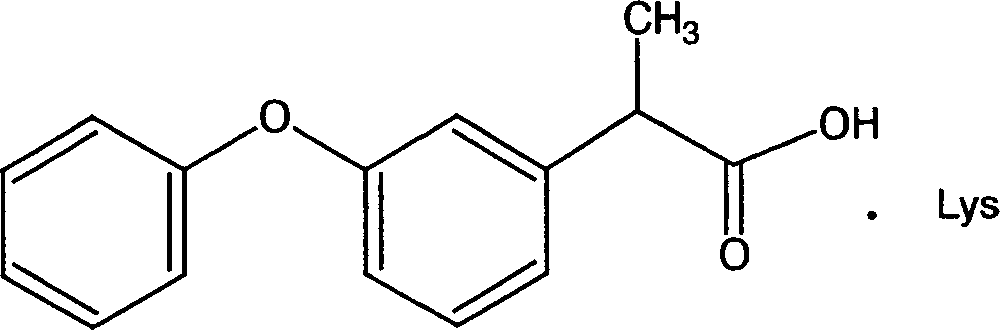
Lysine-ketoprofen and its production
A technology of lysine ketone and lysine, which is applied in the field of amino acid ketoprofen, can solve the problems such as the inability to know the efficacy of arginine ketoprofen ketoprofen, the inability to know the stability, and the like, and achieve increased stability. Sex, improve antipyretic, improve the effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] First add 73.06 grams (0.4mol) of L-lysine and 1000ml of distilled water in the reaction vessel, then add 101.7g (0.4mol) of racemic ketoprofen after dissolving, stir until the solution is clear, freeze-dry to obtain 171.2 grams of white Block or powder is ketoprofen lysine, the yield is 98.0%, and the purity is 99.2%.
[0029] The freeze-drying conditions are: 20℃~-45℃, pre-freezing for 2.5 hours; -45℃~-40℃, sublimation for 3 hours; -40℃~0℃, sublimation for 8 hours; 0℃~30℃, drying for 8 hours; 30℃~35℃, dry for 2.5 hours; vacuum is less than 0.2Mbar, control the maximum product temperature not to exceed 35℃, and the shelf temperature not to exceed 40℃.
[0030] Pharmacodynamic test of lysine ketoprofen
Embodiment 2
[0031] [Example 2] Antipyretic test
[0032] Take 50 ground rabbits weighing 1.6-2.0 kg, half male and half male, and divide them into random groups, 10 rabbits in each group, and administer them by intragastric administration (ig). Negative control group (normal saline 1ml / kg), positive control group (ketoprofen 20mg / kg)), lysine ketoprofen low dose group (3.0mg / kg)), middle dose group (20mg / kg)) , high dose group (80mg / kg)). After measuring the normal body temperature of the rabbit, intravenous injection of inactivated Escherichia coli liquid (about 1106 / kg bacteria, calculated by turbidimetric method) caused fever. After 3 to 4 hours, body temperature was measured as a fever indicator. When the body temperature rose above 1°C, the rabbits in each group were given ig respectively according to the above dosage. Body temperature was measured at 1, 1.5, 2, and 2.5 hours after administration. The degree of body temperature drop at each time point after administration was use...
Embodiment 3
[0035] [Example 3] Analgesic test - hot plate method
[0036] Fifty female mice weighing 19-21 g were taken and divided into random groups, 10 in each group. Divided into 10% gum arabic group (blank group), ketoprofen group (30mg / kg), lysine ketoprofen was divided into 10mg / kg group, 30mg / kg group, 80mg / kg group, ig administration. Adjust the constant temperature water bath hot plate to 55±0.5°C, place the mouse on the hot plate, measure once every 5 minutes, and calculate the average value for 2 consecutive times. The normal pain response latency of each mouse was tested, and the mice licked their hind feet and kicked their legs as pain response indicators. The pain response latency was measured again at 0.5, 1, 1.5, 2, 2.5 hours after ig administration. Those who exceed 60s are counted as 60s.
[0037] The results are shown in Table 2. The results showed that, compared with the blank group, the lysine ketoprofen group had significant differences in the pain thresholds of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com