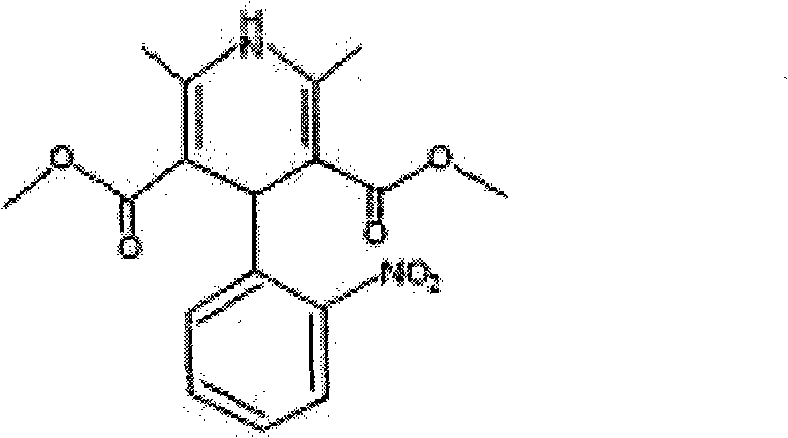
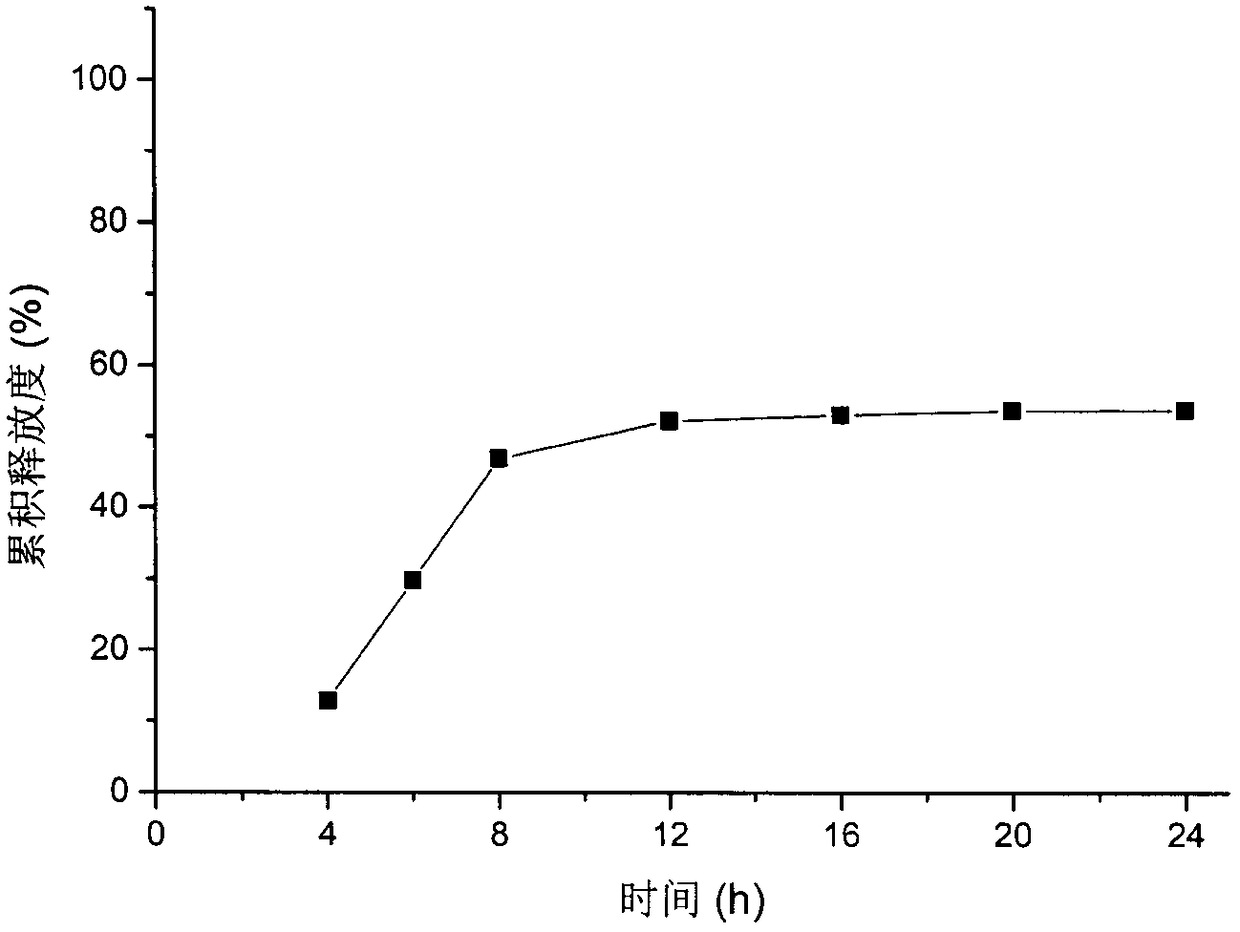
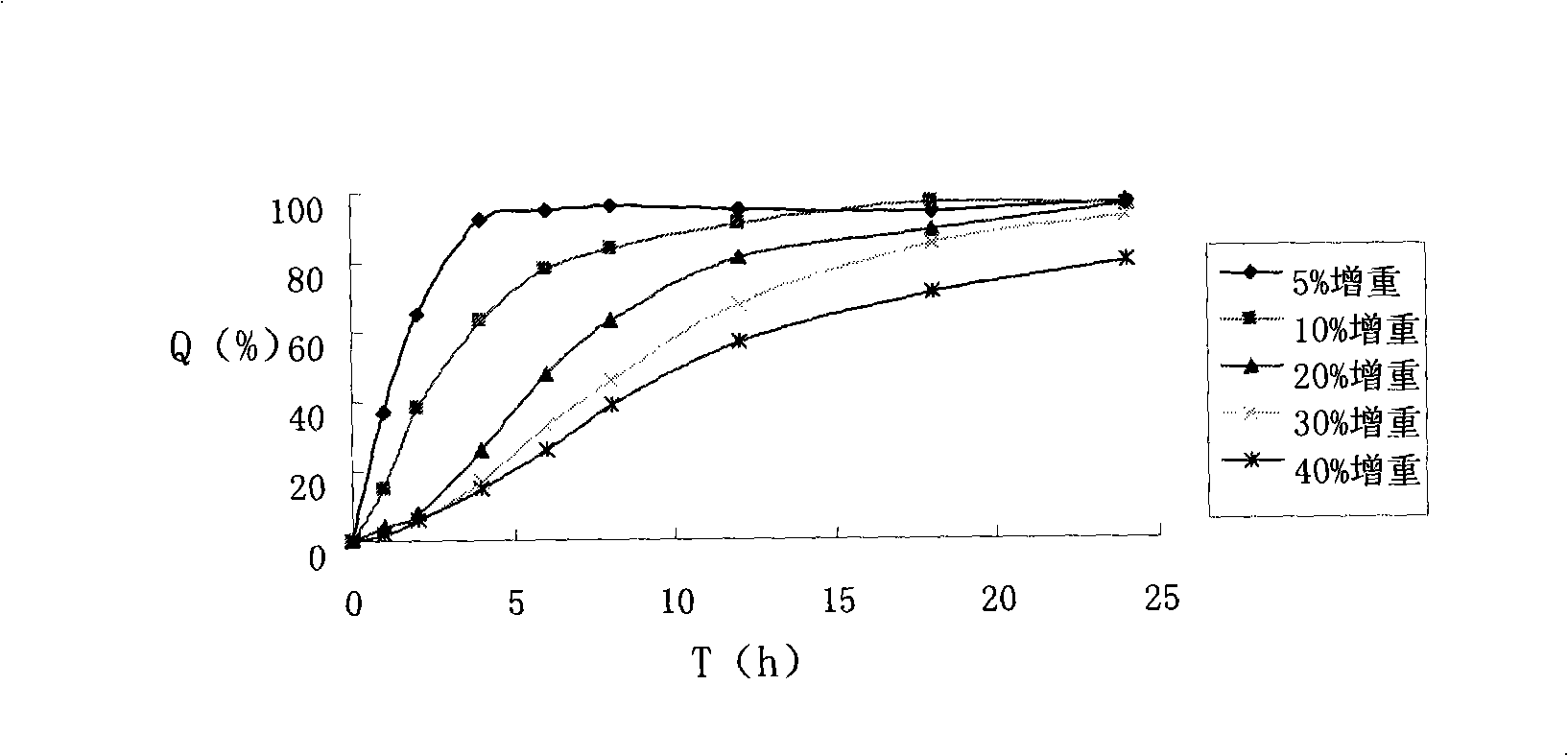
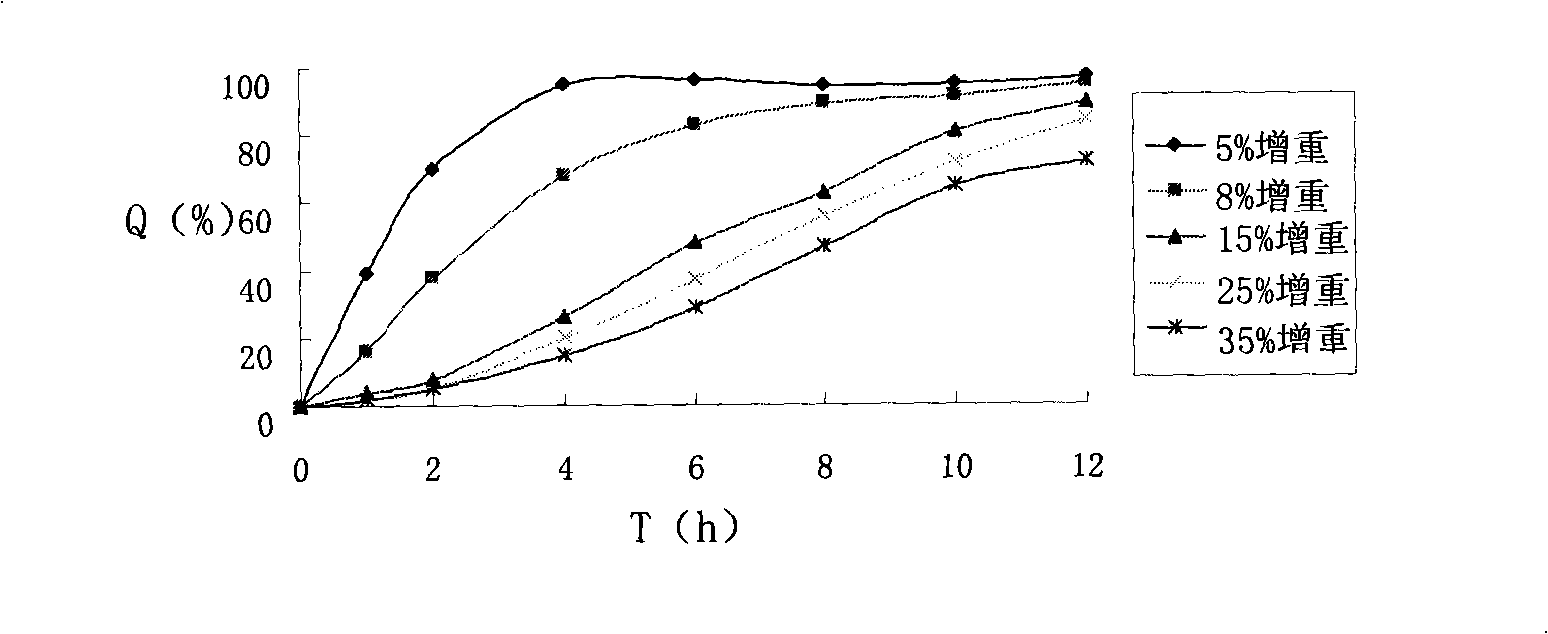
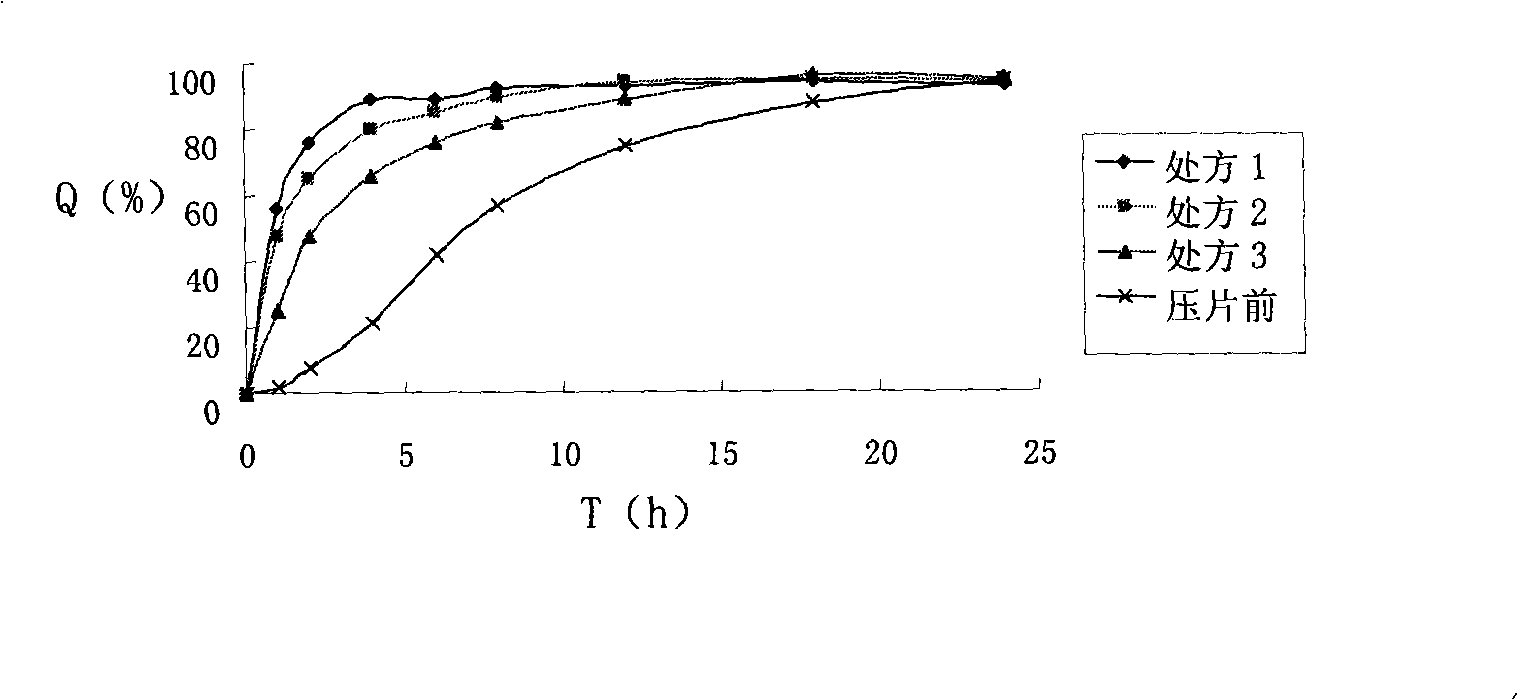
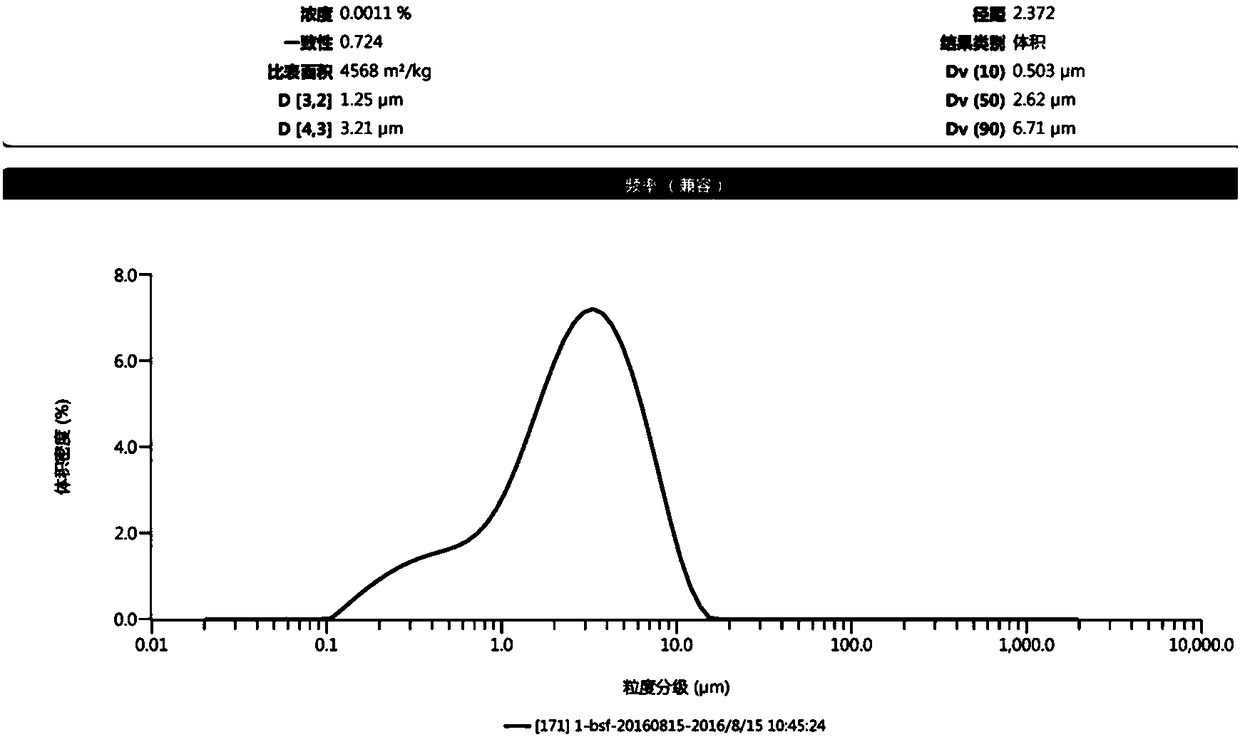
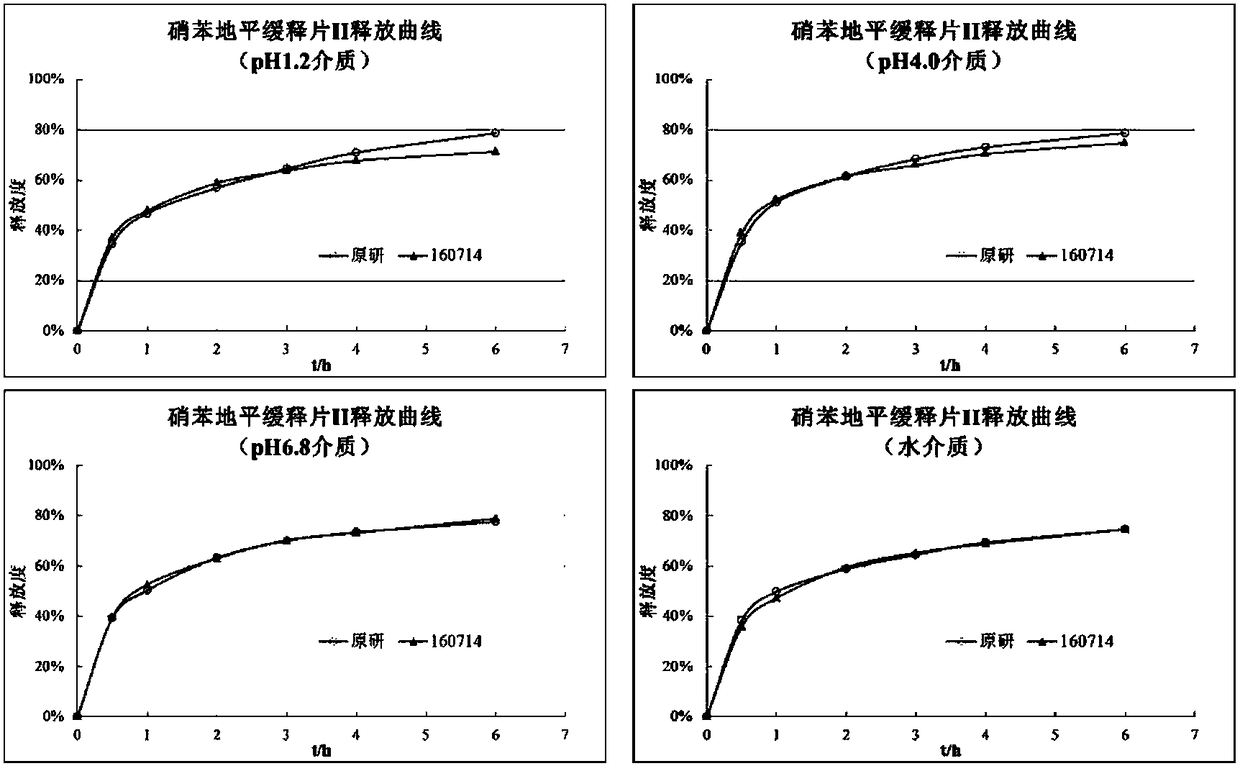
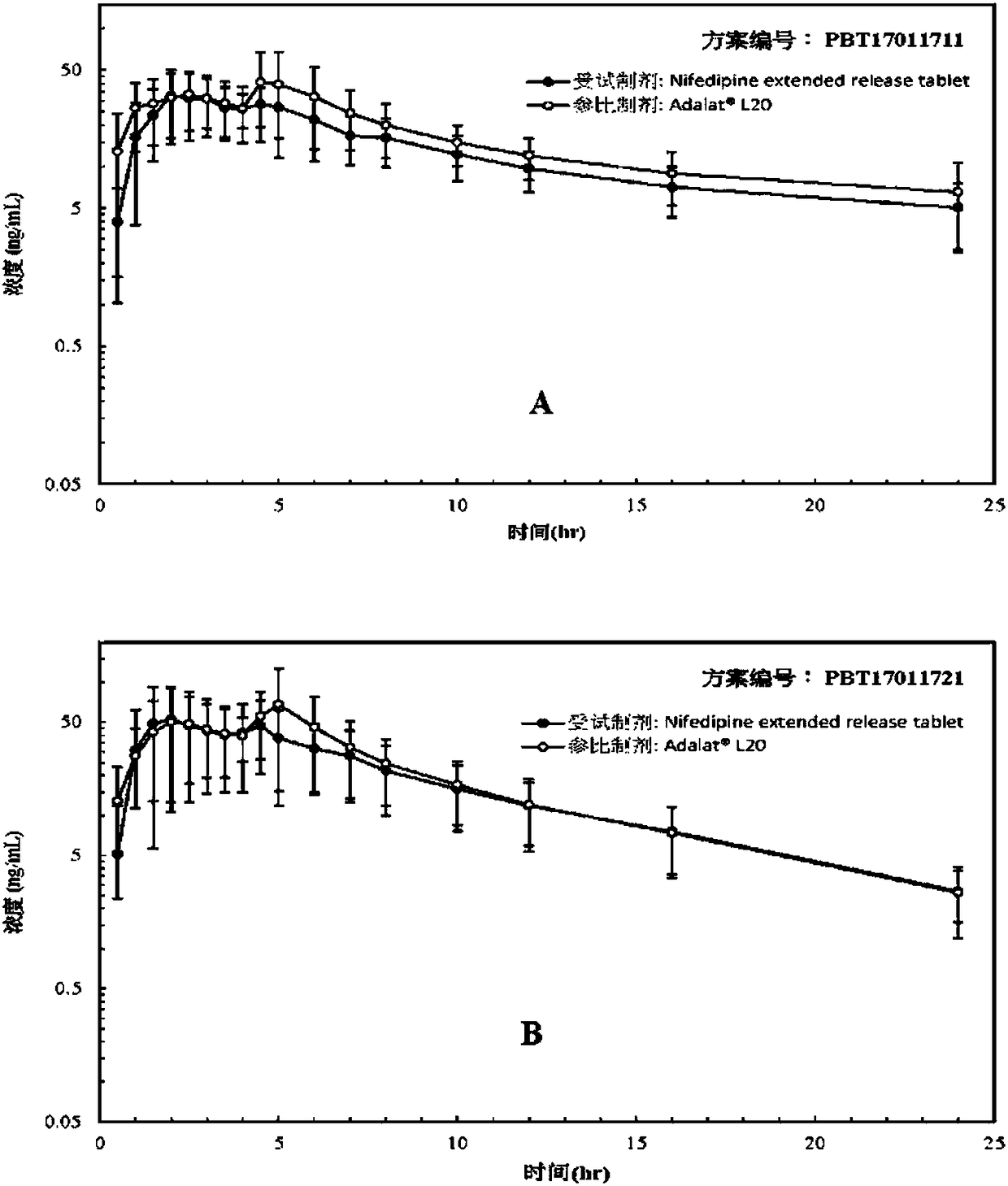
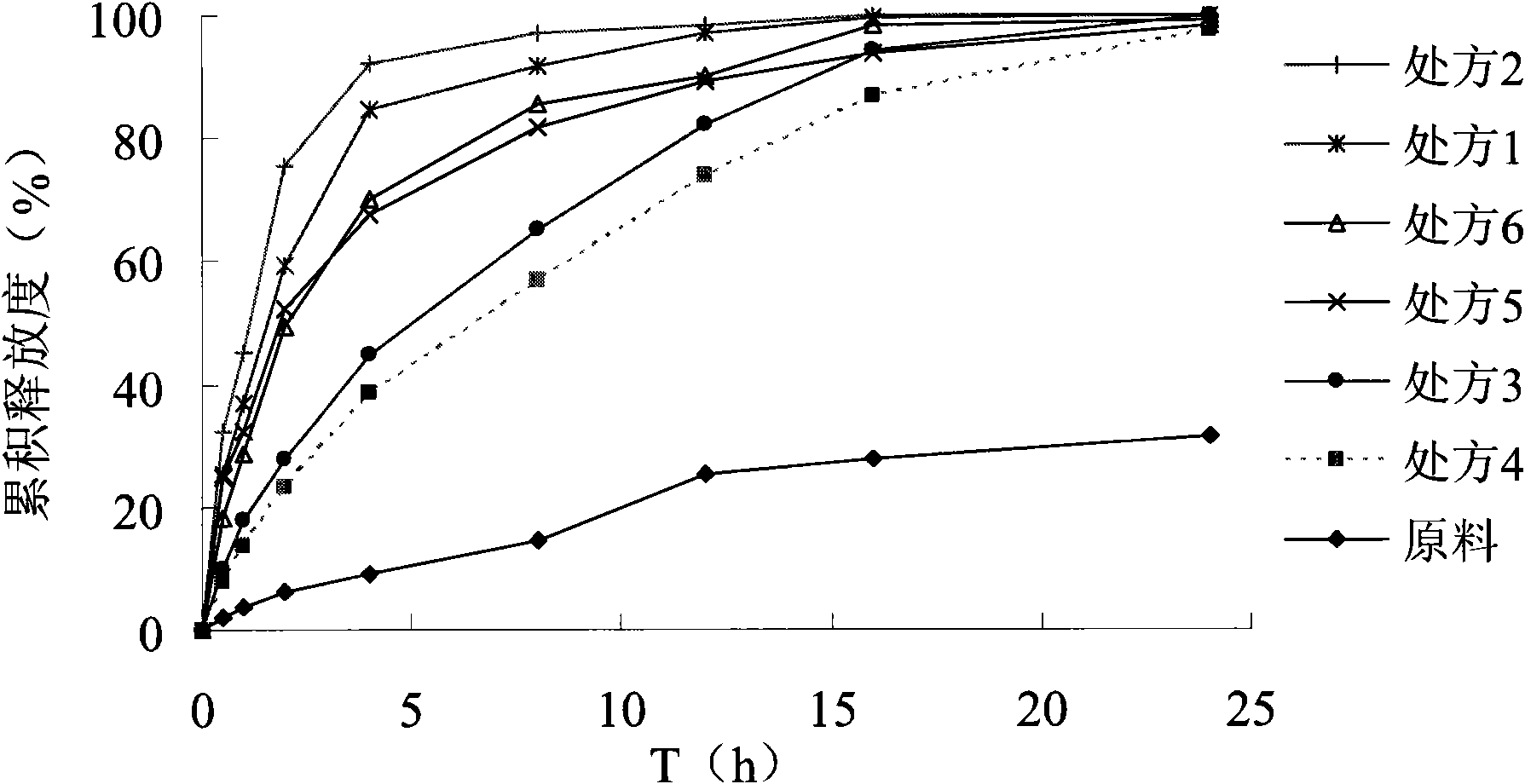
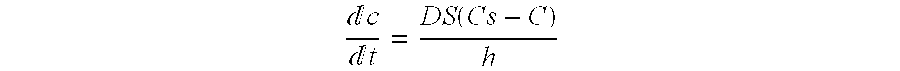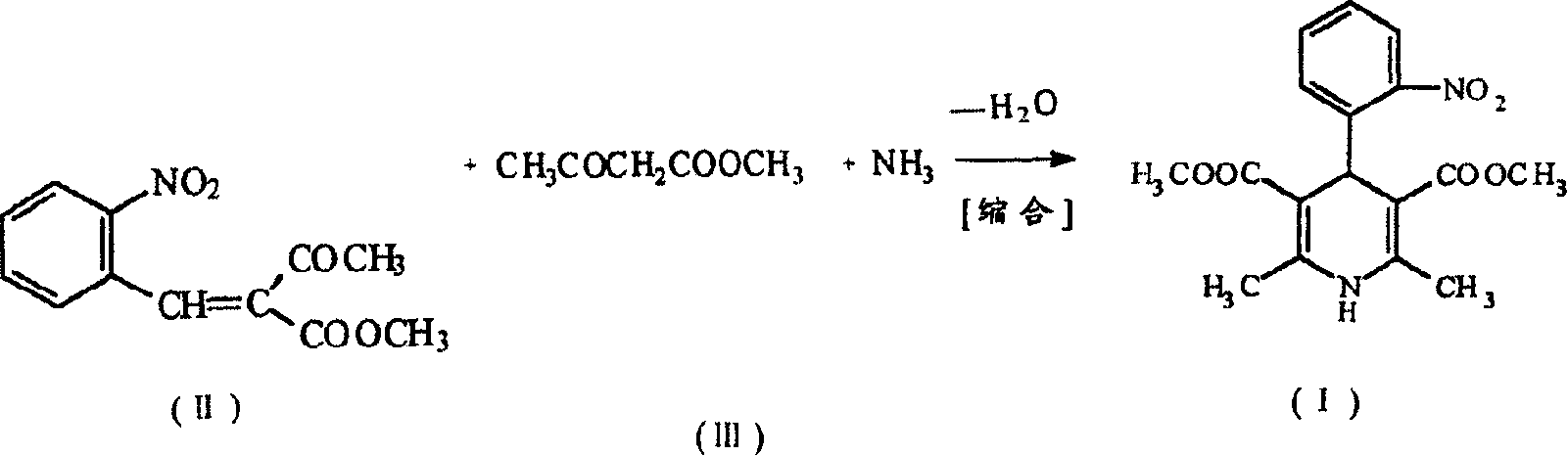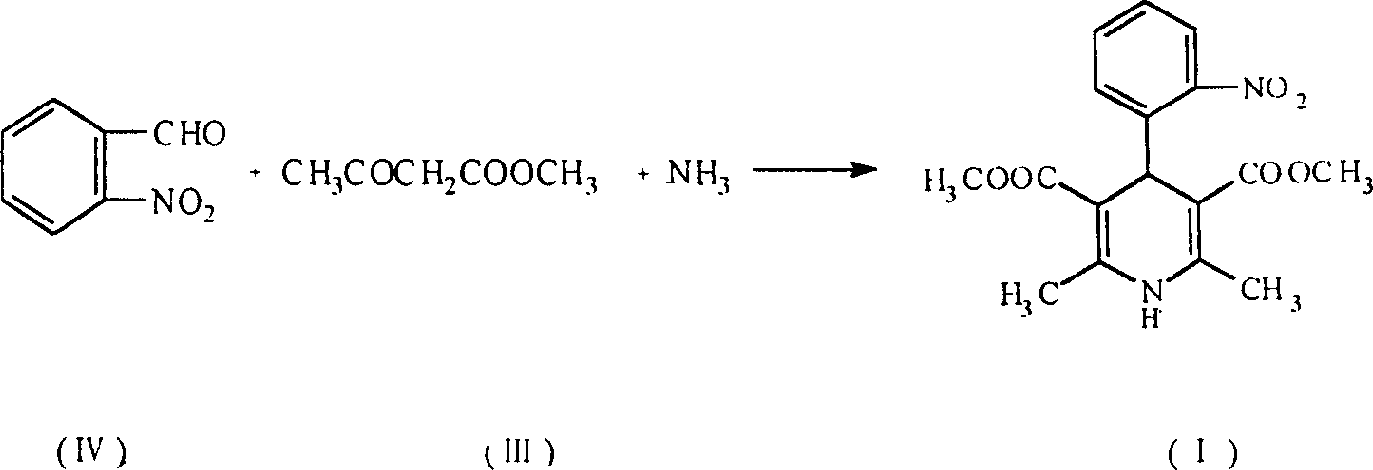Patents
Literature
231 results about "Nifedipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
