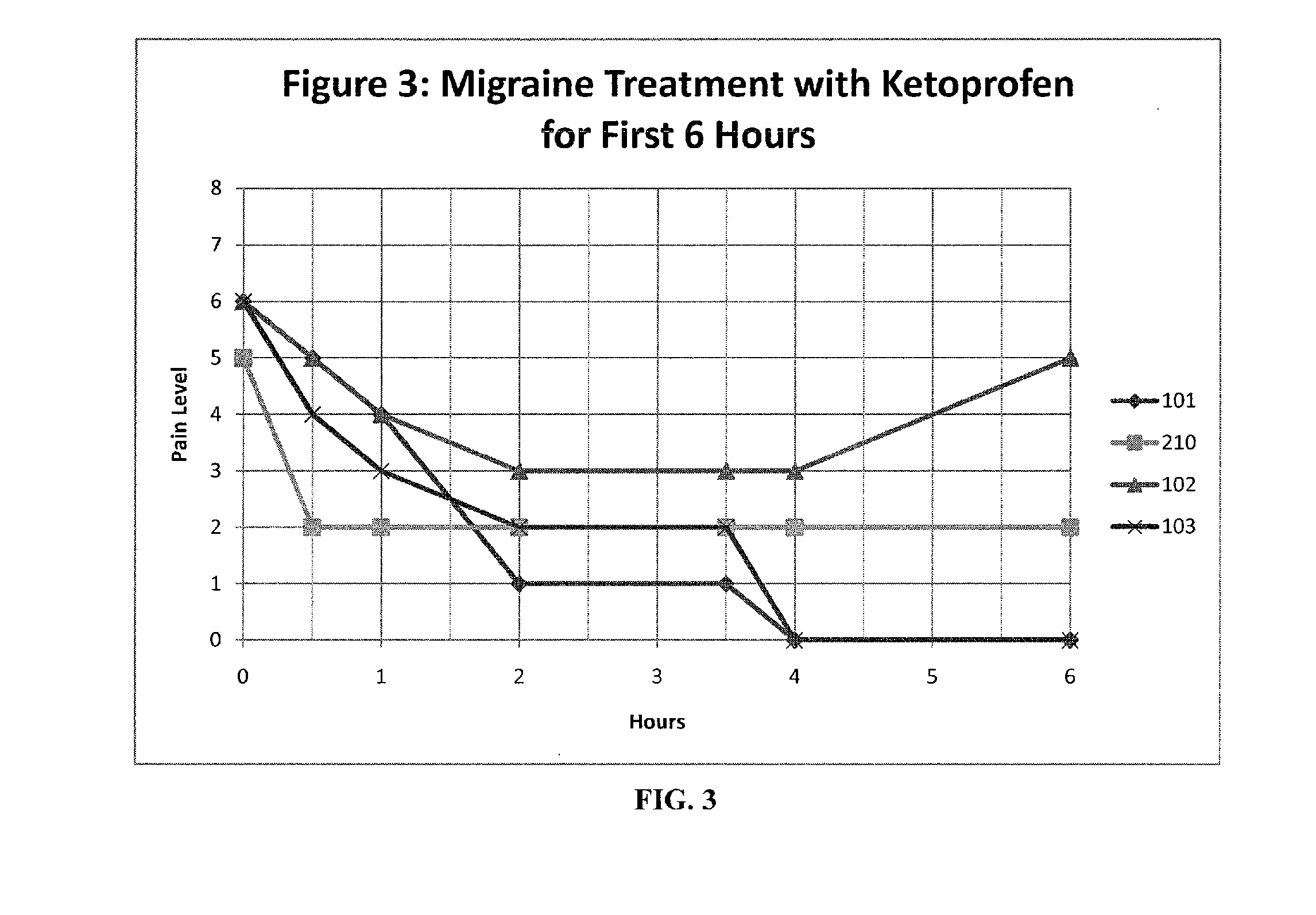
The present invention relates to, among other things, methods for treating trigeminal cephalgias such as migraine and migraine like headaches and other cerebrovascular conditions associated with pain and or inflammation. When non-steroidal anti inflammatory drugs (NSAIDs), such as ketoprofen, are applied locally using specific topical formulations immediate relief of pain is obtained. Intense pain is typically reduced to mild pain or no pain within 30 minutes of application of the topical formulation. The NSAID may be given in combination with other pharmacological agents, such as vasoconstrictors, opioids, decongestants and / or non-opioid migraine drugs, such as triptans and ergots and agents that affect serotonin receptors as agonists, antagonists or partial agonists.
