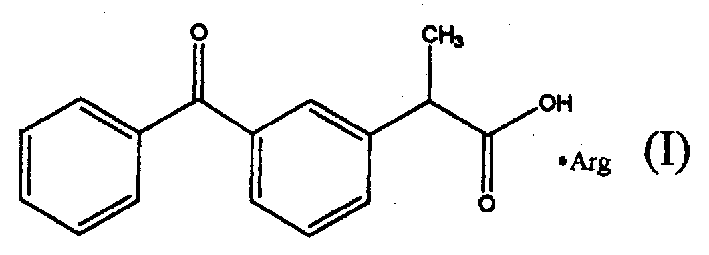
Arginine-ketoprofen with antalgic and inflammation relieving actions and its preparing process
An arginine ketone, arginine technology, applied in the direction of anti-inflammatory agents, organic chemistry, non-central analgesics, etc., can solve the problem of increasing the route of administration, reducing the irritation of the gastrointestinal tract, and limiting the clinical application of ketoprofen Scope and other issues to achieve the effect of improving safety and eliminating stomach pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach : Embodiment 1
[0013] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS Example 1: Preparation of Arginine Ketoprofen
[0014] Add 101.6 g (0.4 mol) of ketoprofen, 69.6 g (0.4 mol) of L-arginine and 1000 ml of distilled water in sequence to the reaction flask, stir to react to a clear liquid, freeze-dry, and the specific conditions are shown in the following table.
[0015] Freeze drying conditions
[0016] Temperature (℃) Time (hours) Remarks
[0017] -40 8 Pre-freeze
[0018] -40 2 vacuum
[0019] -30 2 vacuum
[0020] -20 2 vacuum
[0021] -10 4 Vacuum
[0022] -5 6 Vacuum
[0023] 0 6 Vacuum
[0024] 10 6 Vacuum
[0025] 20 3 Vacuum
[0026] 30 3 was evacuated to obtain 168.8 g of white solid with a yield of 98.6%.
[0027] Using ketoprofen as the reference substance for content determination, the content of the product was 99.8%.
Embodiment 2
[0028] Example 2 Hot plate analgesia test
[0029] 60 female mice weighing 19-21 grams were selected and randomly divided into 6 groups with 10 mice in each group. After measuring the pain threshold before administration by the hot plate method, groups 1-5 were given arginine ketoprofen by gavage. Arabic gum liquid, the dose calculated by ketoprofen is 40.82, 28.41, 20.14, 10mg / kg; the administration concentration is 1.63, 1.14, 0.80, 0.56, 0.39mg / ml, and the administration volume is 0.5ml / 20g; Group 6 In the same way, 0.5ml / 20g of 10% gum arabic was given. Pain thresholds were measured again at 1.5 hours, 3 hours, and 4.5 hours after administration. Calculate the mean value and standard deviation of pain threshold in each group, and do t test to compare whether there is a significant difference between each administration group and the blank control group. The results are shown in the following table.
[0030] Influence of arginine ketoprofen on pain induc...
Embodiment 3
[0035] Effect of Example 3 on the Bacterial Endotoxin Pyrogenic Model in Rabbits
[0036] 30 rabbits with a body weight of 1.85-2.25Kg were selected for the pre-selected pyrogen test, their normal body temperature was measured, and 1ml / kg of bacterial endotoxin solution was injected. , 6 in each group. The first, second and third groups were given 1 dose of ketoprofen arginine ketoprofen calculated as 10, 5, 2.5 mg / kg, and the concentrations were 1 mg / ml, 0.5 mg / ml and 0.25 mg / ml, respectively. The gavage volume was 10ml / kg, and the fourth group was gavaged with 10% gum arabic, 10ml / kg, for the negative control. After gavage, the body temperature of rabbits was measured every 30 minutes, a total of 8 times, and the average body temperature before the bacterial endotoxin was subtracted to obtain the temperature increase value. t-test was used to compare whether there was a significant difference between each administration group and the gum arabic negative control group. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com