Zolpidem tartrate controlled-release pellet and preparation method thereof
A technology for zolpidem tartrate and zolpidem tartrate is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., and can solve the problems of high requirements for production equipment and technical personnel and complex preparation methods , complex preparation process and other problems, to avoid excessive peak-to-valley ratio of blood drug concentration, flexible design, and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
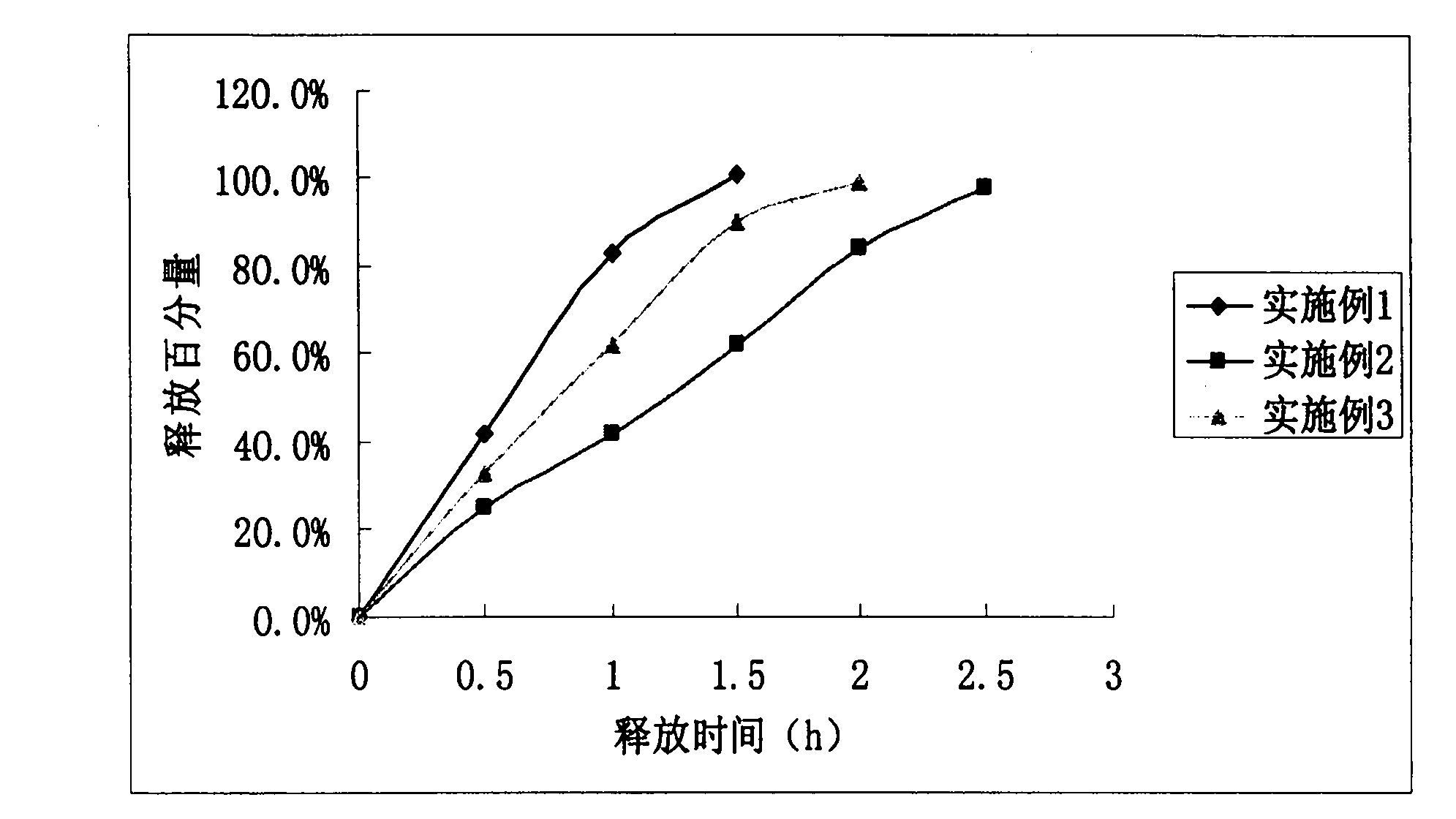
Embodiment 1
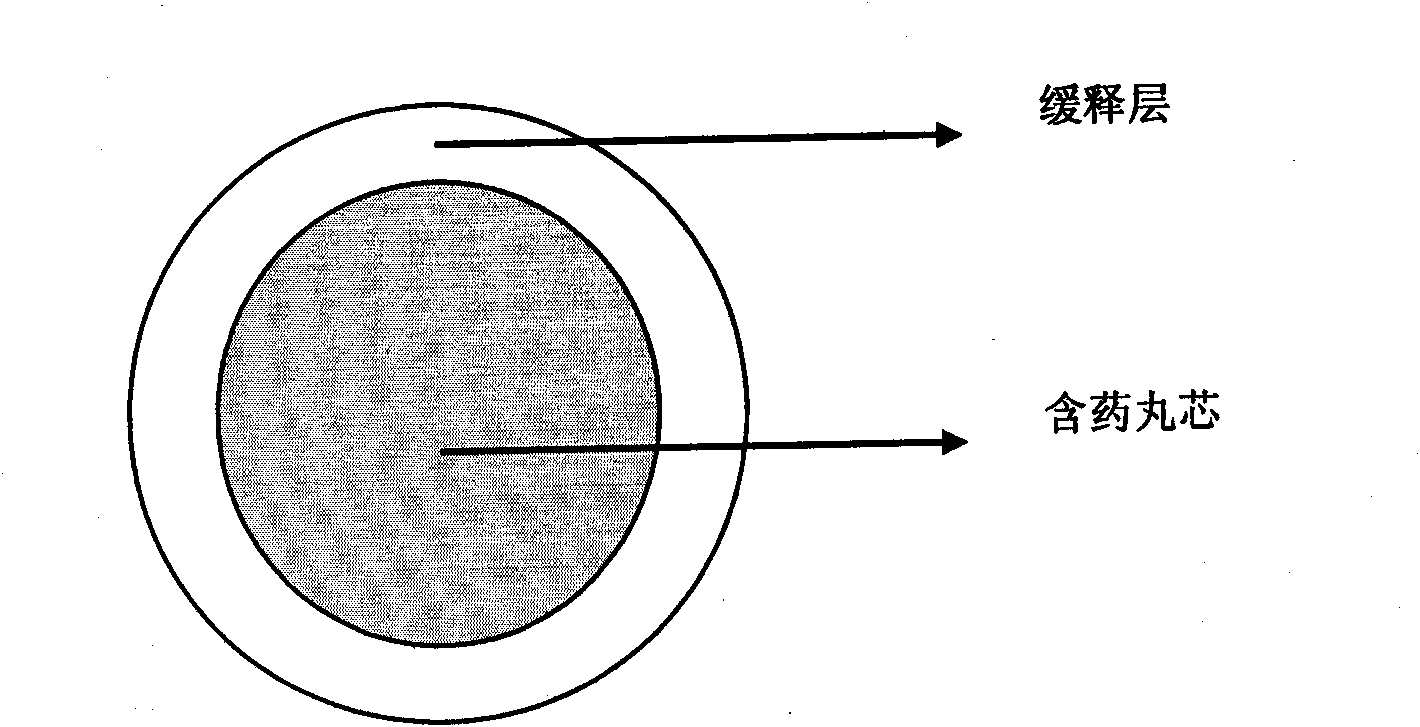
[0047] Embodiment 1 drug-containing 10mg zolpidem tartrate sustained-release pellets
[0048] a. Contains pill core ingredients
[0049] zolpidem tartrate
[0050] zolpidem tartrate
[0051] Preparation method: Weigh the raw and auxiliary materials according to the prescription quantity, mix them evenly, add a binder and mix them evenly to make a soft material; use a granulator (or coating pan) to pick up the mother and roll the pellets to the required mesh size of 15-30 mesh, Dry and sieve the best 18-25 mesh pellets for later use.
[0052] b. Sustained release layer ingredients
[0053] with pill core
[0054] Coating process: Weigh 15g of NE30D, 2g of talc powder and 30g of distilled water, mix them evenly under magnetic stirring, and set aside; weigh 100g of pill cores and place them in a fluidized bed, start the machine, adjust the air inlet temperature to 30°C, and use 5ml / min flow rate, sprayed on the outer layer of drug-containing pelle...