Owner:ACHELIOS THERAPEUTICS
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
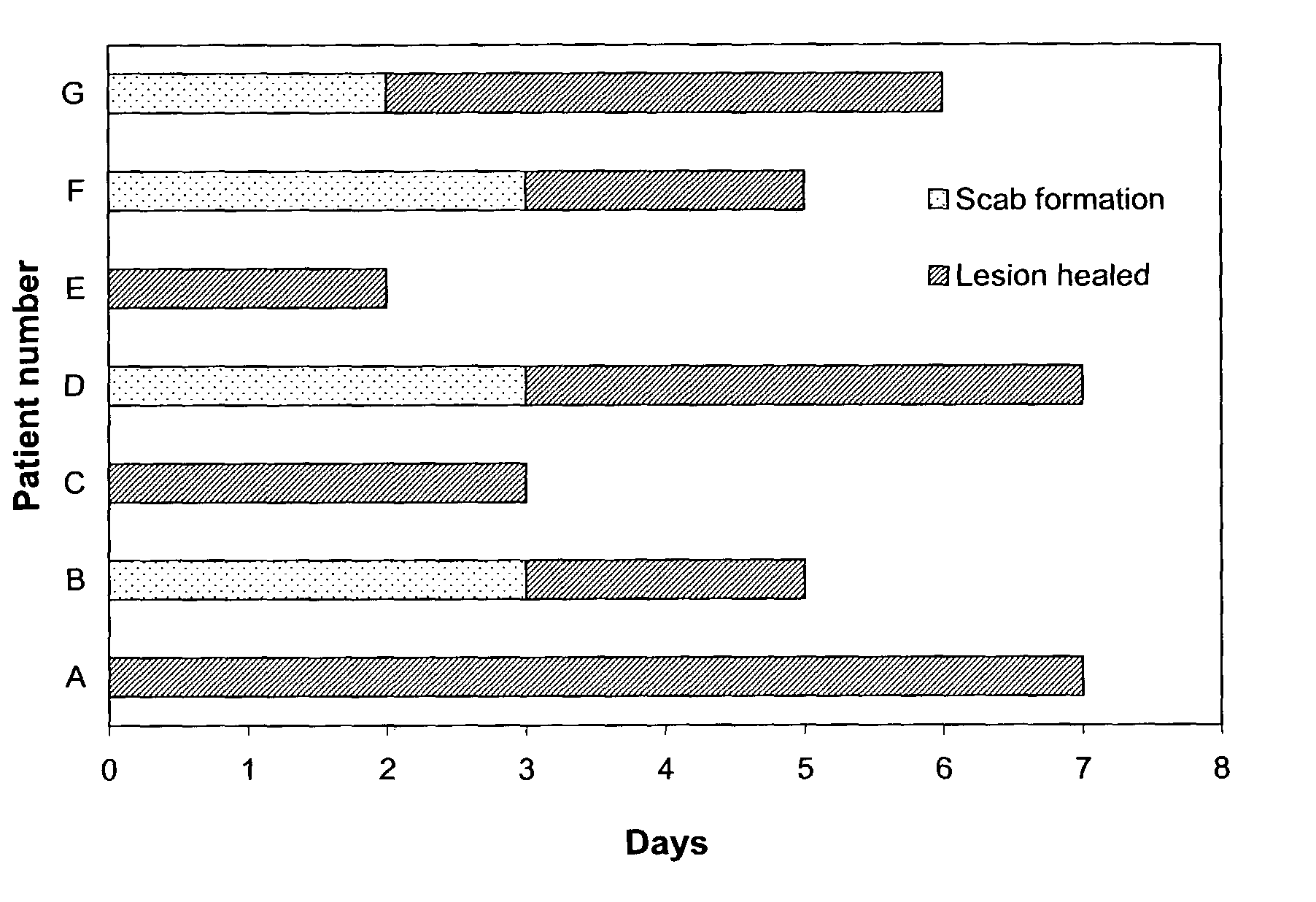
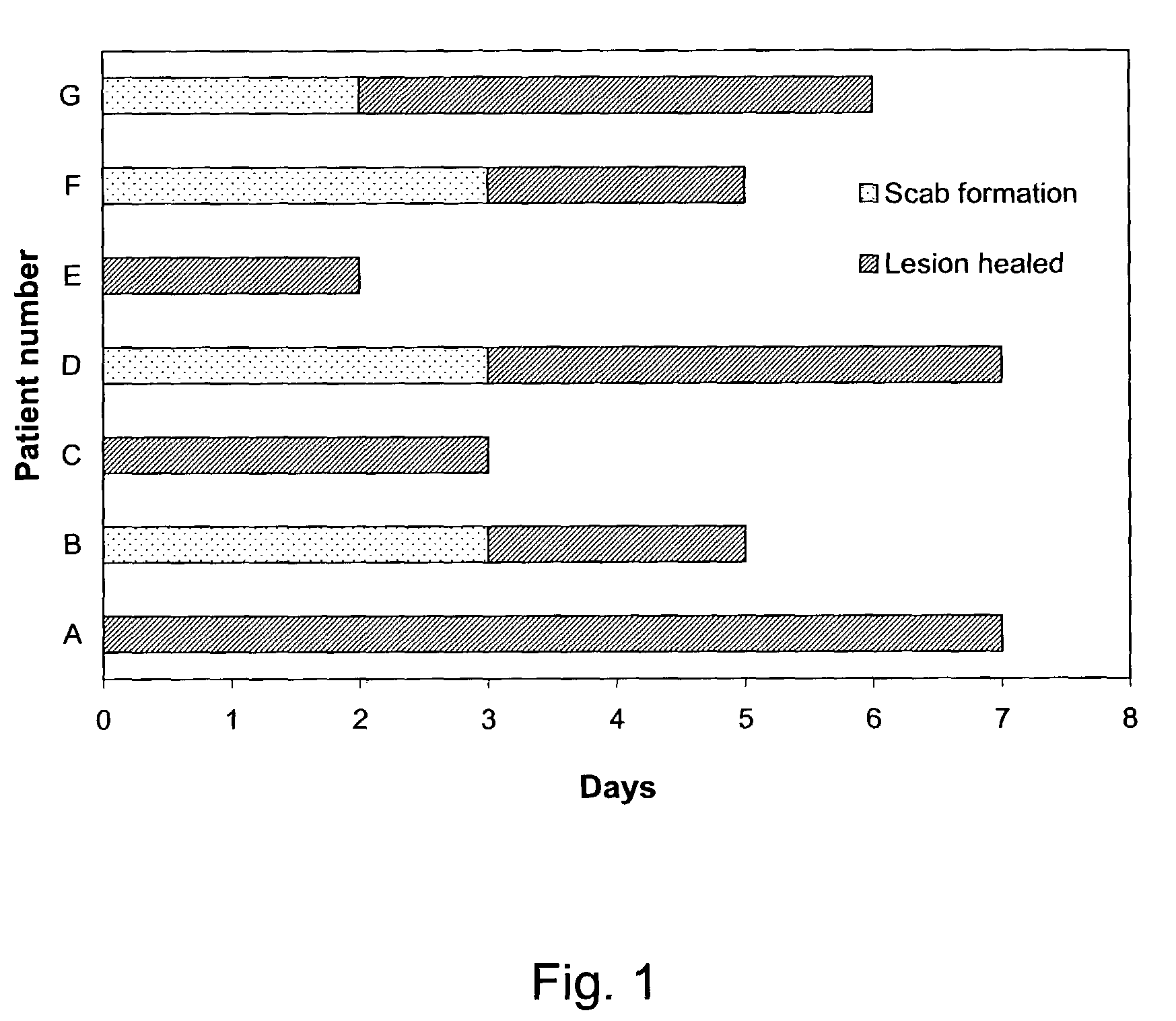
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
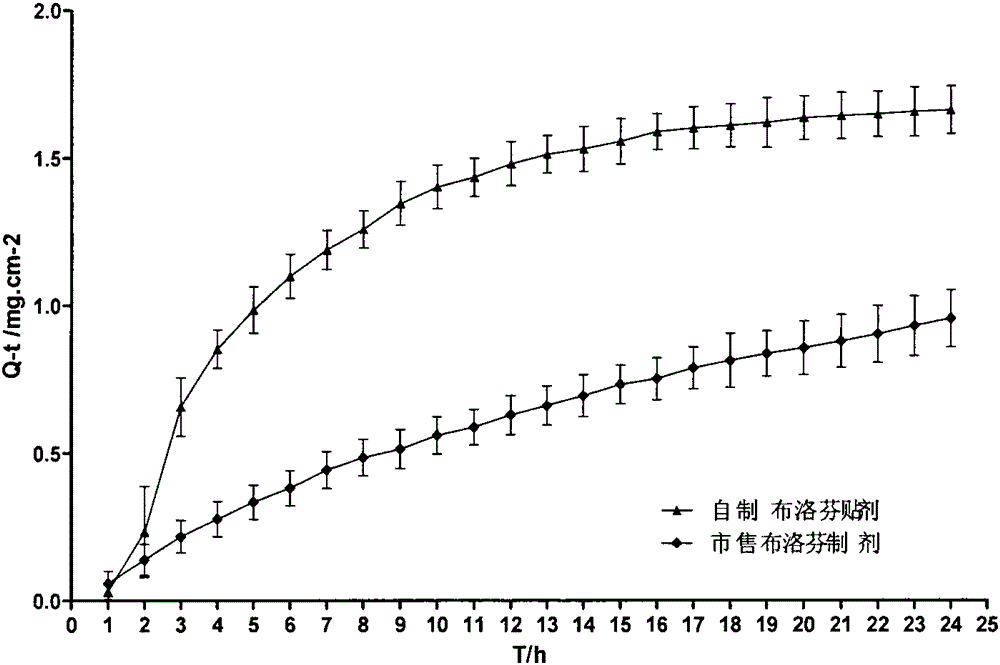
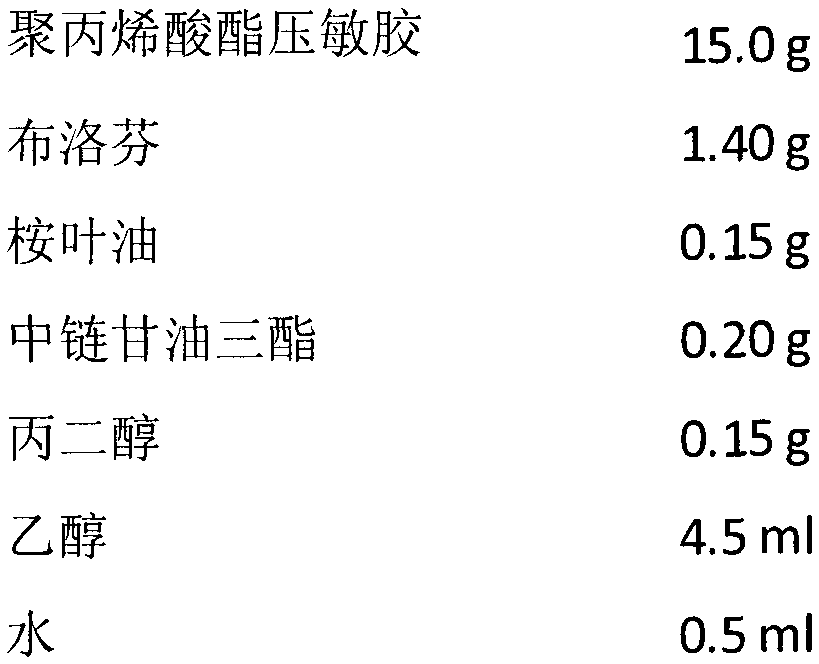
Ketoprofen cataplasm and its preparing method
The present invention belongs to the field of medicine technology, in particular, it relates to a ketoprofen cataplasma preparation and its preparation method. The experiments of initial adhesion, paste content, excipient property, external release and external percutaneous test show that the invented ketoprofen cataplasma preparation can meet the requirements related to pharmacopeia.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Injection liposome microball containing ketoprofen or ketoprofen ester and its preparing process
InactiveCN1709232AIncrease fat solubilitySignificant analgesic and anti-inflammatory effectsOrganic active ingredientsLipid formationMicrosphere
The present invention relates to a lipid microsphere injection containing ketoprofen or ketoprofen ester, its preparation process and prescription. It has good action for stopping pain, resisting inflammation and reducing fever. Said lipid microsphere includes ketoprofen or one ketoprofen ester, injection soybean oil, injection medium chain triglyceride, phospholipids, pure glycerin and injection water. Said invention can be used for preparing pain-stopping and inflammation-relieving medicine.
