Patents
Literature
69 results about "Zolpidem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
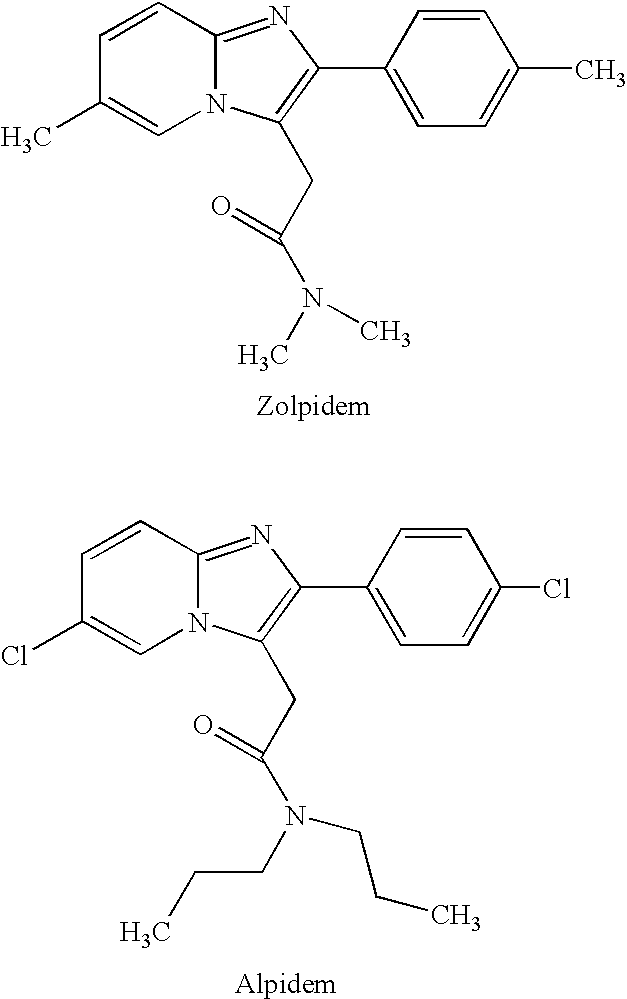
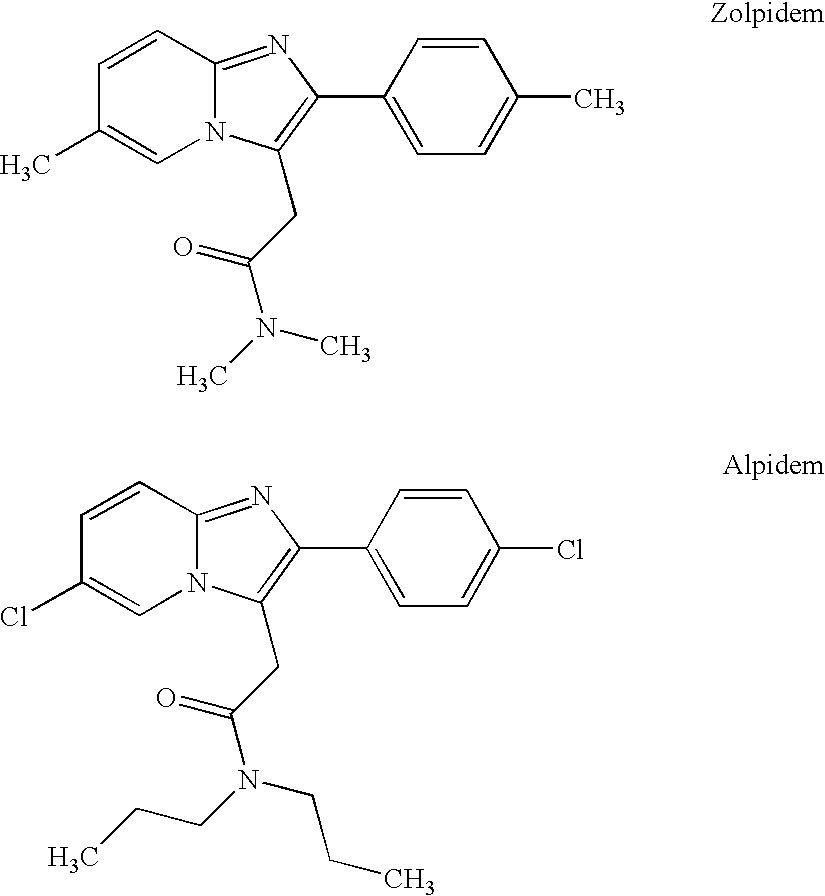
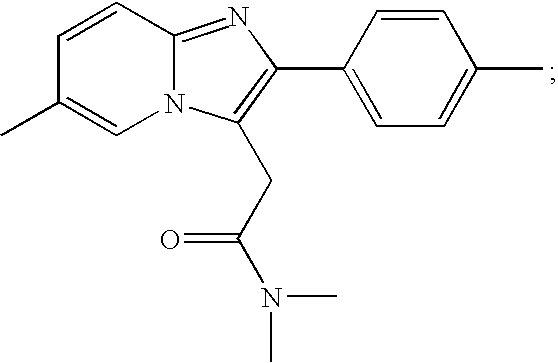
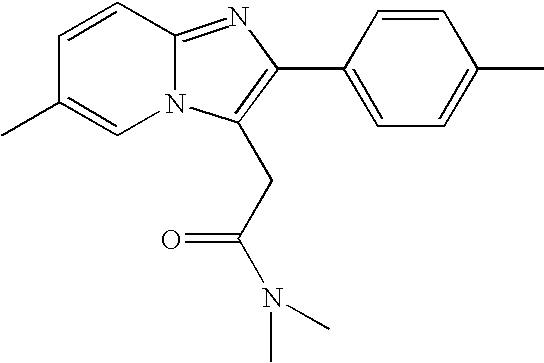
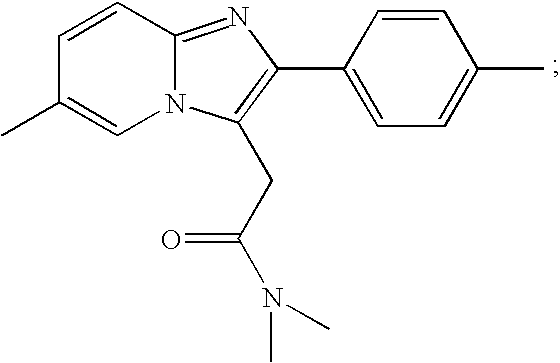
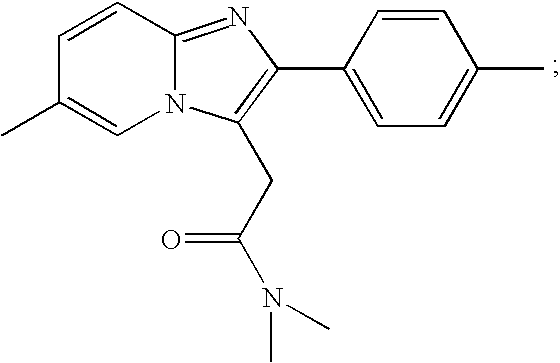
Zolpidem is used for a short time to treat a certain sleep problem (insomnia) in adults.
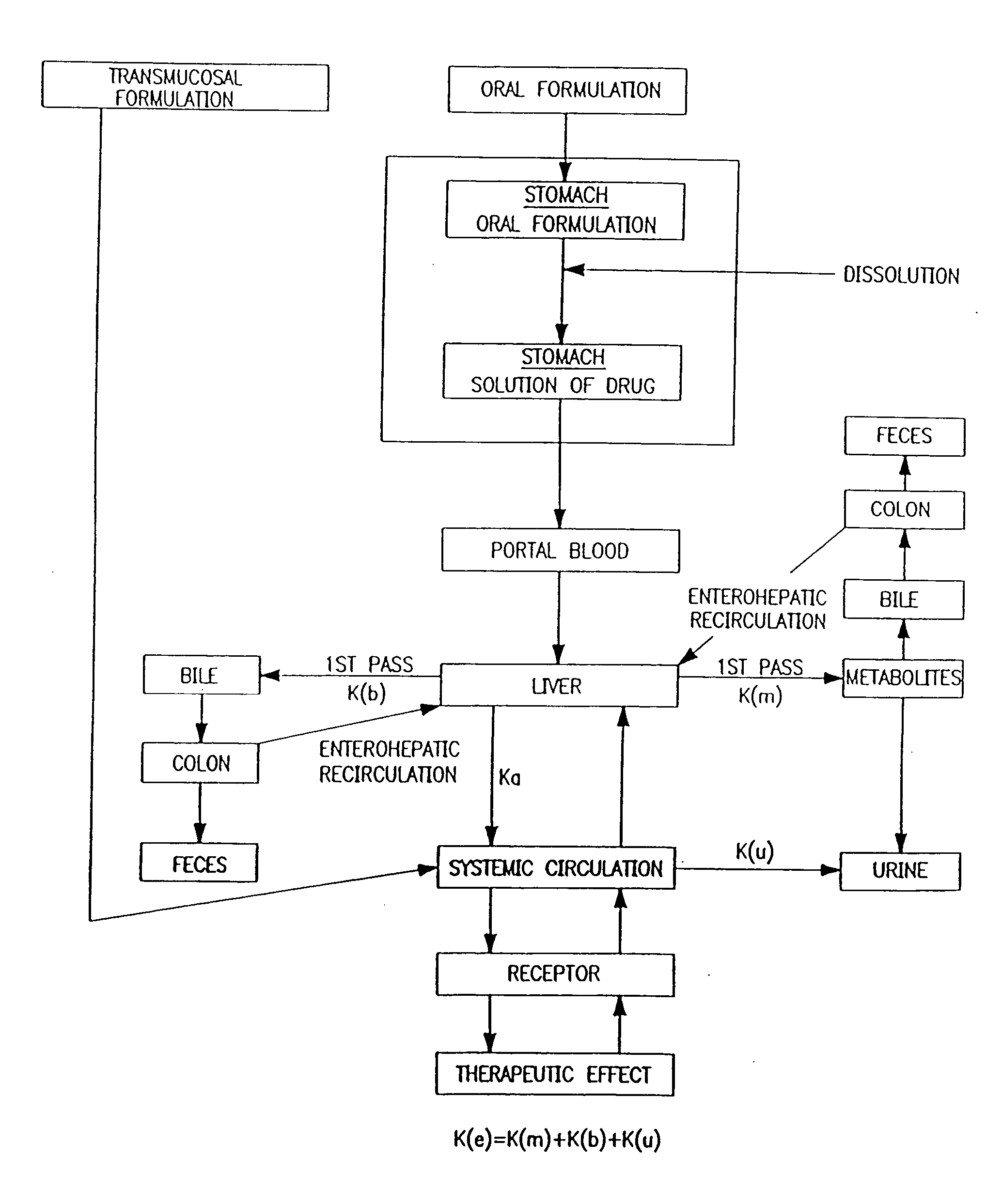
Transmucosal administration of drug compositions for treating and preventing disorders in animals
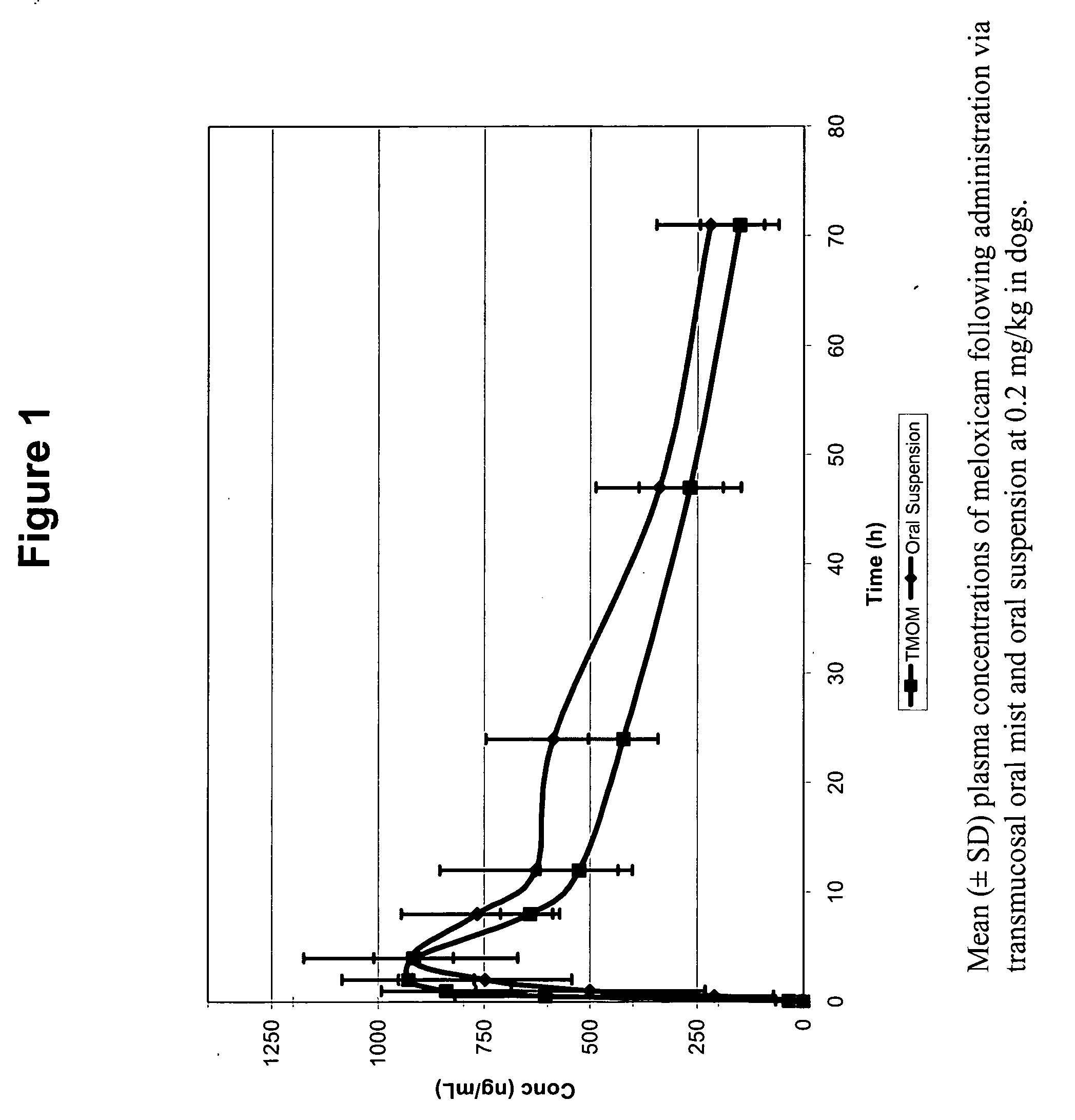
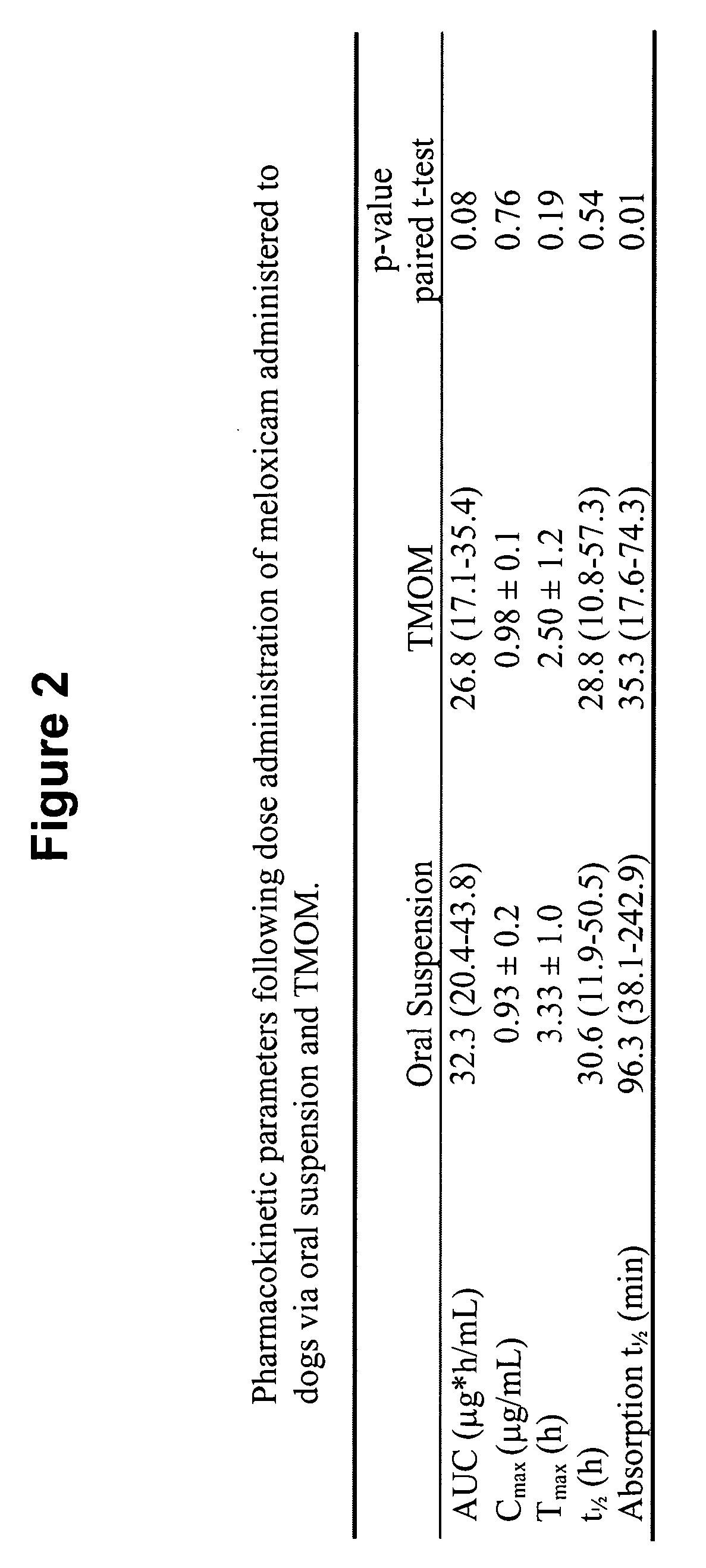
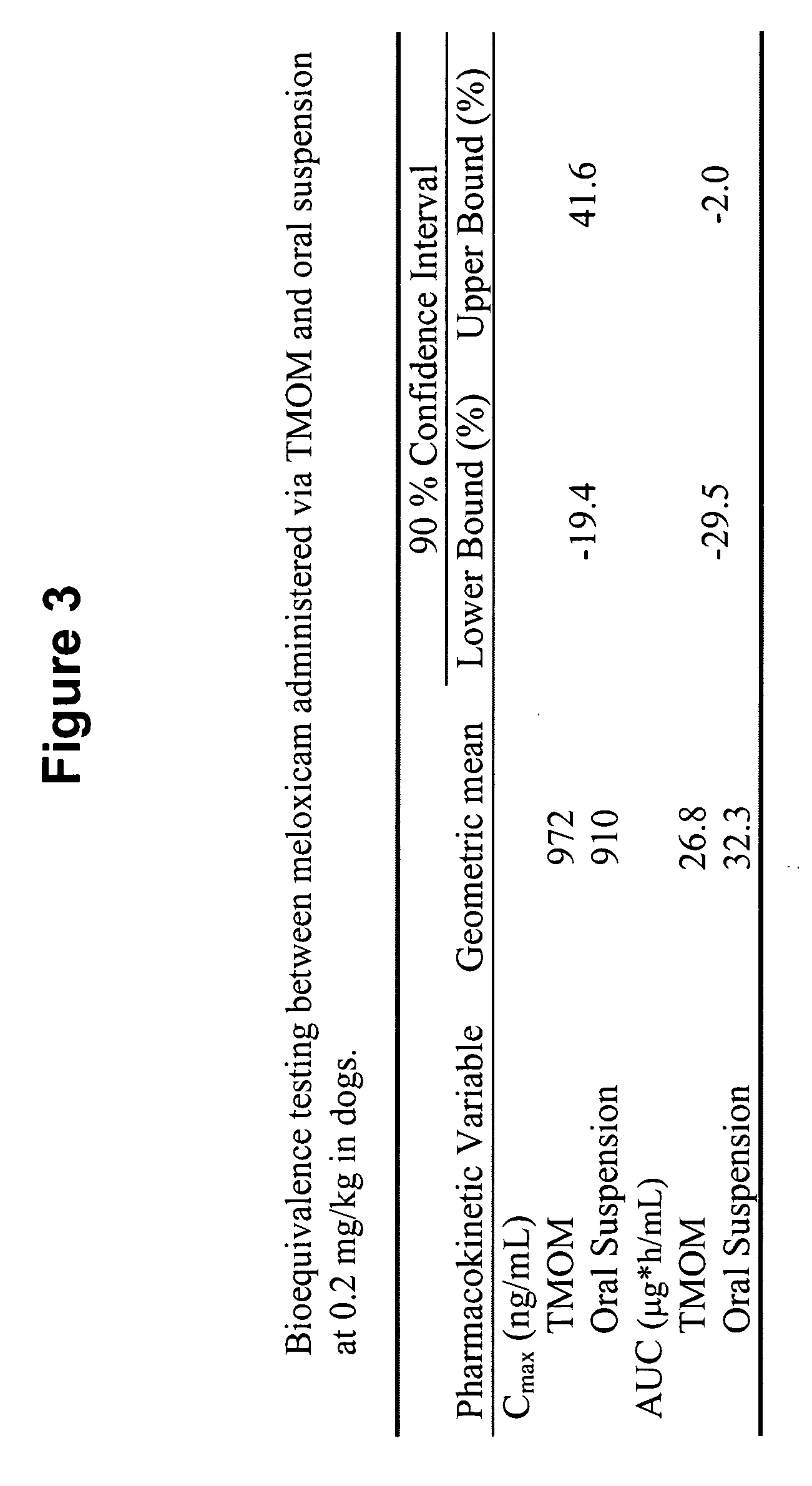
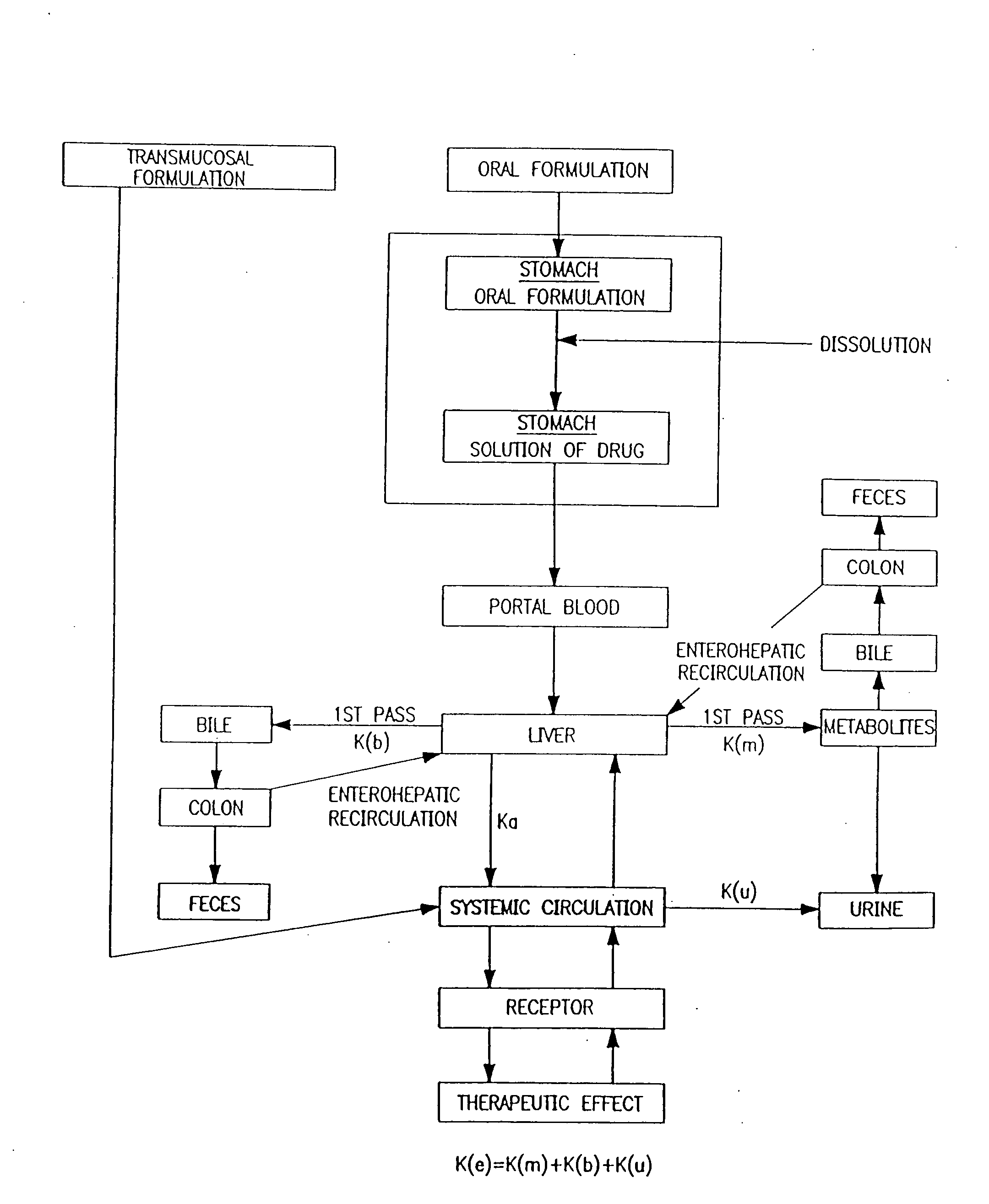
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Buccal, polar and non-polar spray containing zolpidem
InactiveUS20060216240A1Rapid onsetFast absorptionOrganic active ingredientsNervous disorderSolventFast onset
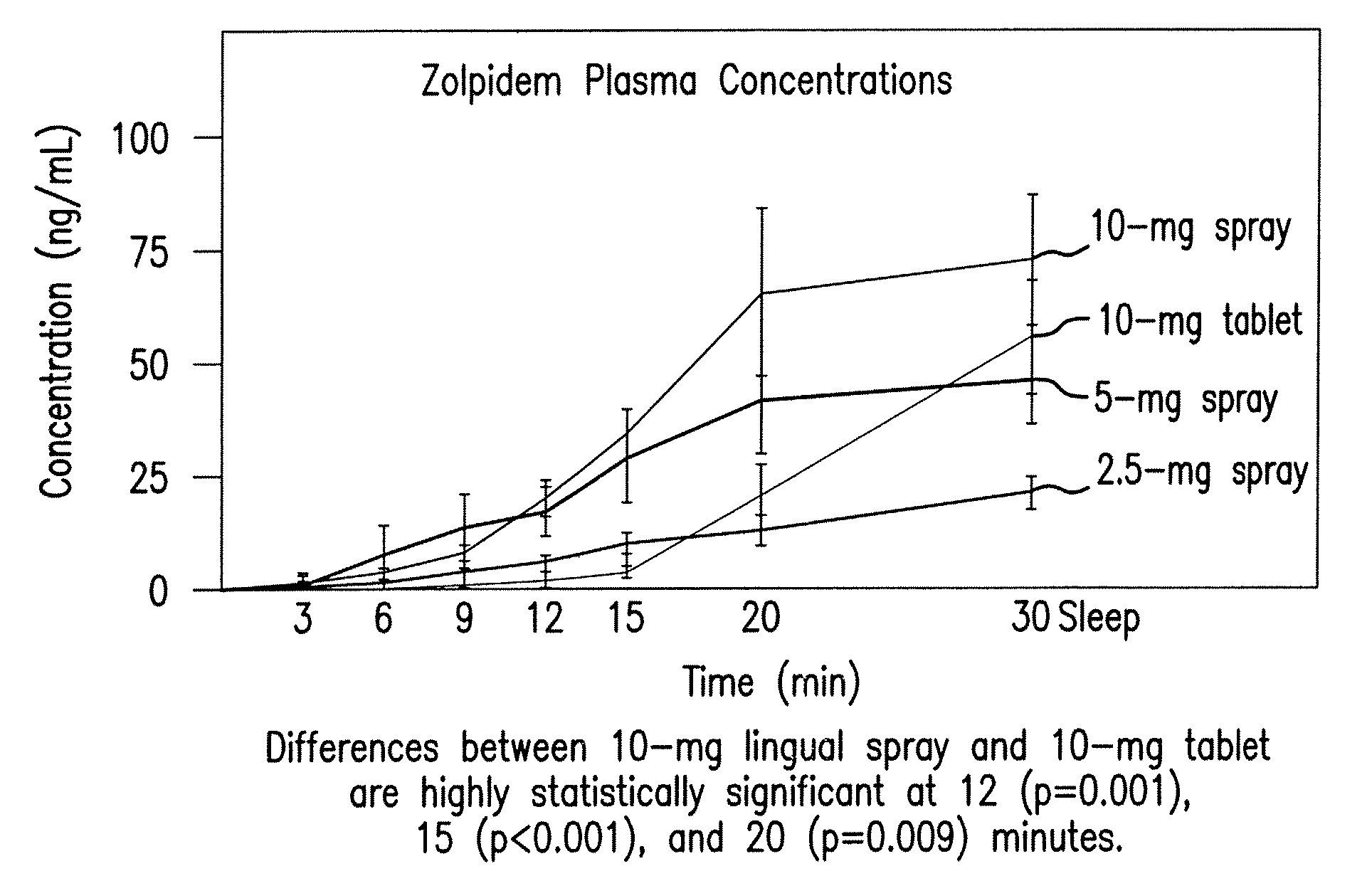
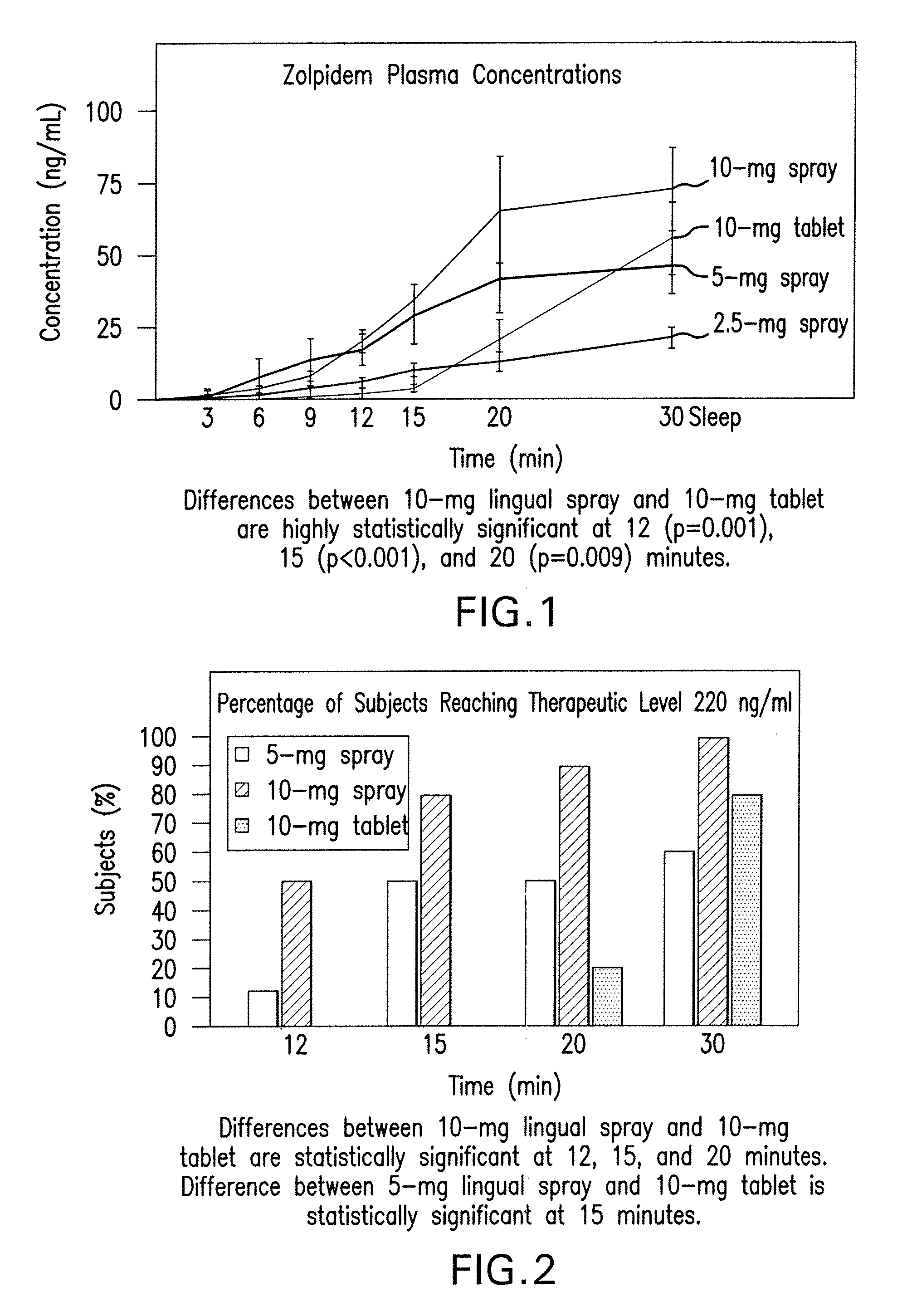
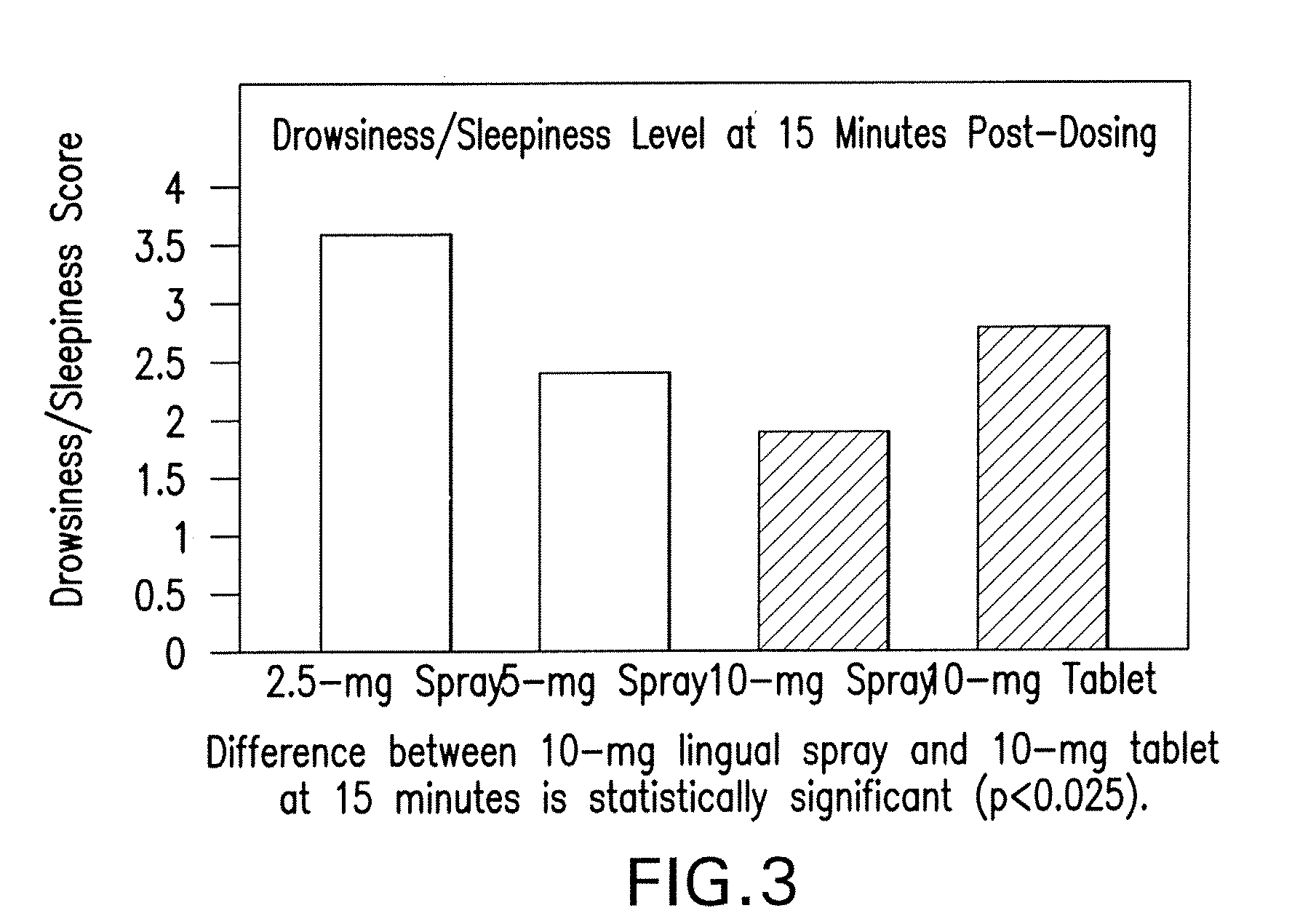
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide zolpidem for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, zolpidem, and optional flavoring agent; formulation II: aqueous polar solvent, zolpidem, optionally flavoring agent, and propellant; formulation III: non-polar solvent, zolpidem, and optional flavoring agent; formulation IV: non-polar solvent, zolpidem, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, zolpidem, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, zolpidem, optional flavoring agent, and propellant.