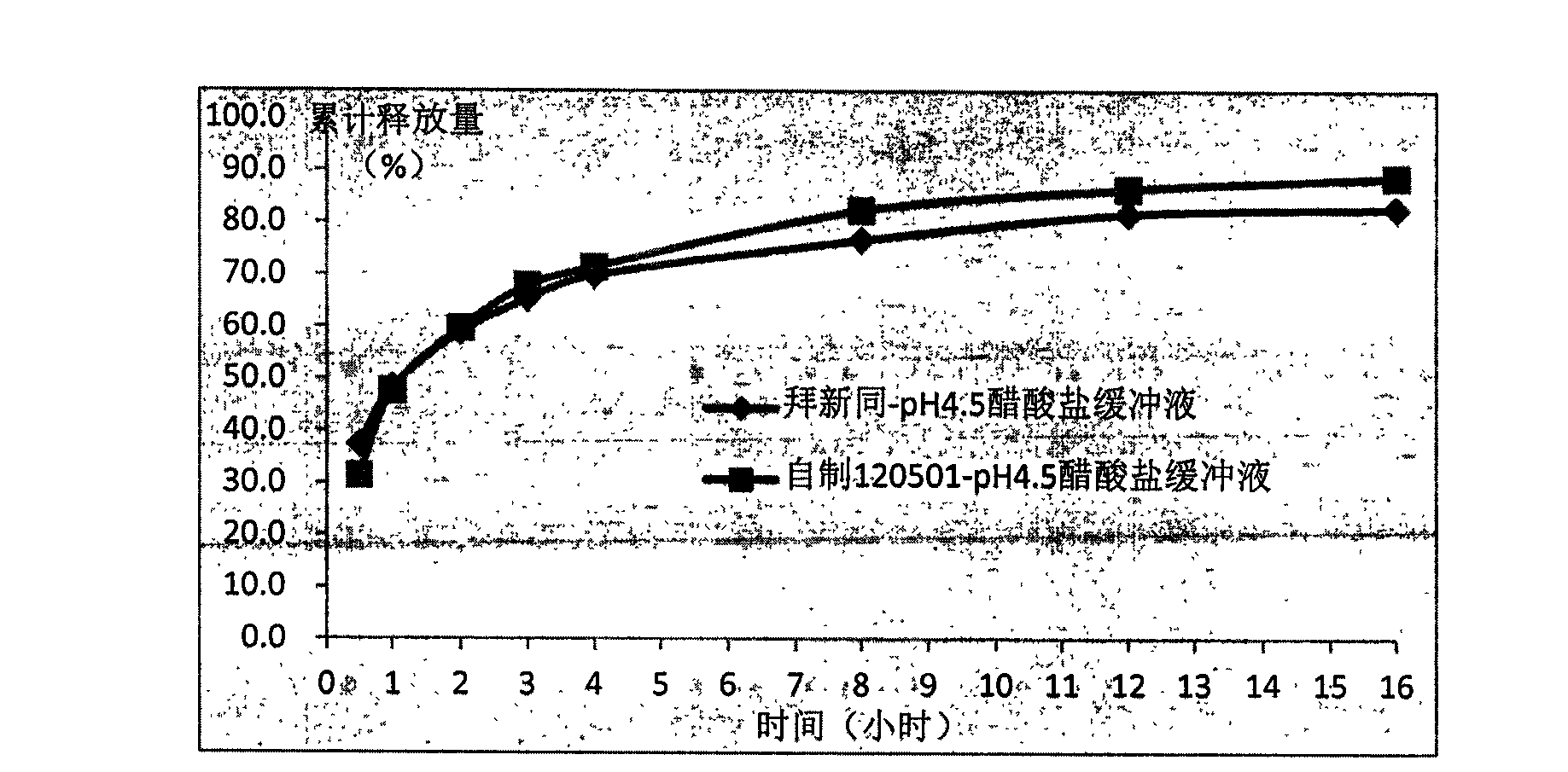
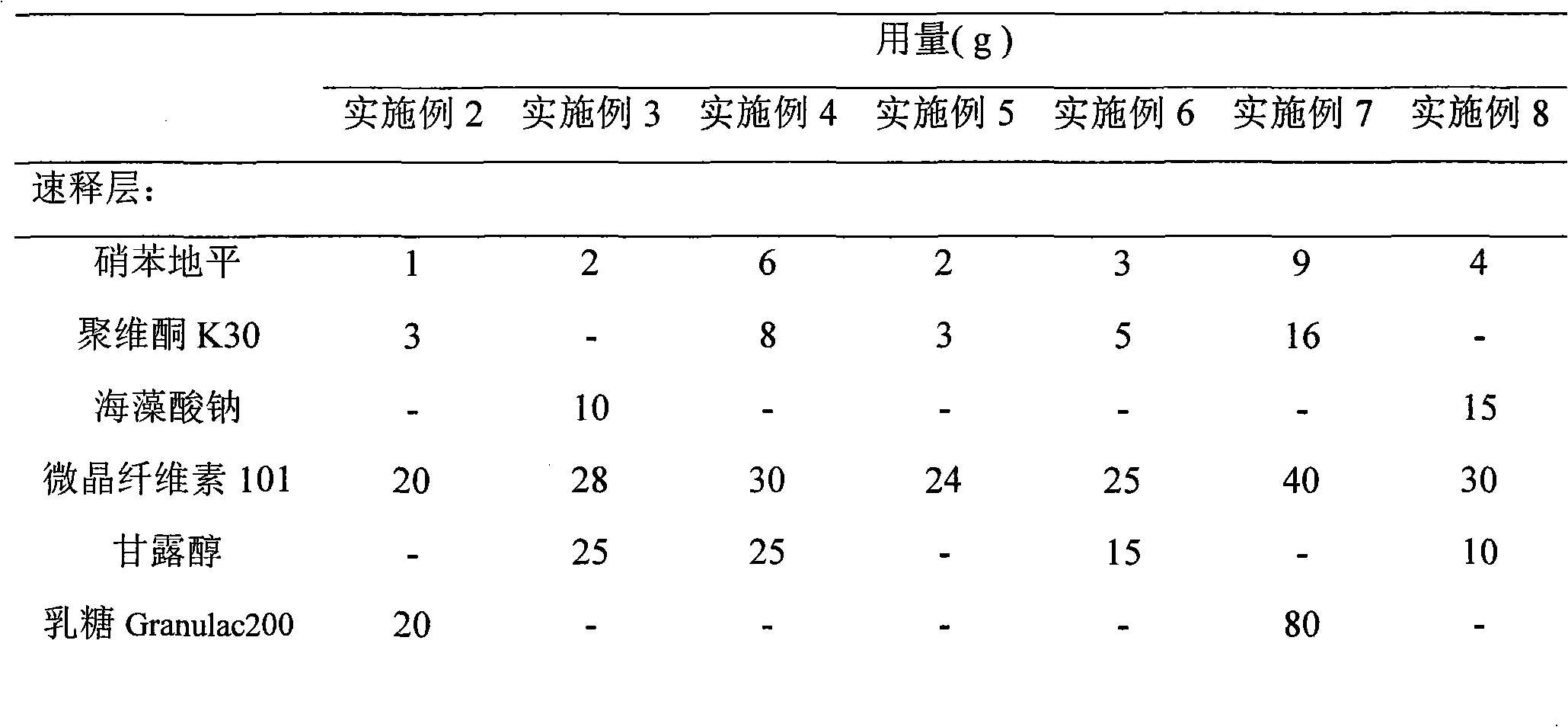
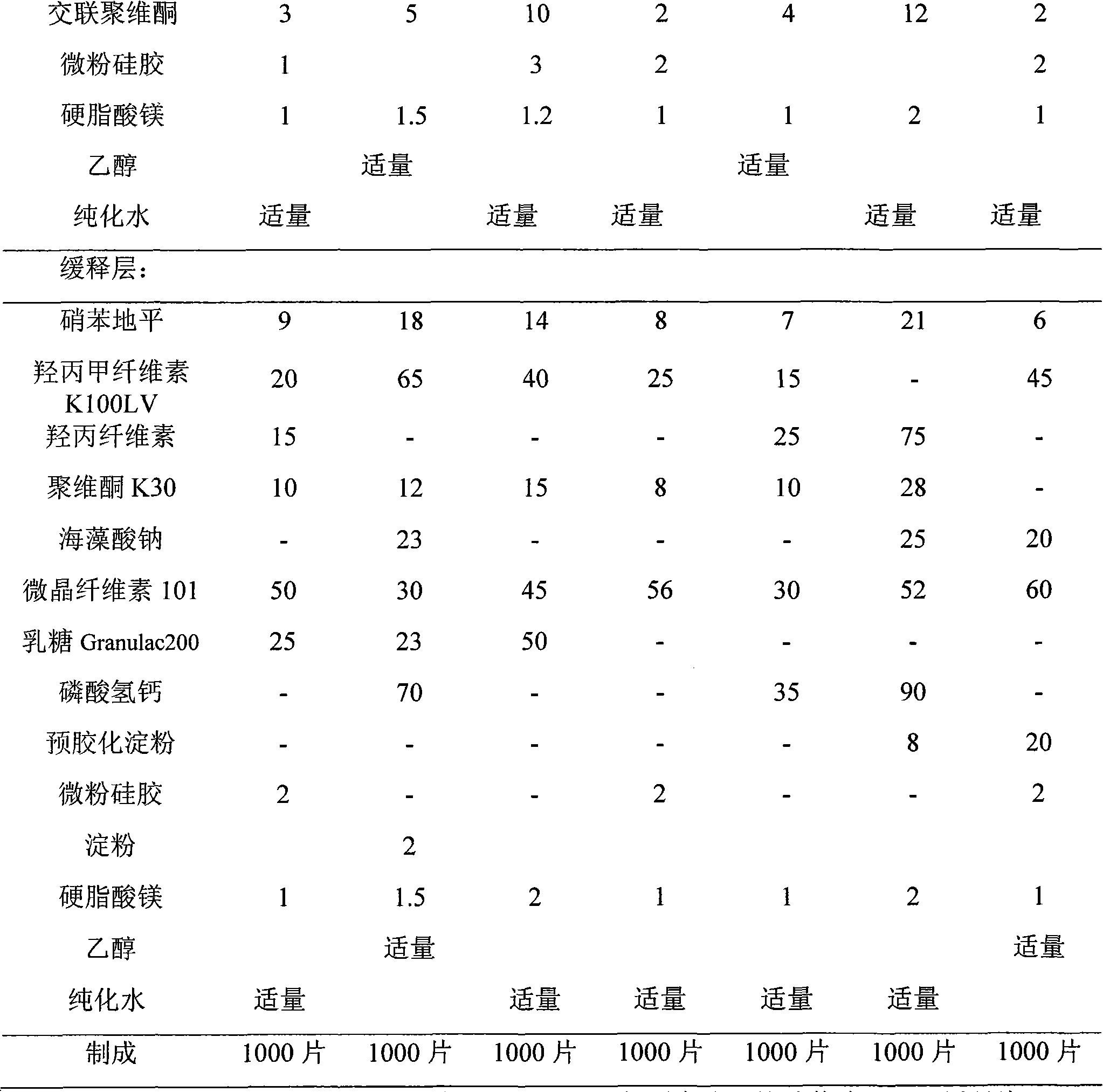
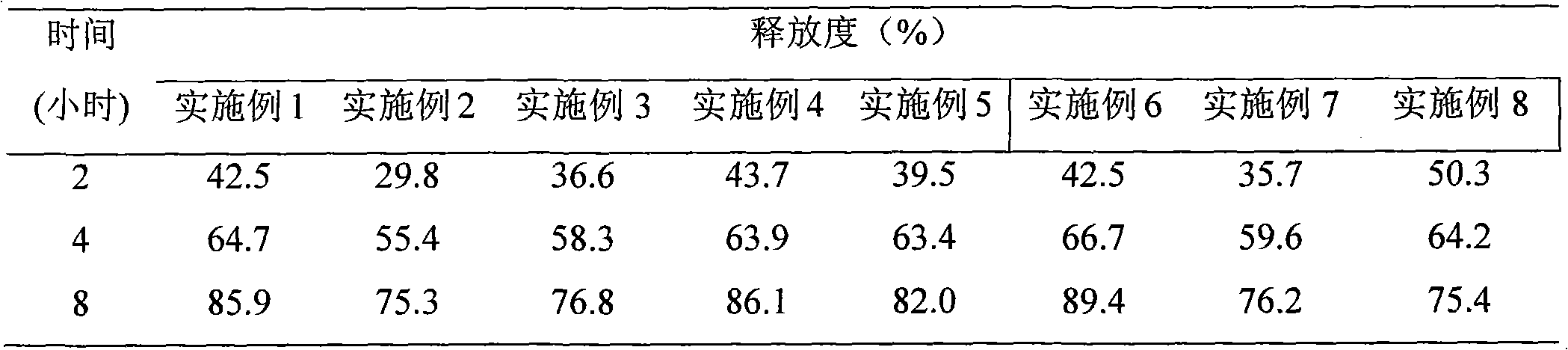
Nifedipine is used alone or in combination with other drugs to treat high blood pressure (hypertension).
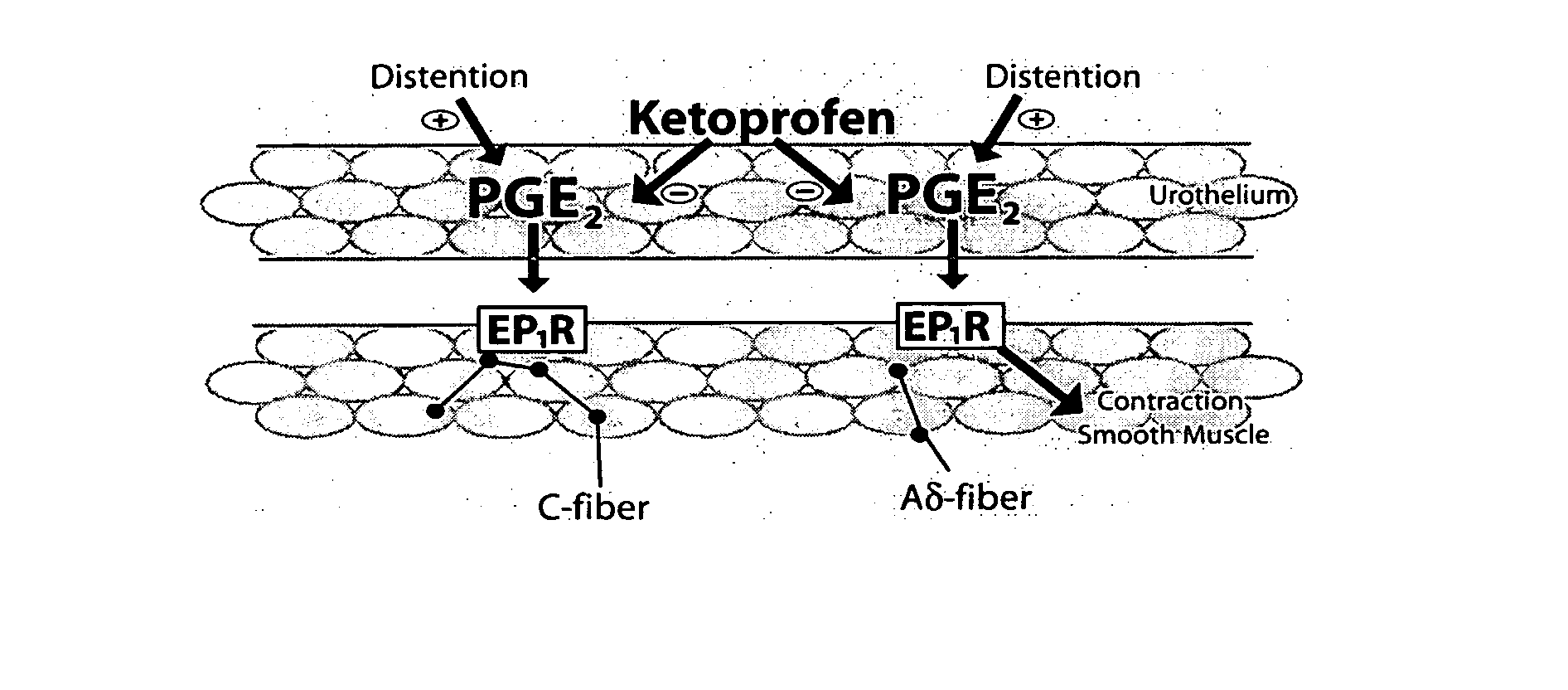
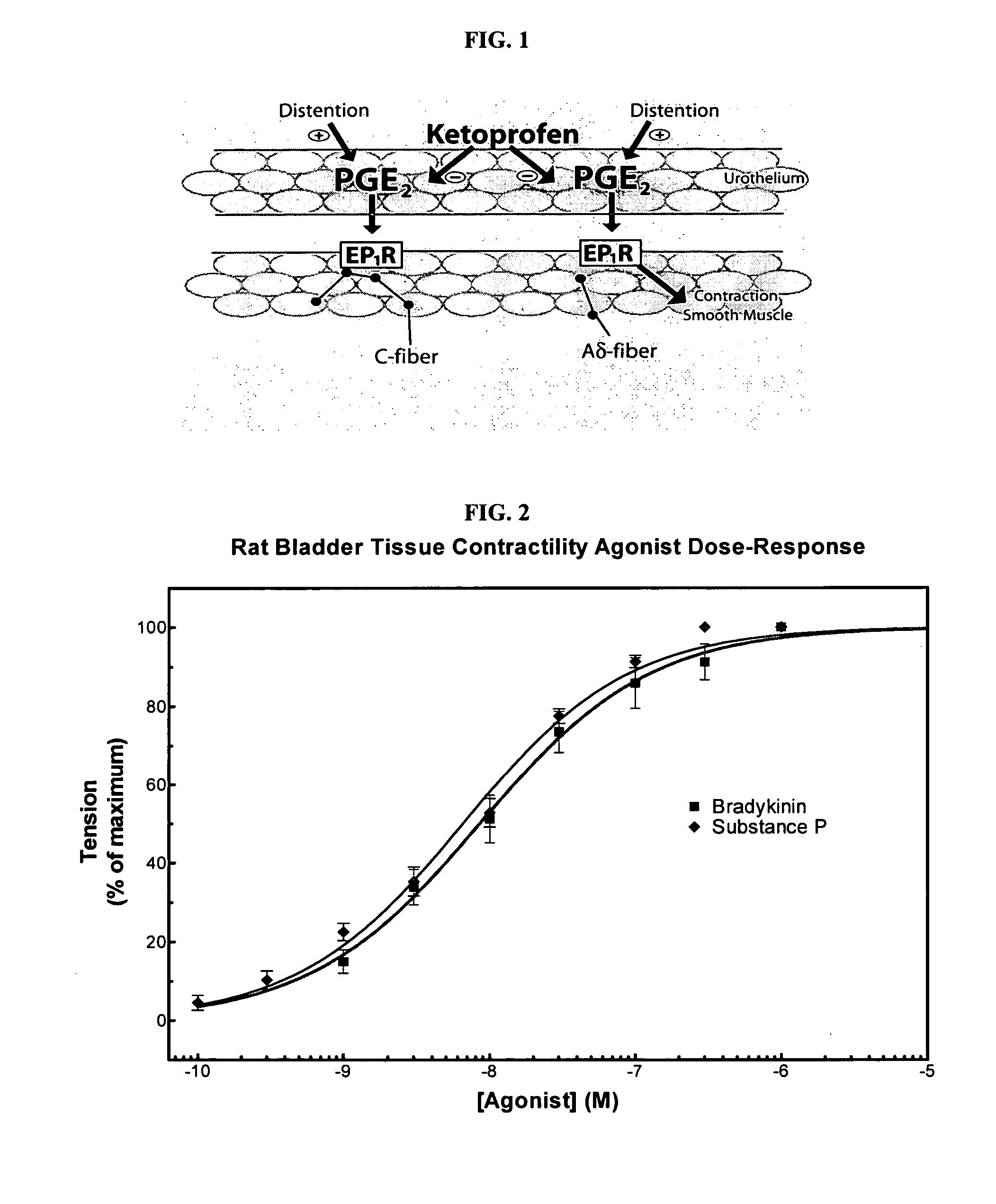
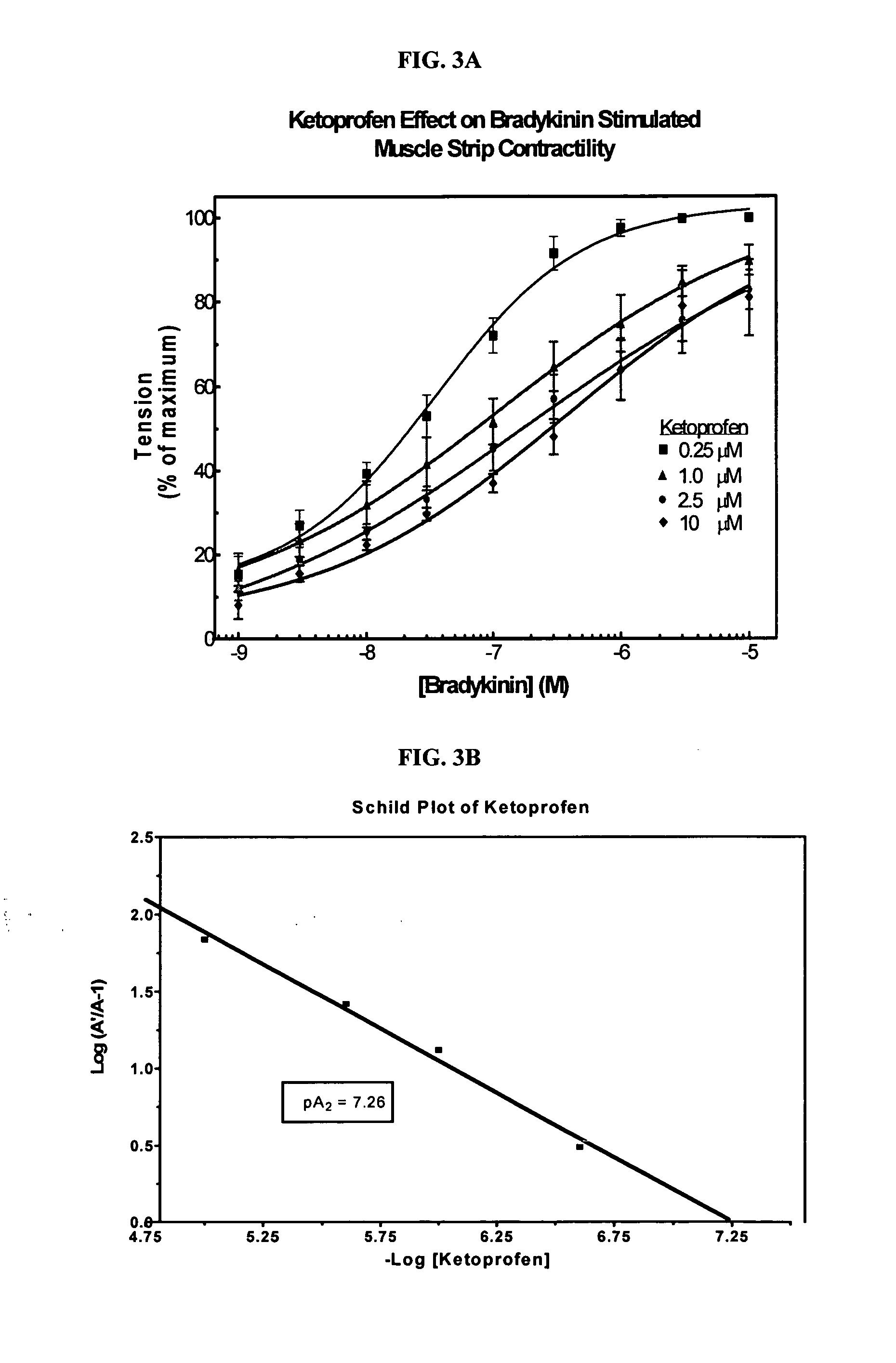
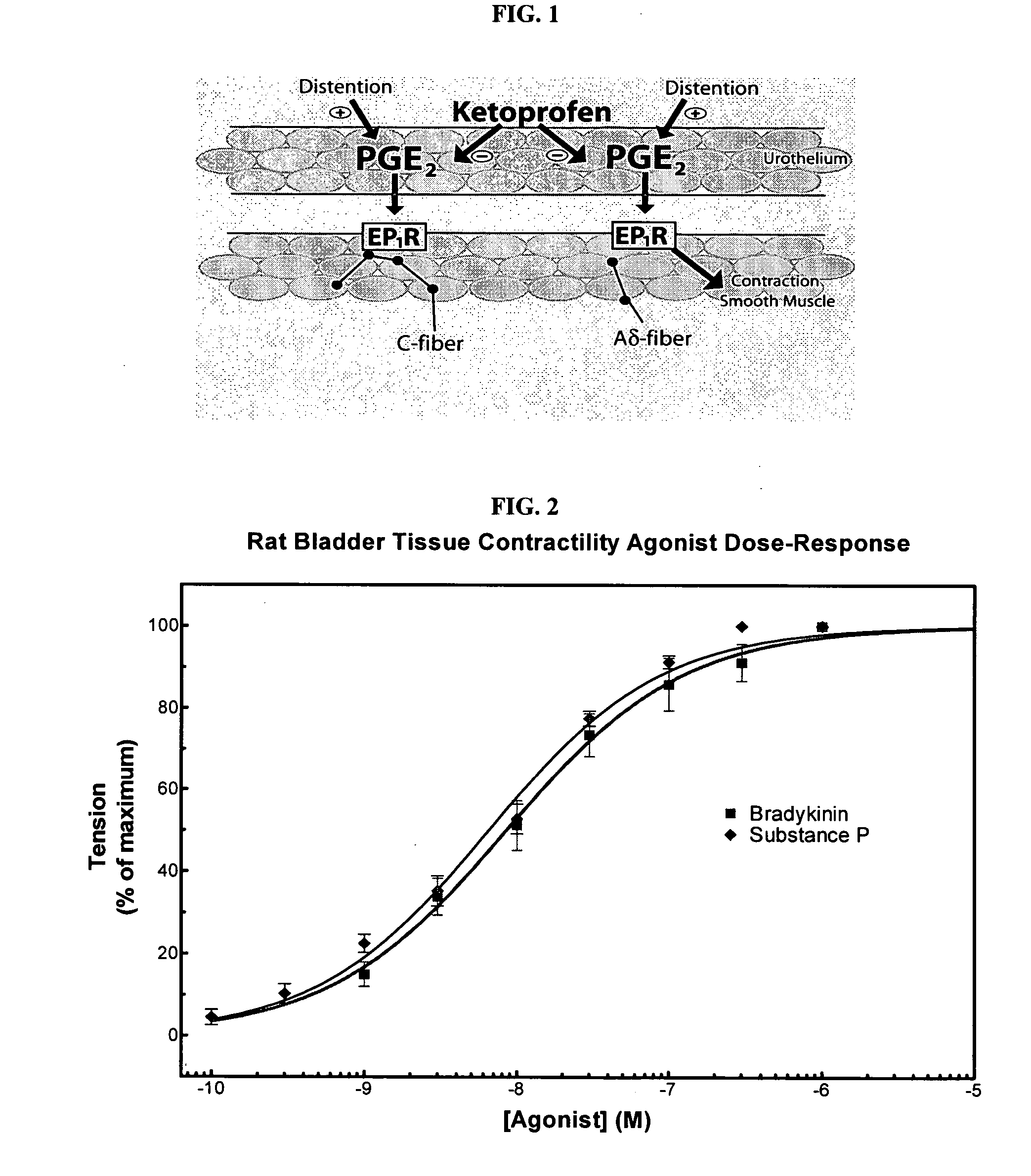
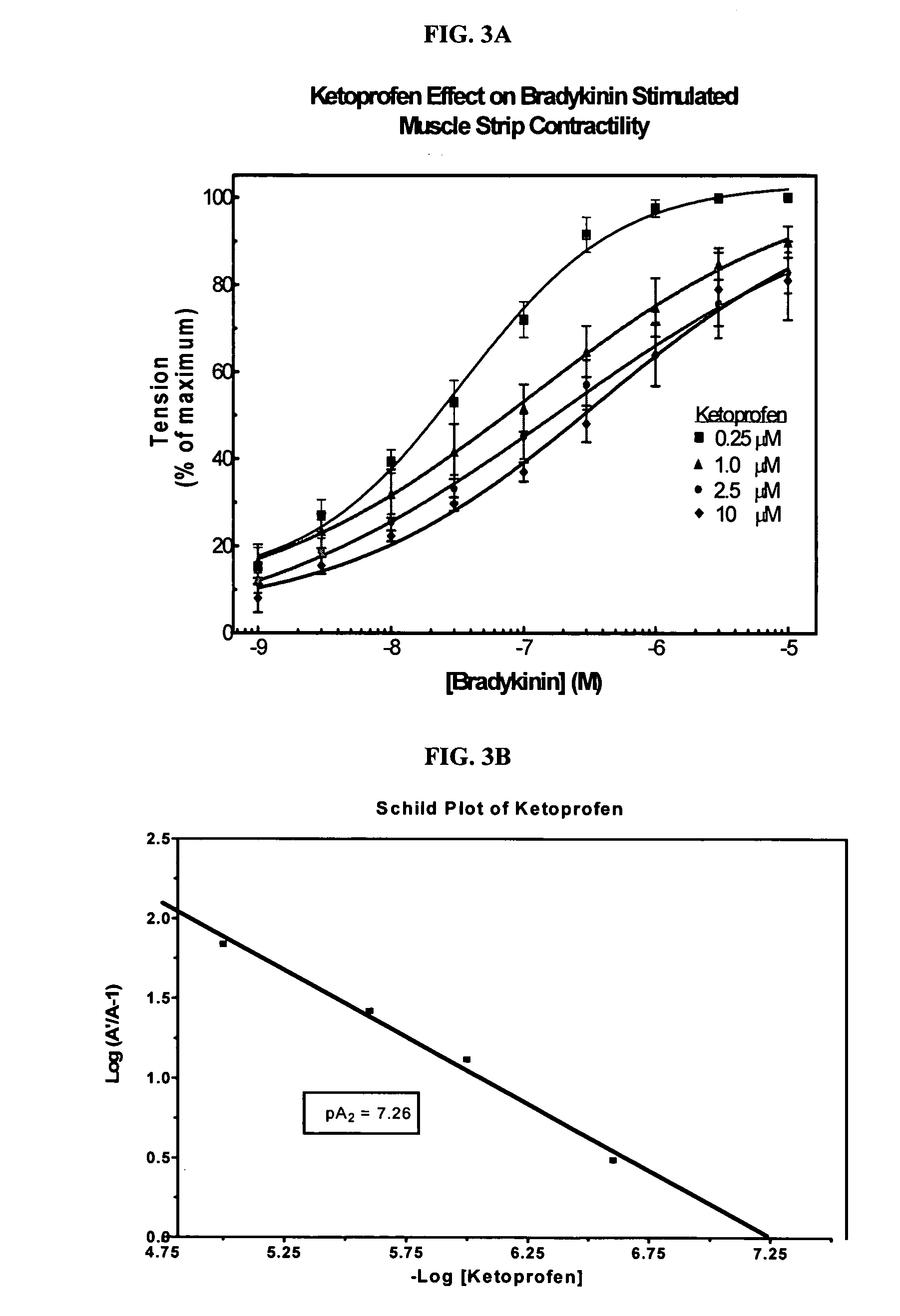
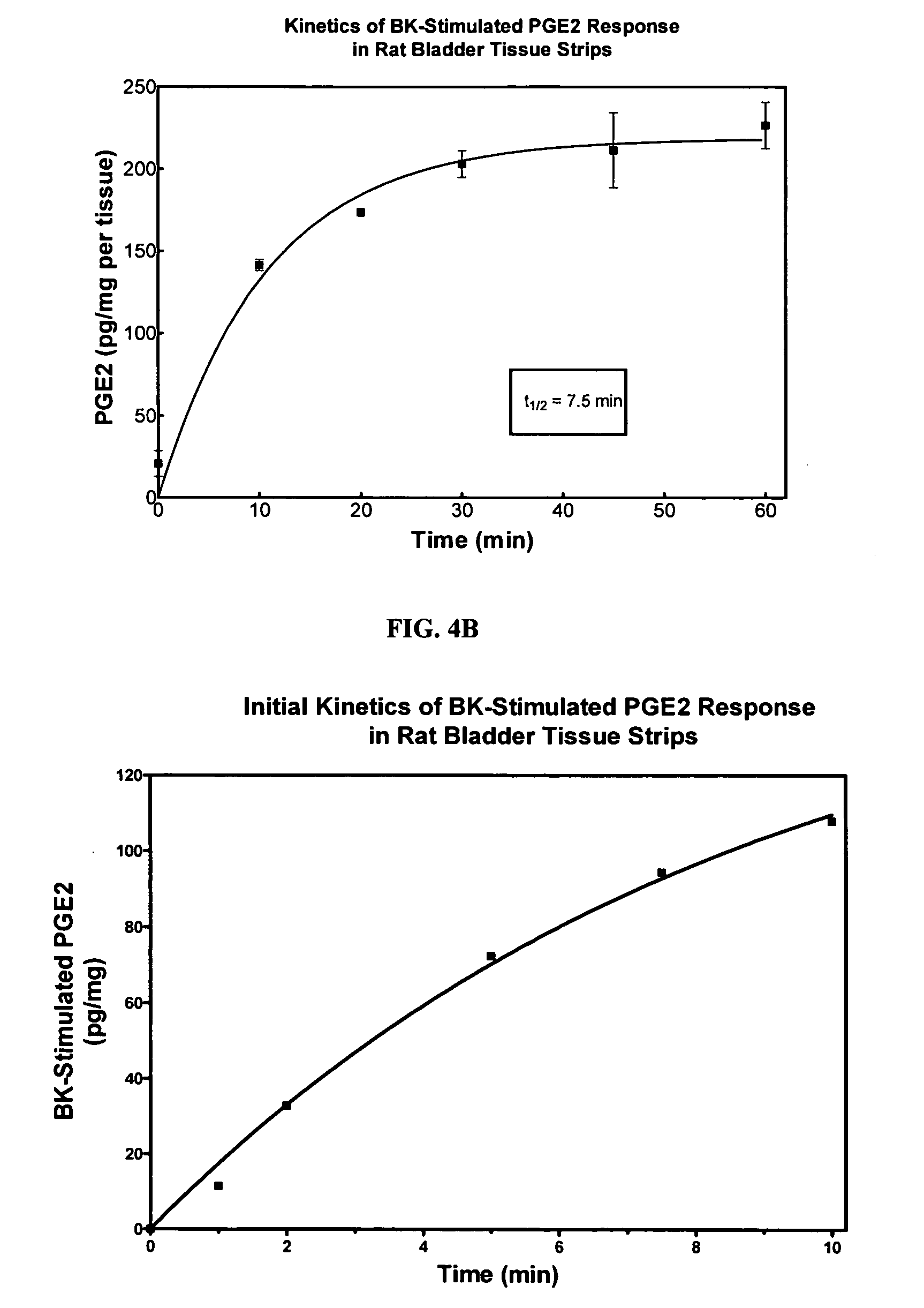
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