Owner:SHENYANG PHARMA UNIVERSITY
Method for catalyzing and splitting 2-aryl ethylformic acid antimer by using Serratieae fat enzyme
InactiveCN101063157AHigh optical purityReduce pollutionMicroorganism based processesFermentationPropanoic acidReaction temperature
The invention discloses a method to resolve 2-aryl radical propanoic acid antimer with Serratieae lipase, which comprises the following steps: touching Serratieae lipase with racemic 2-aryl propionic ester; proceeding enzymatic reaction with temperature at 20-60 deg. c and pH value at 5-11; collecting (S)-2-aryl propionic acid and un-reacting (R)-2-aryl propionic acid; using the (R)-2-aryl propionic acid to enzymatic resolution repeatedly. This invention can leases labor strength and environmental contamination, which can be used to antiphlogistic anodyne firm.
Owner:EAST CHINA UNIV OF SCI & TECH
Long-acting viscose dispersing type transdermal patch and preparing process thereof
InactiveCN105250243AHigh transdermal efficiencyModerate viscosityOrganic active ingredientsAntipyreticTransdermal patchTG - Triglyceride
The invention discloses a long-acting viscose dispersing type transdermal patch and a preparing process thereof and belongs to the medical technical field. The transdermal patch is mainly prepared from bulk pharmaceutical chemicals (such as non-steroid anti-inflammatory drug ibuprofen and salt form thereof, naproxen and salt form thereof, ketoprofen, indometacin and salt form thereof), a penetration enhancer (menthol, oleic acid, medium chain triglyceride, propylene glycol monolaurate, azone, propylene glycol and the like), a dispersing solvent (water, acetone, ethanol, carbinol, ethyl acetate and the like), a polyacrylate pressure-sensitive adhesive (crylic acid, butyl acrylate, crylic acid 2-ethylhexyl ester and the like), a backing layer and a release liner. The long-acting viscose dispersing type transdermal patch and the preparing process thereof have the advantages that drugs can be released from a matrix continuously for 12-48 h, the number of drug residues is low, transdermic absorption property is excellent, and dosing frequency and dosing amount can be reduced; skin irritation is avoided, and adhesion and compliance are high; main drugs and additives are stable in a viscose mechanism; preparing technology is simple, and pollution is avoided.
Owner:CHINA PHARM UNIV
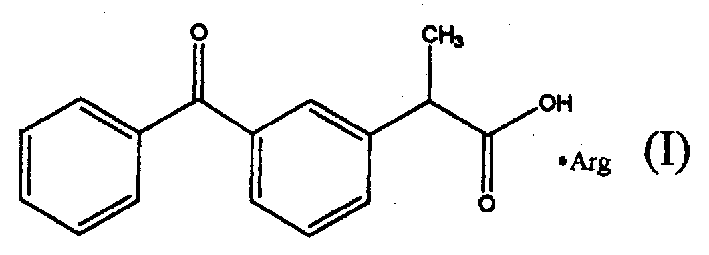
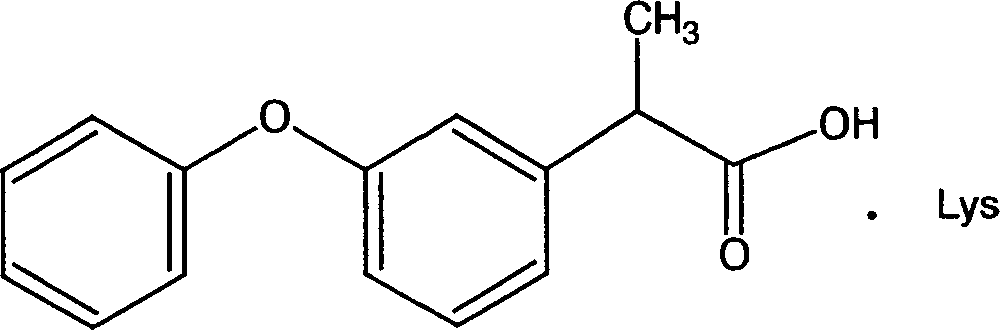
Arginine-ketoprofen with antalgic and inflammation relieving actions and its preparing process