Owner:MAGNA PHARMA INC
Buccal, polar and non-polar spray containing zolpidem
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide zolpidem for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, zolpidem, and optional flavoring agent; formulation II: aqueous polar solvent, zolpidem, optionally flavoring agent, and propellant; formulation III: non-polar solvent, zolpidem, and optional flavoring agent; formulation IV: non-polar solvent, zolpidem, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, zolpidem, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, zolpidem, optional flavoring agent, and propellant.
Owner:MAGNA PHARMA INC
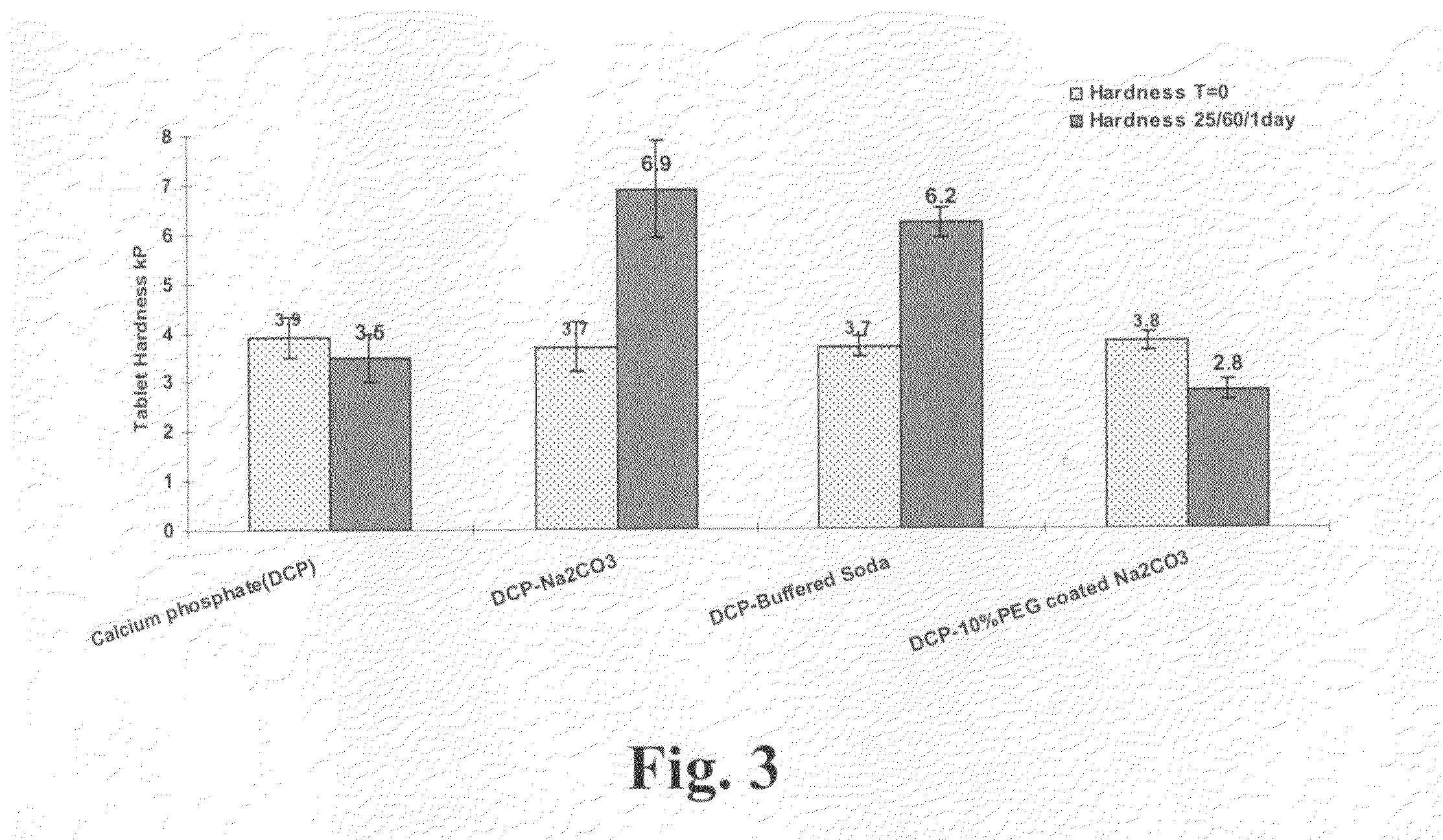
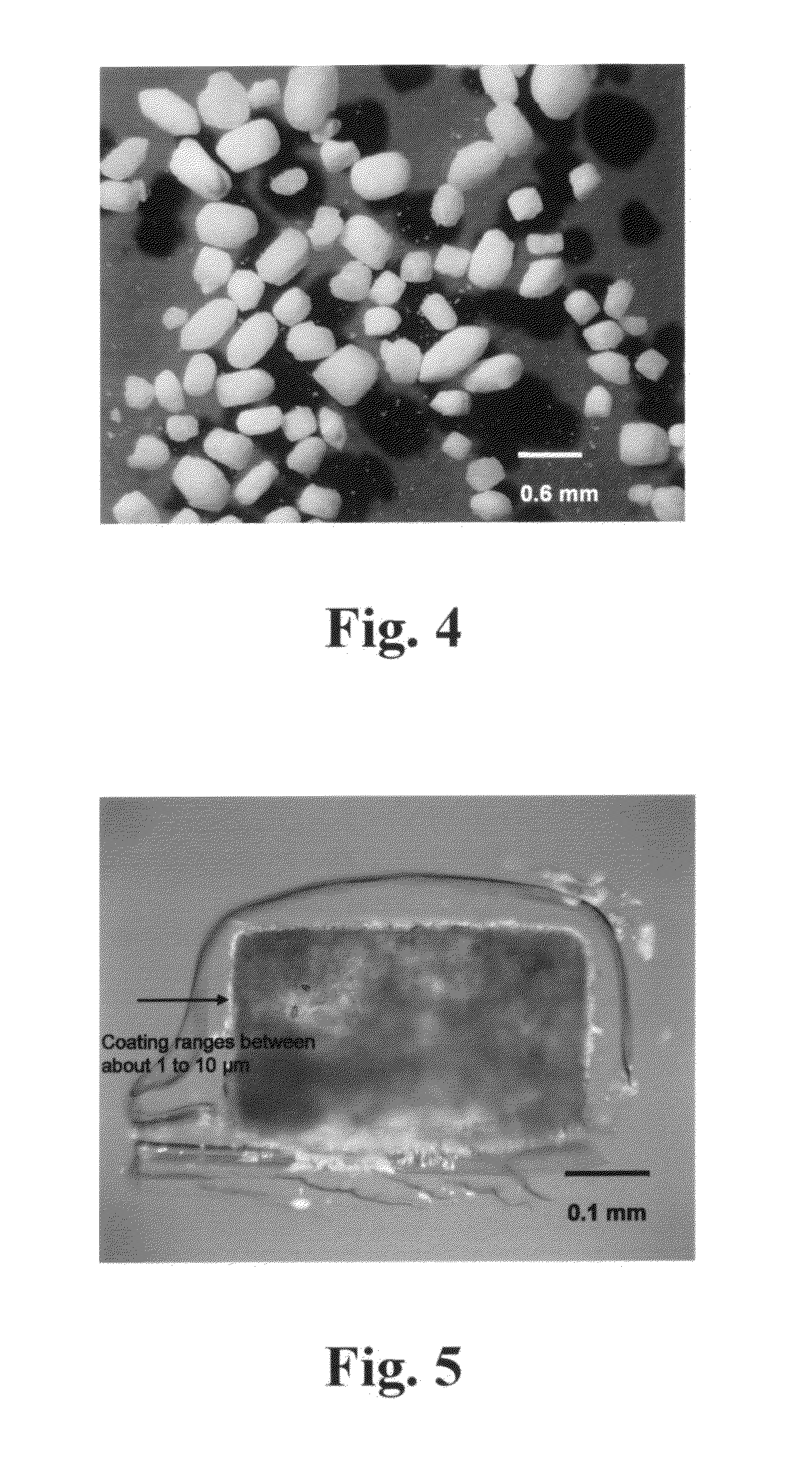
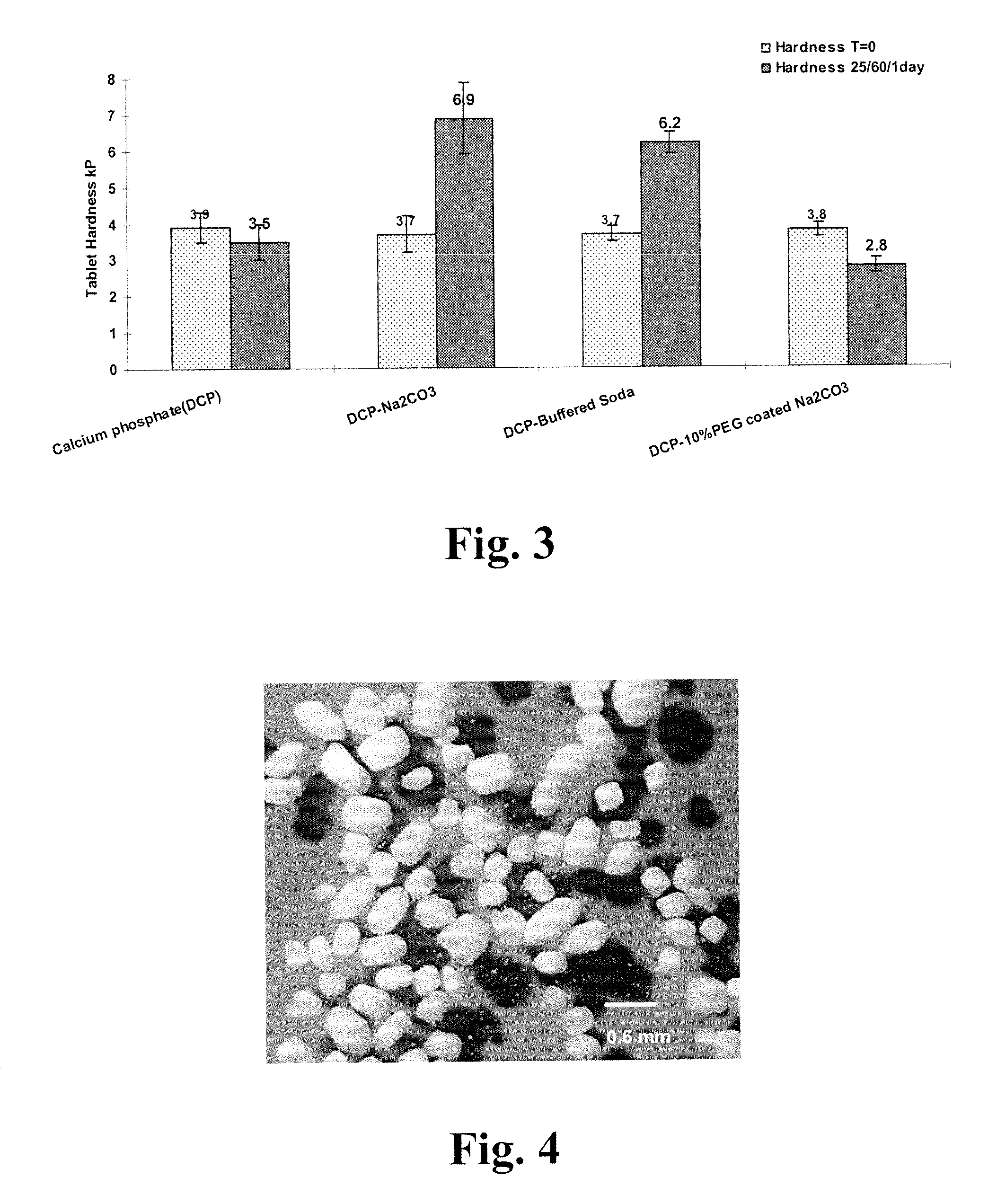
Polyethylene glycol-coated sodium carbonate as a pharmaceutical excipient and compositions produced from the same
Non-effervescent pharmaceutical compositions having at least one particle of carbonate coated by a layer of polyethylene glycol that substantially covers the at least one carbonate particle are described. Compositions are also described where the compositions include a weakly basic therapeutic agent, a first pH-modifying agent having at least one particle of carbonate coated by a layer of polyethylene glycol, and a second pH-modifying agent. The weakly basic therapeutic agent could be, but is not limited to, zolpidem or scopolamine. Compositions including zolpidem and scopolamine are used to treat insomnia and depression, respectively.
