Compound sustained-release pellet tablet containing nifedipine and atenolol and preparation thereof
A sustained-release micropill tablet and technology for sustained-release micropellet, which are applied in the field of nifedipine and atenolol compound sustained-release micropellet tablets and their preparation, can solve the problem of process stoppage, difficult control of drug release behavior, equipment damage, etc. problem, to achieve good mixing uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
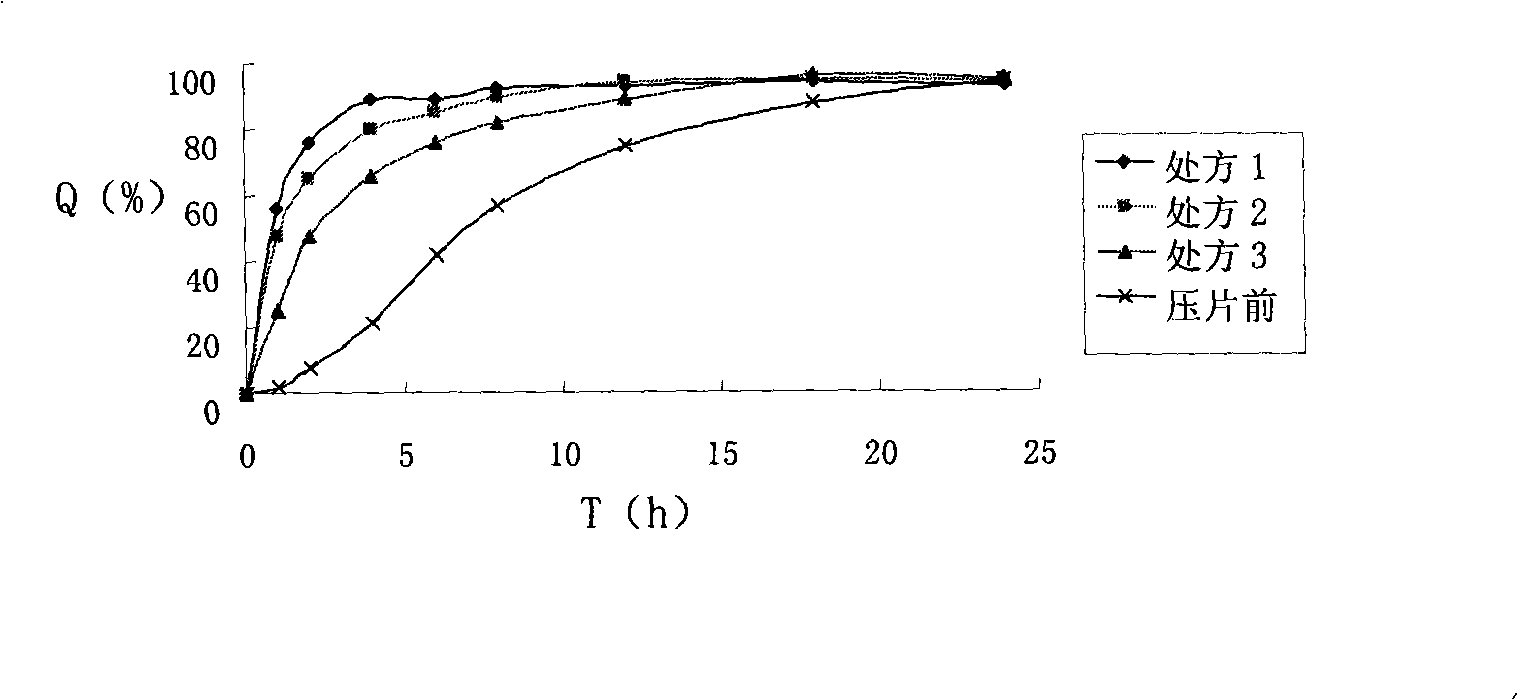
[0051] Take by weighing 5g atenolol, 5g microcrystalline cellulose and 3g tartaric acid, mix homogeneously, use the 2% aqueous solution of povidone K30 as the soft material of binding agent system, extrude and spheronize and prepare atenolol containing pill core, sieve Take 30-40 mesh for later use.