The present invention relates to the arginine-ketoprofen with antalgic and inflammation-relieving functions, relative isomers and its preparing process. Its solubility in water is solved to reduce its by-effect and it can provide a new application approach.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Platinum complexes
InactiveCN101469004AGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSulfate radicalsChlorogenic acid
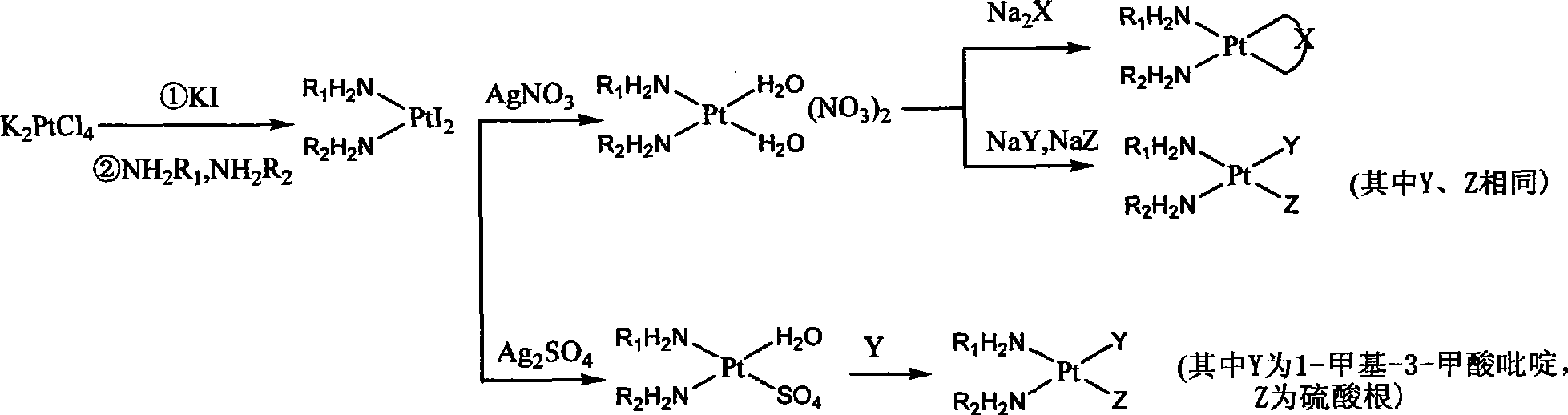
The invention relates to platinum complexes of the upper right formula and pharmaceutically acceptable salts or solvates, wherein R1 and R2 are respectively and independently selected from H or C1-C4 alkyls, or R1 and R2 are coupled as shown by the lower right formula. X is selected from a 3,4-dinicotinic acid radical or a cis form aconitic acid radical; and Y which has the same case with Z is selected from a cinnamic acid radical, a ketoprofen acid radical, a chlorogenic acid radical, a caffeic acid radical or a ferulic acid radical; or Y is different from Z, wherein Y can be 1-methyl-3-pyridine carboxylic acid, and Z can be a sulfate radical. The invention also relates to a preparation method for the platinum complexes and application of the platinum complexes to the preparation of anticancer drugs.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Nonsteroidal antiinflammatories with nitric oxide donors and its preparation method
InactiveCN101053662ALittle side effectsMild reaction conditionsAntipyreticAnalgesicsIndometacinSide effect
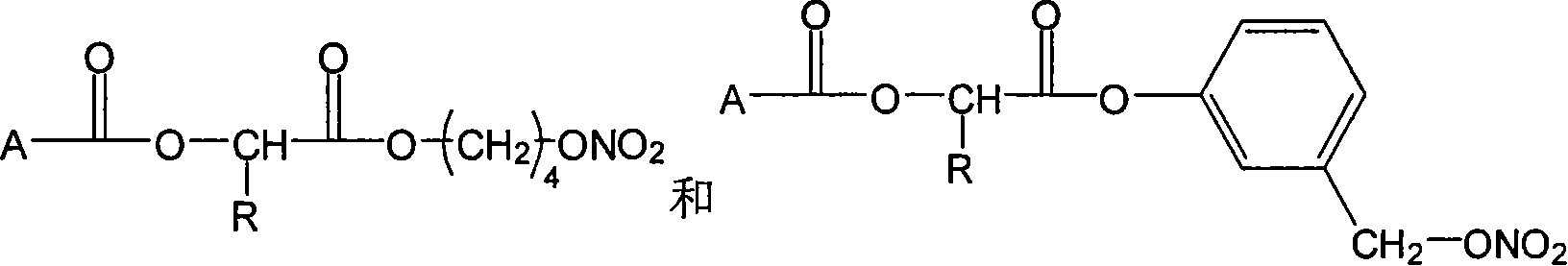
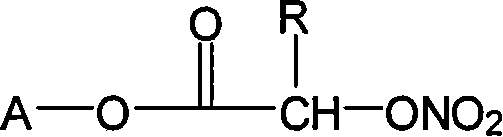
The invention relates to a non-steroidal anti-inflammatory drug with nitrogen oxide donor and a method for preparing same, which can be used to eliminate inflammation, relieve fever and stop pain, and can decrease the frequently seen side effect thereof on the gastrointestinal tract. The structure of which is A-O(X)-CO-O(y)-B-ONO2,wherein, A is the non-steroidal anti-inflammatory group; B is the connection group, when x=0, y=1; when x=1 then y=0. The the non-steroidal anti-inflammatory groups includes aspirin, diclofenac, indometacin, lumiracoxib, brufen, ketoprofen, naproxen, piroxicam, and meloxicam. The preparation process are that the bromhydrin (or hydroxybenzene) reacts with silver nitrate into hydroxy nitrate, then reacts with bromo acid into the connection group of nitrogen oxide donor, then connects with the non-steroidal anti-inflammatory drug; or the non-steroidal anti-inflammatory drug condensates with bromo acid into a bromide intermediate, then reacts with silver nitrate into the non-steroidal anti-inflammatory drug with nitrogen oxide donor.