Owner:TRANSCEPT PHARMA
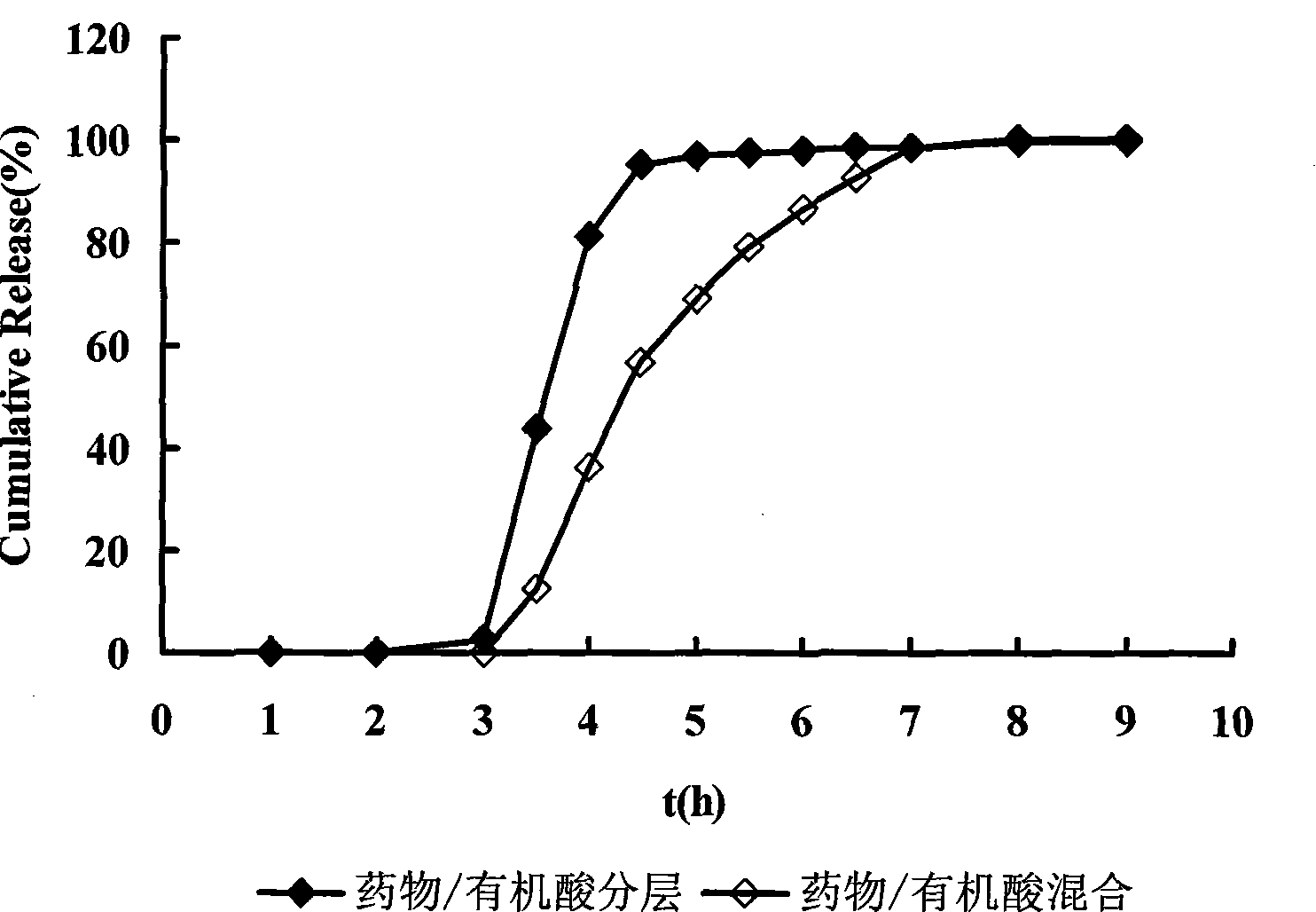
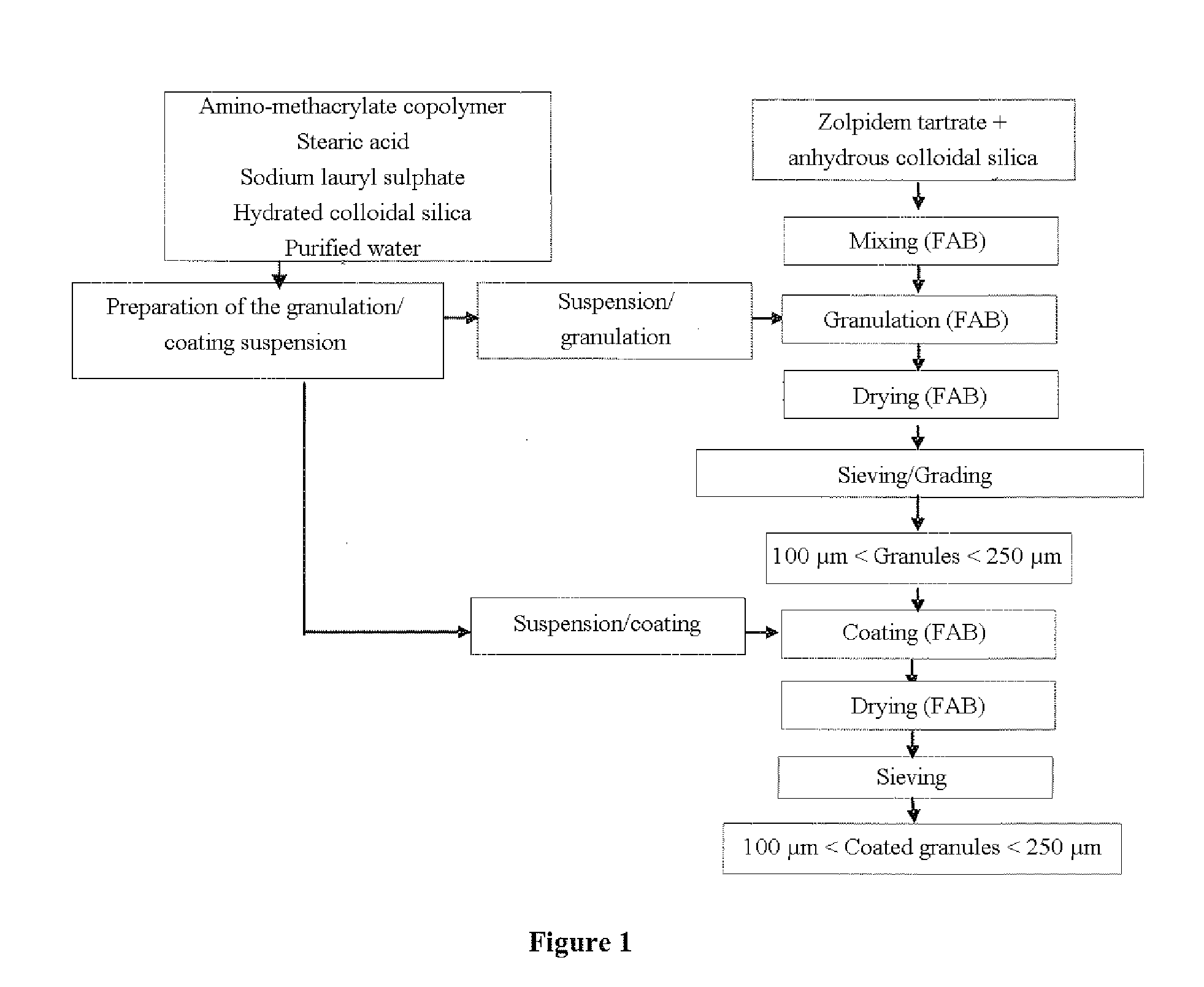
Timing pulsed release micro-pill of zolpidem salt
InactiveCN101574328ARegulated releaseIncrease elasticityOrganic active ingredientsNervous disorderTime lagGlutaric acid
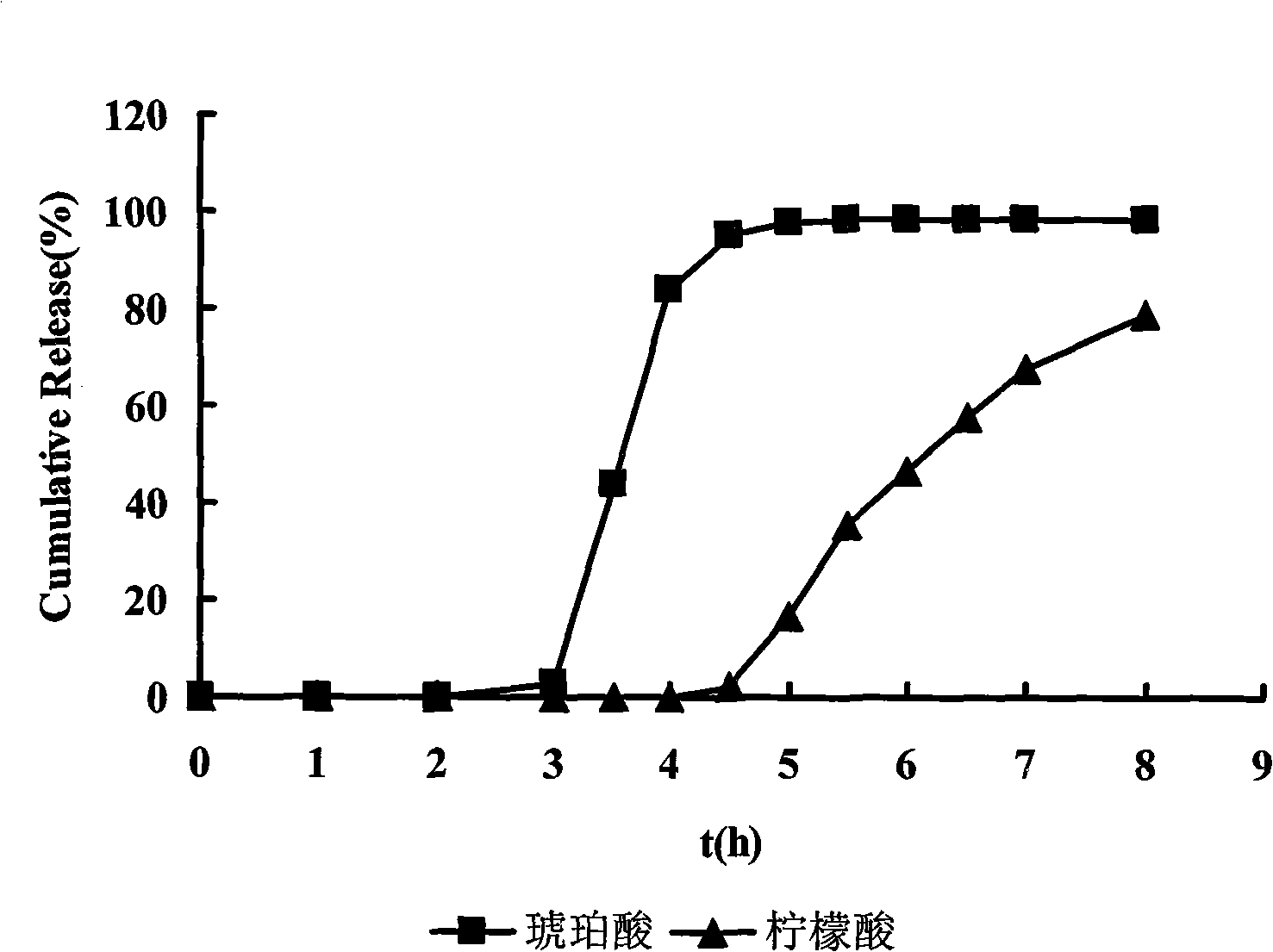
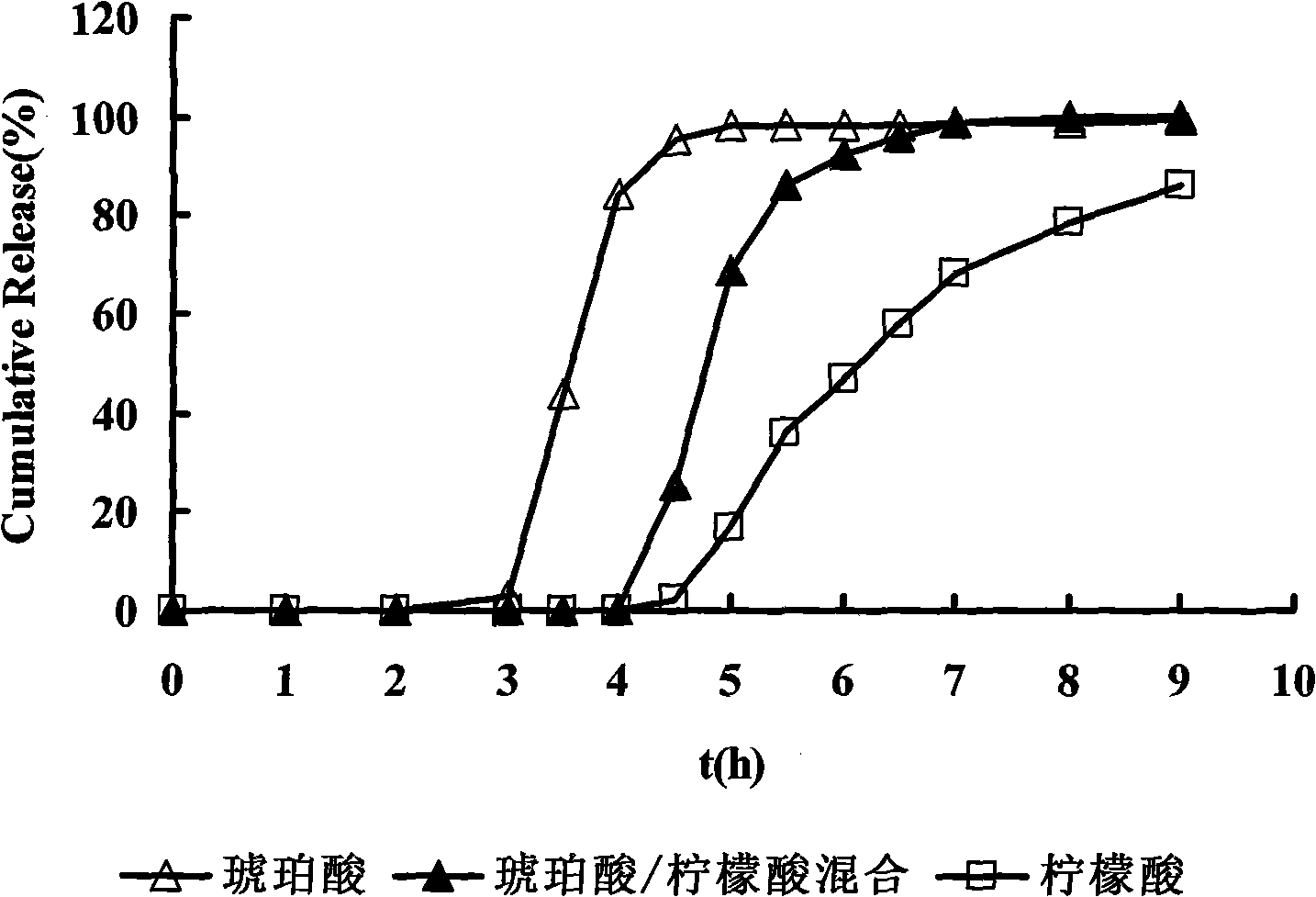
The invention relates to the field of pharmaceutical preparations, in particular to a timing pulsed release micro-pill of a zolpidem salt, which is characterized in that the center of the micro-pill is a blank pill core, an external time-lag layer is coated by low-permeability acrylic resin containing quaternary ammonium salt radicals, and a medicament layer and an organic acid layer are arranged between the blank pill core and the time-lag layer, wherein the organic acid layer contains a mixture of two organic acids, and the mixture of the two organic acids is prepared by mixing one of succinic acid, glutaric acid and adipic acid, and one of citric acid, malic acid and tartaric acid. Combining with sleep characteristics of early awakening crowds, the prepared micro-pill has certain release time lag and releases quickly after a period of time lag so that a patient obtains effective blood concentration on the point of awakening so as to prolong the sleep time of the patient.
