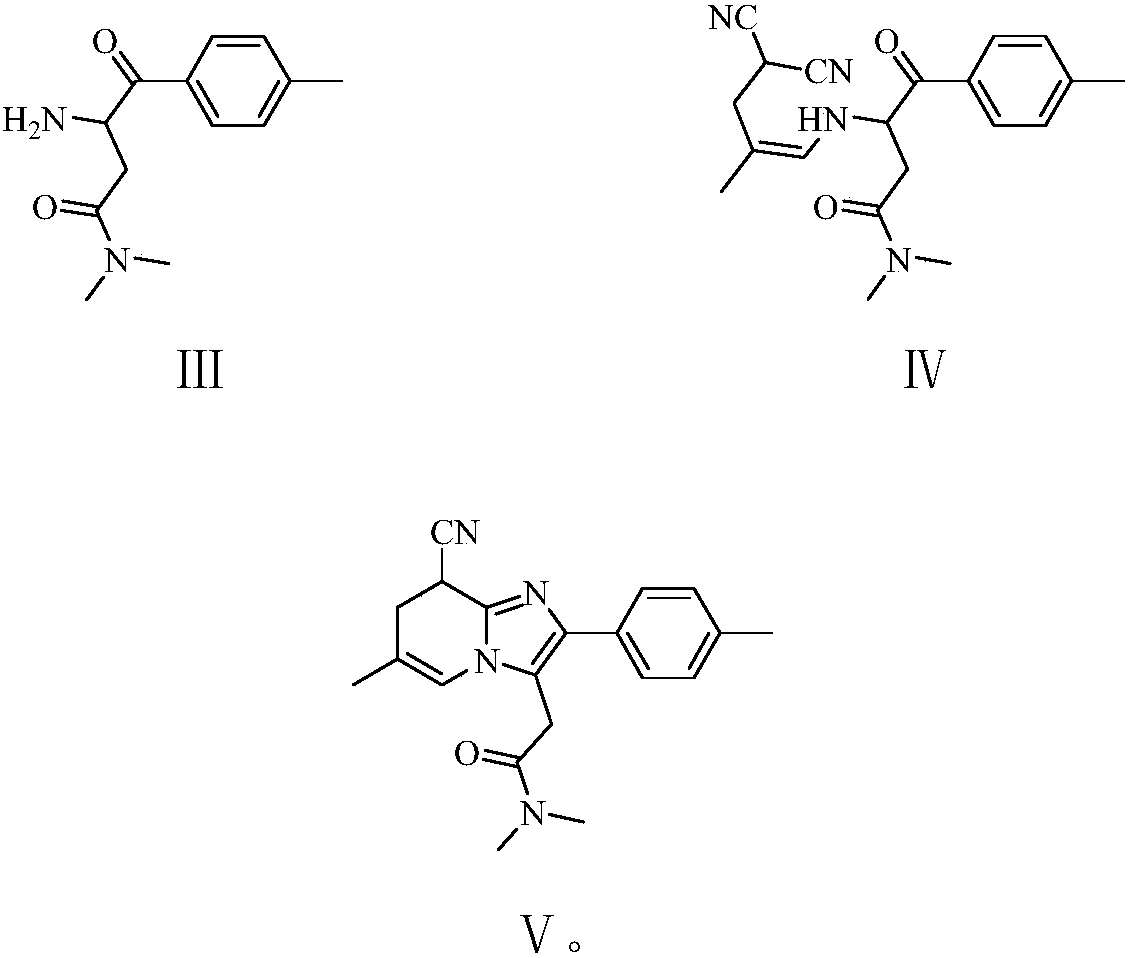
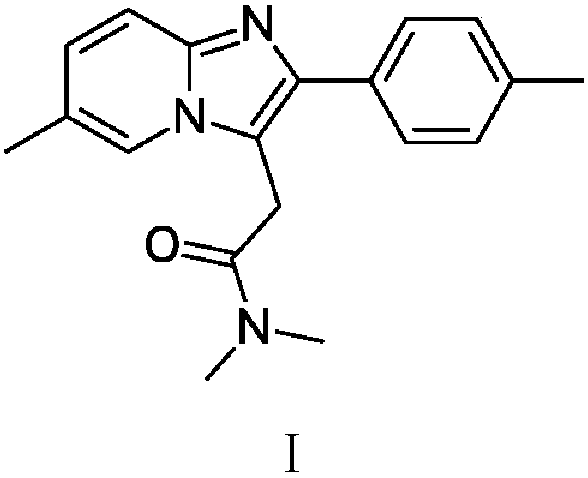
Preparation method of zolpidem
A technology for zolpidem and a compound is applied in the field of preparation of zolpidem, which can solve the problems of unsuitable industrial production, poor atom economy, large amount of process waste water and the like, and achieves the effects of short preparation period, short operation period and short technological process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
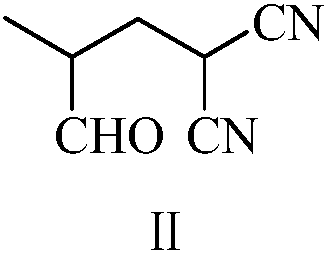
[0056] Example 1: Preparation of 2-methyl-4,4-dicyano-n-butyraldehyde (II)
[0057] To a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 100 g of tetrahydrofuran, 33.0 g (0.5 mole) of malononitrile, 35.0 g (0.5 mole) of 2-methacrolein, and 0.8 g of DBU, The reaction was stirred at 50 to 55°C for 4 hours. The solvent was recovered by distillation, and then distilled under reduced pressure (90-110° C. / 3-5 mmHg) to obtain 66.4 g of 2-methyl-4,4-dicyano-n-butyraldehyde, with a yield of 97.6% and a gas phase purity of 99.8%.
Embodiment 2
[0058] Example 2: Preparation of 2-methyl-4,4-dicyano-n-butyraldehyde (II)
[0059] In a 500 ml four-necked flask connected with stirring, a thermometer, and a reflux condenser, add 100 g of acetonitrile, 33.0 g (0.5 mole) of malononitrile, 38.5 g (0.55 mole) of 2-methacrolein, and 0.8 g of DBN, The reaction was stirred at 55 to 60°C for 4 hours. The solvent was recovered by distillation, and then distilled under reduced pressure (90-110° C. / 3-5 mmHg) to obtain 66.2 g of 2-methyl-4,4-dicyano-n-butyraldehyde, with a yield of 97.4% and a gas phase purity of 99.6%.
Embodiment 3
[0060] Example 3: Preparation of 2-methyl-4,4-dicyano-n-butyraldehyde (II)
[0061] Into a 250 ml four-necked flask connected with a stirring, thermometer, and reflux condenser, 33.0 g (0.5 mole) of malononitrile, 35.0 g (0.5 mole) of 2-methacrolein, and 0.8 g of DBU were stirred at 50 to 55 ° C. React for 5 hours. Then vacuum distillation (90-110° C. / 3-5 mmHg) yielded 66.0 g of 2-methyl-4,4-dicyano-n-butyraldehyde with a yield of 97.1% and a gas phase purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com