Owner:江苏吴中苏药医药开发有限责任公司
Method of utilizing continuous flow microreactor to synthesize benzophenone derivative
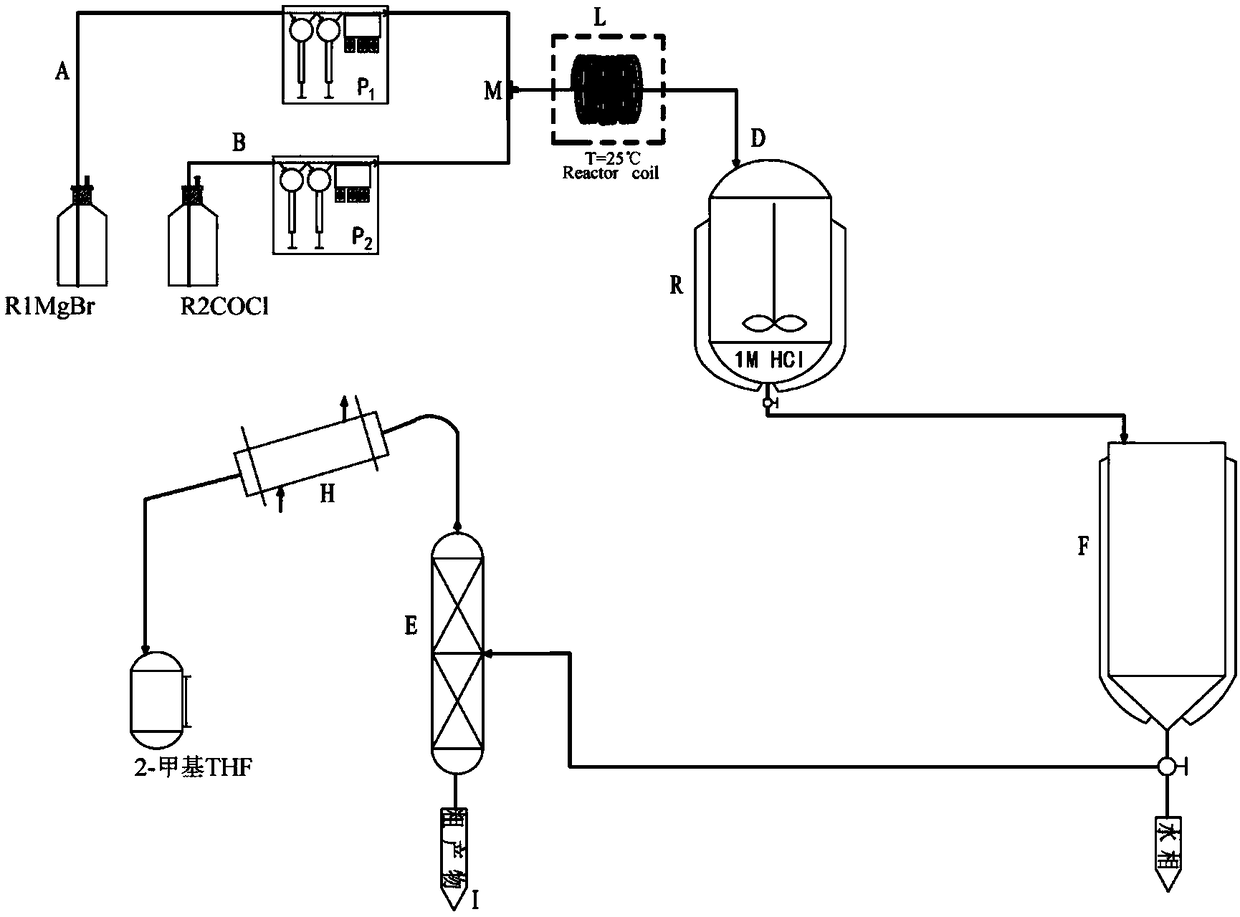
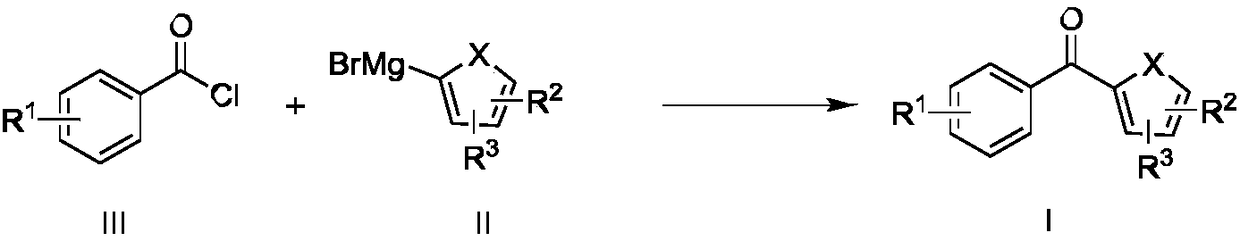
ActiveCN108409516AShort reaction timeImprove reaction efficiencyProcess control/regulationCarboxylic acid nitrile preparationEnvironmental resistanceGrignard reagent
The invention belongs to the technical field of organic synthesis, and particularly relates to a method of utilizing a continuous flow microreactor to synthesize a benzophenone derivative. The methodincludes: using aryl Grignard reagent and acyl chloride as raw materials; at normal temperature, continuously synthesizing the benzophenone derivative in the microreactor; recycling a reaction solvent2-methyl tetrahydrofuran. Problems of environmental pollution and reaction operation safety caused by the fact that conventional Fridel-Crafts reaction is excessively dependent on reagents like aluminum trichloride, ferric trichloride and zinc dichloride are avoided, the defect that novel catalytic processes are expensive in catalytic reagent and harsh in operation condition is made up, and continuous synthesis of a medical intermediate ketoprofen nitrile is realized efficiently. The method has the advantages of high operation convenience, high reaction safety, high yield, high efficiency andreaction solvent reusability and is environment-friendly and efficient.