Owner:CHINA PHARM UNIV
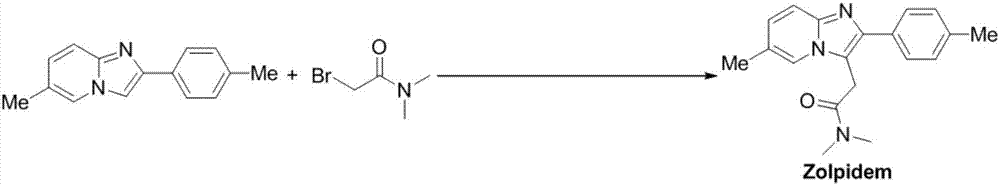
Process for preparing zolpidem
InactiveUS20070027180A1Rapid onsetEliminate the problemBiocideOrganic chemistryZolpidemPolymer science
Owner:DR REDDYS LAB LTD
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Compositions and methods for treating middle-of-the night insomnia
Owner:TRANSCEPT PHARMA
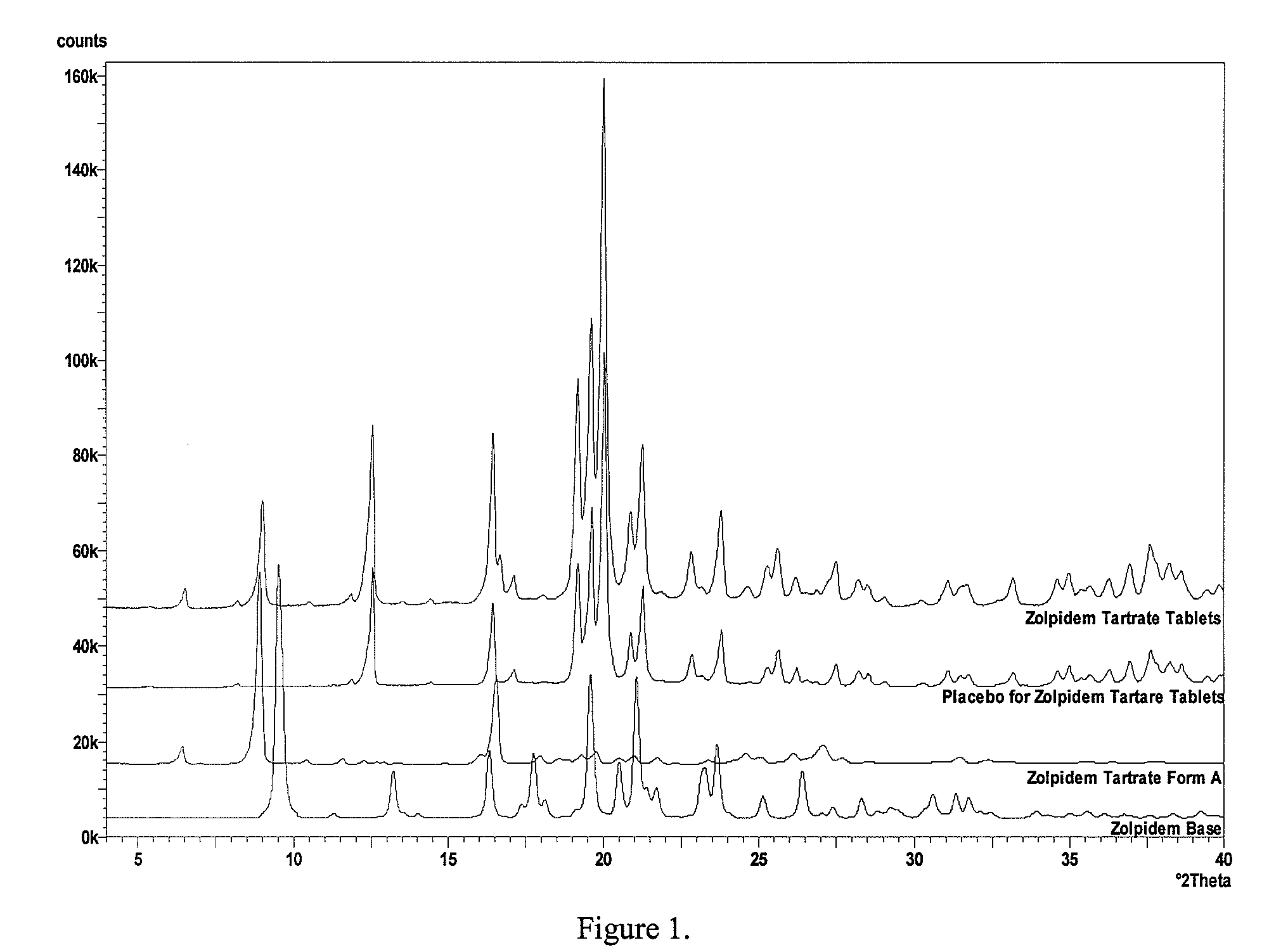
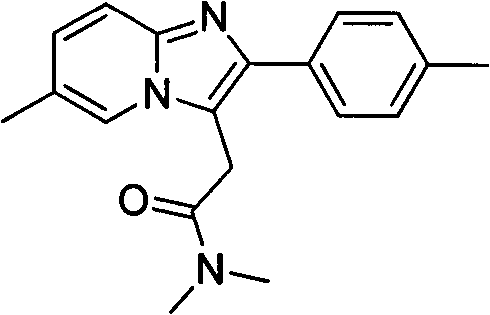
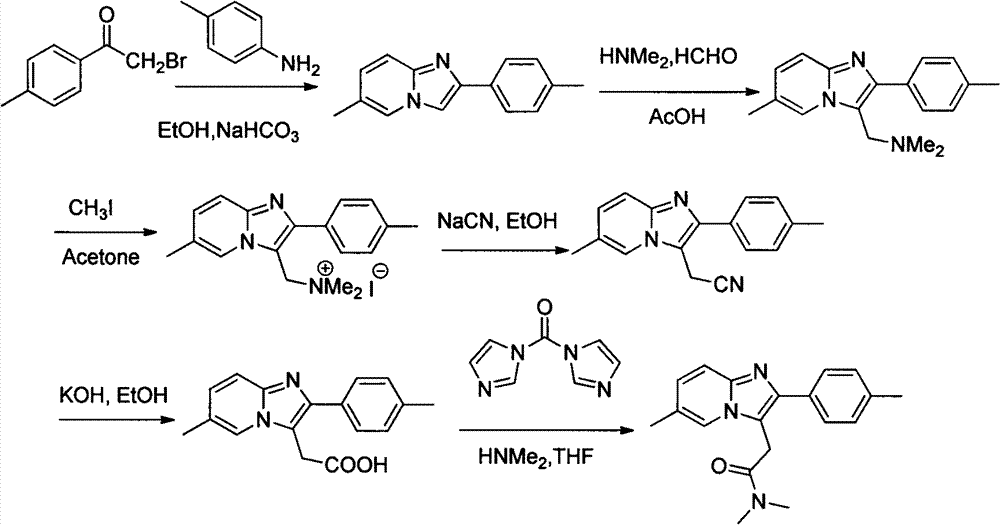
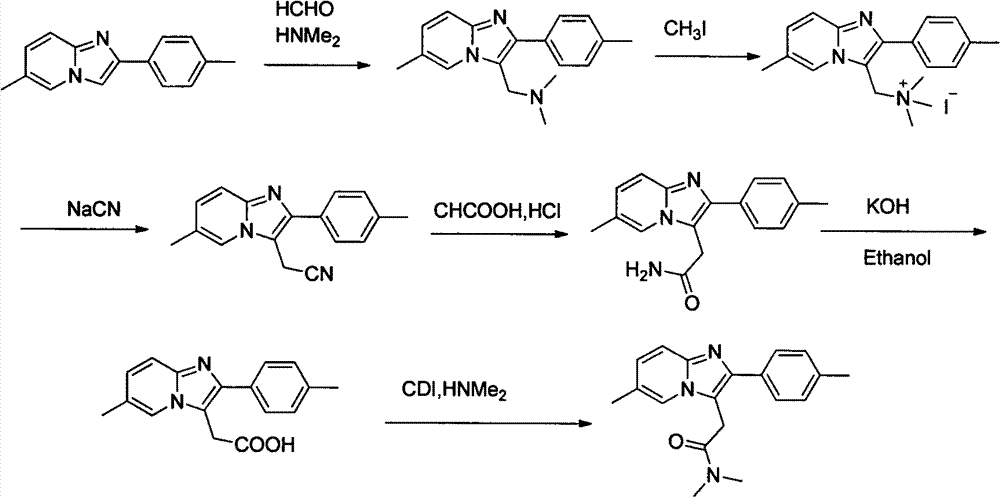
Processes for the Preparation of Zolpidem and its Hemitartrate
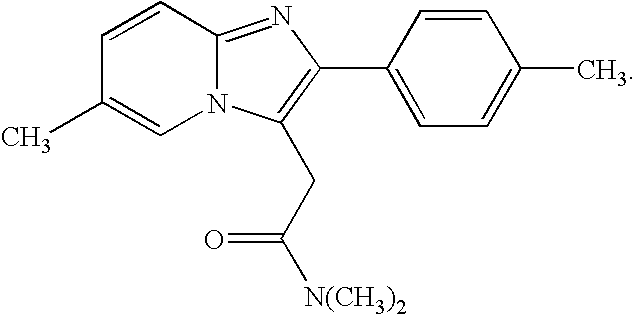
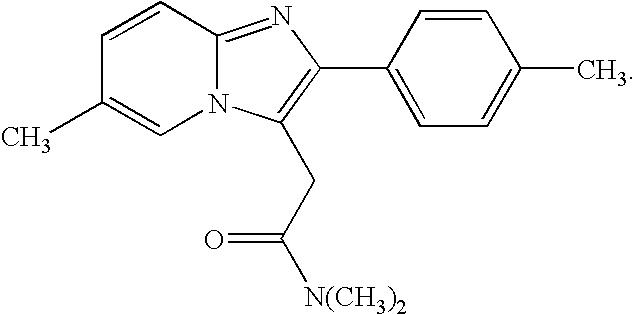
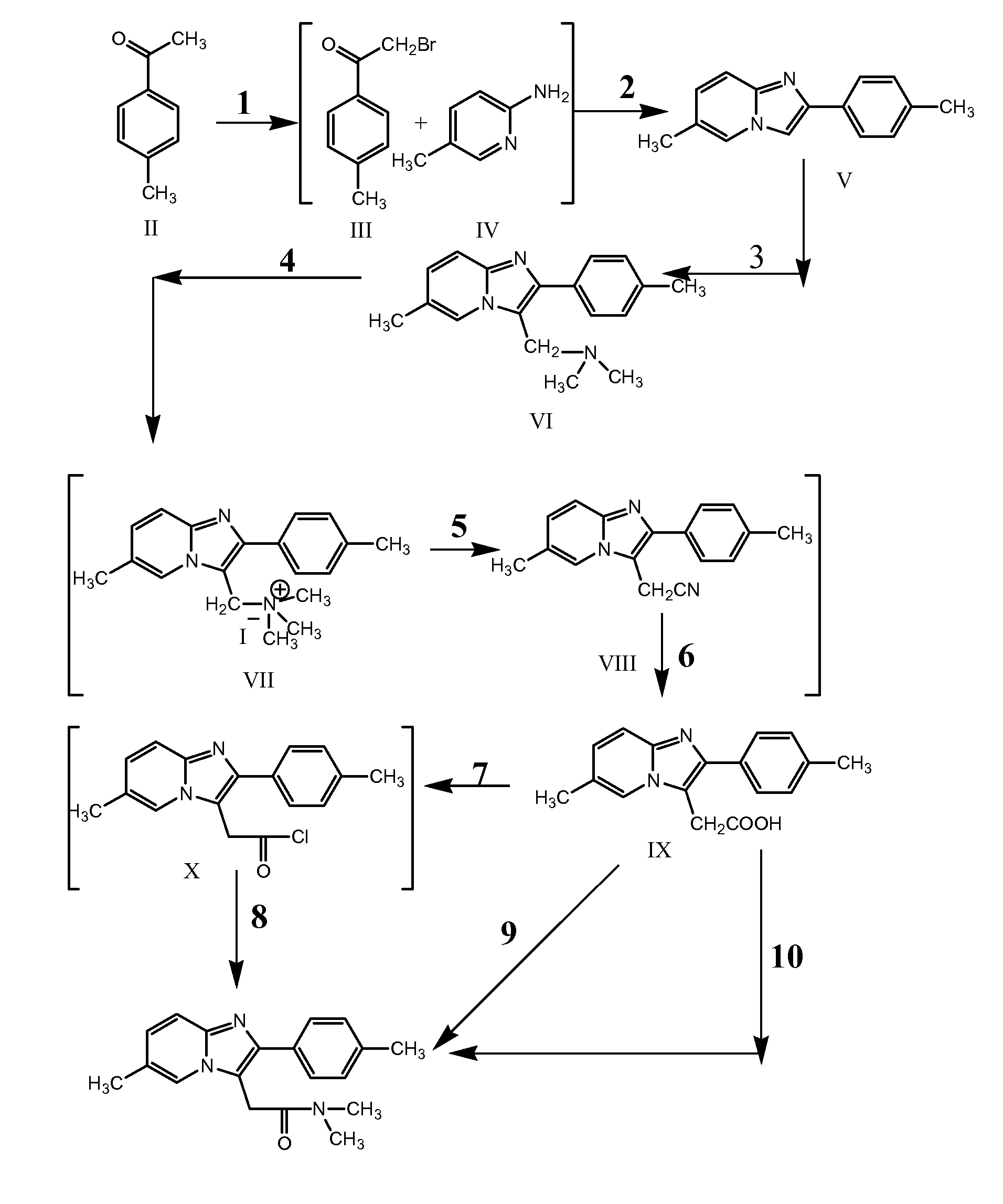
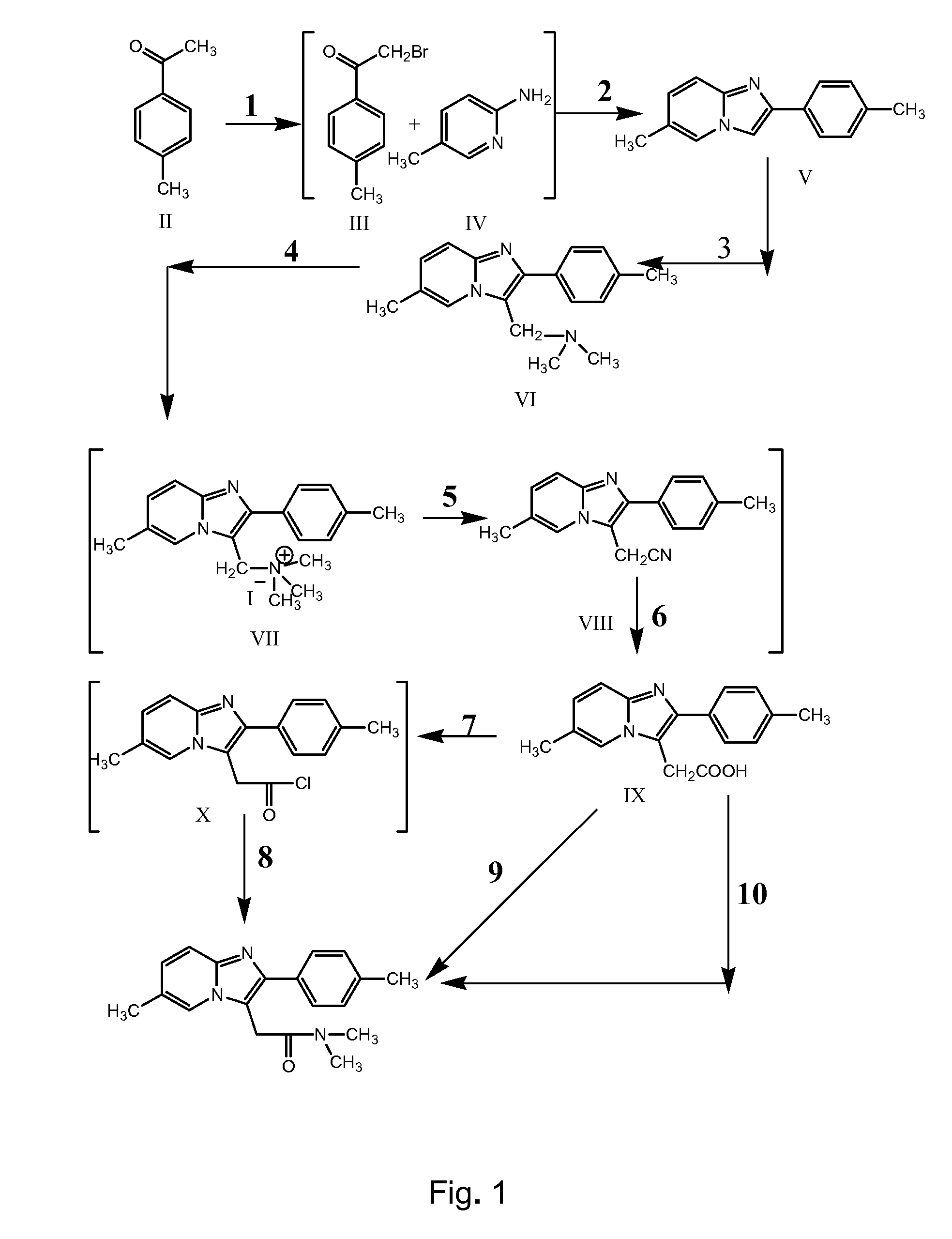
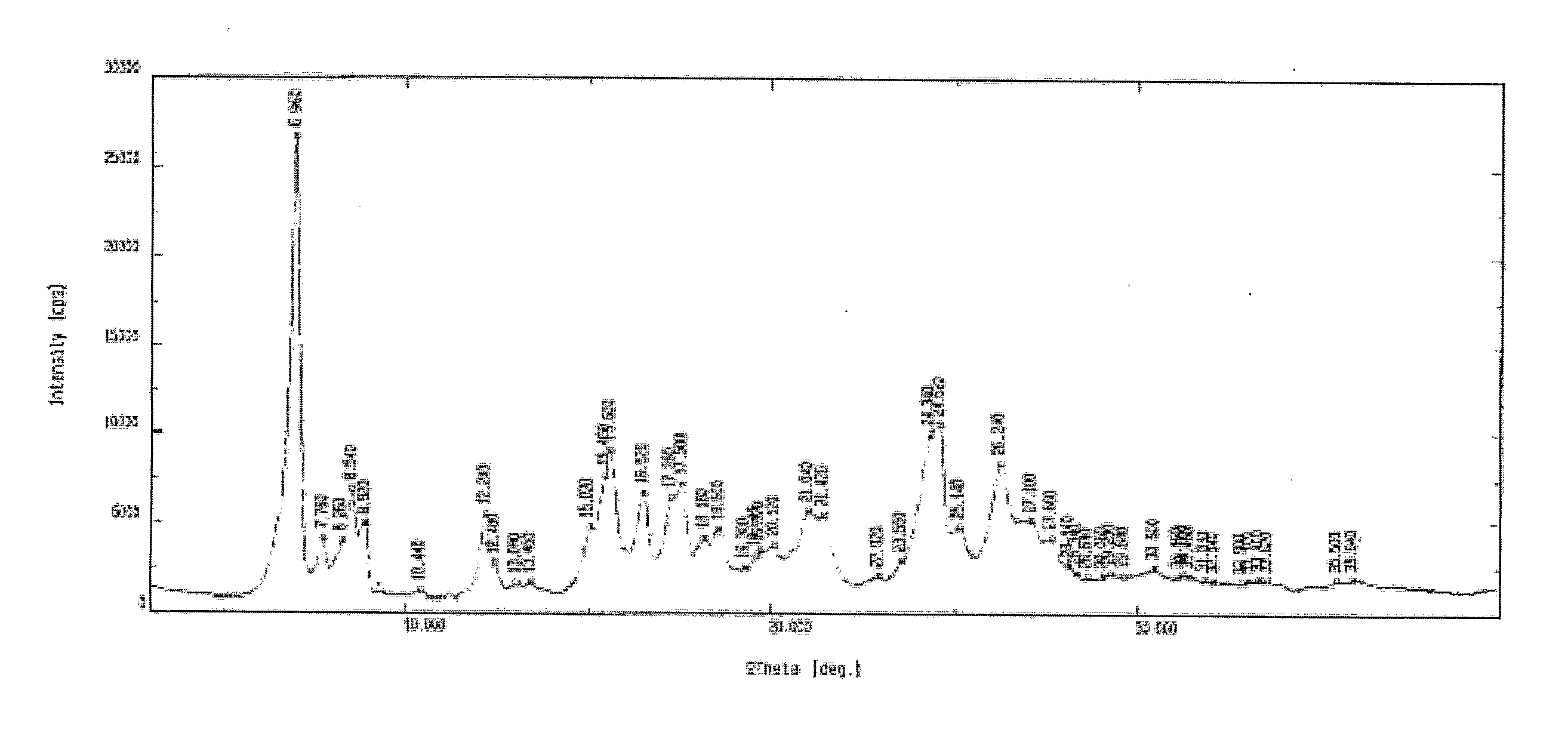
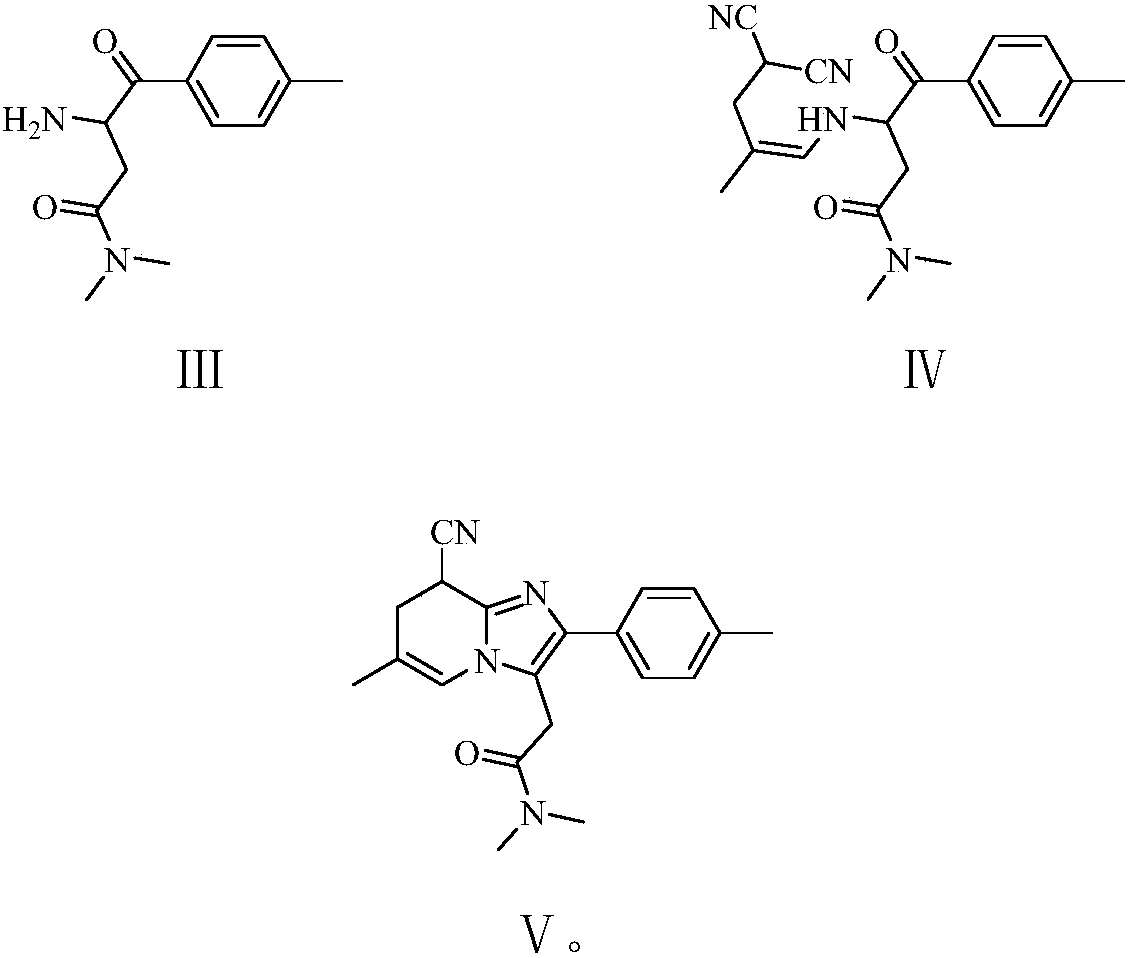
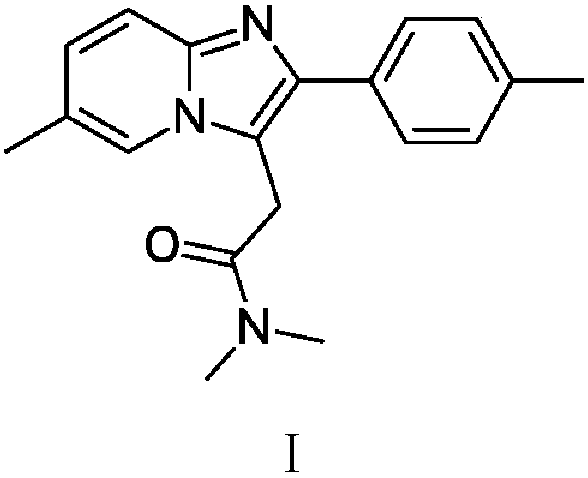
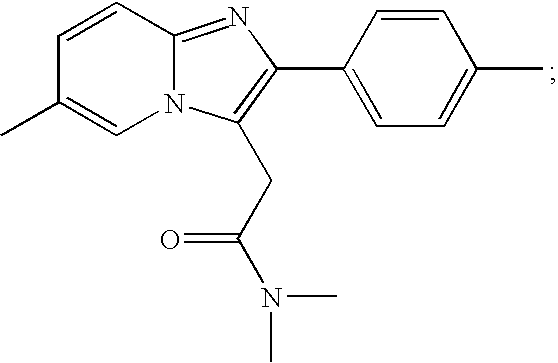
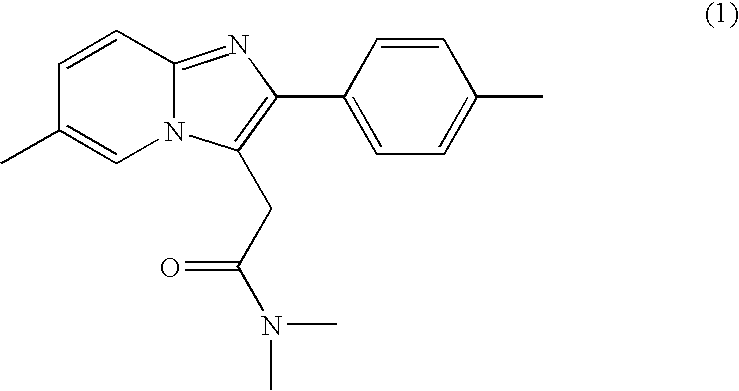
The invention relates to the preparation of a non-hygroscopic polymorphic form of zolpidem hemitartrate, designated as Form I, and pharmaceutical compositions including it. The invention also relates to use of the compositions for treating anxiety, sleep disorders and convulsions. The invention also relates to a process for the preparation of zolpidem or pharmaceutically acceptable salts thereof by condensing 3-bromo-N,N-dimethyl-4-oxo-4-p-tolyl-butyramide with 2-amino-5-methylpyridine in a polar aprotic solvent.