InactiveUS20070248639A1Inhibiting pain/inflammationPrevent spasmsBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
ActiveUS20060263393A1Inhibits pain/inflammation and spasmInhibiting pain/inflammationBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
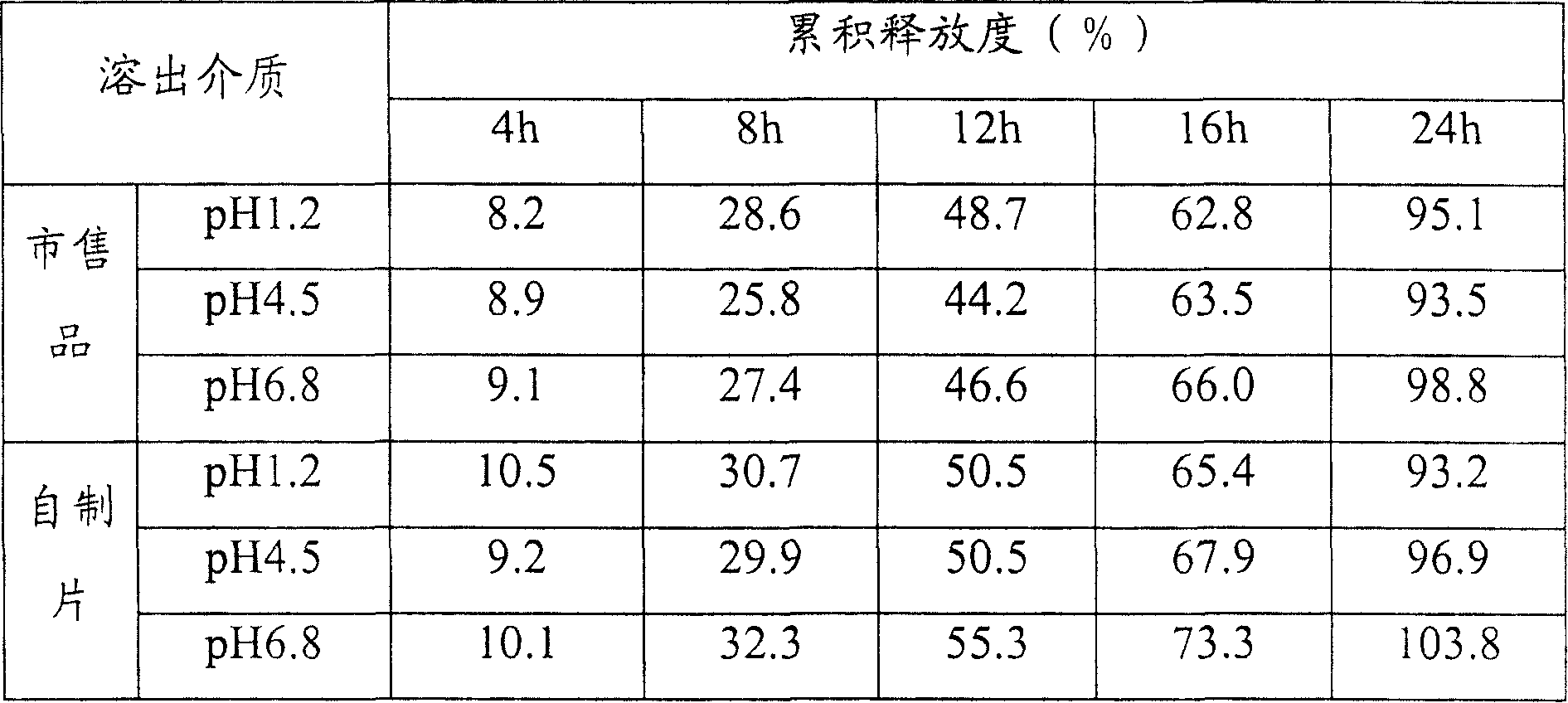
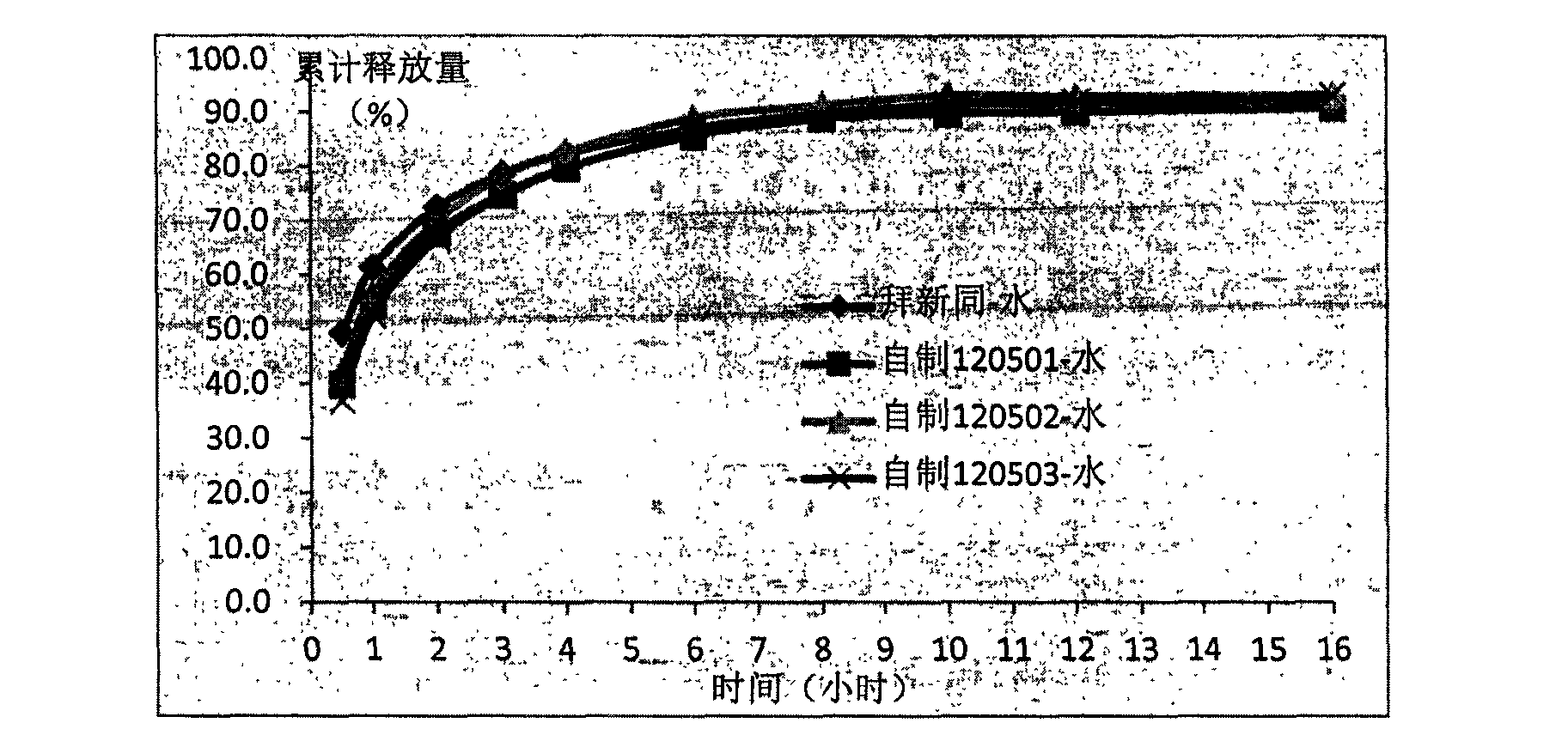
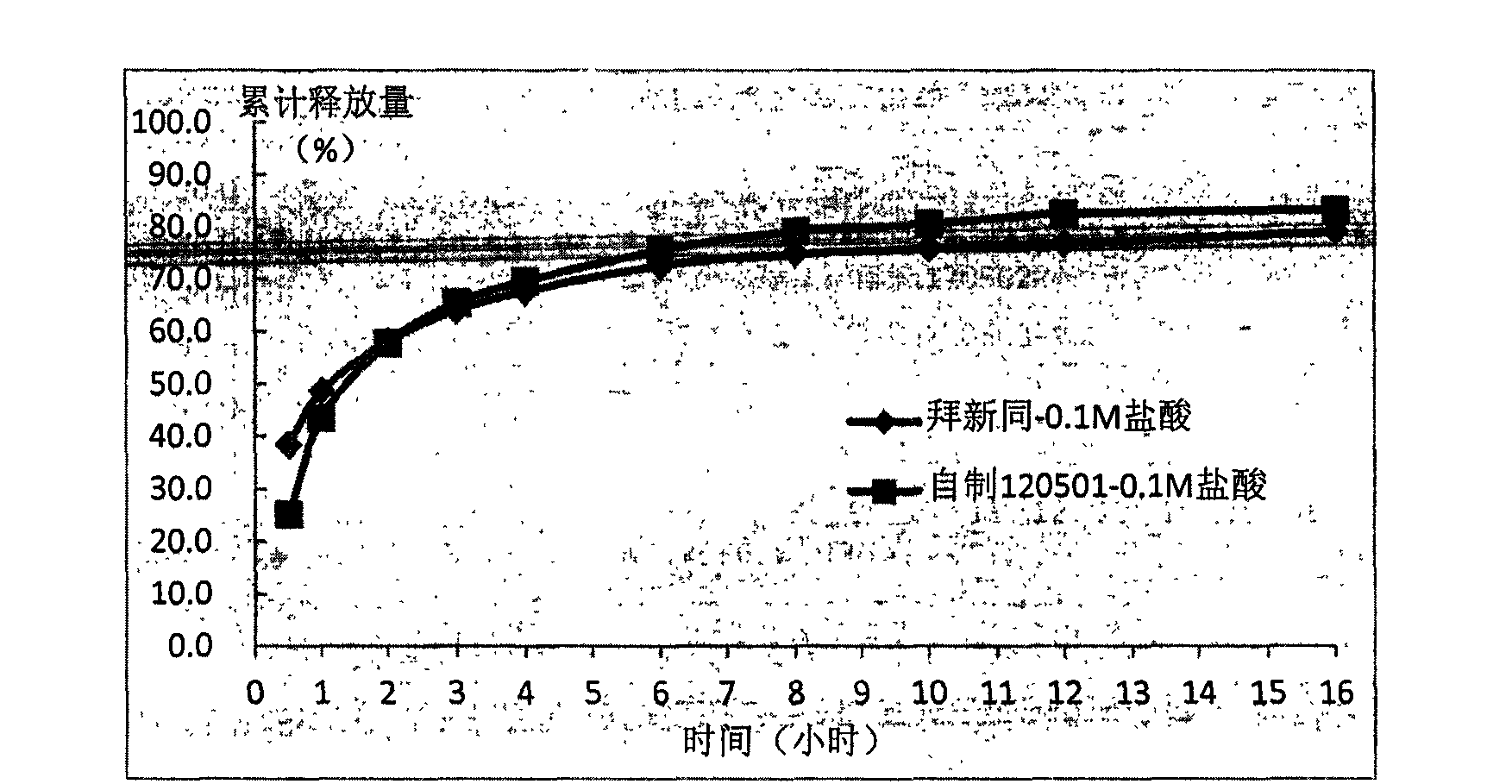
Controlled release pharmaceutical tablets containing an active principle of low water solubility
It is described a new method for the preparation of pharmaceutical tablets carrying poorly soluble in water principle; this method allows to obtain tablets with fast and / or slow release of the active principle. The peculiar feature is the fact that the poorly soluble in water active principle (es: nifedipine) is treated with a surfactant, during the granulation phase or whatever during the preparation process; the obtained product, subjected to a compression, produces pharmaceutical tablets which show high bioavailability of the carried active principle. This procedure can be used to prepare polymeric matrixes (with modified release), formed by tablets with one or more layers. The procedure of manufacture and the characteristics of the new finished tablet are described.