Owner:JIANGNAN UNIV
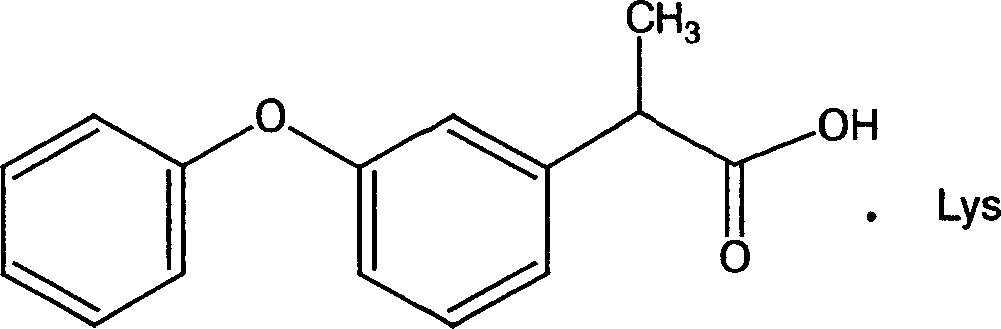
Lysine-ketoprofen and its production
InactiveCN1939893ASolve solubilityImprove antipyreticOrganic active ingredientsOrganic compound preparationSolubilitySide effect
A lysine ketoprofen with antipyretic, analgesia and anti-inflammatory functions and its production are disclosed. The process is carried out by proportioning lysine with ketoprofen in 1:1mol to obtain the final product. Its advantages include higher pharmacodynamic strength, better solubility, safety and stability and low side effect.
Owner:BEIJING SAISHENG PHARMA
Methods for the treatment of neuropathic pain and other disorders using R(-)-ketoprofen
InactiveUS6620851B2Treat or prevent tinnitus or ringing in the earsBiocideSenses disorderDiseaseNeuropathic pain
Owner:SEPACOR INC
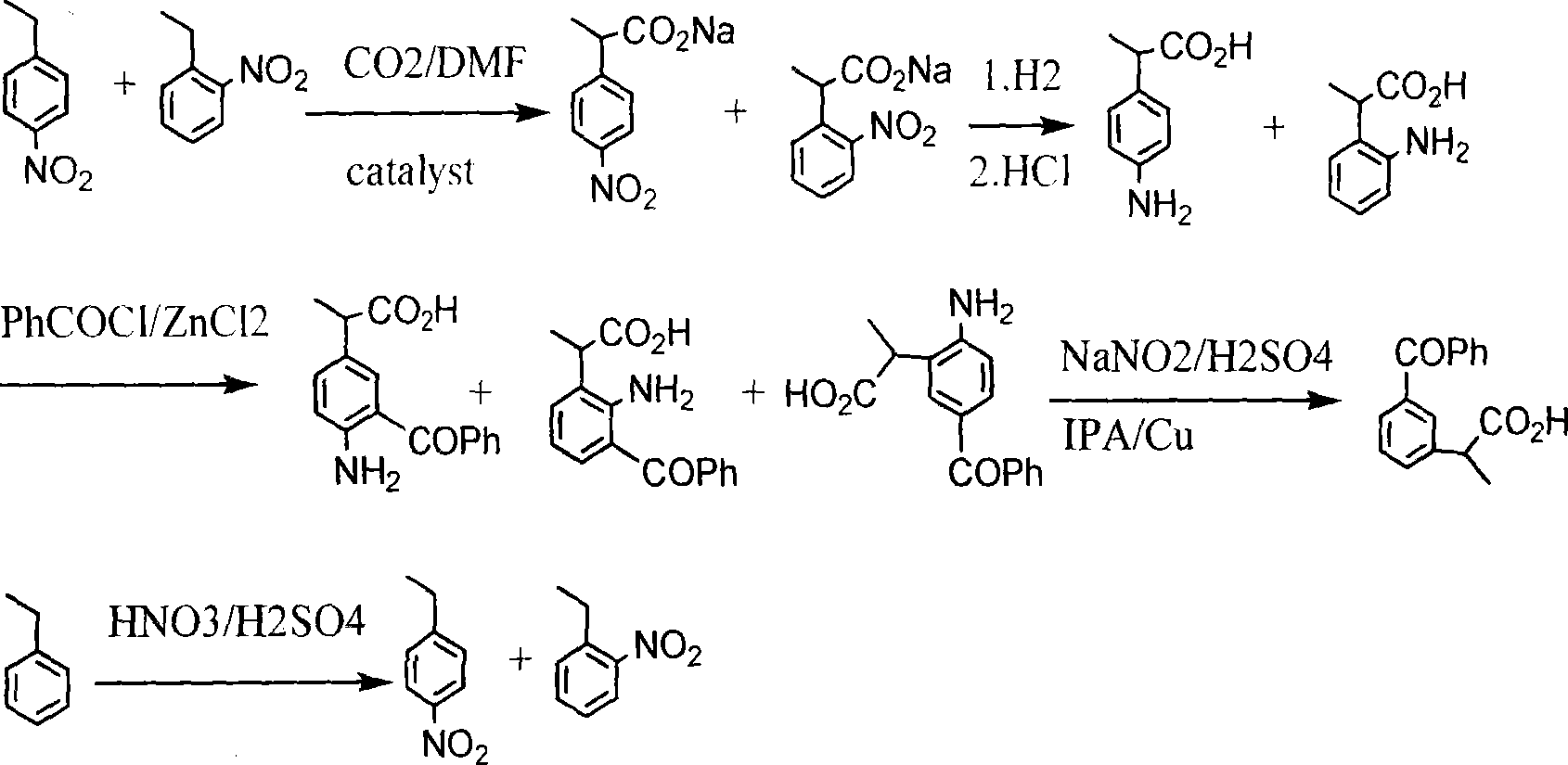
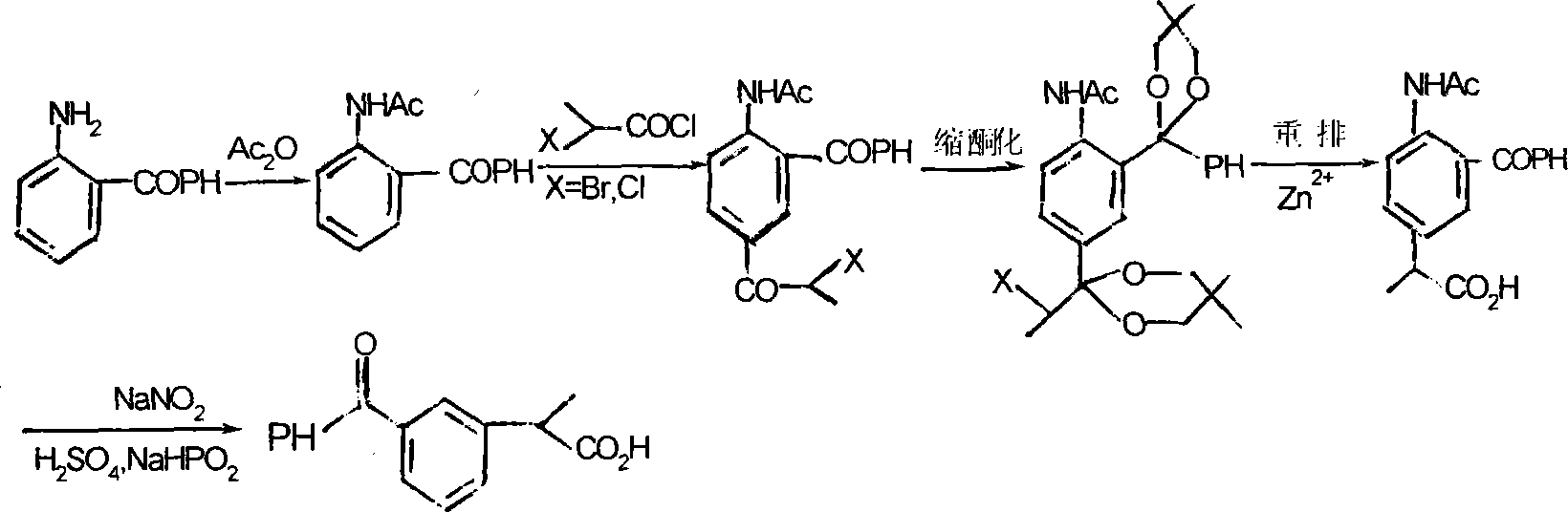
Method for synthesizing ketoprofen by using ethylbenzene as raw material
ActiveCN101250103ACost savingsCut costsOrganic compound preparationChemical recyclingBenzoyl chlorideWastewater
A method for synthesizing ketoprofen from ethylbenzene relates to a preparation method of organic drug, in particular to a method for using ethylbenzene as raw material to synthesize ketoprofen. The inventive method comprises synthesizing o-nitro ethylbenzene and p-nitro ethylbenzene via ethyl benzene, directly processing carboxylation reaction, hydrogenation reduction reaction and benzoylation reaction on the mixture of o-nitro ethylbenzene and p-nitro ethylbenzene without separation, and processing deamination reaction to obtain crude product of ketoprofen, and purifying to obtain the ketoprofen product. The invention directly processes o-nitro ethylbenzene and p-nitro ethylbenzene with cheap carbon dioxide without separation, via carboxylation reaction, while the reaction is safe and environment friend and can save cost. Aminophenyl and benzoyl chloride are directly treated via friedel craft reaction, with less reaction process, low cost, little waste discharge. And the invention uses copper powder as catalyst and uses isopropanol as reducer, thereby saving cost and avoiding the treatment to phosphorus waste water.