Owner:RANBAXY LAB LTD
Anti-insomnia compositions and methods
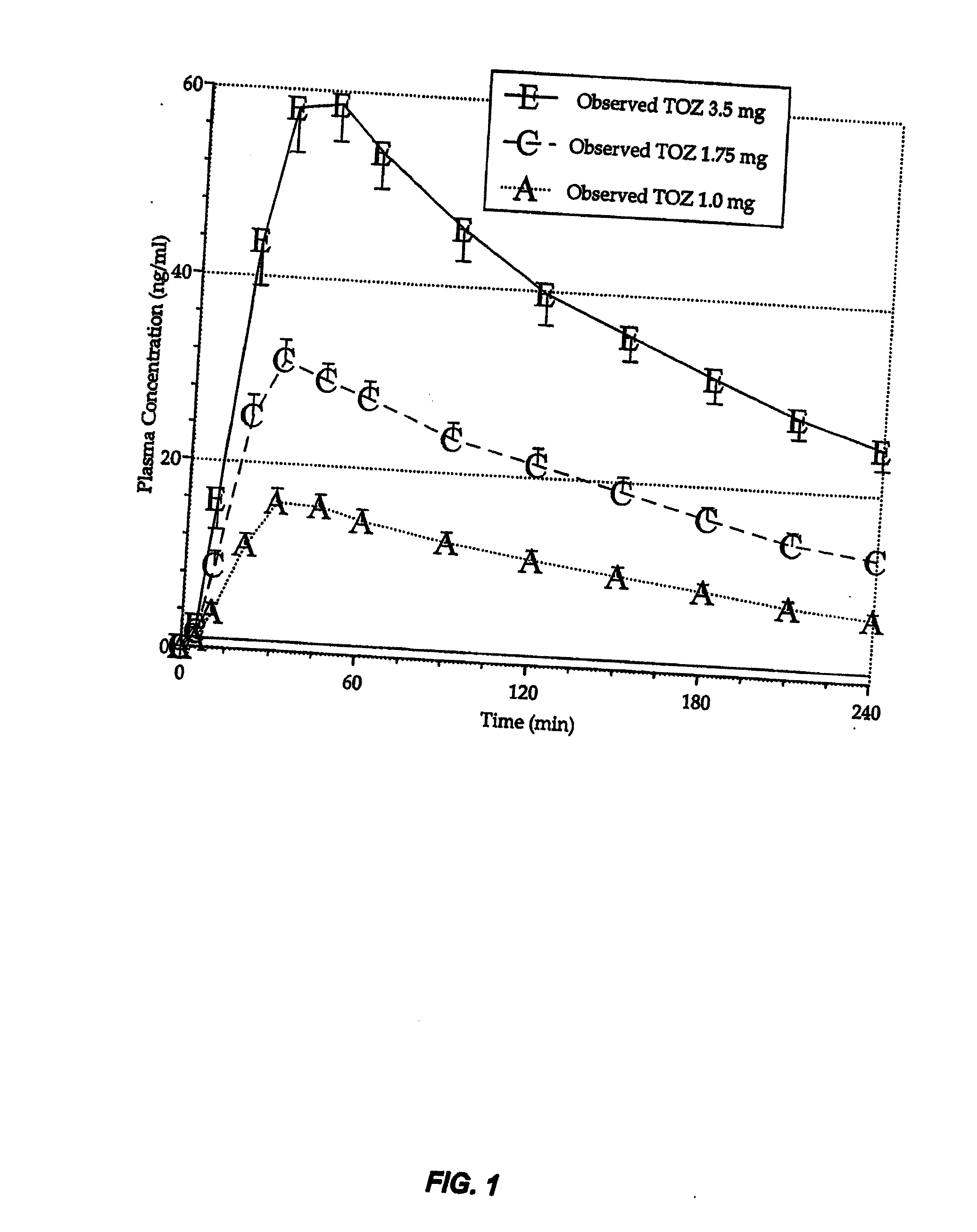
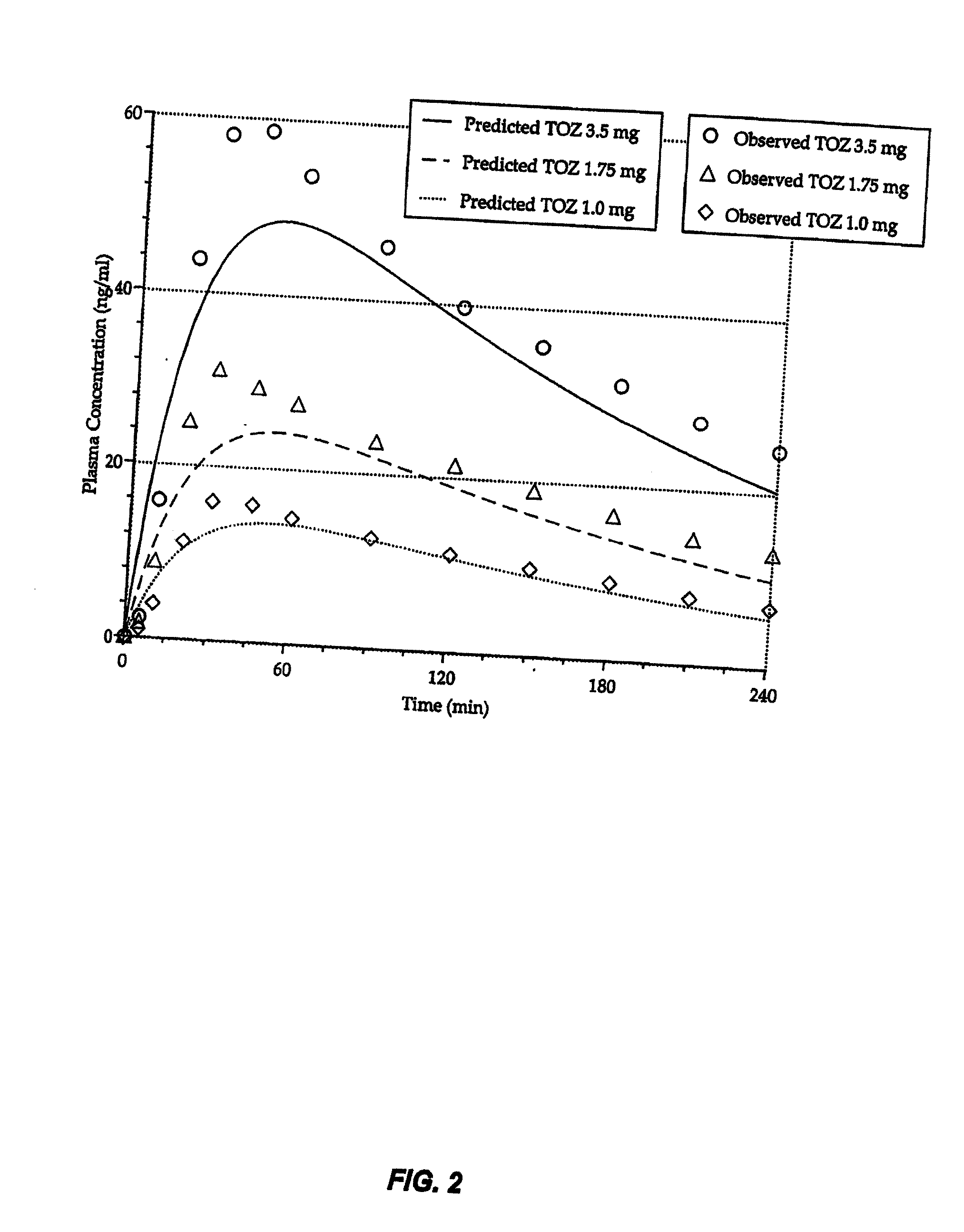
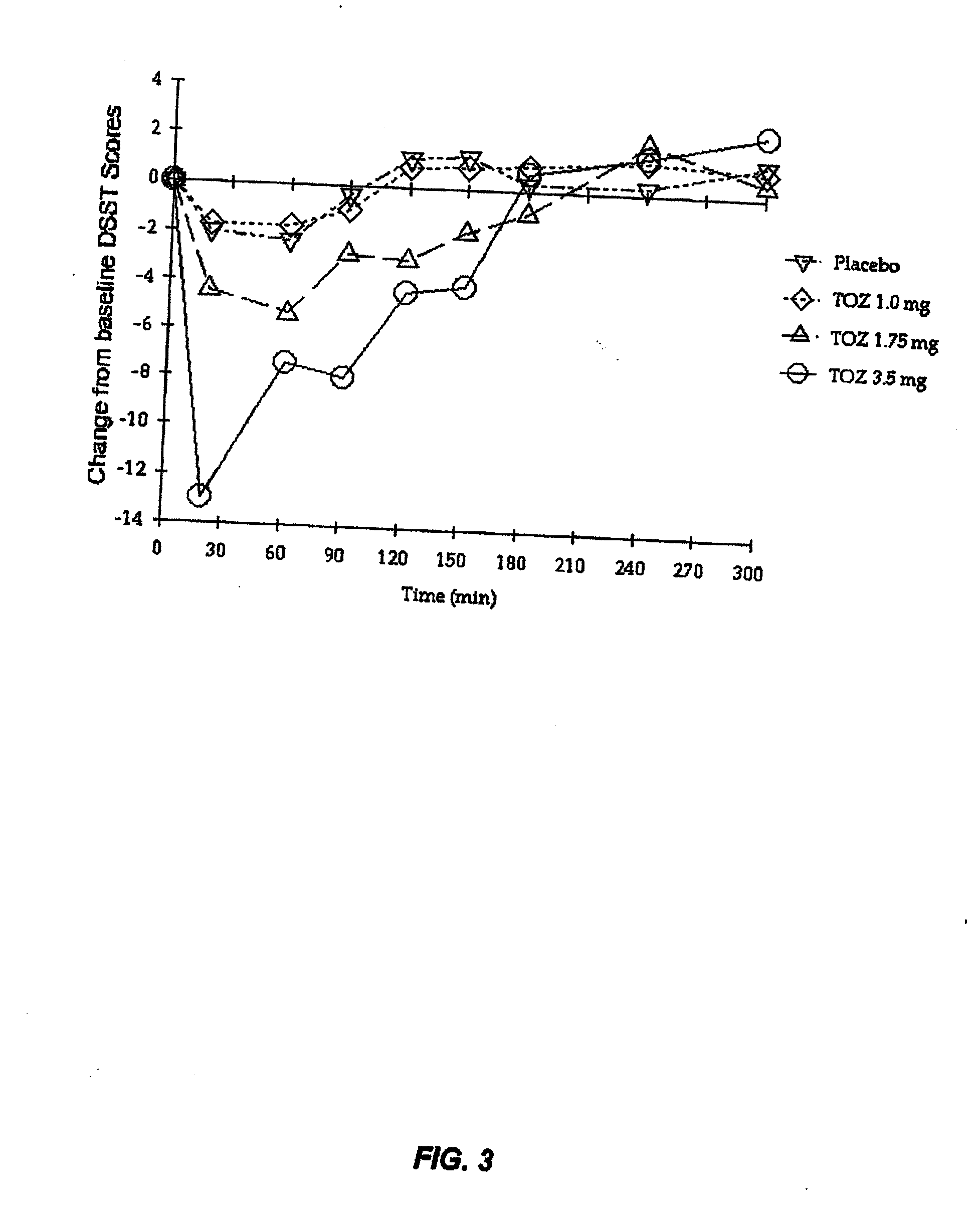
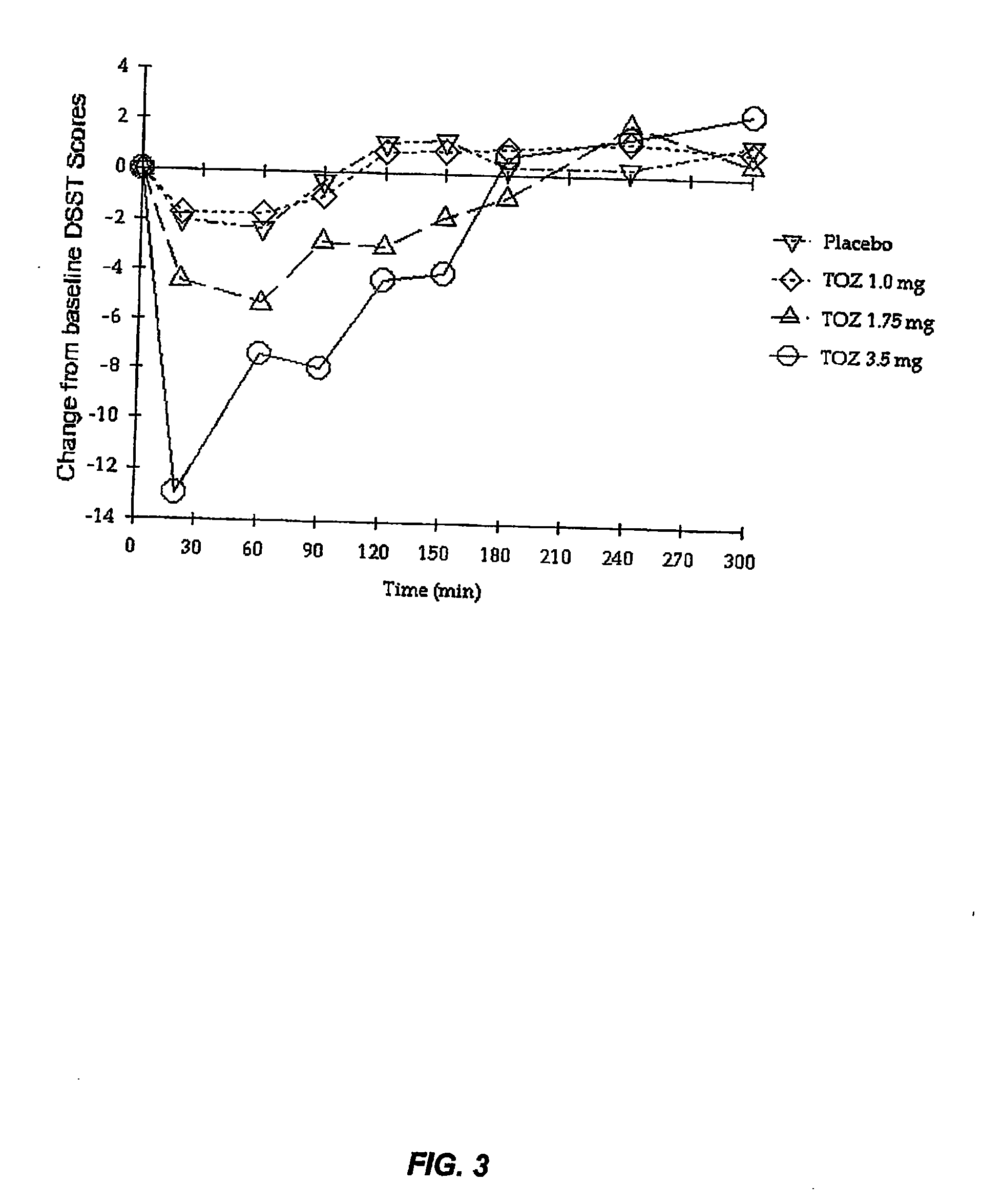
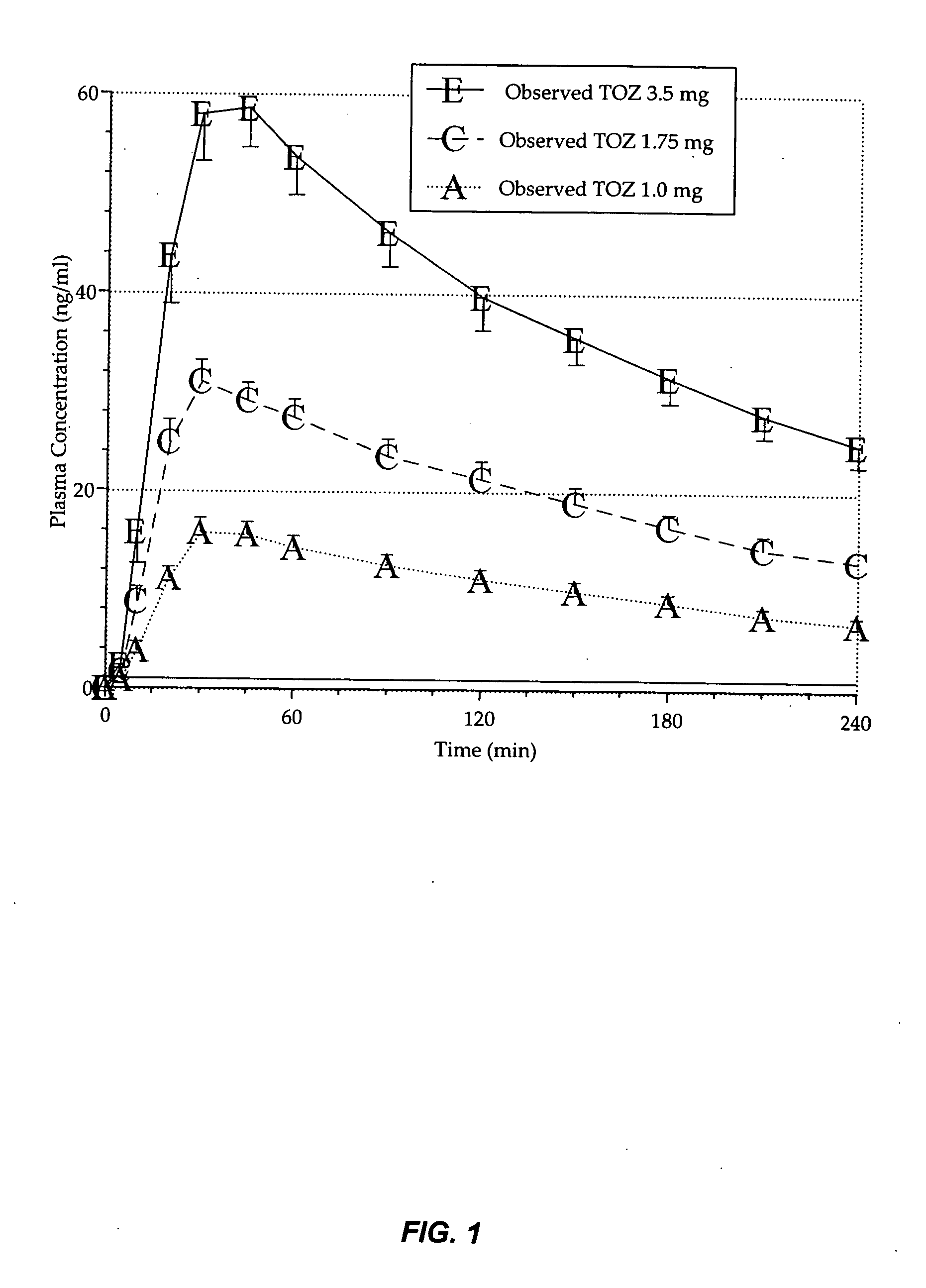
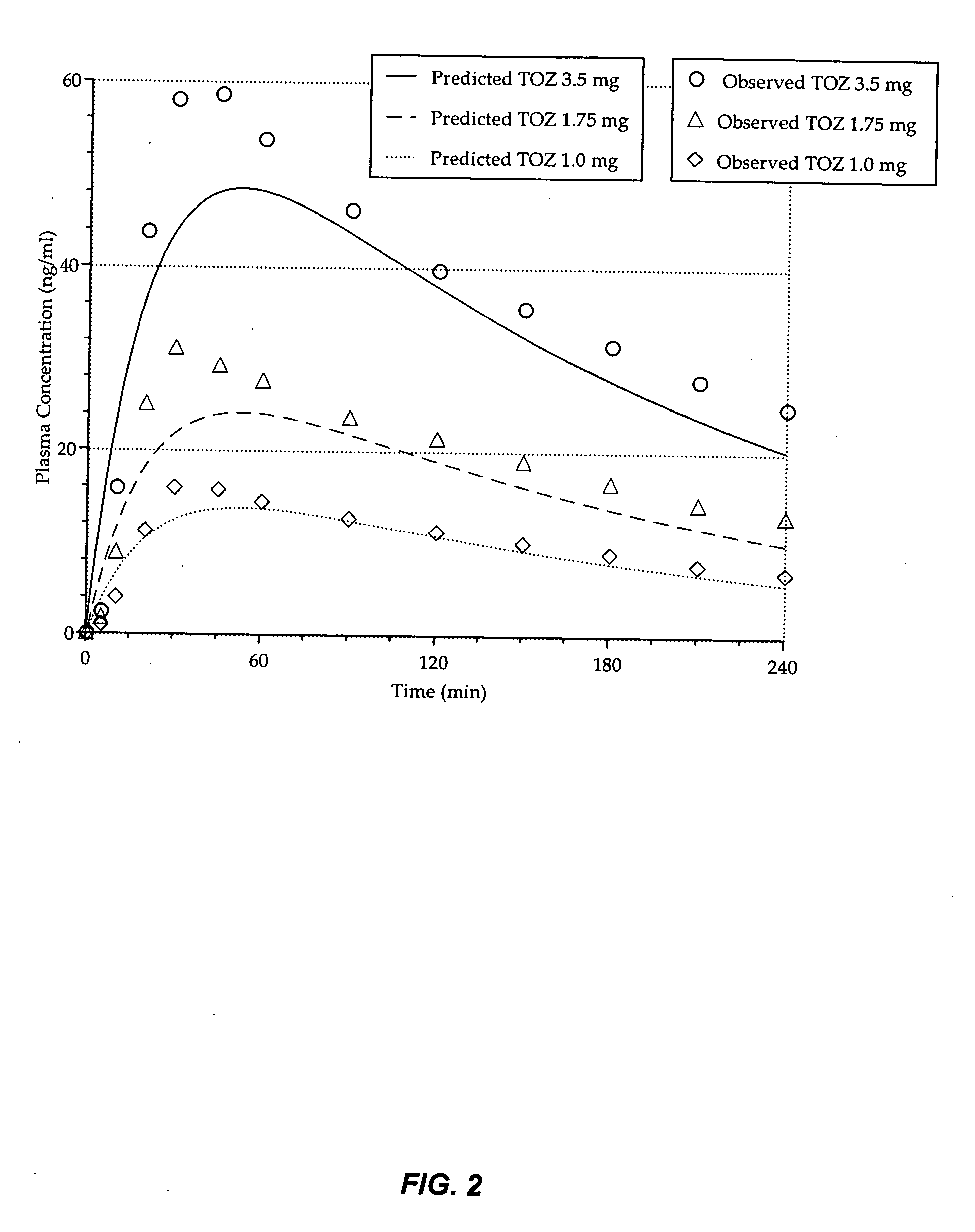
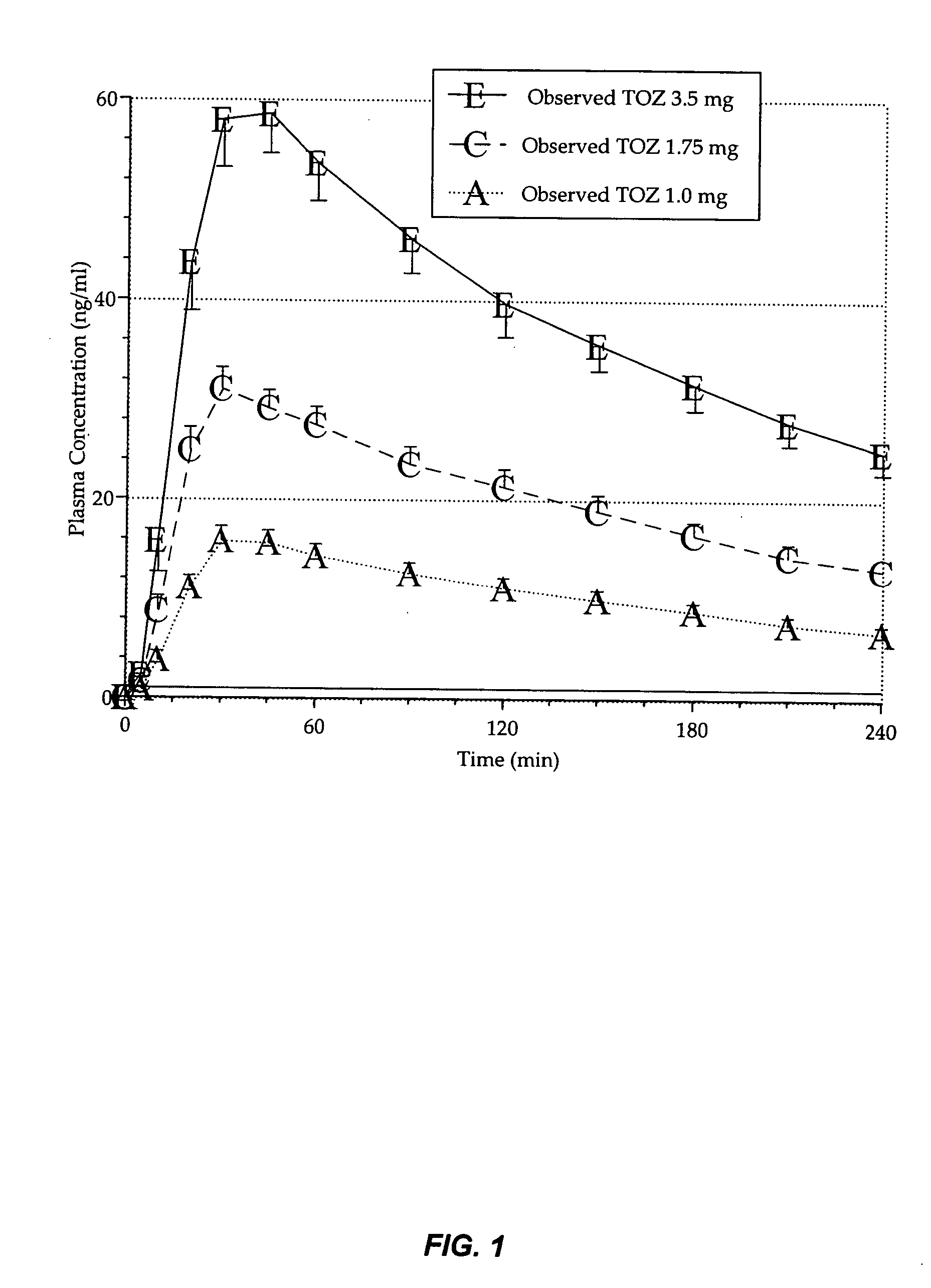
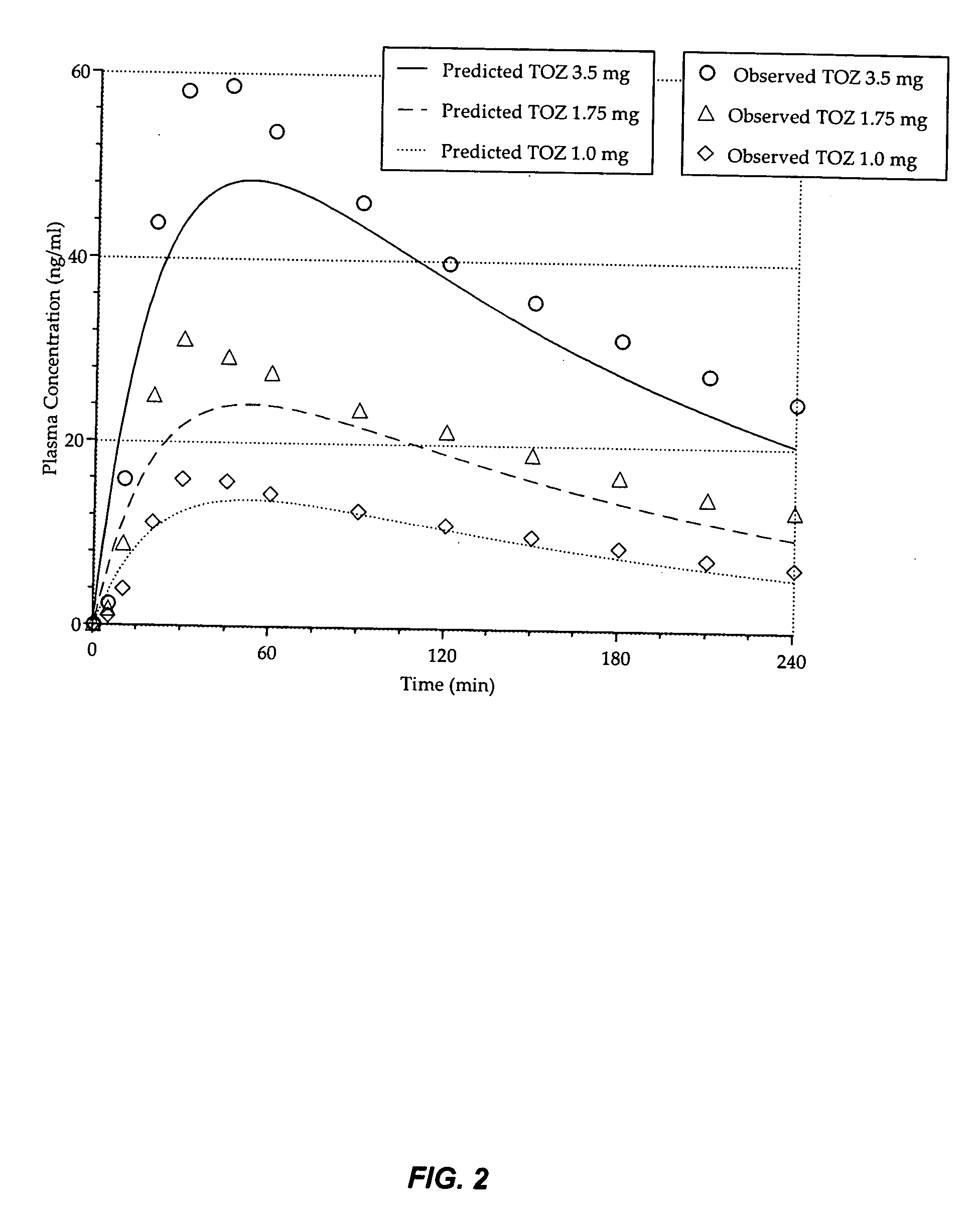
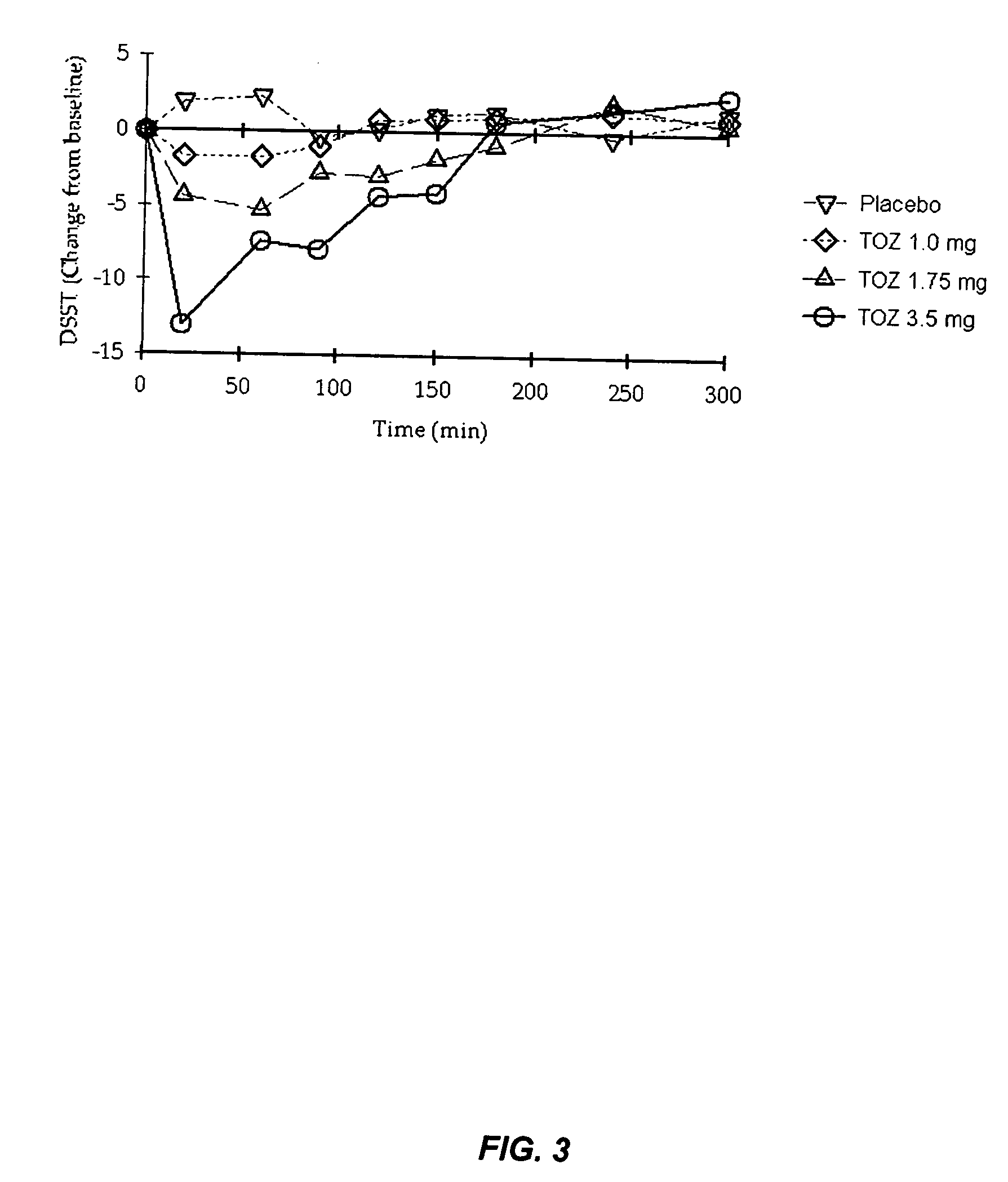
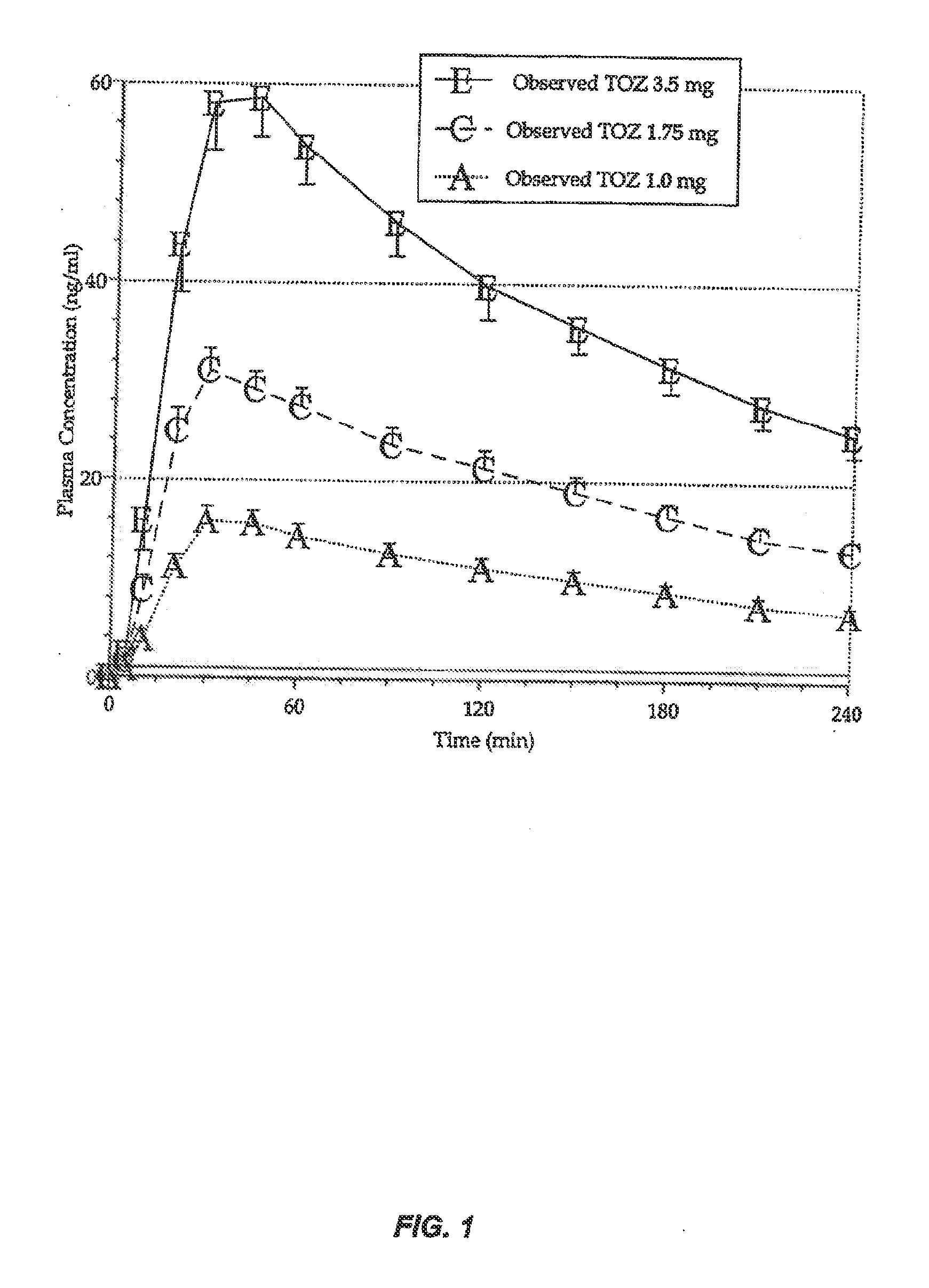
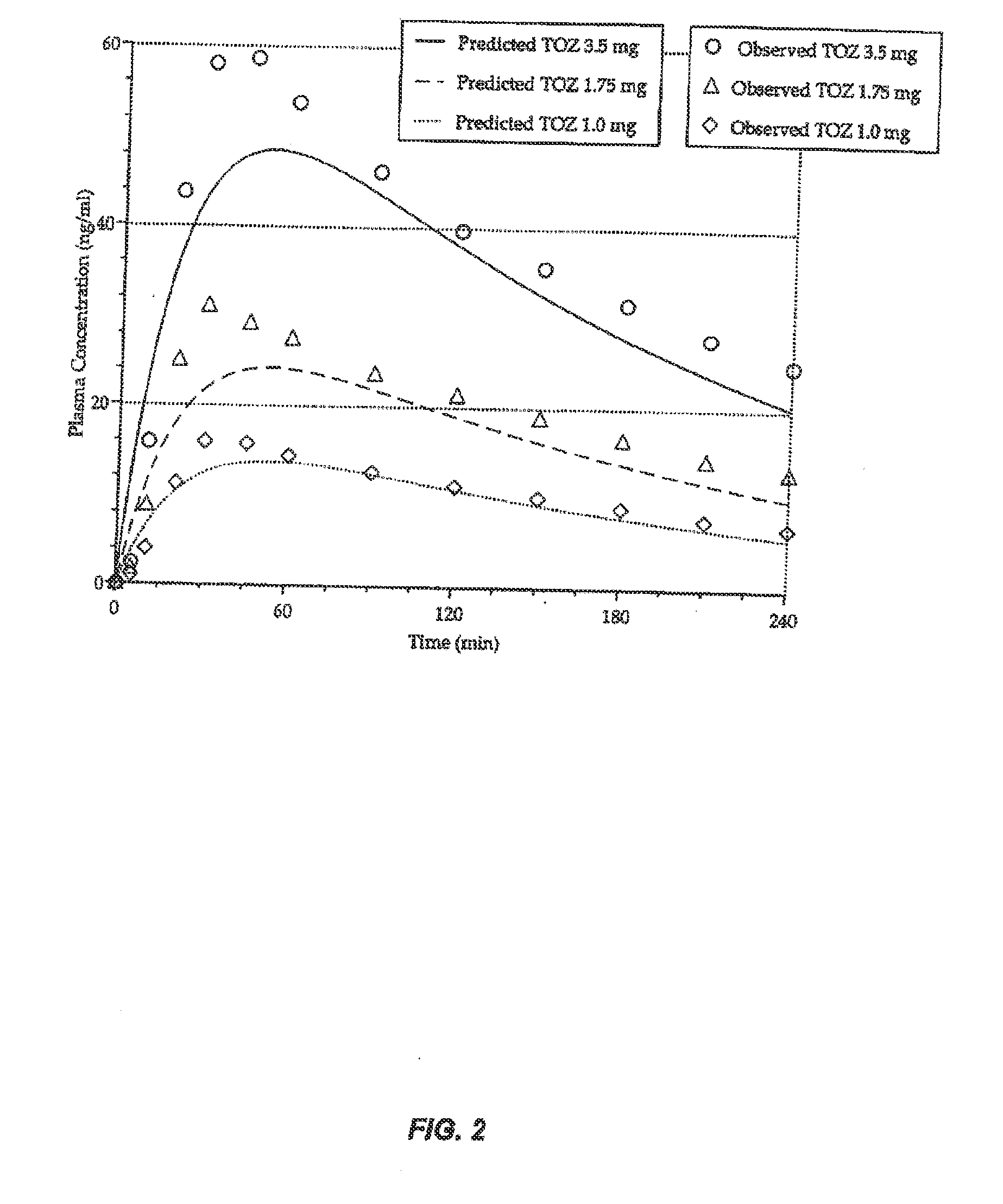
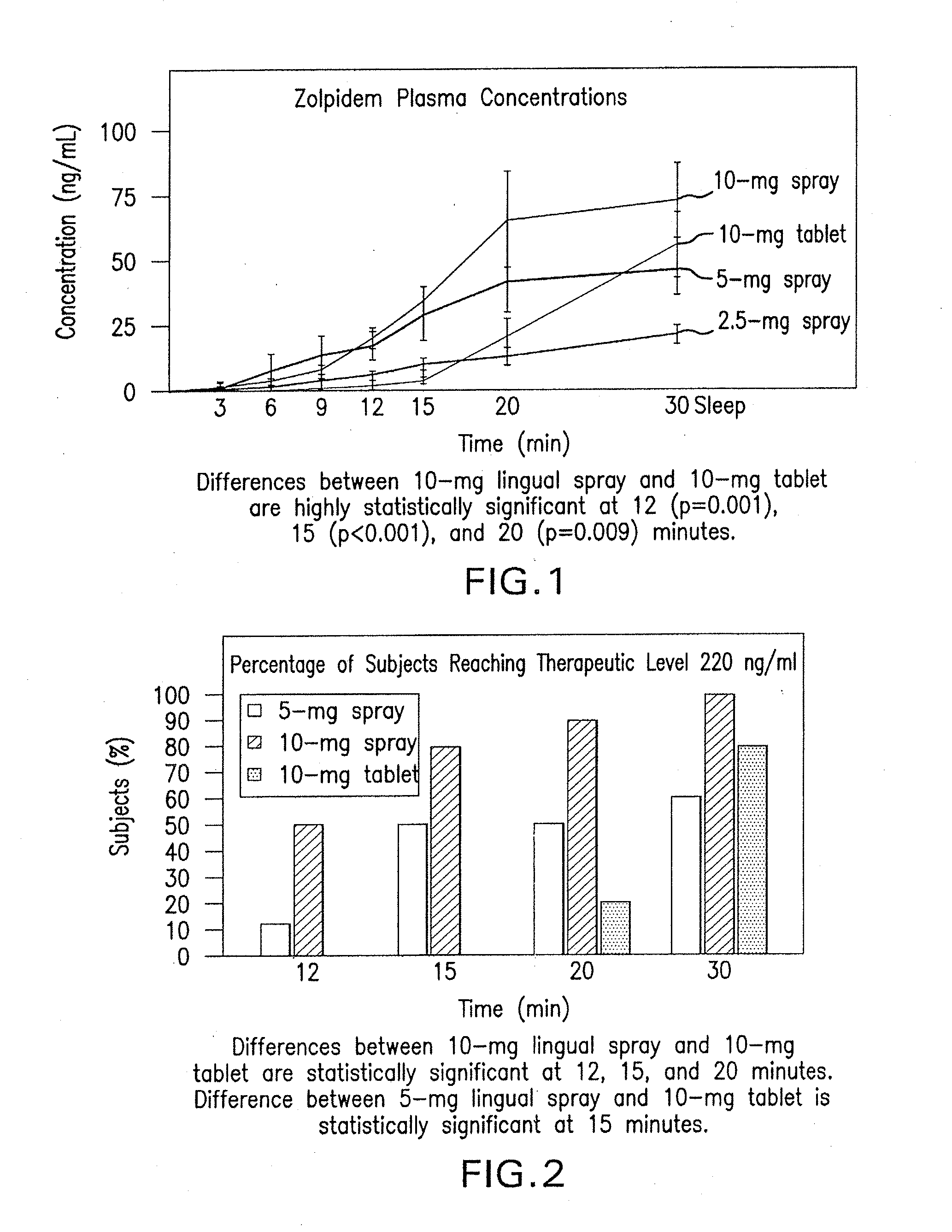