Owner:JAGOTEC AG
Nifedipine sustained-release tablet
InactiveCN102125531AOrganic active ingredientsPharmaceutical delivery mechanismNifedipineSustained Release Tablet
The invention provides a nifedipine sustained-release tablet which contains a physiological effective dose of nifedipine and release blocker and a pharmaceutically acceptable excipient, wherein the nifedipine is dispersed in the release blocker; the release blocker is selected from macromolecular substances which can be swelled in water, such as hydroxypropyl methyl cellulose, sodium alginate, carboxymethyl cellulose, methyl cellulose and xanthan gum, and the amount of the used release blocker is 5-30 percent of the weight of the sustained-release tablet; and the nifedipine sustained-release tablet contains 15-30mg of nifedipine and also contains several of filler, a disintegrating agent, a surfactant, an adhesive, a lubricant and a glidant as the excipient. The nifedipine sustained-release tablet can be used for treating hypertension and angina pectoris.
Owner:上海中邦斯瑞生物药业技术有限公司
Nifedipine sustained release tablet
InactiveCN101966164AOrganic active ingredientsPharmaceutical delivery mechanismProlonged-release tabletMagnesium stearate
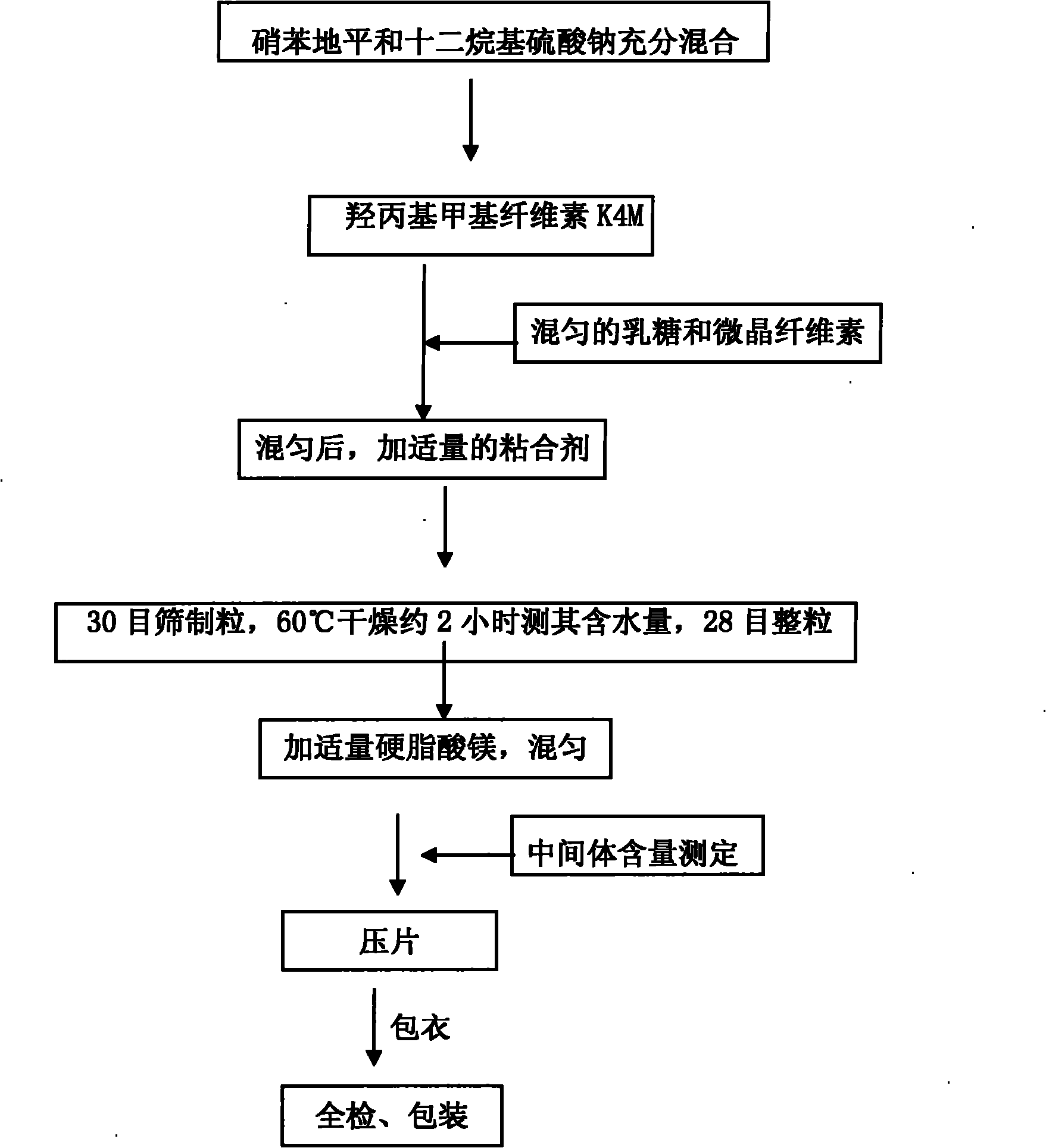
The invention relates to a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet is characterized by comprising a tablet core and film coating liquid, wherein the tablet core comprises the following components in percentage by mass: 10 to 12 percent of nifedipine (Nif), 6.5 to 7.5 percent of hydroxypropyl methyl cellulose K4M, 45 to 47 percent of lactose, 34 to 35 percent of microcrystalline cellulose, 0.48 to 0.55 percent of lauryl sodium sulfate, a proper amount of 95 percent ethanol and a proper amount of magnesium stearate; and the film coating liquid comprises the following component in percentage by mass: 5.85 to 6.55 percent of Opadry II and 92.86 to 93.82 percent of distilled water. The preparation method comprises the following step of: mixing the nifedipine and polymer by utilizing the imported polymer and dispersion technology to form a hydrophilic matrix tablet so as to fulfill the aim of sustained release. Compared with an ordinary tablet, the sustained release tablet can continuously act for 12 hours relaxatively, is released in a sustained way and has less and slighter adverse reaction.
Owner:ANHUI YONSENT PHARMA
Nifedipine osmotic pump controlled release tablet and preparation method thereof
InactiveCN102138912AMaintain blood levelsGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineControlled Release Tablet
The invention provides a nifedipine osmotic pump controlled release tablet comprising a drug-containing layer tablet core, a booster layer tablet core, a coating membrane and a single drug-release pore on the surface of the controlled release tablet at one side of the drug-containing layer tablet core. The nifedipine osmotic pump controlled release tablet provided by the invention has stable medicament release velocity, basically realizes zero drug release within 0-20h and basically fully releases drug; therefore, the dosing number of a patient is reduced, and more stable blood drug concentration can be realized after a patient takes the drug. The nifedipine osmotic pump controlled release tablet provided by the invention is a safe, effective, stable, controllable and conveniently-applied medicament new preparation for clinically treating hypertension.
Owner:CHINA PHARM UNIV
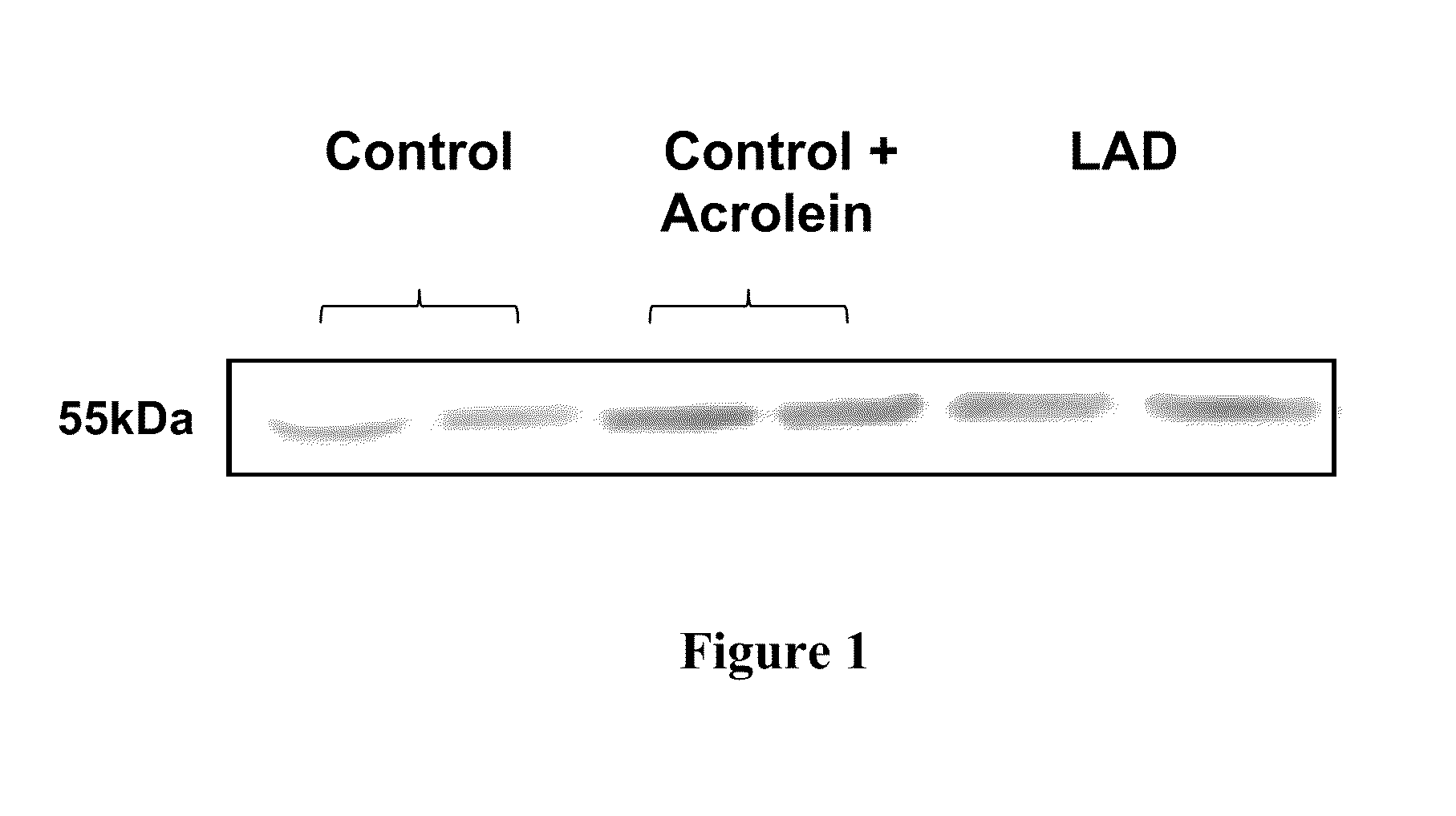
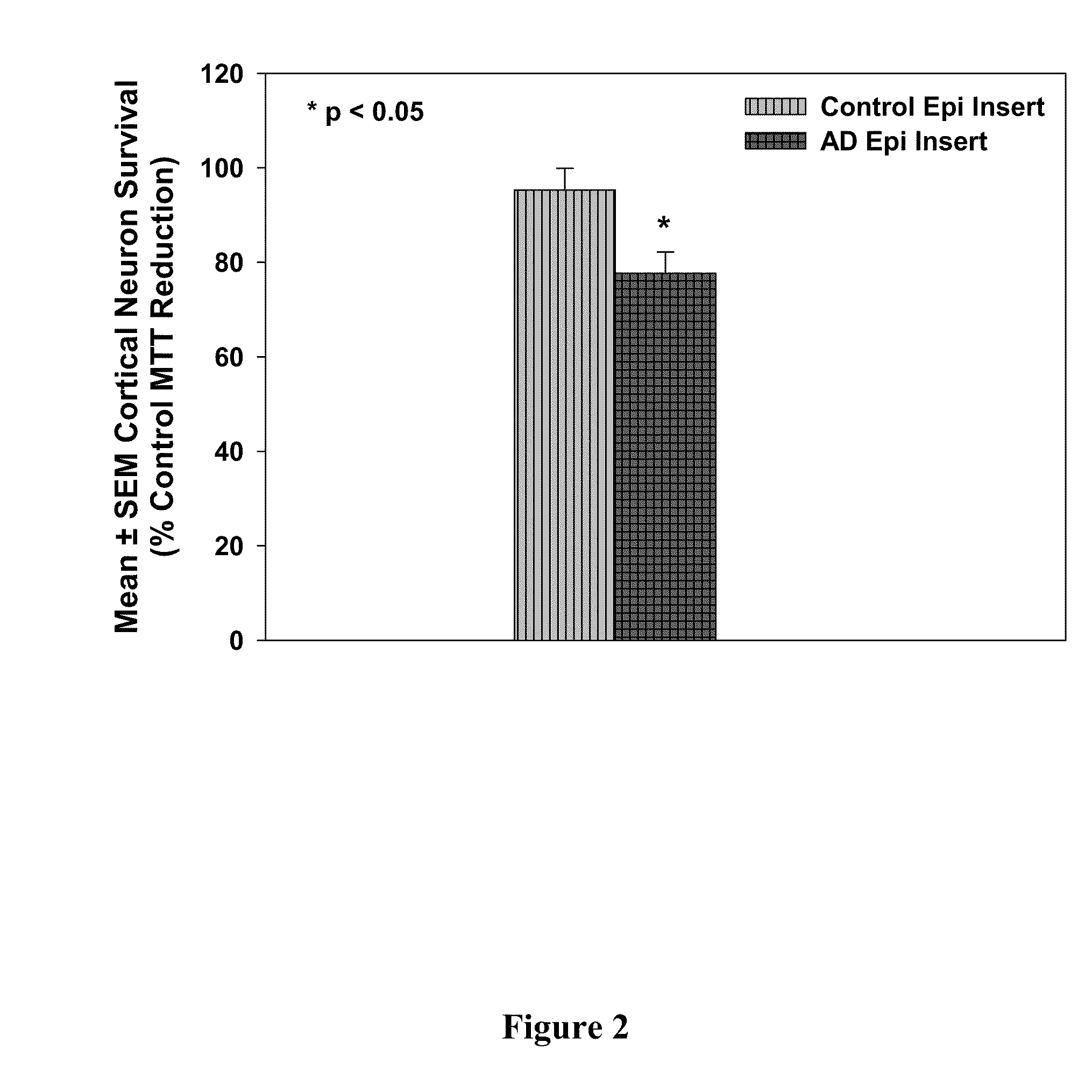
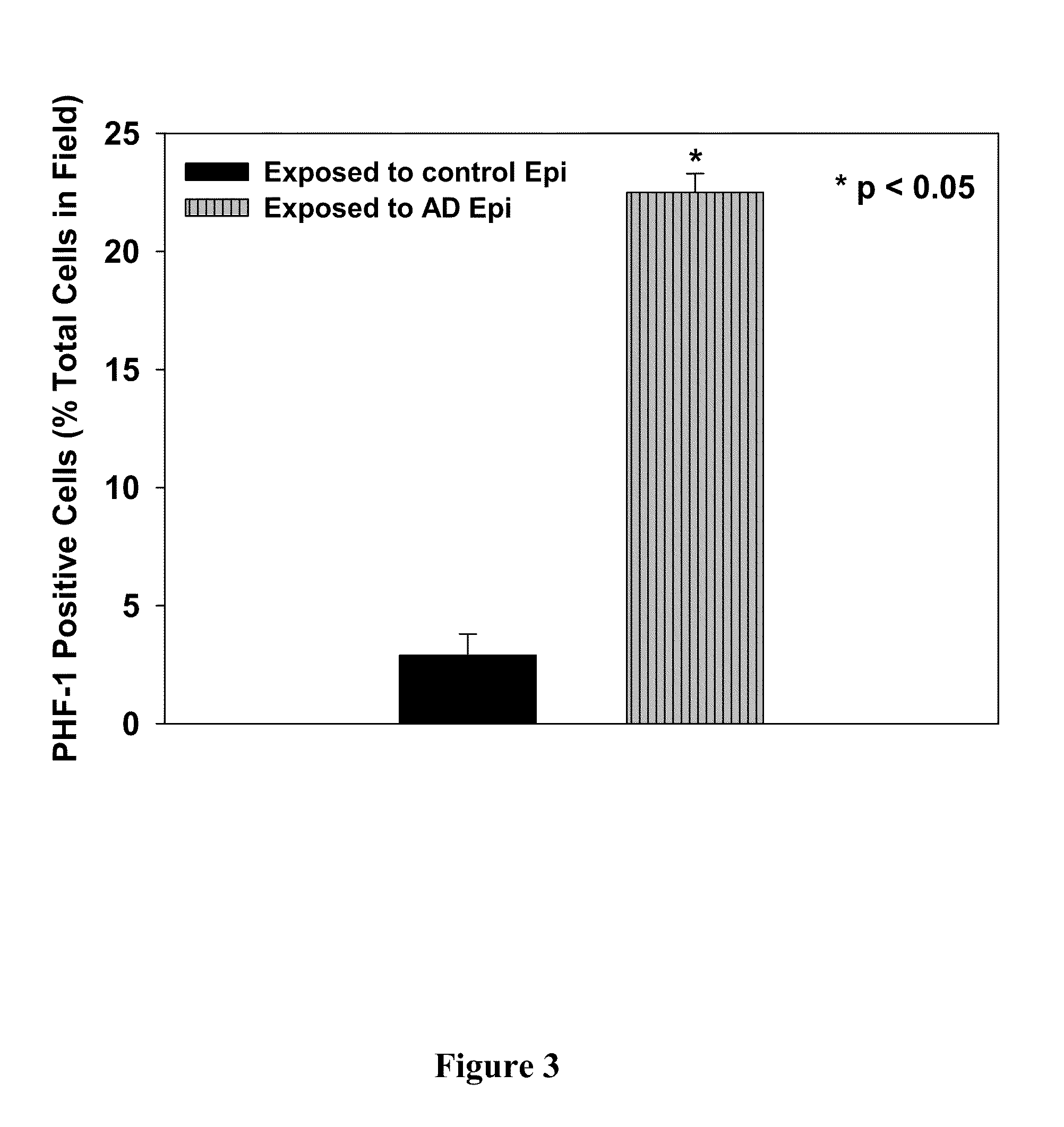