Owner:HUBEI XUNDA PHARMA
Gel for topical delivery of NSAIDs to provide relief of musculoskeletal pain and methods for its preparation
ActiveUS9012402B1Improve tolerancePromote formationOrganic active ingredientsPeptide/protein ingredientsStainingPatient compliance
A drug-delivery system is described which can serve as a platform for the topical delivery of a wide variety of therapeutic agents to the skin. Specifically, a topical external analgesic gel contains ketoprofen, a skin penetration enhancer / cosolvent, a thickening agent and a base to adjust pH. The formulation uses a relatively small number of safe components and is easy to prepare with a high yield of finished product. The chemical stability of ketoprofen in the gel and the physical stability of the gel itself ensure a satisfactory shelf-life for the product. The gel is aesthetically pleasing (i.e., easy water-washability, non-irritating to skin, non-staining of clothing, etc.) and has proven to provide rapid relief of musculoskeletal pain, thereby helping to ensure patient compliance.
Owner:BLANCHARD JAMES
Ketoprofen gel and preparation method thereof
InactiveCN103156803AGood transdermal effectQuick effectOrganic active ingredientsAntipyreticAntiinflammatory EffectKetoprofen
The invention relates to a ketoprofen gel and a preparation method thereof. The ketoprofen gel is prepared from the following components: 2-5 % of ketoprofen, 0.5-2 % of matrix material, 2-4 % of a transdermal enhancer, 0.5-5 % of a neutralizer, 0.05-0.2 % of an anti-oxidant, 20-50 % of ethanol and the balance being water. The ketoprofen gel provided by the invention solves problems of stimulation of ketoprofen preparations on gastrointestinal tract in a background technology, is used externally, has advantages of good transdermal effect, quick effect and good analgesic and anti-inflammatory effects, and can play drug effects for a long time.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com