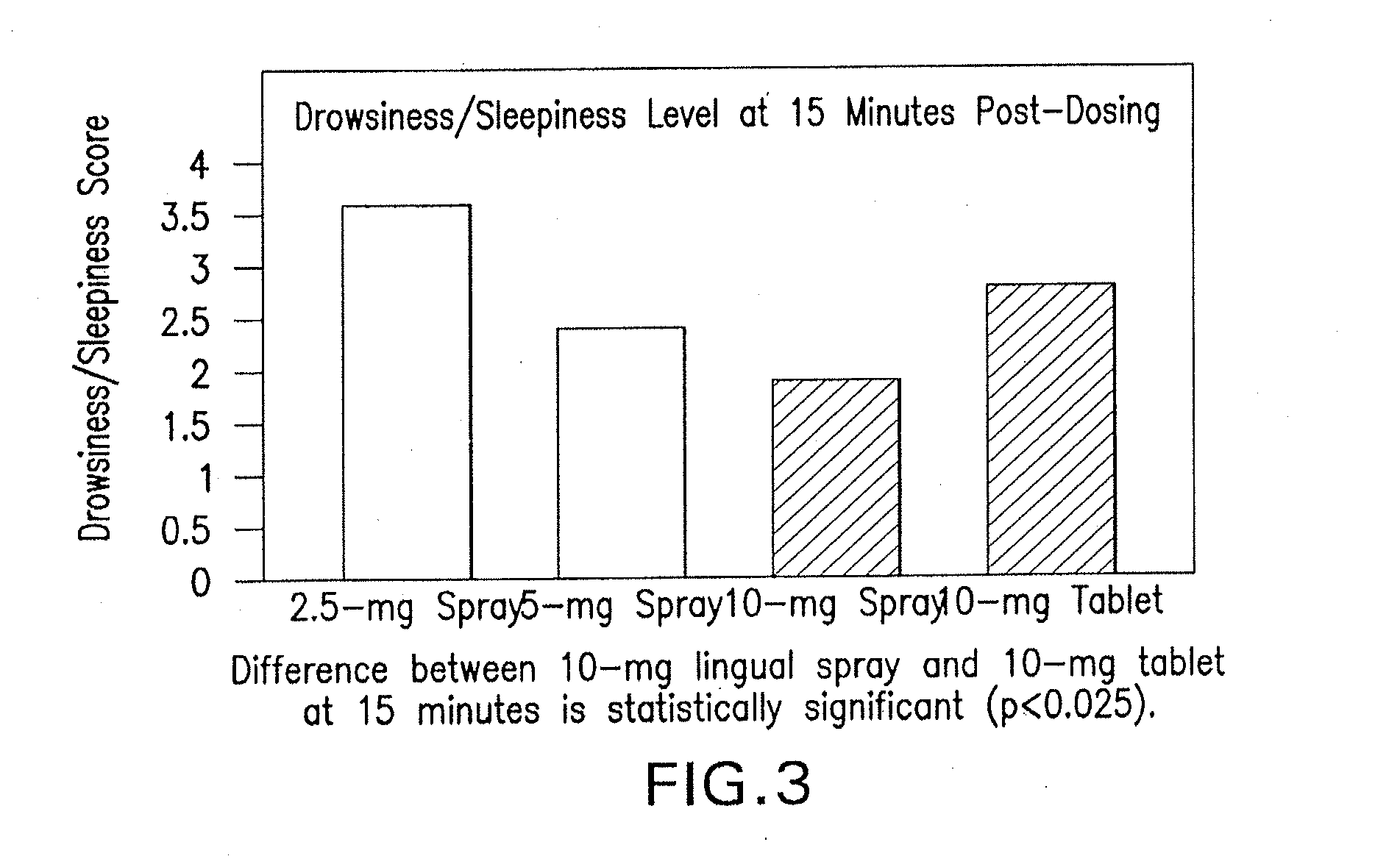
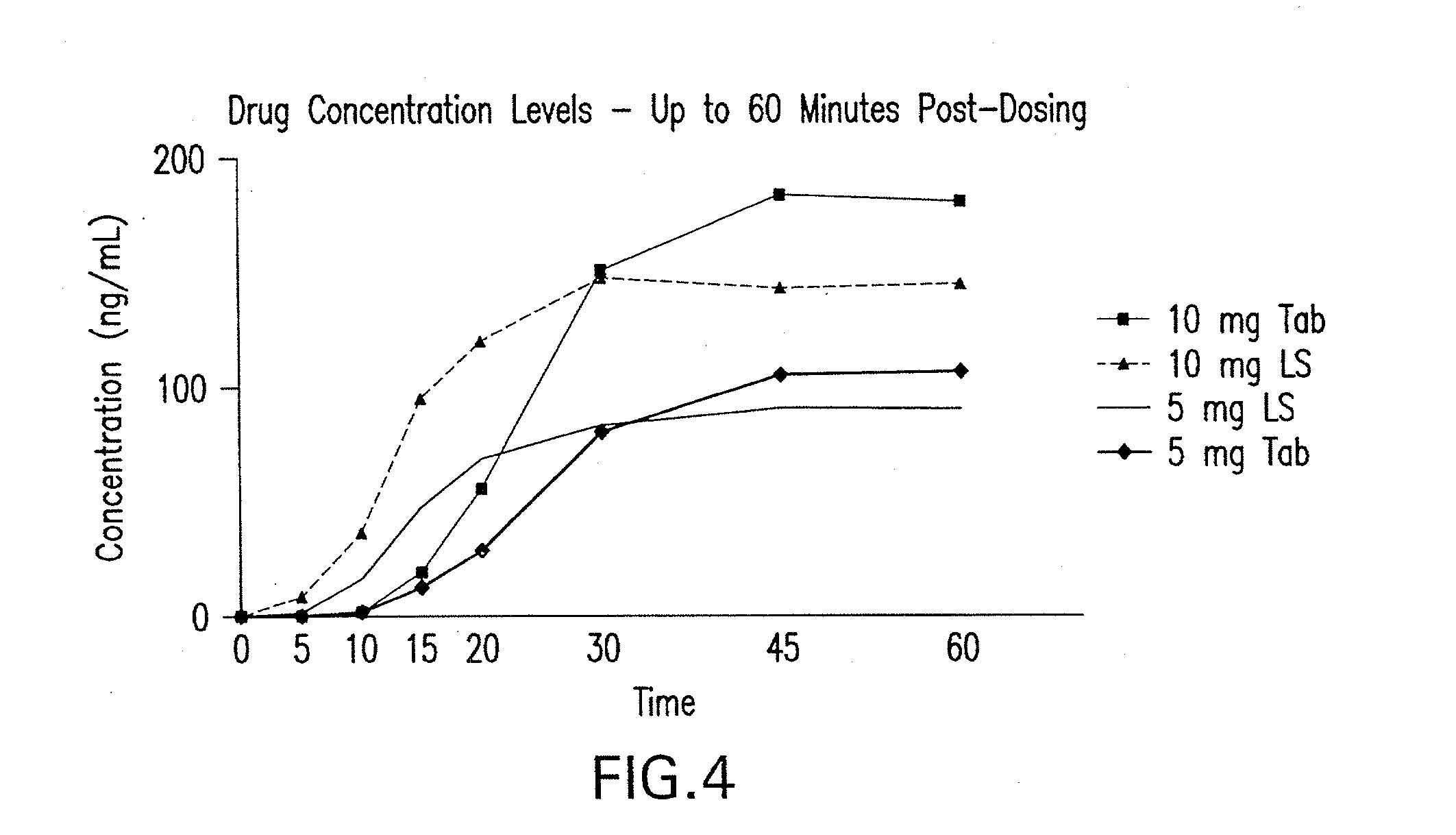
Compositions of zolpidem, and methods for their manufacture and use for treating insomnia. The compositions are formulated as oral sprays for transmucosal absorption of zolpidem. The methods of treatment in some cases involve night-time dosing administration to achieve therapeutic zolpidem blood levels within 20 minutes or less, tapering off to less than 20 ng / ml within less than five hours, in some cases less than four hours, post dosing.