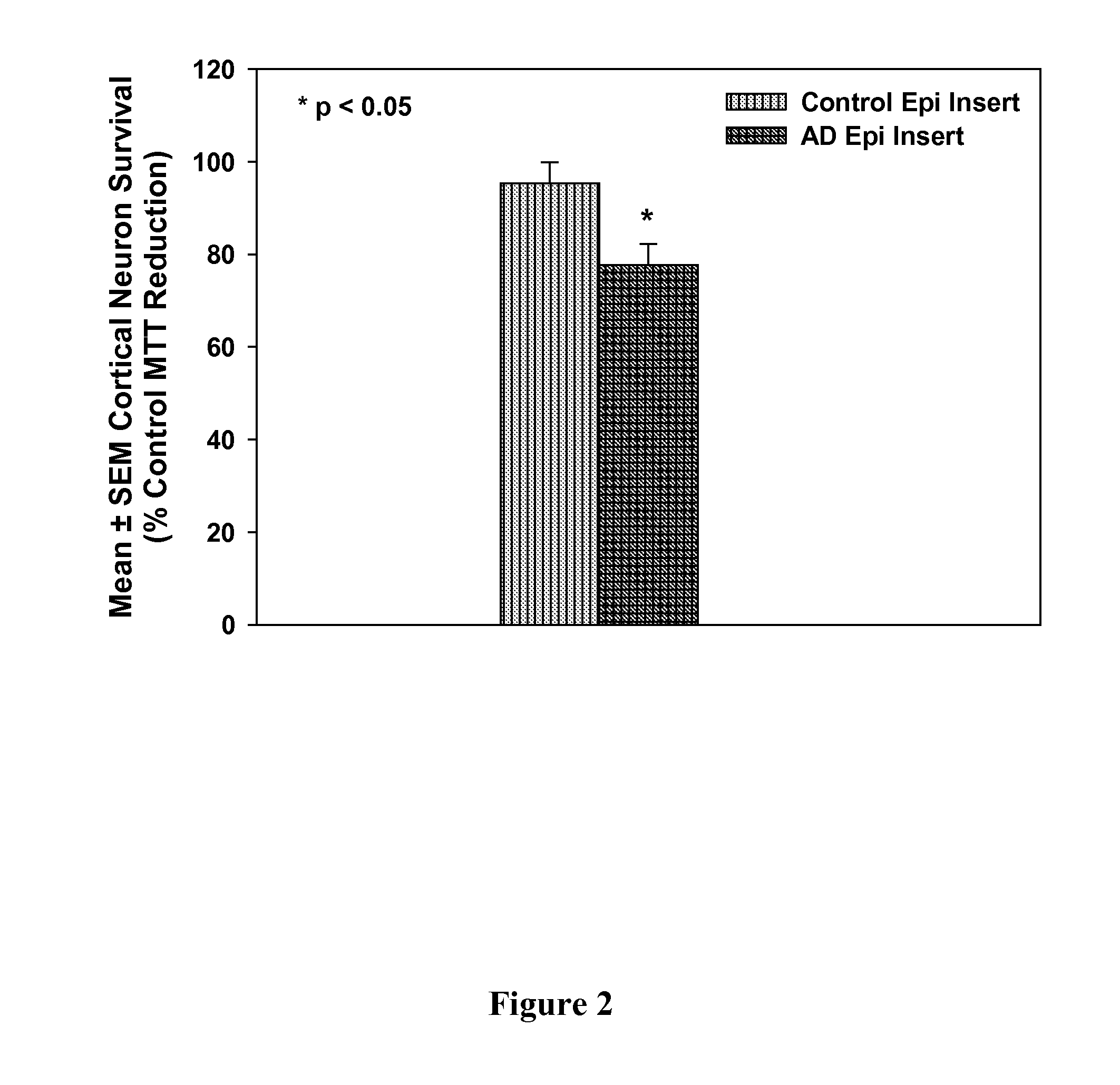
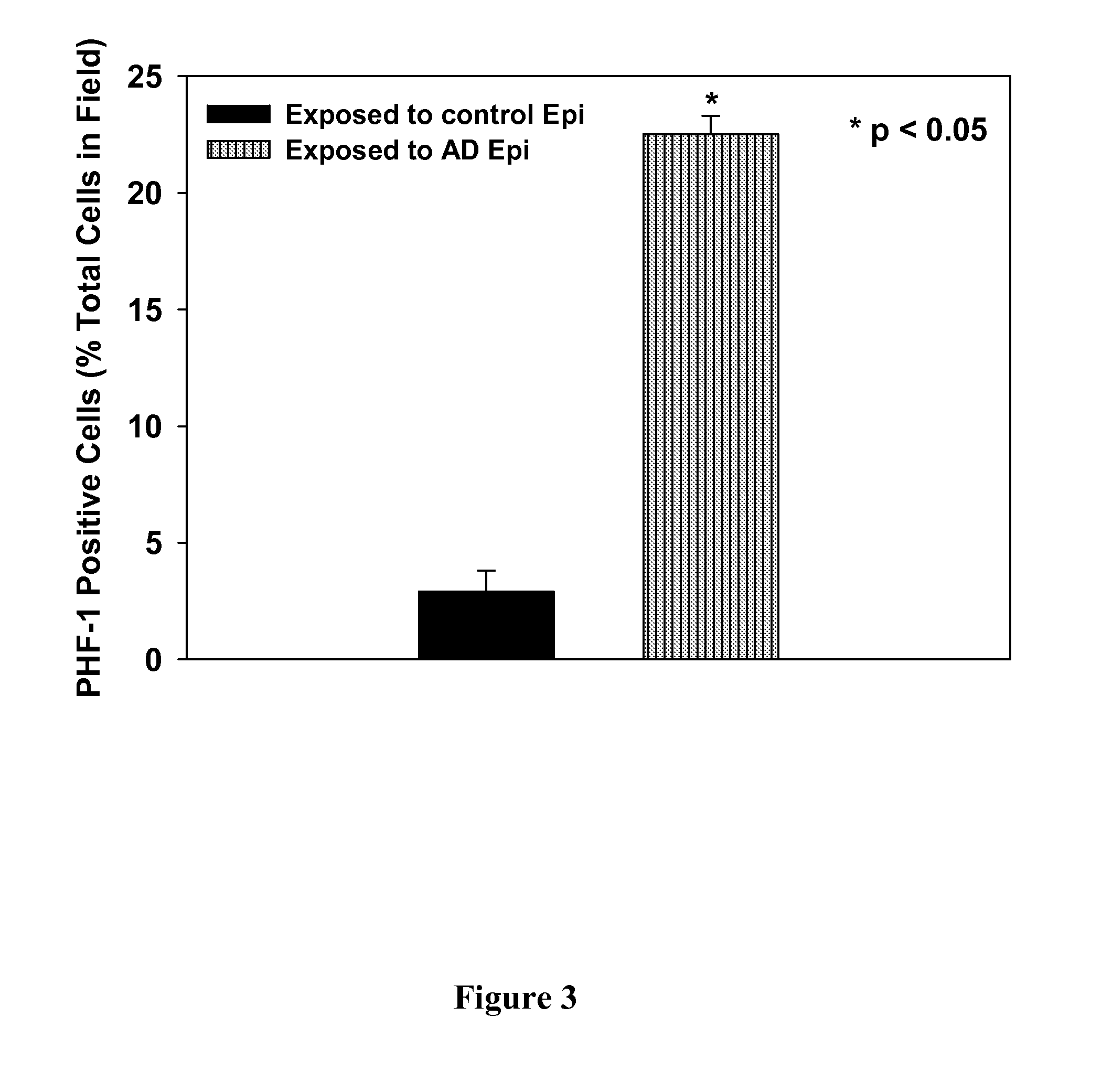
Treatment of mci and alzheimer's disease
ActiveUS20110118299A1Avoid it happening againReduce processBiocideNervous disorderNifedipineMild cognitive impairment (MCI)
The present invention provides, among other things, therapeutic compositions and methods that can effectively treat, slow or prevent a neurological disease (e.g., a neurodegenerative disease, e.g., mild cognitive impairment (MCI) or Alzheimer's disease (AD)), in particular, based on therapeutically effective amount of nifedipine, oxidized or nitroso nifedipine derivatives, lactam (e.g., a compound of formula (Ic) or (Ic-i), e.g., NFD-L1), thyroxine (T4), triiodothyronine (T3) and combinations thereof.
Owner:UNIV OF KENTUCKY RES FOUND
Nifedipine controlled-releasing tablet and preparation method thereof
ActiveCN101167700AShort time lagQuick effectOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineMedicine
The invention discloses nifedipine controlled release medicament, which contains a pastille layer and a boosting layer with the proportion of 1: 0.5-3 by weight, wherein the pastille layer contains nifedipine and carrying agent which is the homopolymer of vinylpyrrohdone and / or copolymer of vinylpyrrohdone and is 40-99% of the pastille layer, the boosting layer includes infiltration promoting polymer which is 10-80% of the boosting layer by weight, insoluble polymer which is 10-80% of the boosting layer by weight, and the other component is osmotic pressure accelerant The rate of medicament releasing controlled by nifedipine can make the medicament release the nifedipine in 24 hours by administering drug once a day.
Owner:OCEAN STAR INT
Nifedipine controlled release compositions and preparation methods therefor
InactiveUS20080095840A1Short lag timeImprove stabilityPill deliveryCapsule deliveryNifedipineWater insoluble
A nifedipine controlled release composition is provided comprising a drug-layer and a push-layer at a ratio of 1:0.5˜3 by weight. The drug-layer contains nifedipine and 40˜99 percent by weight of the drug-layer of hydrophilic polyvinylpyrrolidone homopolymer and / or copolymer carrier. The push-layer comprises about 10 to 80 percent by weight of the push-layer of osmopolymers, about 10 to 80 percent by weight of the push-layer of water-insoluble polymers, and about 5 to 50 percent by weight of the push-layer of osmagents. The composition is used in osmotic pump tablets for controlled release of nifedipine useful for administration once a day.
Owner:OCEAN STAR INT
Capsule for treating hypertension and coronary disease and its preparing method
InactiveCN1709426AEasy to prepareSimple recipeOrganic active ingredientsUnknown materialsSalvia miltiorrhizaNifedipine
The present invention relates to a Chinese medicine capsule preparation for effectively curing hypertension and coronary heart disease. Said capsule preparation is made up by using 14 Chinese medicinal materials of gastrodia root, uncaria stem and thorn, chrysanthemum flower, tortoise plastron, lyceum berry and others and adding nifedipine through a certain preparation process.
Owner:段世荣
Topical composition and method for treating occlusive wounds
The present invention provides a topical composition comprising about 6% to about 15% nifedipine and about 6% to about 15% pentoxifylline for treating severe vascular occlusive wounds. The present invention also provides a method and a kit for treating the vascular occlusive wound by applying the composition to the open wound, and cleaning and dressing the wound at least once daily.