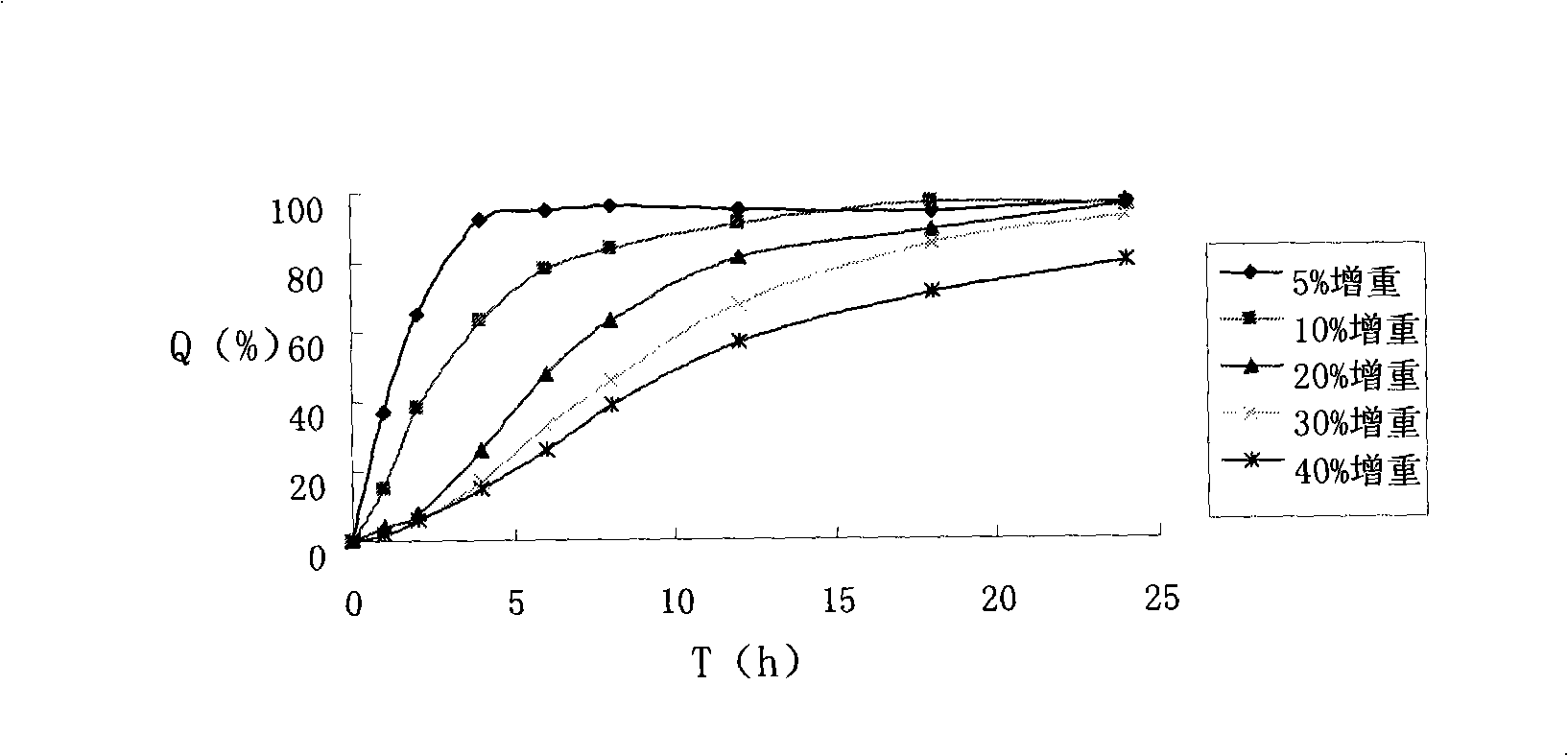
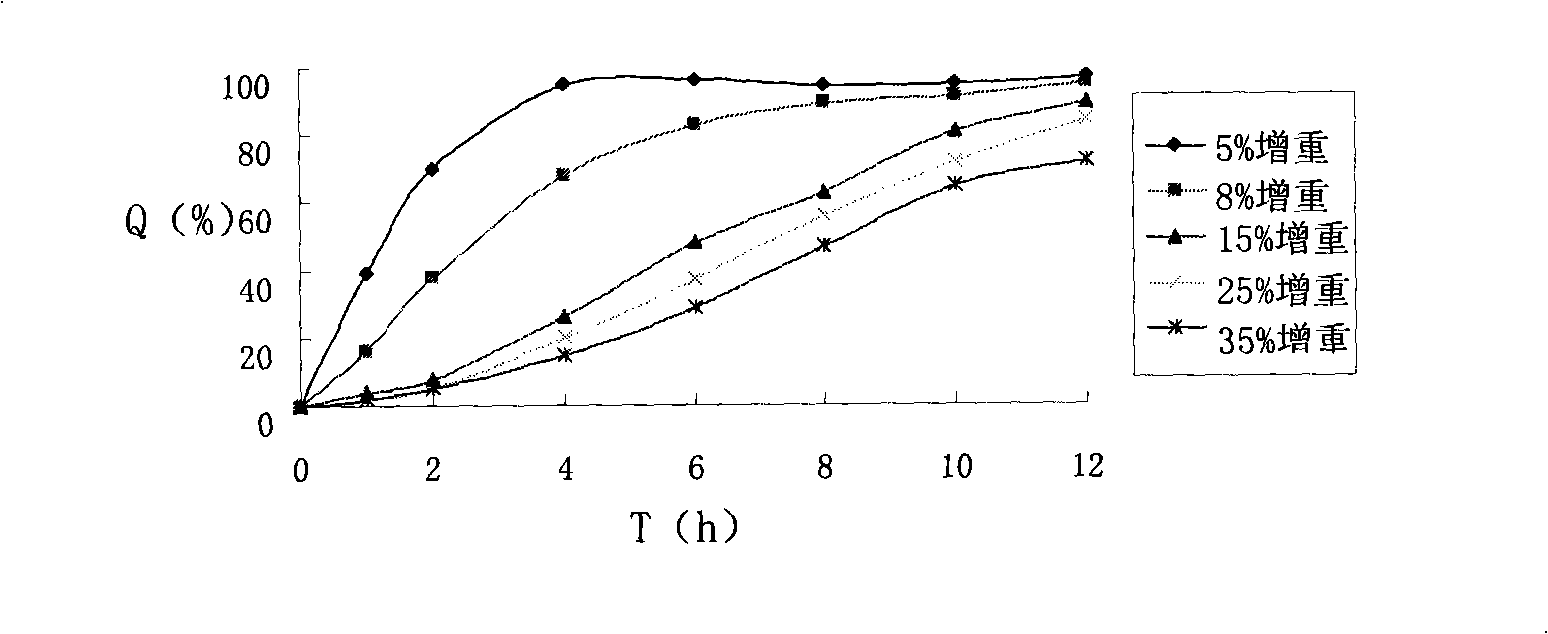
[0052] Take above-mentioned 10g atenolol drug-containing pellet cores in the fluidized bed. Add 0.267g of talc powder and 0.002g of bright blue lake into 15ml of water, mix and stir to obtain a suspension, add 0.533g of methyl methacrylate: butyl acrylate 1:1.5 polymer, 15ml of water and 15ml of ethanol before use In the coating solution, adjust the wind speed, temperature and coating solution flow rate to ensure a good fluidized state, and the coating weight increases by about 8%, to prepare atenolol sustained-release pellets with a drug content of 35.6%.
[0053] Take by weighing 10g of nifedipine and 20g of polyethylene glycol 6000 to prepare a solid dispersion, take by weigh...
Embodiment 2
[0059] Take by weighing 5g atenolol, 5g microcrystalline cellulose and 3g tartaric acid, mix homogeneously, use the 2% aqueous solution of povidone K30 as binder system soft material, centrifugal granulation coating prepares atenolol containing pill core , sieve 30-40 mesh for later use.
[0060] Take the above-mentioned 10g atenolol-containing pill core in a centrifugal granulation coating machine. Add 0.833g of talcum powder and 0.002g of bright blue color lake into 15ml of water and mix and stir to obtain a suspension, which consists of 1.667g of methyl methacrylate: ethyl acrylate 1:2 polymer, 15ml of water and 15ml of