Owner:MAGNA PHARMA INC
Method for preparing zolpidem
The invention discloses a method for preparing zolpidem. The method comprises the step of subjecting 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine, which serves as a raw material, to a reaction with 2-bromo-N,N-dimethylacetamide under the action of photocatalysis, thereby obtaining the zolpidem. Compared with the prior art, the method has the advantages that environment-friendly and pollution-free LED light serves as an energy source, so that an environment-friendly sustainable development idea is embodied; and during the preparation of the zolpidem, five steps in the prior art are shortened to one step. According to the method, the preparation route is short, the preparation method is simple, the production cost is low, the product yield is high, the consumption of a solvent and the pollution to environments during blowdown are reduced, the implementation is easy, and thus, the industrialization is facilitated.
Owner:NANJING NORMAL UNIVERSITY
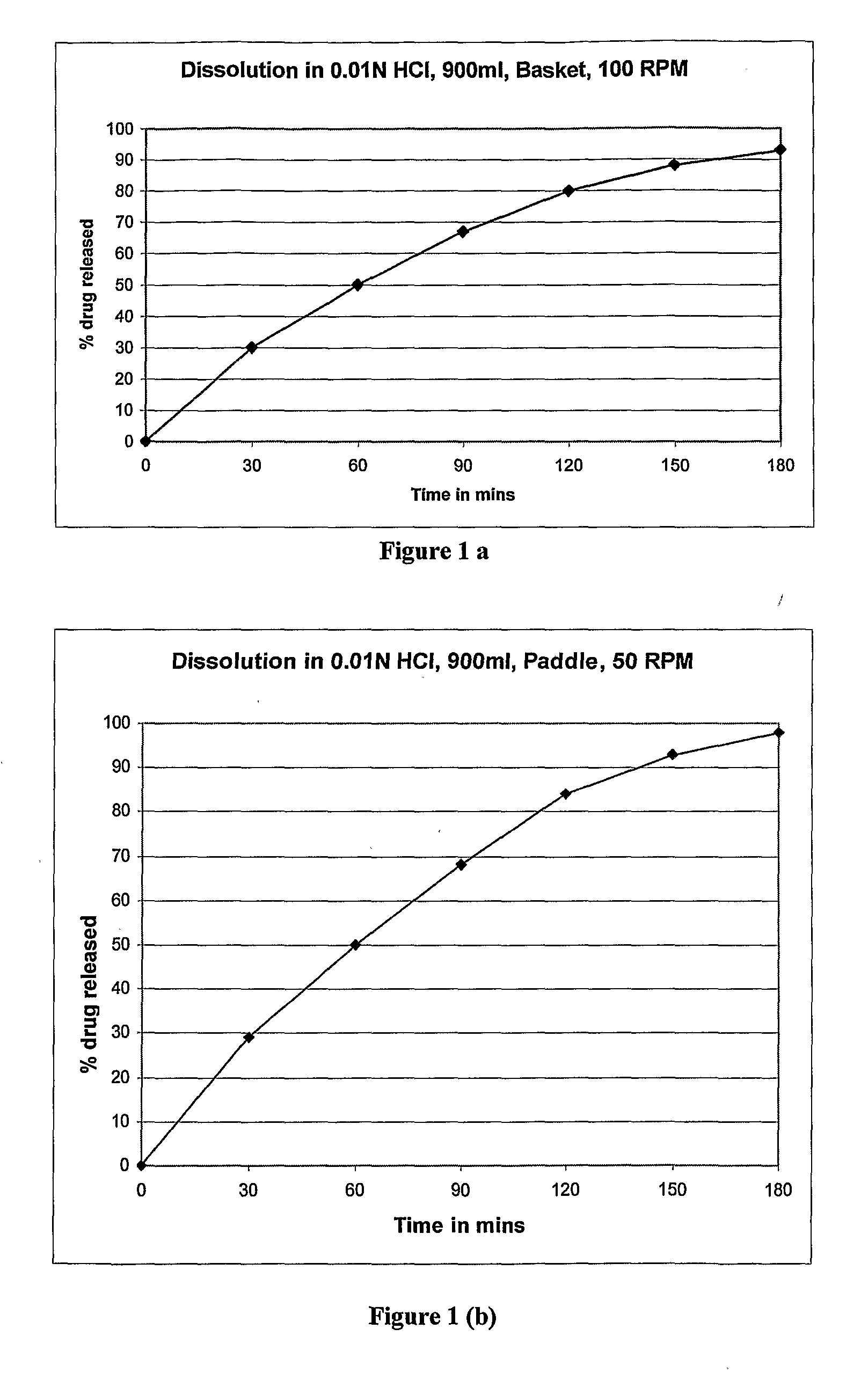
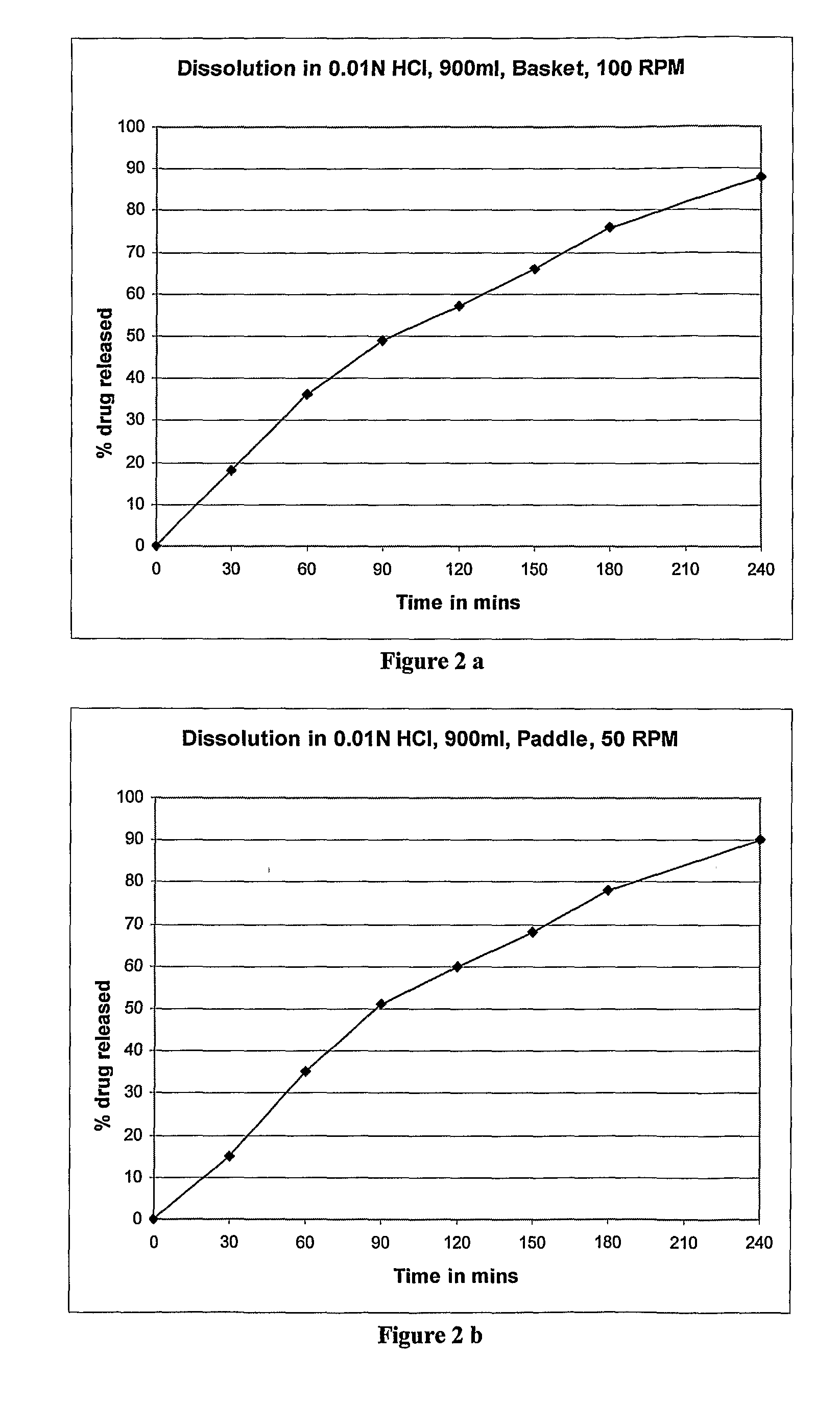
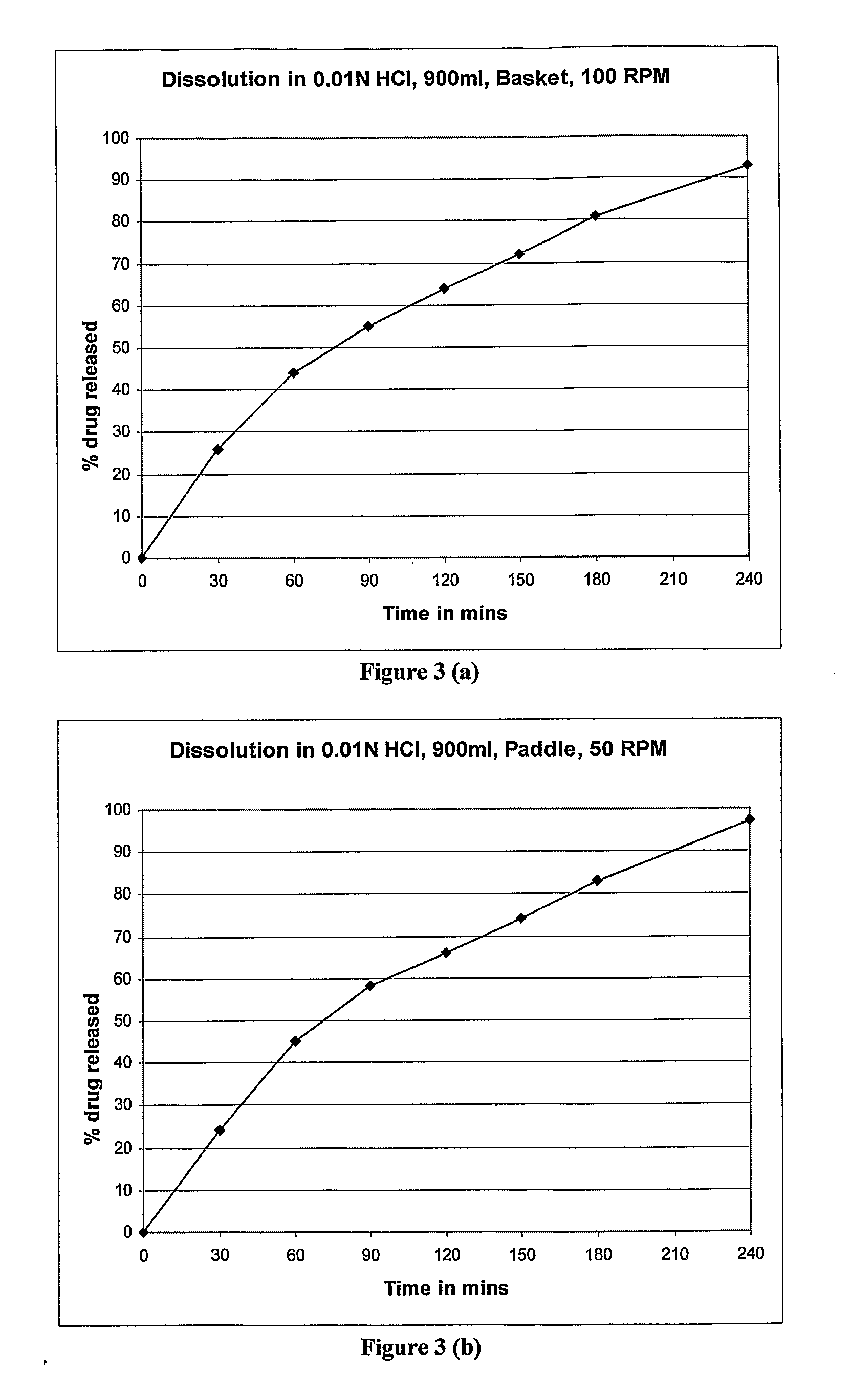
Controlled release dosage forms of zolpidem
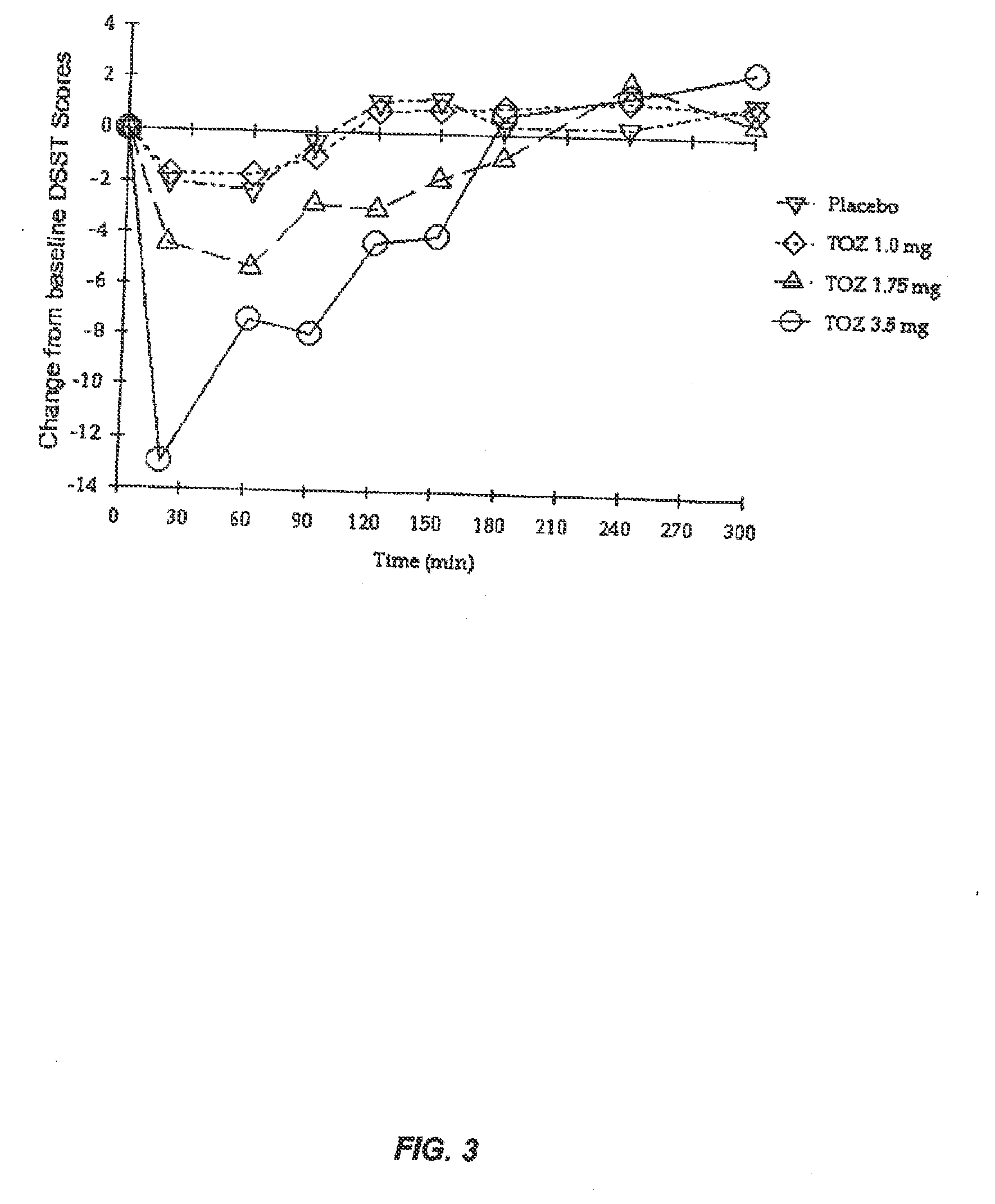
A controlled release dosage forms comprising zolpidem or a salt thereof to release zolpidem to induce rapid onset of sleep, and continue to release zolpidem in a controlled manner to maintain effective plasma concentrations over an extended period of time to improve sleep maintenance. The pharmaceutical controlled-release dosage form of zolpidem or a salt thereof having a dissolution profile when measured in a type I or II dissolution apparatus according to the U.S. Pharmacopoeia in 0.01M hydrochloric acid buffer at 37° C., such that less than 40% is released at the end of 30 minutes.
Owner:LUPIN LTD
Stabilized Zolpidem Pharmaceutical Compositions
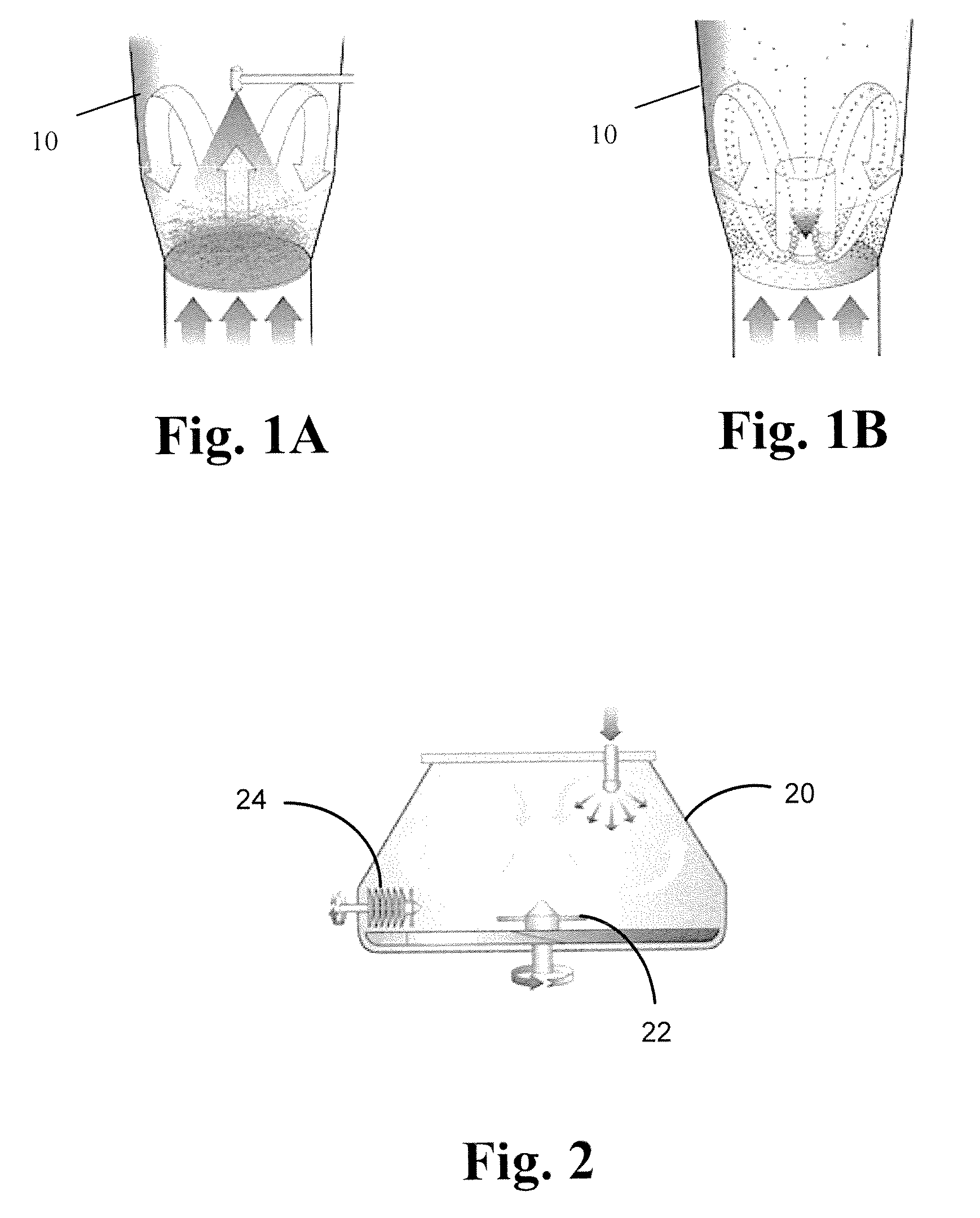
Pharmaceutical compositions for buccal delivery of zolpidem comprising an effective amount of zolpidem and a carbonate and bicarbonate buffer system in an amount sufficient to raise the pH of saliva to at least 8.5 irrespective of starting pH, and wherein the carbonate forms a coating on the bicarbonate wherein the amount of carbonate coating is at least 30% (w / w) of the total buffer amount are described. Methods of treating insomnia in a subject are described, the method including the step of administering to the subject the pharmaceutical composition of the present invention, wherein the administering is on an as-needed basis. Method of treating MOTN insomnia in a subject are also described.