Owner:FOOTE MARY ANN +1
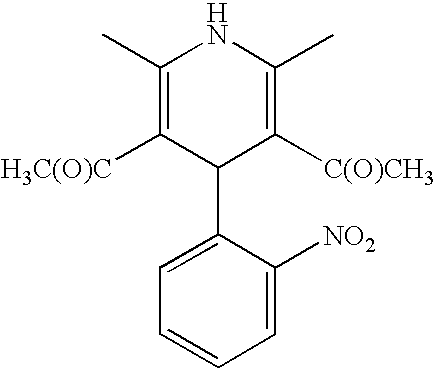
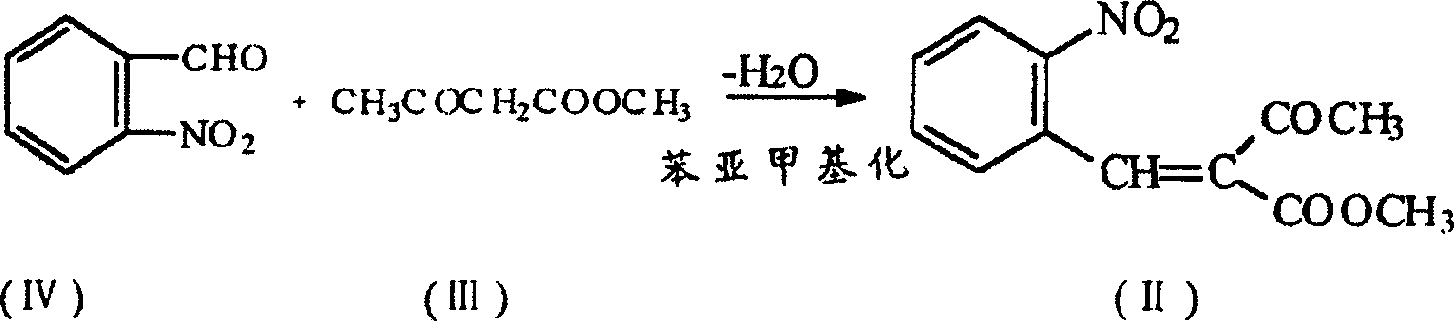
Prepn process of nifedipine
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde,the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
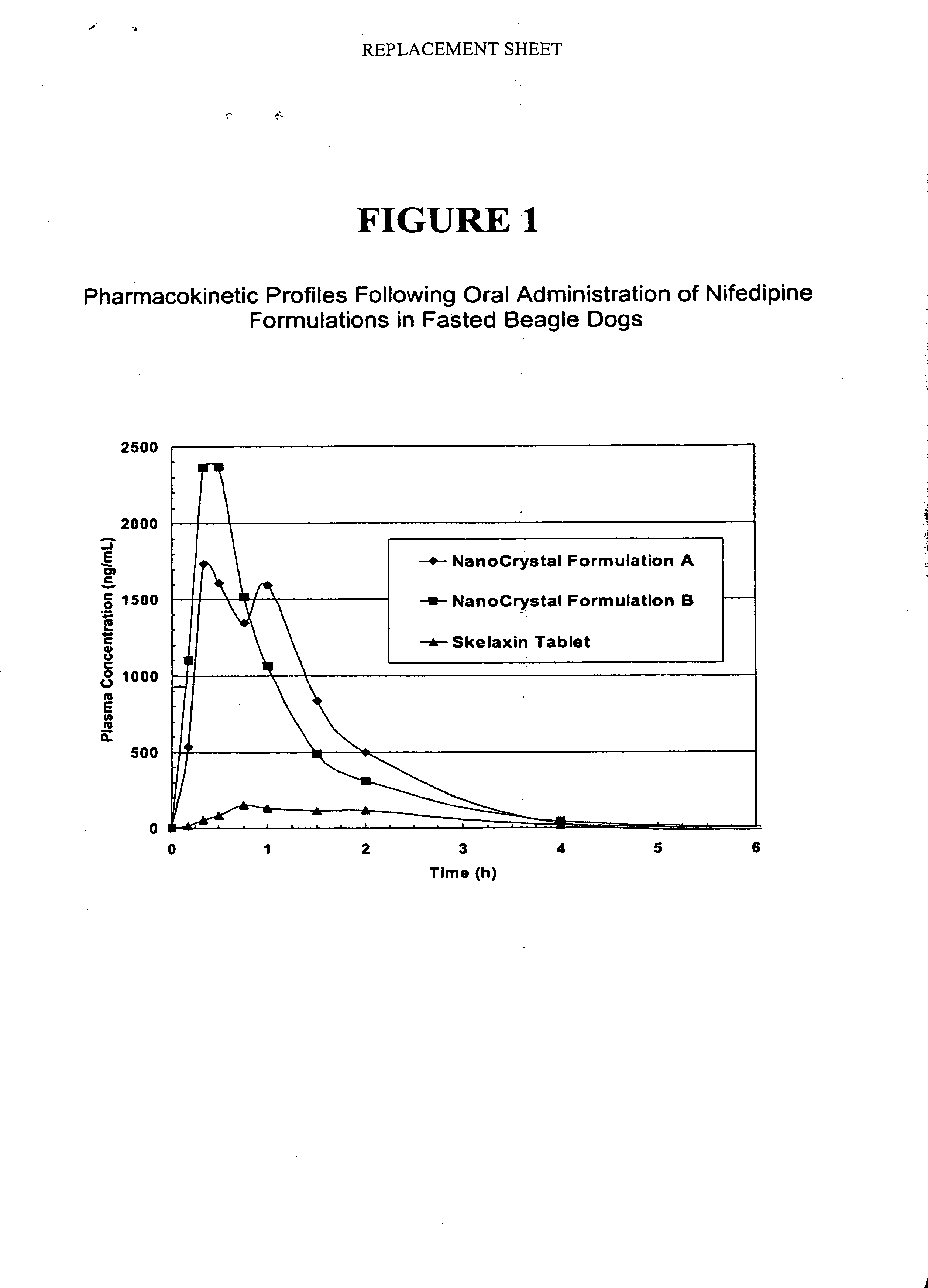
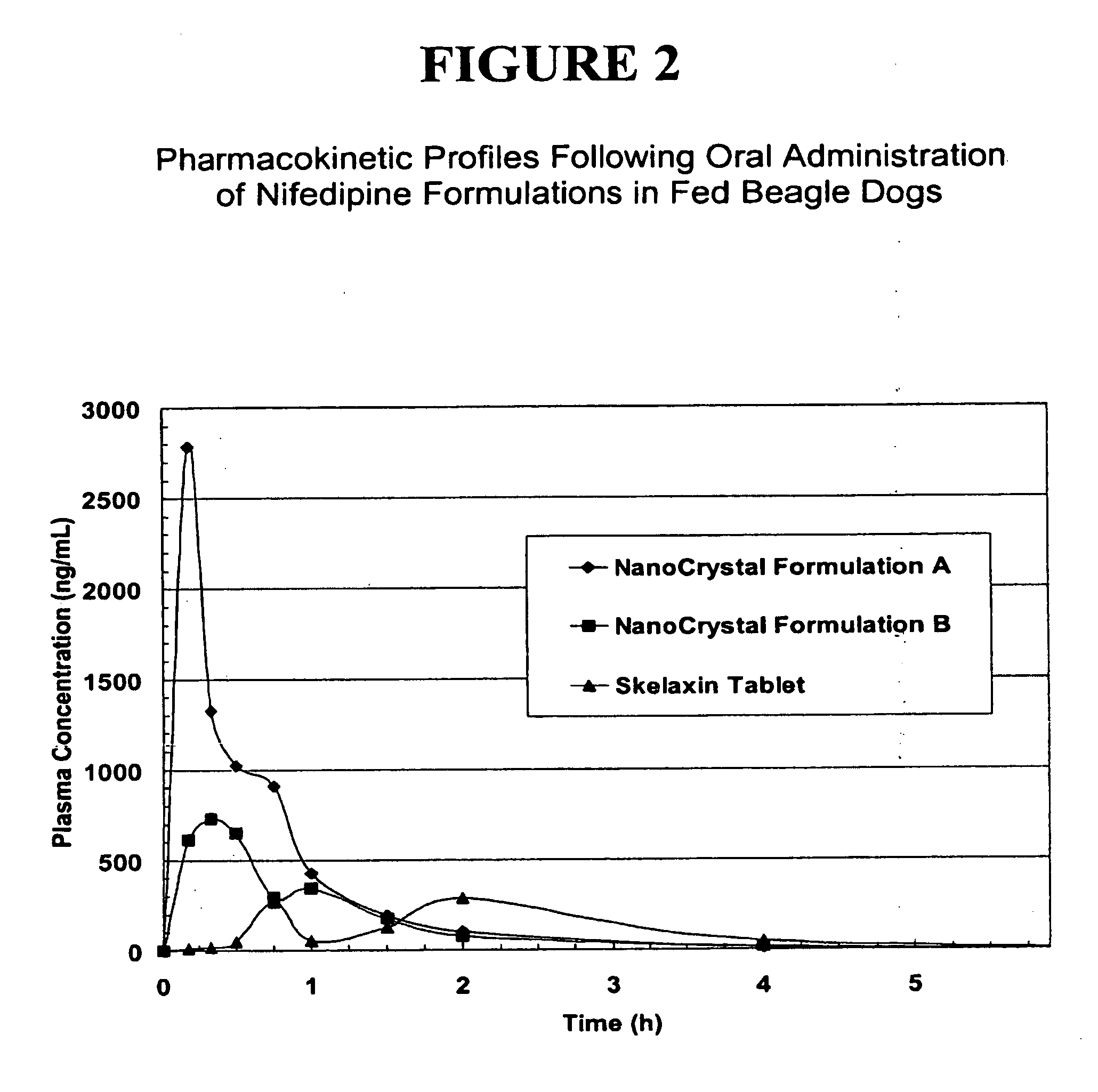
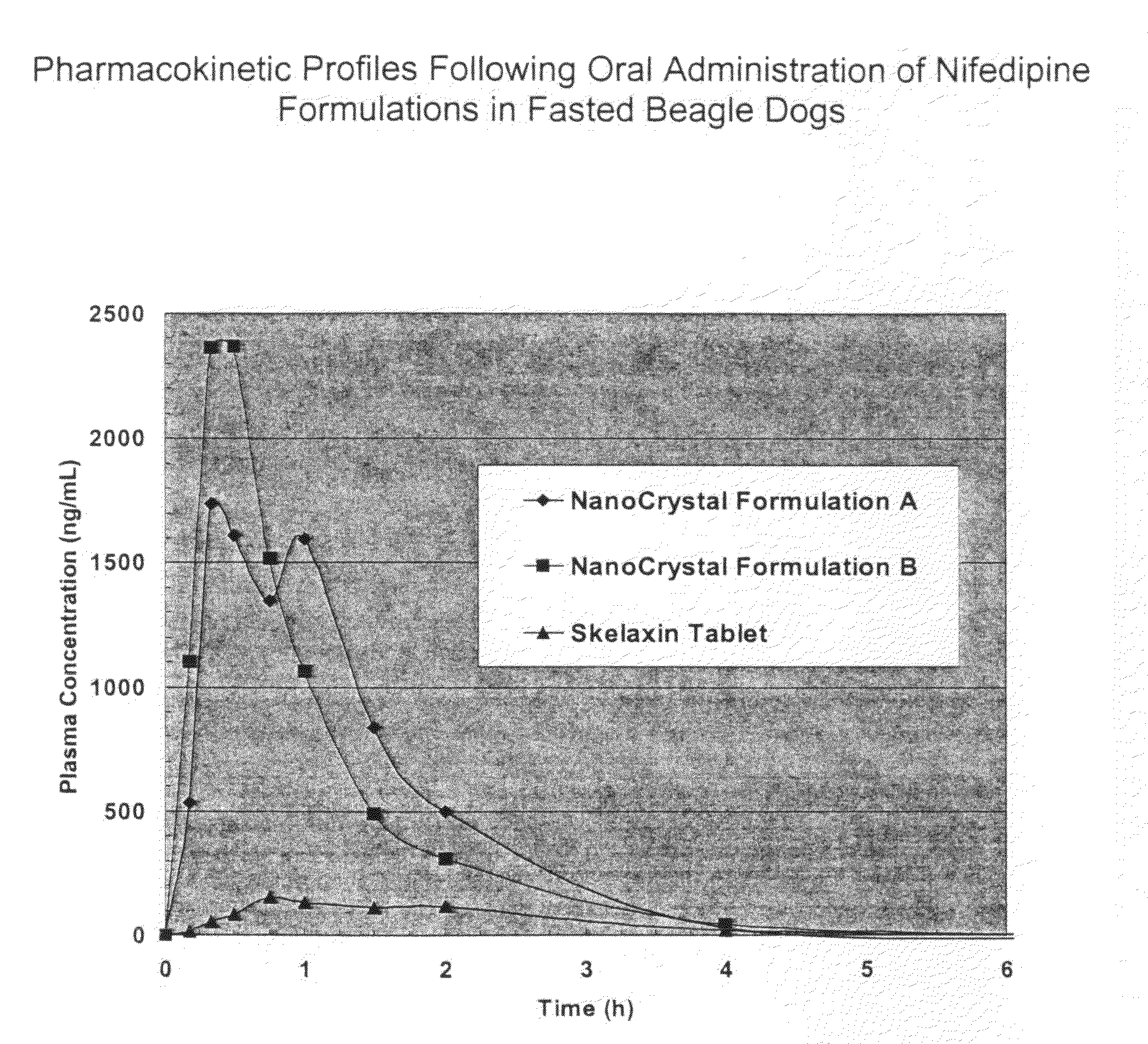
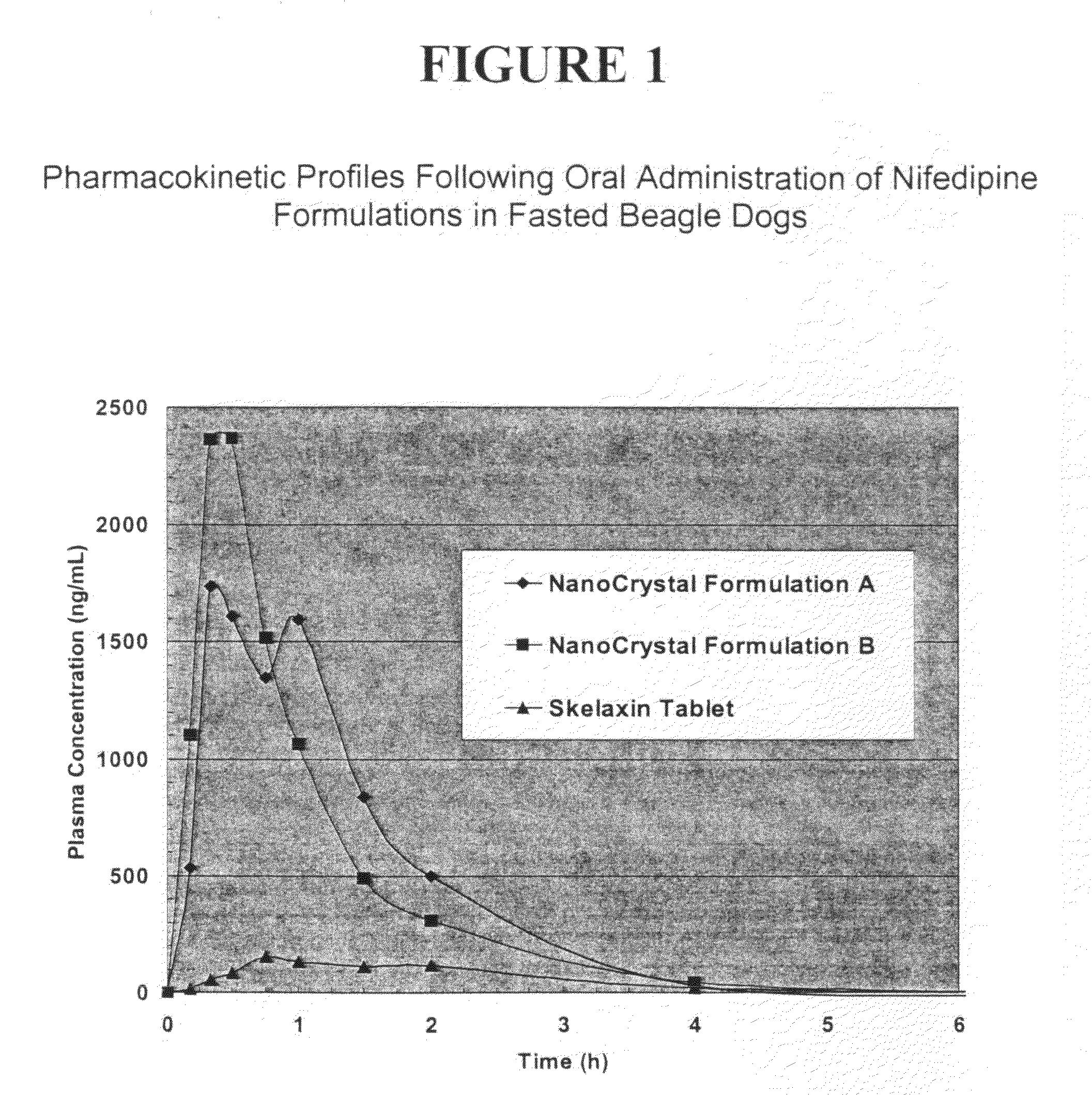
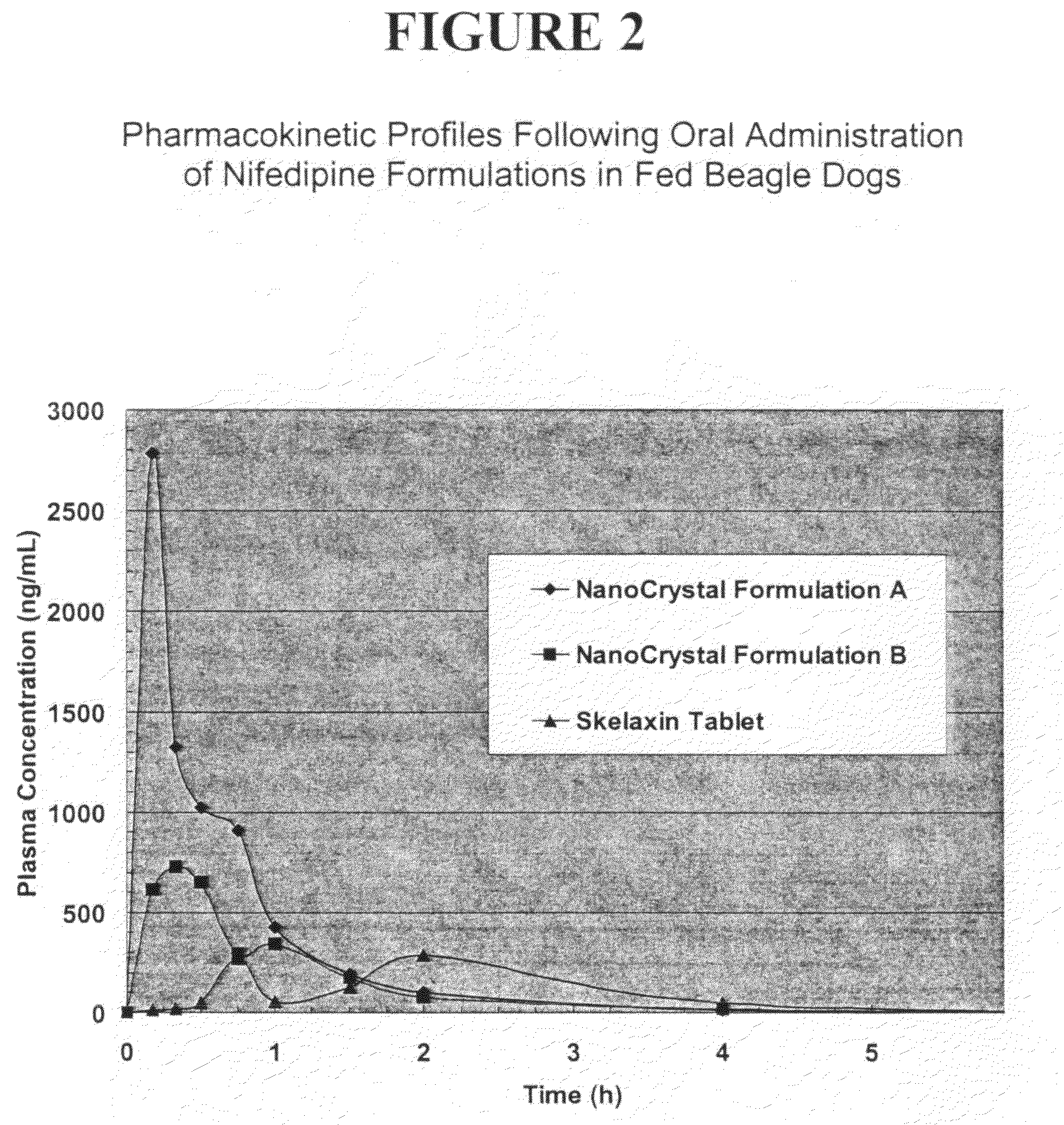
Novel nifedipine compositions
The present invention is directed to nanoparticulate compositions comprising nifedipine. The nifedipine particles of the composition have an effective average particle size of less than about 2 microns.
Owner:ELAN PHRMA INT LTD
Treatment of mci and alzheimer's disease
InactiveUS20100292281A1Elevated level of complexHigh expressionBiocideNervous disorderNifedipineMinimal cognitive impairment
The present invention provides, among other things, therapeutic compositions and methods that can effectively treat, slow or prevent mild cognitive impairment (MCI) or Alzheimer's disease (AD), in particular, based on therapeutically effective amount of nifedipine, oxidized or nitroso nifedipine derivatives, thyroxine (T4), triiodothyronine (T3) and combinations thereof.
Owner:UNIV OF KENTUCKY RES FOUND
Ointment for treating hemorrhoid and its preparing method
InactiveCN1813766AEliminate illnessIncreased secretionsOrganic active ingredientsAerosol deliveryNifedipineExternal Hemorrhoid
The present invention discloses an ointment for curing hemorrhoids. It is characterized by that 89%-98% of Vaseline, 0.1%-0.9% of nifedipine, 0.5%-4% of hydrocortisone, 1%-6.04% of lidocaine and 0.01%-0.07% of menthanol are successively added into a sterile container, and uniformly stirred so as to obtain the invented product.
Owner:杨树强
Sustained release tablet for treating cardiovascular diseases and preparation method thereof
ActiveCN103845299AReduce usageAvoid potential harmOrganic active ingredientsPharmaceutical delivery mechanismVascular diseaseSustained Release Tablet
The invention discloses a nifedipine sustained release tablet for treating cardiovascular diseases and a preparation method thereof. Sustained release granules are prepared by adopting low-melting-point sustained release materials including glyceryl behenate, polyethylene glycol 4000 and nifedipine; use of an organic solvent is avoided; the nifedipine sustained release tablet disclosed by the invention is good for the environmental protection; potential hurt to patients caused by residual organic solvents is also avoided; the sustained release materials and active medicines form granules similar to solid dispersoid, so that sustained release of nifedipine is kept for a long time, and furthermore, rapid release of medicines in rapid release granules is not influenced. The nifedipine sustained release tablet disclosed by the invention is prepared by adopting the conventional granulating process; the complex production equipment and the production process are avoided; energy is saved; the production efficiency is greatly increased.
