Method for catalyzing and splitting 2-aryl ethylformic acid antimer by using Serratieae fat enzyme
A technology of Serratia and arylpropionic acid, which is applied in the field of separation of 2-arylpropionic acid enantiomers, can solve the problems of increased catalyst cost, complicated treatment process, and failure to meet the high purity requirements of the pharmaceutical industry. To achieve the effect of reducing labor intensity and environmental pollution and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Cultivate wild bacteria:
[0047] The wild Serratia S. marcescens ECU1010 (strain collection number: CGMCC No. 1219) was cultured in the fermentation medium, the culture time was 24 hours, the temperature was 30 ° C, and the shaking speed was 160 rpm, and then the supernatant was collected by centrifugation. 2000ml of enzyme solution, concentrated to 300ml by ultrafiltration, added 58.2 grams of ammonium sulfate until the saturation of ammonium sulfate was 35% to precipitate protein;
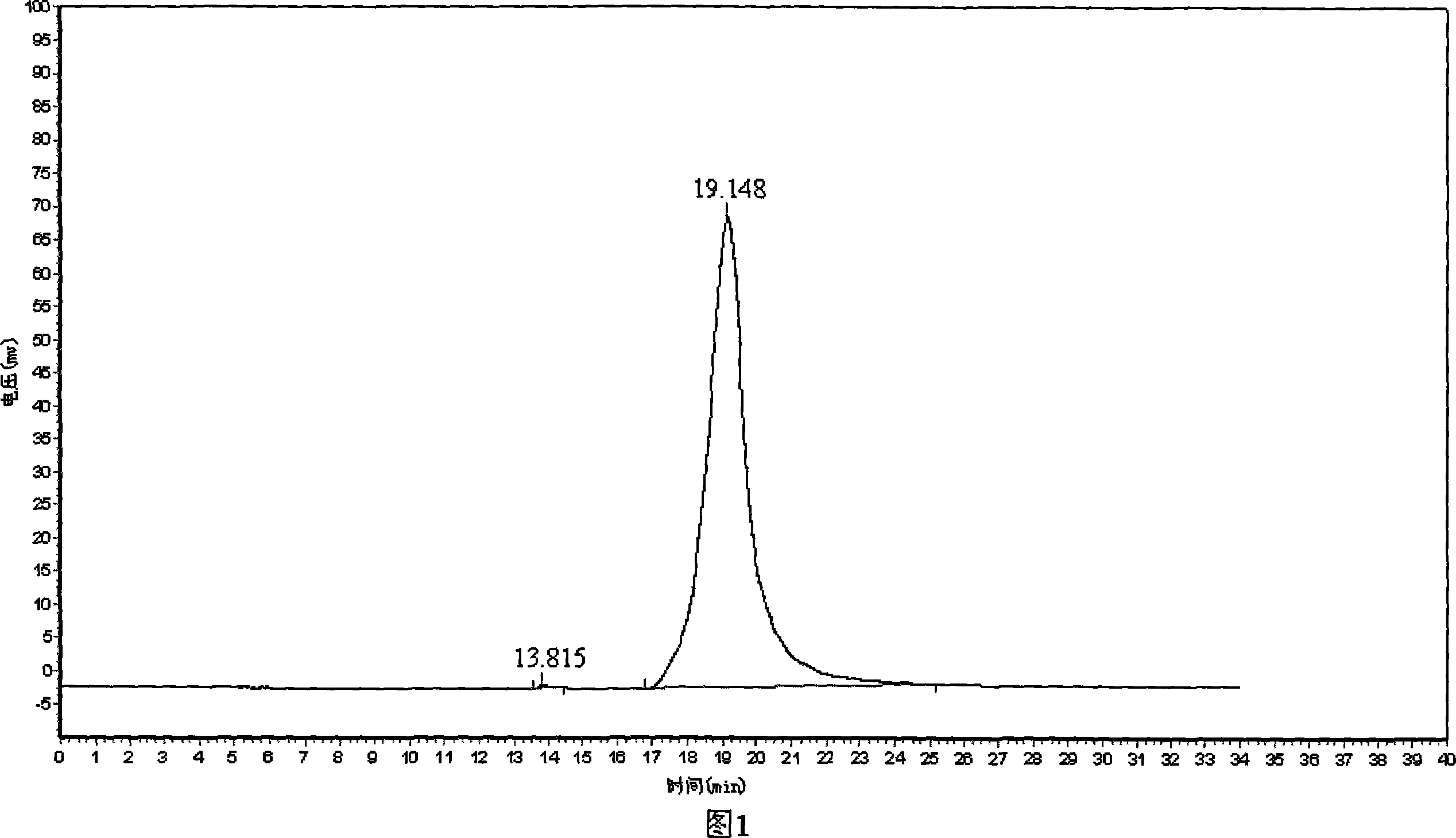
[0048] Using DEAE-Toyopearl 650M ion chromatography, then using Sephadex G150 gel chromatography, and then using Pheneyl-Toyopearl 650M hydrophobic chromatography to purify to obtain SDS electrophoresis pure protein, and HPLC detection shows that its purity is almost 100%, see figure 1.