Owner:SPI PHARMA
Polyethylene glycol-coated sodium carbonate as a pharmaceutical excipient and compositions produced from the same
Non-effervescent pharmaceutical compositions having at least one particle of carbonate coated by a layer of polyethylene glycol that substantially covers the at least one carbonate particle are described. Compositions are also described where the compositions include a weakly basic therapeutic agent, a first pH-modifying agent having at least one particle of carbonate coated by a layer of polyethylene glycol, and a second pH-modifying agent. The weakly basic therapeutic agent could, but is not limited to, be zolpidem or scopolamine. Compositions including zolpidem and scopolamine are used to treat insomnia and depression, respectively.
Owner:TRANSCEPT PHARMA
Method for determining residual sedative type veterinary medicaments in mutton
InactiveCN103954721AMeet Residue Analysis RequirementsHigh recovery rateComponent separationPerphenazinePromethazine
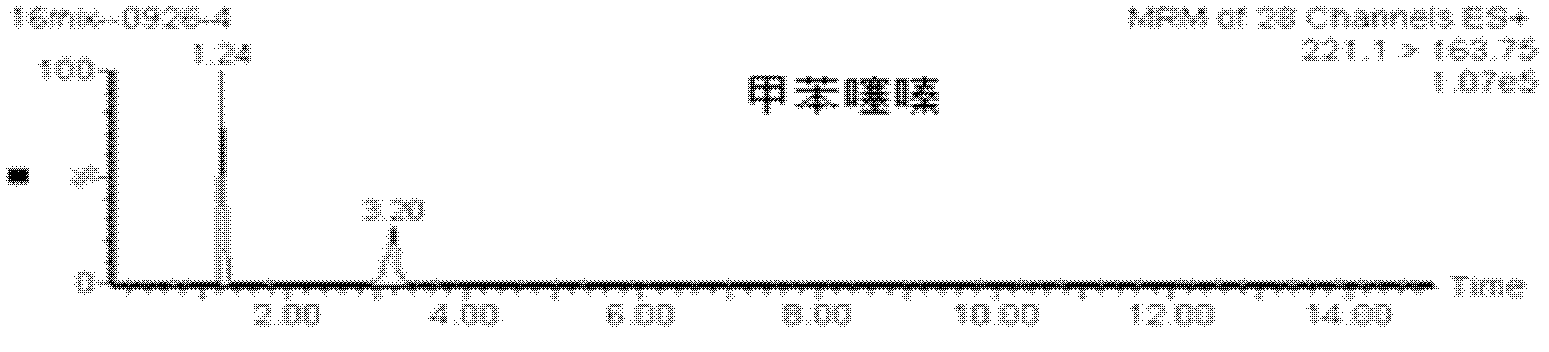
The invention relates to a method for determining residual medicaments in mutton, and in particular relates to a method for determining multiple sedative type medicaments in mutton at the same time. The residual sedative type veterinary medicaments refer to zolpidem, haloperidol, chlordiazepoxide, promethazine, nitrazepam, chlorpromazine hydrochloride, perphenazine, fluphenazine hydrochloride, clonazepam, xylazine hydrochloride, propionylpromazine, carazolol, acepromazine, droperidol and azaperone. The method has the advantage that the residual amounts of 15 types of sedative type medicaments in mutton are determined through high-resolution liquid chromatography-tandem mass spectrometry. The method is high in sensitivity and high in recovery rate, and can meet the detection requirements on veterinary medicaments.
Owner:GANSU AGRI UNIV
Zolpidem-based orodispersible pharmaceutical tablet
The present invention is directed to oral pharmaceutical forms for rapid disintegration which make it possible to prevent possible misuse of the zolpidem present therein. The present invention thus relates to a zolpidem-based orodispersible tablet formulation intended to prevent abuse of use of the tablet at the expense of a third party.
Owner:SANOFI SA
Method for preparing compound zolpidem
ActiveCN103360387AAvoid the disadvantages of long and high costHigh yieldOrganic chemistryChemical synthesisBiochemical engineering
The invention belongs to the field of chemical synthesis, and relates to a method for preparing a compound zolpidem. The invention provides an effective new method for preparing zolpidem by a trimaceral serially reaction. The method for preparing the zolpidem provided by the invention has the advantages that the reaction step is short, the condition is moderate, atom economy and environmentally friendly are achieved, the yield is high, the cost is low, industrialized production is applicable, and the defects in the prior art that the synthetic route is long and the cost is high are overcome.
Owner:JIANGSU HANSOH PHARMA CO LTD
Stabilized zolpidem pharmaceutical compositions
Owner:SPI PHARMA +1
Compositions and methods for treating middle-of-the-night insomnia
The present invention provides compositions having a therapeutically effective amount of zolpidem, carbonate buffer, bicarbonate buffer, and a mixture comprising large and fine particles of silicon dioxide. Compositions having a therapeutically effective amount of zolpidem, carbonate buffer, bicarbonate buffer, and sodium stearyl fumarate are also described.
Owner:SINGH NIKHILESH +1
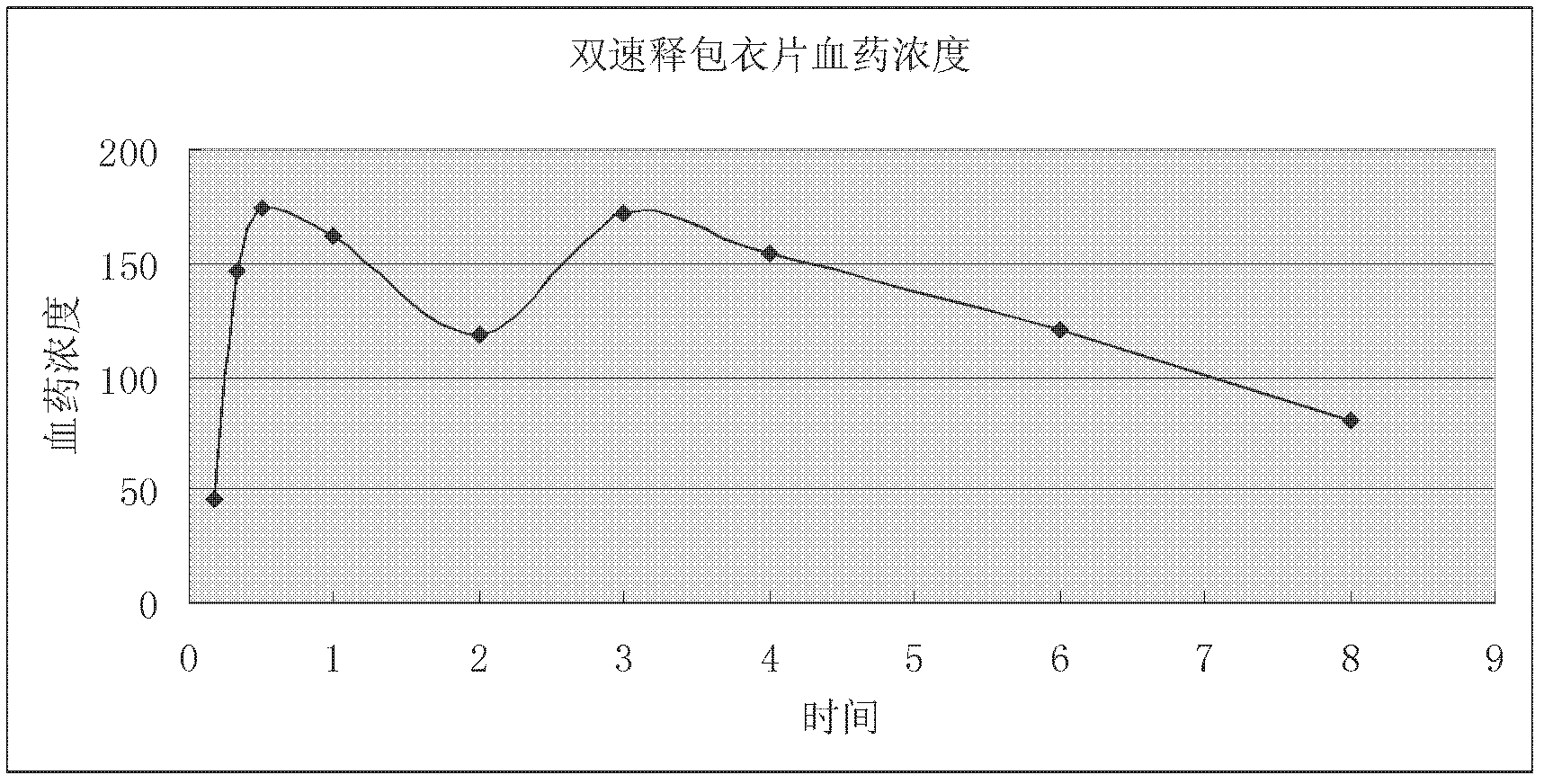
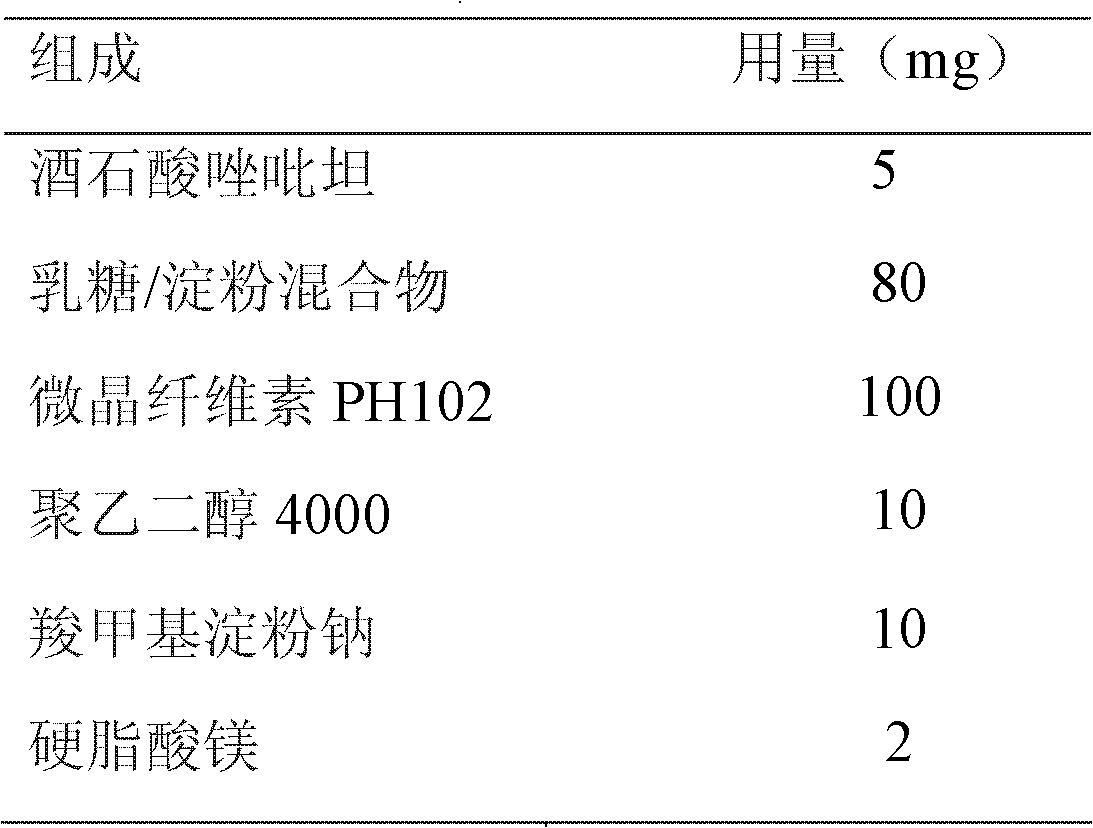
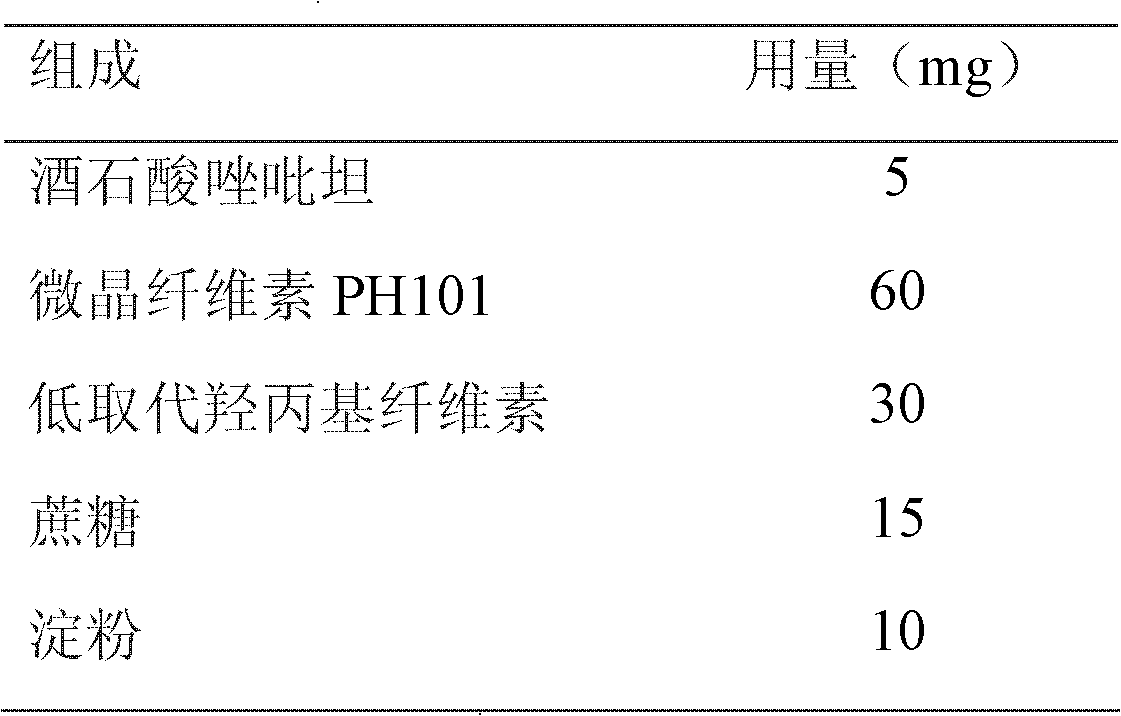
Two-phase release preparation containing zolpidem or salt of zolpidem and preparation method thereof
ActiveCN102552107ASleep longerAvoid hydrolysisPowder deliveryOrganic active ingredientsHydrogenFast release
The invention discloses a two-phase release preparation containing zolpidem or salt of zolpidem, which is characterized by comprising a normal medicine-containing fast release preparation and enteric fast release preparation and being suitable for respectively releasing zolpidem or salt of zolpidem in two preset periods of time. More than 90% dose of the normal medicine-containing fast release preparation is released within 30 minutes in 0.01M of hydrochloric acid buffer solution at 37 DEG C, and more than 90% dose of the enteric fast release preparation is released within 30 minutes in phosphate buffer solution of 6.8 potential of hydrogen (pH) at 37 DEG C. The two-phase release preparation containing zolpidem or salt of zolpidem is specific to the sleep characteristics of a patient with sleep difficulties and early waking, and has effects on patients with both sleeping difficulties and early waking simultaneously.
Owner:吉林天衡药业有限公司
Process for preparing zolpidem hemitartrate and tartrate polymorphs
A method for preparing a polymorph of a hemitartrate salt of a compound having the structure as follow comprises dissolving a free base form of the compound in a liquid medium comprising an alcohol and a tartrate derivative to form a solution comprising the compound, the alcohol, and the tartrate derivative; heating the solution to a temperature sufficient to dissolve the compound and the tartrate derivative; and cooling the solution to a temperature sufficient to precipitate the hemitartrate salt of the compound.
Owner:MALLINCKRODT INC
Preparation method of zolpidem
ActiveCN110272414ASimple and fast operationLow costOrganic chemistryElimination reactionRaw material
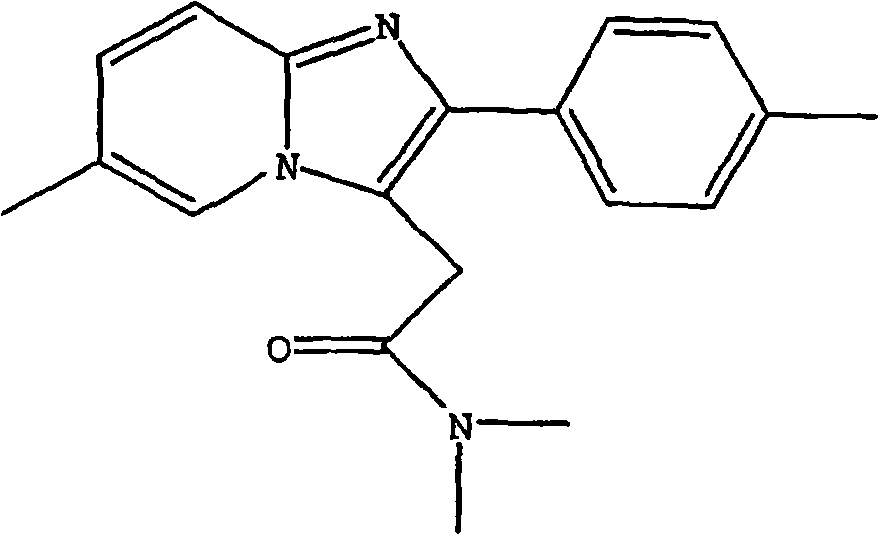
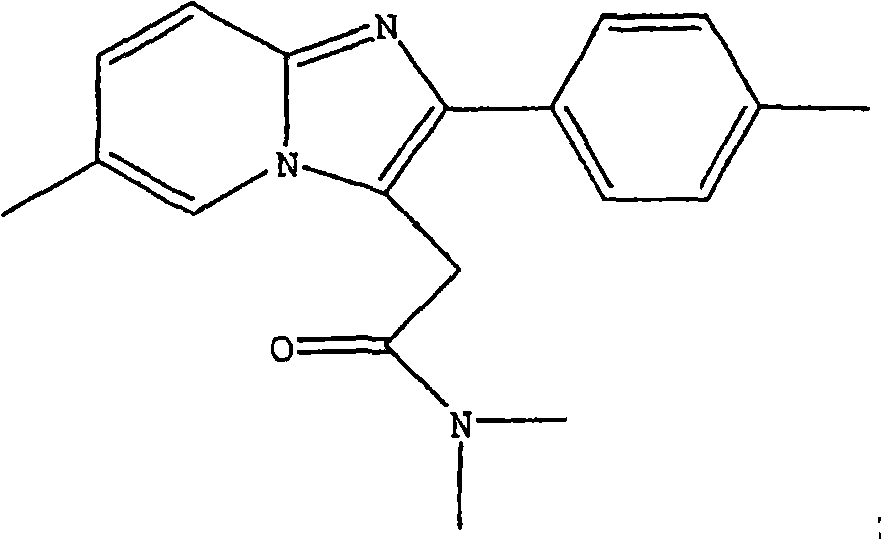
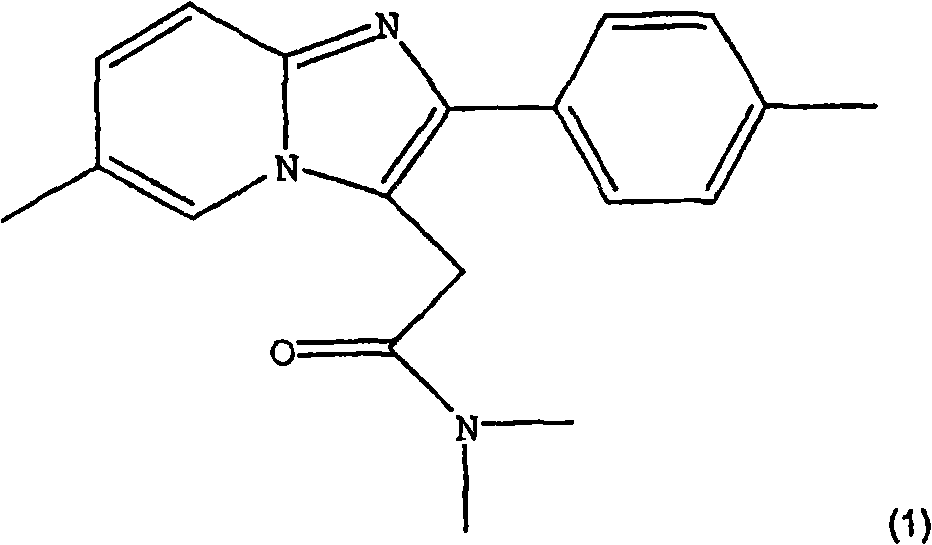
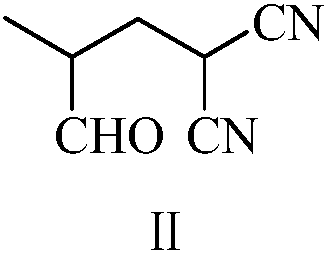
The invention provides a preparation method of zolpidem. The method comprises the following steps: malononitrile and 2-methacrolein which are used as raw materials undergo a 1,4-addition reaction to prepare 2-methyl-4,4-dicyano-n-butyraldehyde, the 2-methyl-4,4-dicyano-n-butyraldehyde and N,N-dimethyl-4-p-methylphenyl-4-oxo-3-amino-n-butyramide undergo a two-stage dehydration condensation reaction, and the obtained reaction product and an acid binding agent undergo an elimination agent to prepare the zolpidem. The method of the invention has the advantages of cheap and easily available raw materials, short process flow, mild reaction conditions, simplicity in operation, low cost, low amount of three wastes, greenness, environmental protection, good selectivity, high yield and high purity of the product, and facilitation of industrial production of the zolpidem.
Owner:XINFA PHARMA
Process for Preparing Zolpidem Hemitartrate and Tartrate Polymorphs
A method for preparing a polymorph of a hemitartrate salt of a compound having the structure: comprising dissolving a free base form of the compound in a liquid medium comprising an alcohol and a tartrate derivative to form a solution comprising the compound, the alcohol, and the tartrate derivative; heating the solution to a temperature sufficient to dissolve the compound and the tartrate derivative; and cooling the solution to a temperature sufficient to precipitate the hemitartrate salt of the compound.
Owner:MALLINCKRODT INC
Anti-insomnia compositions and methods
Compositions of zolpidem, and methods for their manufacture and use for treating insomnia. The compositions are formulated as oral sprays for transmucosal absorption of zolpidem. The methods of treatment in some cases involve night-time dosing administration to achieve therapeutic zolpidem blood levels within 20 minutes or less, tapering off to less than 20 ng / ml within less than five hours, in some cases less than four hours, post dosing.
Owner:MAGNA PHARMA INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com