Embodiment 2
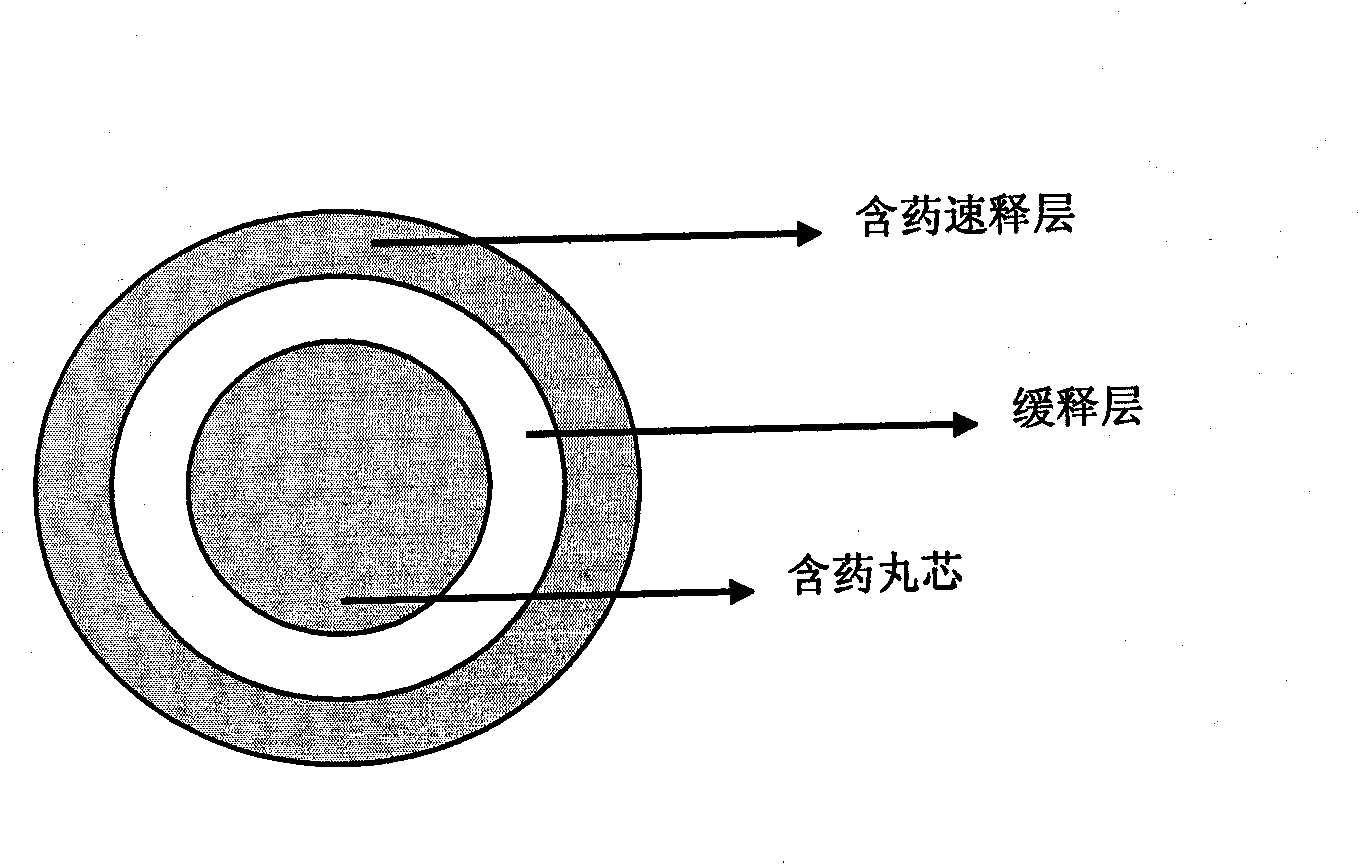
[0055] Embodiment 2 drug-containing 15mg zolpidem tartrate sustained-release pellets
[0056] a. Contains pill core ingredients
[0057] Blank base pellets (16-20 mesh)
[0058] Preparation method: Dissolve the prescribed amount of zolpidem tartrate, tartaric acid and hypromellose in 100g of distilled water; take 200g of blank base pellets in a fluidized bed, adjust the inlet air temperature to 45°C, and spray at a flow rate of 7ml / min Spray on the outer layer of the base pill until it is completely sprayed, dry for 15 minutes, take out and sieve to remove fine powder for later use.
[0059] b. Sustained release layer ingredients
[0060] with pill core
[0061] Coating process: weigh L30D-5550g, talc powder 5g, triethyl citrate 3g and distilled water 100g, mix evenly under magnetic stirring, and set aside; weigh 180g of pill core and place it in a fluidized bed, start the machine, adjust The air inlet temperature is 30°C, and the coating is carried out a...
Embodiment 3
[0062] Example 3 Drug-containing 10mg zolpidem tartrate sustained-release pellets
[0063] a. Contains pill core ingredients
[0064] Blank base pellets (18-22 mesh)
[0065] Preparation method: Dissolve the prescribed amount of zolpidem tartrate, tartaric acid and hypromellose in a mixed solution of distilled water and ethanol; take 300 g of blank base pills in a fluidized bed, adjust the inlet air temperature to 50°C, and keep the bed temperature At 40-45°C, spray on the outer layer of the base pellet at a flow rate of 8ml / min until it is completely sprayed, dry for 15 minutes, take out and sieve to remove fine powder for later use.
[0066] b. Sustained release layer ingredients
[0067] with pill core
[0068] with pill core
[0069] Coating process: Weigh 80g of ethyl cellulose 25% aqueous dispersion, 60000.5g of polyethylene glycol, 2.0g of hypromellose and 80g of distilled water, mix evenly under magnetic stirring, and set aside; weigh 260...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com