Method of utilizing continuous flow microreactor to synthesize benzophenone derivative
A technology of benzophenone and microreactor, which is applied in chemical instruments and methods, preparation of organic compounds, chemical/physical/physicochemical reactors, etc. It can solve the problems of demanding equipment, severe heat release, and low yield. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
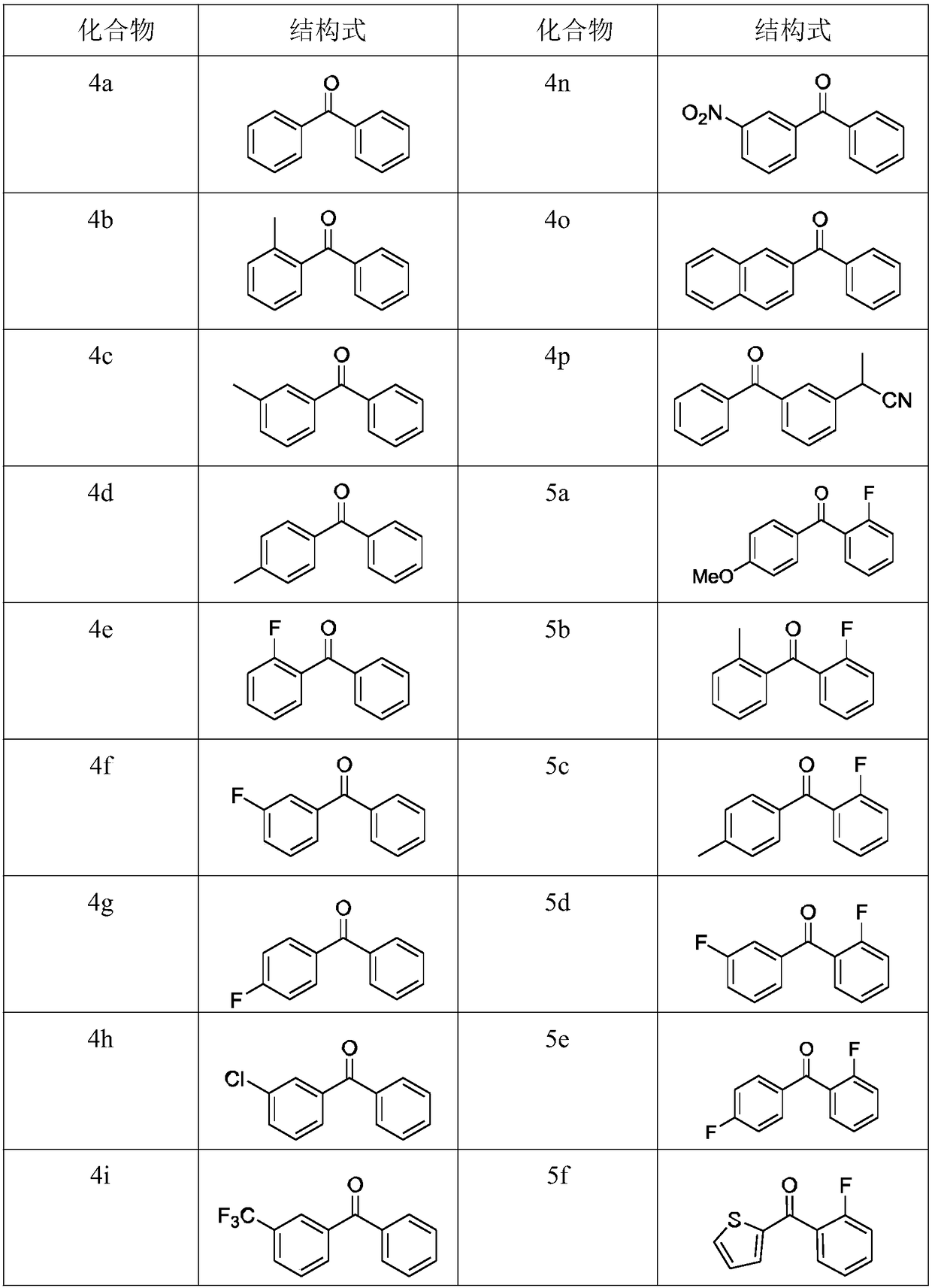
Examples
Embodiment 1
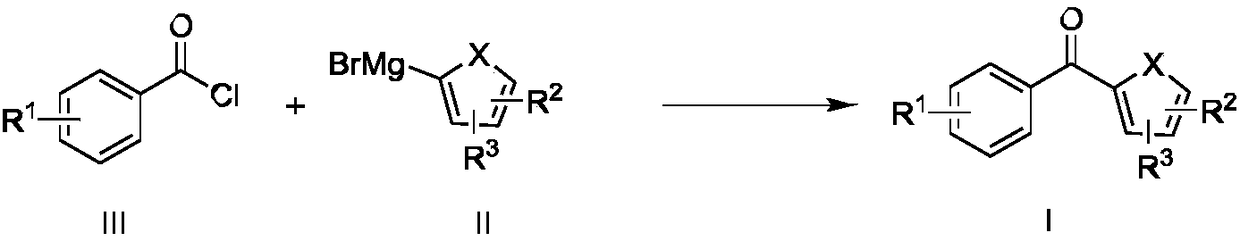
[0055] Enlarge the synthesis raw material drug cyanoketoprofen (KPN), the chemical reaction formula is as follows:
[0056]
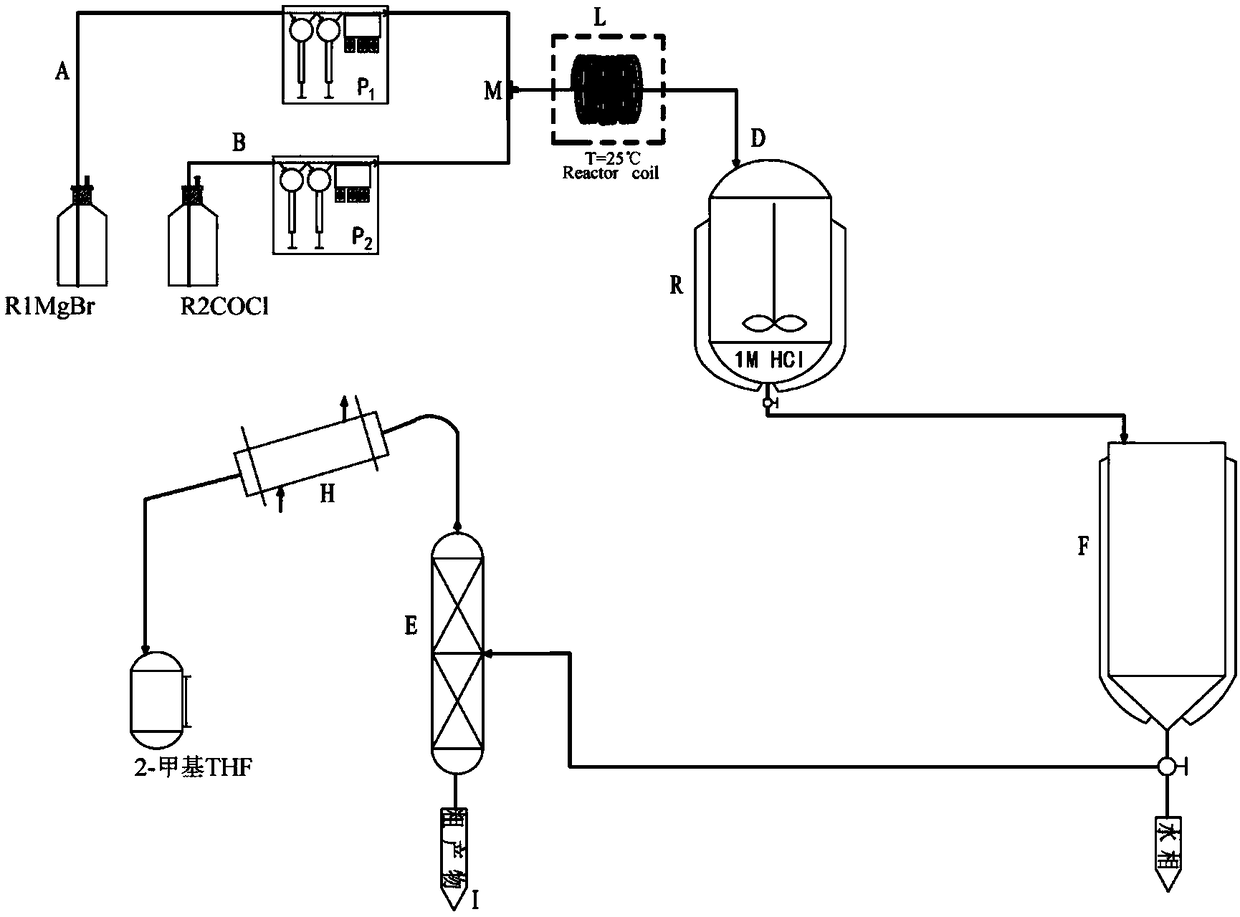
[0057] A solution of 3-(1-cyanoethyl)benzoyl chloride (260mmol) in 2-methyltetrahydrofuran (650mL) and a solution of phenylmagnesium bromide (390mmol) in 2-methyltetrahydrofuran (650mL) were passed through material channel A , B, through metering pump P 1 ,P 2 , flow into the mixing module M with a preset temperature of 25°C at a flow rate of 1mL / min and mix. After staying in the reaction module L for 1h, the reaction solution flows out from the outlet D, and the reaction solution flowing out of the outlet D is collected in a device equipped with 1mol / L hydrochloric acid solution in the reactor R, quenched by stirring, poured into the stratifier F to separate the water phase, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, and concentrated in the distillation tower E to obtain the crude product , the solvent 2-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com