Owner:YABAO PHARMA BEIJING
Double-layer sustained-release nifedipine tablet and preparation method thereof
The invention relates to a double-layer sustained-release nifedipine tablet and a preparation method thereof, and the double-layer sustained-release nifedipine tablet is characterized by consisting of an immediate-release layer and a slow-release layer, wherein the nifedipine-containing proportion of the immediate-release layer and the slow-release layer, by weight, is 1:1 to 1:15. The invention belongs to medicine preparation technical field. The purpose of the invention is to provide a double-layer sustained-release nifedipine tablet capable of good patient compliance, little side-effect, fast acting and enduringly keeping stable effective plasma concentration. In addition, an another purpose of the invention is to provide a preparation method for double-layer sustained-release nifedipine tablet, and the method has the advantages of good reappearance of the preparation technology, high production efficiency, and is suitable for industrialized mass production and has good release homogeneity of the prepared double-layer sustained-release nifedipine tablet.
Owner:COSCI MED TECH CO LTD
Novel nifedipine compositions
The present invention is directed to nanoparticulate compositions comprising nifedipine. The nifedipine particles of the composition have an effective average particle size of less than about 2 microns.
Owner:ALKERMES PHARMA IRELAND LTD
Prepn. process of nifedipine
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde, the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
Compound Atenolol-Nifedipine slow releasing prepn
InactiveCN1452966AEasy to take medicineAvoid missing dosesOrganic active ingredientsPharmaceutical delivery mechanismActive componentMedicine
The present invention relates to compound slow-release Atenolol-Nifedipine preparation and its medical application. The compound delayed preparation has active components Atenolol and Nifedipine anddelaying material for delayed release. The preparation includes also pharmacologically acceptable carrier.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Nifedipine double-layer osmotic pump tablet and preparation method thereof
PendingCN108338976AFacilitated releaseReduce the number of dosesOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineMedicine
The invention provides a nifedipine double-layer osmotic pump tablet. The nifedipine double-layer osmotic pump tablet includes a medicated-layer tablet core, a boosting-layer tablet core, a semipermeable membrane and single drug-releasing holes arranged in the surface of the semipermeable membrane at one side of a medicated layer. The nifedipine double-layer osmotic pump tablet is simple in preparation process and low in cost and has stable drug-releasing rate; zero-level release is basically realized in 416 hours, and the the drug release is basically complete; the purpose of administration of preparation once per day is achieved, and the compliance of a patient is improved. The invention provides a preparation method of a nifedipine controlled-release tablet.
Owner:CHINA PHARM UNIV
Orally disintegrating Nifedipine prepn and its recipe
InactiveCN1403082AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsPill deliveryNifedipineCurative effect
The recipe of orally disintegrating Nifedipine preparation includes Nifedipine, solid dispersing carrier, stuffing, disintegrating agent, corrective, lubricant, etc. The present invention aims at providing one kind of orally disintegrating Nifedipine preparation with simple preparation process, easy administration, quick effect and obvious treating effect. The present invention reaches the said aim via the synergetic effect of solid dispersing carrier and disintegrating agent. After being taken, the preparation of the present invention is disintegrated in oral cavity into fine powder and this is favorable to the resolution and absorption of the medicine. The preparation is easy to take, especially for special patient with dysphagia.
Owner:ZHEJIANG UNIV
Compound sustained-release pellet tablet containing nifedipine and atenolol and preparation thereof
The invention relates to the pharmaceutical preparation field, in particular to a compound controlled release pellet tablet of nifedipine and atenolol, and is characterized in that: controlled release pellet of nifedipine, controlled release pellet of atenolol and blank pellet are pelletized to obtain the compound controlled release pellet tablet of nifedipine and atenolol, wherein the controlled release pellet of nifedipine and the controlled release pellet of atenolol are respectively obtained from drug contained core coated with acrylic resin; the weight of coating of the controlled release pellet of nifedipine is added by 10 to 30 percent while the weight of coating of the controlled release pellet of atenolol by 8 to 25 percent; the blank pellet comprises microcrystaline cellulose and one of macrogol 6000, macrogol 4000 and stearic acid or the mixture of one or two of the macrogol 6000, the macrogol 4000 and the stearic acid, wherein the blank pellet takes up 50 to 70 percent of the total weight of the compound controlled release pellet tablet of nifedipine and atenolol.
Owner:CHINA PHARM UNIV
Nifedipine sustained release tablets and preparation method thereof
ActiveCN108186593AIn line with industrial production conditionsBreaking down the technical barriers of original researchOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineSustained Release Tablet
The invention discloses a nifedipine sustained release tablet and a preparation method thereof. The grain diameter D90 of nifedipine is 5-10 mu m, and a tablet core is prepared from, in percentage byweight, 25% of nifedipine, 5%-10% of retardant composition, 0.5%-1.5% of tween 80 and the balance of other auxiliary materials. In order to guarantee the storage quality of the tablet core, the tabletis colored and coated, and the weight gain of a coat is 4%-5%. Initiative formula composition is adopted, a sustained release mode is innovated, and the nifedipine sustained release tablet capable ofcontinuously releasing a drug within 12 h is prepared, two times of human body pre-BE (bioequivalence) (12 cases, empty stomach / full stomach) research prove the BE of the product, and besides, a dissolution method related in vitro and in vivo is developed. The drug release principle and process monopoly of the nifedipine sustained release tablet are broken through, the adopted formula and preparation process are simpler and more controllable, facilitate industrial production in China and provide more clinic choices for patients and doctors, and the medication burden of the patients is reduced.
Owner:南京百思福医药科技有限公司
Nifedipine composition and preparation method thereof
InactiveCN102028687AImproved release propertiesStable blood concentrationOrganic active ingredientsPharmaceutical product form changeCelluloseNifedipine
The invention belongs to the technical field of medicine, and relates to a nifedipine composition and a preparation method thereof. The novel nifedipine composition contains nifedipine, solid dispersion carrier hydroxy propyl cellulose and a release speed regulator, and is prepared by hot-melt extrusion technology. The composition has the advantages of simple preparation process, short production cycle, high repeatability and no organic solvent residues, and is easy for industrial mass production; and the prepared composition can slowly and constantly release the nifedipine to play a role in controlling the medicament concentration in a body and reducing occurrence of side effect.
Owner:ZHEJIANG ANGLIKANG PHARMA
Application of combination of tetracycline medicine and fluconazole in preparation of antifungal product, and product thereof
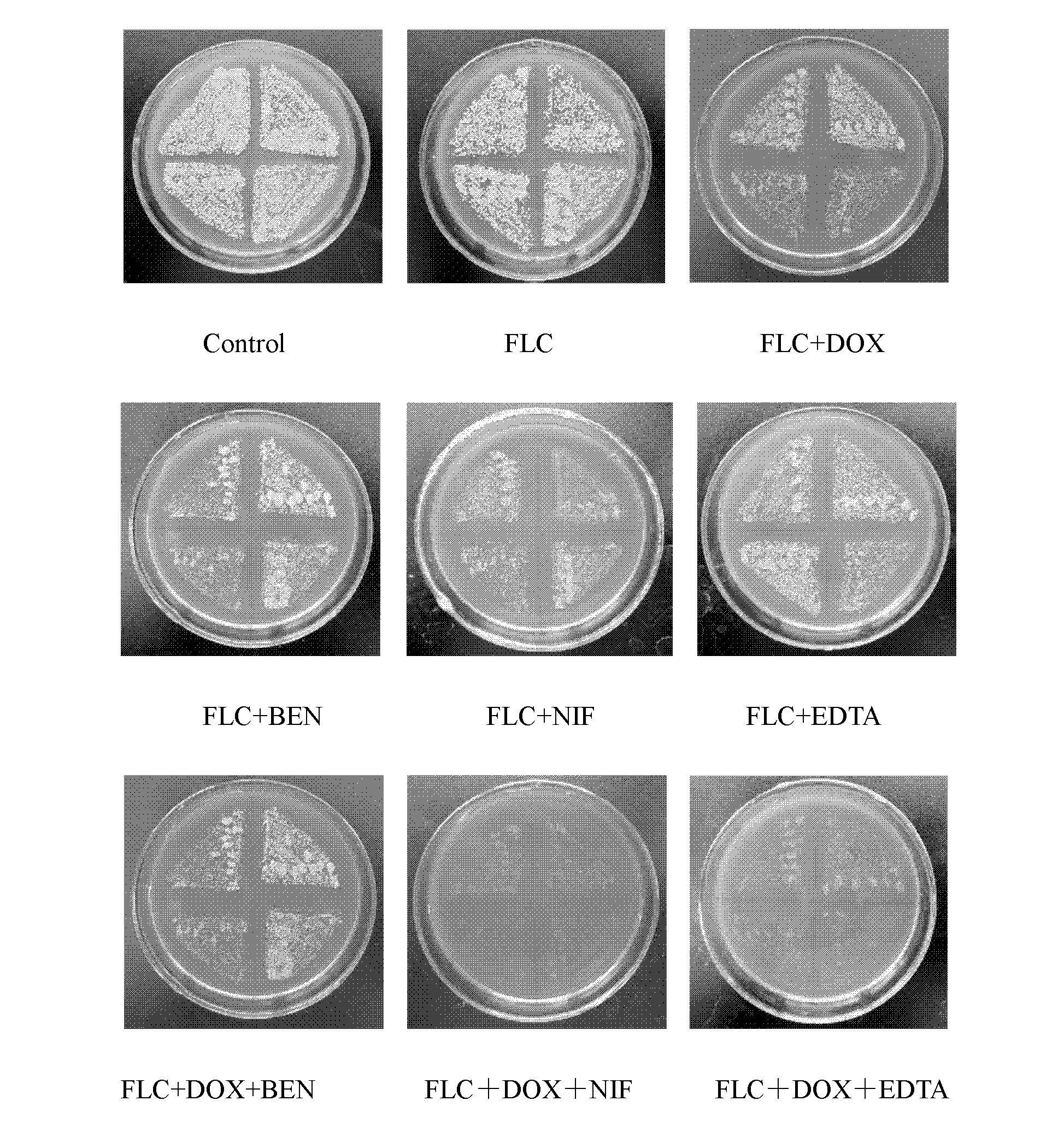
InactiveCN102626415AMIC lowerIncrease concentrationAntimycoticsTetracycline active ingredientsCalcium modulationAntimicrobial drug
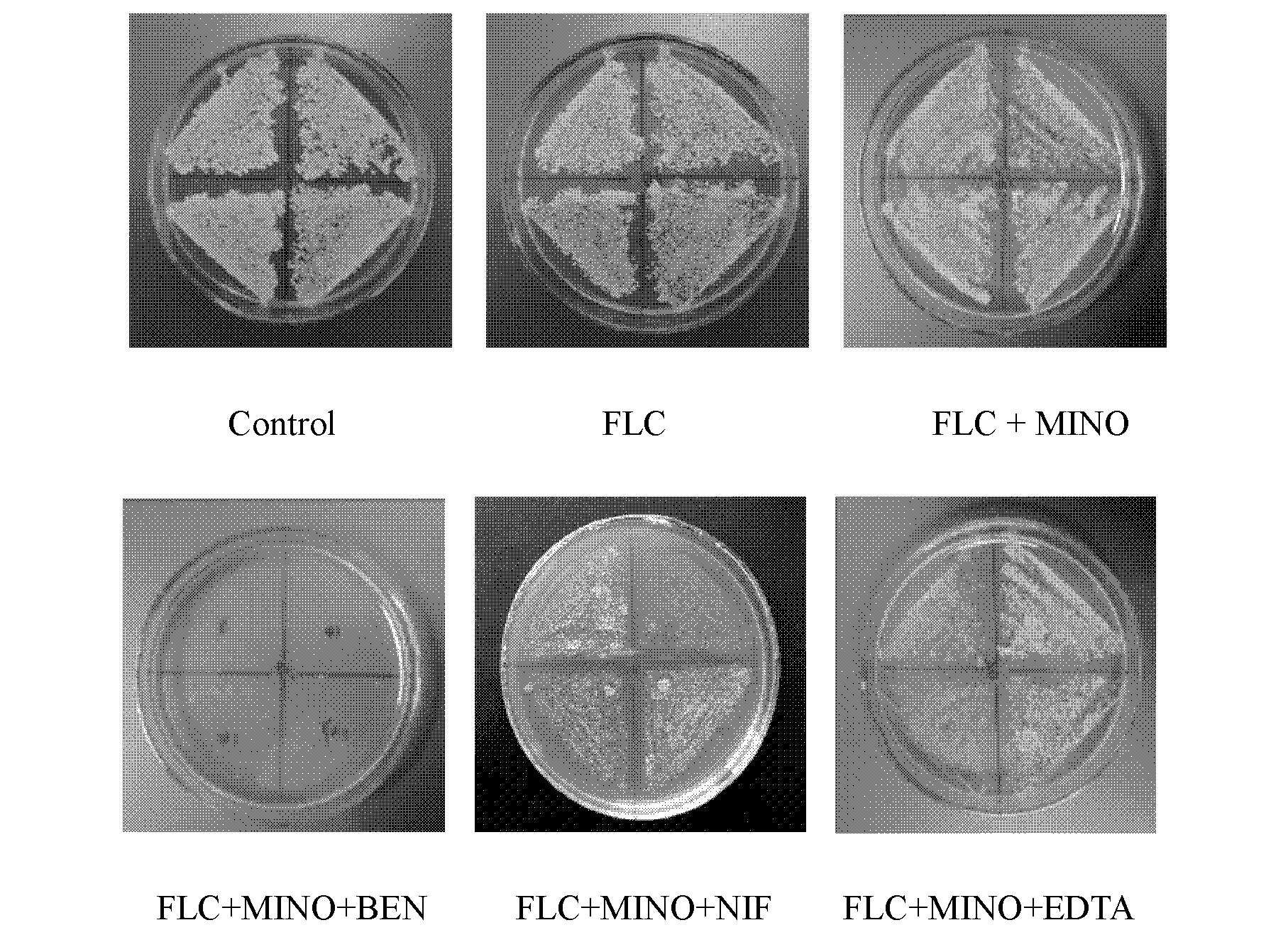
The invention discloses an application of a combination of a tetracycline medicine and fluconazole (FLC) in the preparation of an antifungal product, and the product thereof, and concretely discloses a synergistic effect of three cell calcium regulators of a non-selective calcium channel retarding agent benidipine (BEN), a selective L-type calcium channel retarding agent nifedipine (NIF) and a calcium ion chelating agent ethylene diamine tetraacetic acid (EDTA) to the FLC and tetracycline antifungal medicines of doxycycline (DOX) and minocycline (MINO), and an application of the three cell calcium regulators in Candida albicans resisting, wherein a synergistic antifungal effect can be generated by the combined application of the FLC, the DOX and the MINO. Results confirm that the combined application of the FLC and the DOX / MINO can generate the synergistic antifungal effect, and has a substantial killing effect on drug-resistant Candida albicans; the BEN, the NIF and the EDTA can obviously enhance the combined antifungal effect of the FLC and the DOX / MINO to the Candida albicans, and can obviously reduce the lowest effective concentration during the combined application of the FLC, the MINO and the DOX; and the combined application of the three calcium regulators and the FLC can enhance the antifungal effect.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
Treatment of MCI and Alzheimer's disease
The present invention provides, among other things, therapeutic compositions and methods that can effectively treat, slow or prevent a neurological disease (e.g., a neurodegenerative disease, e.g., mild cognitive impairment (MCI) or Alzheimer's disease (AD)), in particular, based on therapeutically effective amount of nifedipine, oxidized or nitroso nifedipine derivatives, lactam (e.g., a compound of formula (Ic) or (Ic-i), e.g., NFD- L1), thyroxine (T4), triiodothyronine (T3) and combinations thereof.
Owner:UNIV OF KENTUCKY RES FOUND
Nifedipine sustained release tablets preparation method
InactiveCN101190207AQuick effectStrong medicineOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletNifedipine
The invention discloses a preparation method of a sustained release tablet of nifedipine: (1) nifedipine and ethyl vitamin are prepared into frame type particles according to the ratio of 1:0.1-100, and nifedipine and acrylics acid resin are prepared into frame type particles according to the ratio of 1:0.1-100; (2) nifedipine and PEG are heated to be melted according to the ratio of 1:0.1-100 and then are prepared into solid dispersion, and then the solid dispersion is crushed into particles; (3) the frame type particles are mixed with the particles described in the (2) step according to the ratio of 1:1-10, and then are compressed into tablets. The preparation method overcomes the disadvantage of the traditional technique of complexity and being difficult to be industrialized; the technique of the invention is simple in technique and easy to be controlled, and the sustained release tablet of nifedipine prepared by the preparation method can remarkably reduce the adverse reaction of the medicine, enhance the compliance of patients as well as improve clinical curative effect.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD
Nifedipine double-layer osmotic pump medicinal composition and preparation technology thereof
ActiveCN102178677AMedication safetyDrug safetyOrganic active ingredientsPill deliveryAdhesiveSolvent
The invention provides a nifedipine double-layer osmotic pump medicinal composition and a preparation technology thereof. The composition comprises a double-layer tablet core and a controlled release coating membrane thereof, wherein the double-layer tablet core is composed of pharmaceutically acceptable excipients and comprises one or more than one of a macromolecular suspending aid substance, an osmotic pressure active substance, a permeation enhancer, a dissolving aid substance, a colorant, a macromolecule swelling substance, a lubricant, a flow aid, an adhesive and a solvent; and the controlled release coating membrane is composed of one or more than one of a membrane forming material, a pore-forming agent and a plasticizer. The nifedipine double-layer osmotic pump medicinal composition prepared by the invention can effectively control a medicament to release in a specific time so that the medicament taking time can be decreased.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Orally administrable nifedipine pellet and process for the preparation thereof
The present invention relates to a fast-release as well as a prolonged release type of nifedipine pellets and the process for the preparation thereof. The fast-release type of nifedipine pellets comprises a particulate core which is covered by a nifedipine coating layer. The particulate core comprises water-soluble or water-insoluble excipient(s) and a pharmacologically acceptable carrier. The nifedipine coating layer comprises an effective amount of nifedipine dissolved in organic solvent(s). This nifedipine coating layer can further be mixed with a suspension which comprises an adhesive, an emulsifier, and a dispersant. The preferred composition of the fast-release type of nifedipine includes 20-70% of the particulate core, 3-15% of nifedipine, 1-20% of emulsifier, 1-20% of adhesive, and 1-30% of dispersant. The prolonged-release type of nifedipine pellets comprises, in addition to the particulate core and the nifedipine coating layer, a surface coating layer which is made of at lease one consisting of hydroxypropylcellulose, hydroxypropylmethylcellulose and ethylcellulose. This surface coating layer further comprises a plasticizer which is selected from the group consisting of triethylcitrate, triacetin, and diethyl phthalate.
Owner:LEE FANG YU +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com