ethanol before use In the coating solution, adjust the wind speed, temperature and coating solution flow rate, and the coating weight increases by about 25%, to prepare atenolol sustained-release pellets with a drug content of about 30.77%.
[0061] Take by weighing 10g of nifedipine and 20g of polyethylene glycol 4000 to prepare a solid dispersion, take by...
Embodiment 3
[0067] Take by weighing 5g atenolol, 10g microcrystalline cellulose and 3g tartaric acid, mix homogeneously, use the 2% aqueous solution of povidone K30 as binder system soft material, fluidized bed one-step preparation atenolol contains pill core, Sieve 30-40 mesh for later use.
[0068] Take above-mentioned 10g atenolol drug-containing pellet cores in the fluidized bed. Add 0.5g of talc powder and 0.002g of bright blue color lake into 15ml of water and mix and stir to obtain a suspension, add 1g of methyl methacrylate: butyl acrylate 1:2 polymer, 15ml of water and 15ml of ethanol before use In the coating liquid, adjust the wind speed, temperature and coating liquid flow rate to ensure a good fluidized state, and the weight of the coating is increased by about 15%, to prepare atenolol sustained-release pellets with a drug content of 24.15%.
[0069] Take by weighing 10g of nifedipine and 40g of polyethylene glycol 6000 to prepare a solid dispersion, take by weighing 10g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com