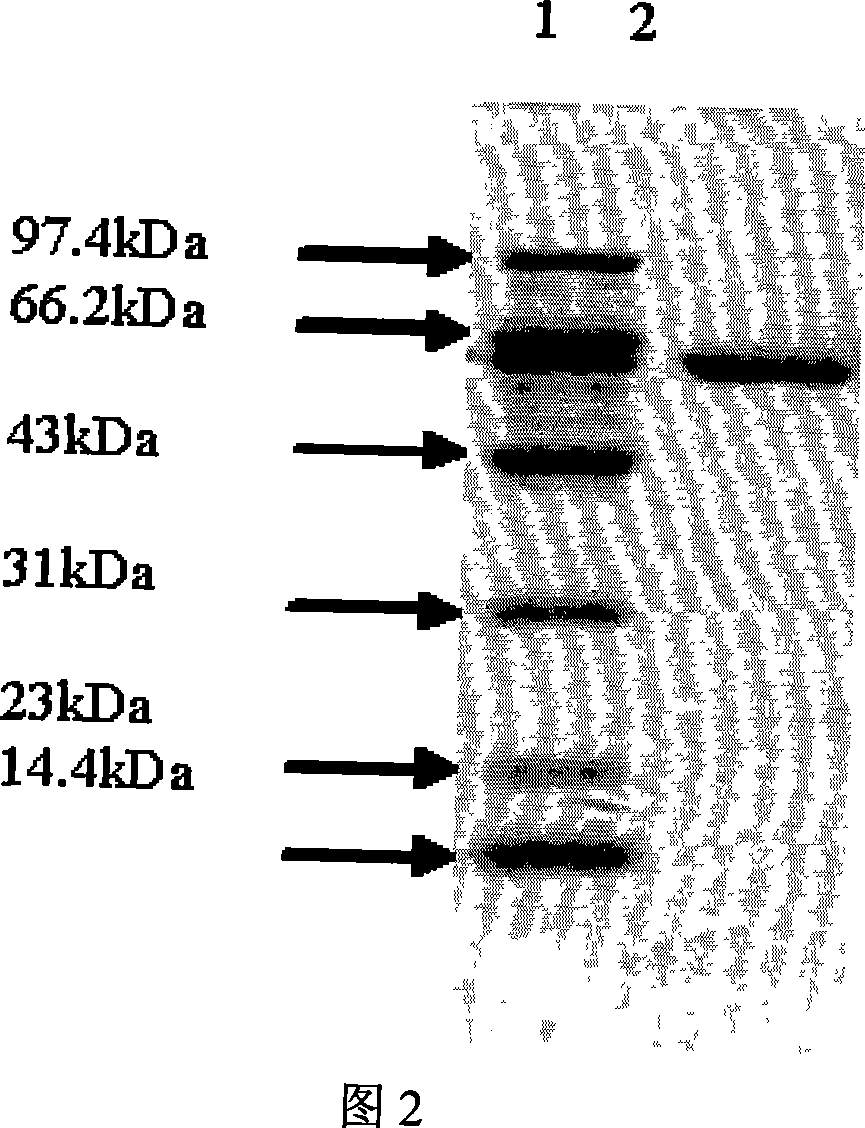
[0049] The biochemical experiments of this enzyme show that the molecular weight of its subunit is 65kDa, as shown in Figure 2 lipase SDS-PAGE electrophoresis map; the isoelectric point is 4.2, as shown...
Embodiment 2
[0051] Extraction of S. marcescens ECU1010 lipase gene and acquisition of recombinant strain BL21(DE3) / pBCLipE1:
[0052] (1) Acquisition of S. marcescens ECU1010 lipase gene
[0053] The wild bacterium S. marcescens ECU1010 (strain collection number: CGMCC No. 1219) was cultivated, and the cultivation method was as follows: the solid plate was placed in an incubator at 30° C. for inverted cultivation;
[0054] The components and contents of said culture medium are:
[0055] Liquid medium (LM): (g / L) tryptone 10g, beef extract 5g, NaCl 5g, pH 7.0
[0056] Solid medium (SM): LM+1.5% (w / v) agar powder
[0057] All reagents used were domestic analytical grade.
[0058] Total bacterial DNA was extracted using standard methods (see Nucleic Acids Res. 1993, 21:2260-2263). Then, using the total DNA as a template, the amplification primers P1 and P2 of the lipase gene were designed according to the literature J Bacterial.
[0059] P1: 5' ccg cat acc aat aac gtt tca tca 3' (SEQ ID...
Embodiment 3~8
[0118] Using the lipase produced by the S.marcescens ECU1010 of Example 1 and the recombinant bacterium BL21(DE3) / pBCLipE1 of Example 2 as a biocatalyst, split the racemic ketoprofen esters of different carbon chain lengths to obtain optical Pure (S)-ketoprofen.
[0119] Reaction condition is: the enzyme liquid 1ml that embodiment 1 and 2 obtains, wherein contains the enzyme amount of 6U, adds 2.8mg racemic ketoprofen axetil, makes its final concentration be 10mM, then adds 50ul of DMSO (dimethyl methylene Sulfone) (final concentration is 5% (v / v)) to aid in dissolution, and reacted for 24 hours at 30° C. under the condition of 1000 rpm.
[0120] The obtained sample was acidified with 6mol / L HCl to pH = 1, then extracted with an equal volume of ethyl acetate, and then extracted with a chiral chromatographic column Chiracel OJ-H column (Daicel Japan, Φ0.45×25cm), and separated by n-hexane-iso Propanol-acetic acid (volume ratio is 90: 10: 0.2) is mobile phase (1ml / min), on high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com