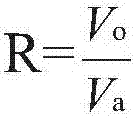Patents
Literature
155 results about "Butyramide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyramide is the amide of butyric acid. It has the molecular formula C₃H₇CONH₂. It is a white solid that freely soluble in water and ethanol, but slightly soluble in diethyl ether. At room temperature, butyramide is a crystalline solid and in contrast to butyric acid, it is devoid of unpleasant, rancid smell.
Food products containing a fruit component
InactiveUS20070092623A1Continuous refreshing feelingHigh acceptanceAlcoholic beverage preparationFood preparationMenthoneFruit juice
A fruit juice-containing food product containing following components (a) and (b) in addition to a fruit component and a base having sweetness; and a method for reinforcing a flavor of a fruit juice-containing food product. (a) one or more refreshing feeling substances selected from the group consisting of menthol, menthone, camphor, pulegol, isopulegol, pulegone, cineol, mint oil, peppermint oil, spearmint oil, eucalyptus oil, and fractions there of. (b) one or more cool feeling substances selected from the group consisting of 3-1-menthoxypropane-1,2-diol, N-ethyl-p-menthane-3-carboxamide, 3-1-menthoxy-2-methylpropane-1,2-diol, p-menthane-3,8-diol, 2-1-menthoxyethane-1-ol, 3-1-menthoxypropane-1-ol, 4-1-menthoxybutane-1-ol, cyclic carboxamides, acyclic carboxamides,N,2,3-trimethyl-2-isopropyl butanamide, a menthoxy alkanol (alkyl group having 2 to 6 carbons), a menthoxy alkyl ether (alkyl group having 1 to 6 carbons), and a menthoxy alkanediol (alkyl group having 3 to 6 carbons).
Owner:TAKASAGO INTERNATIONAL CORPORATION
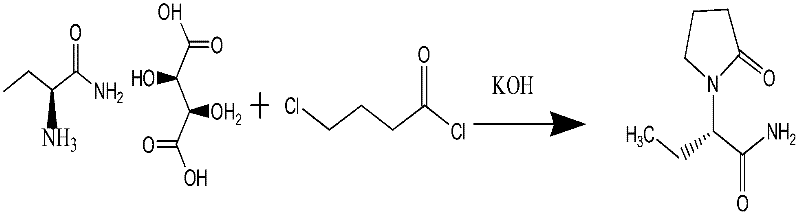
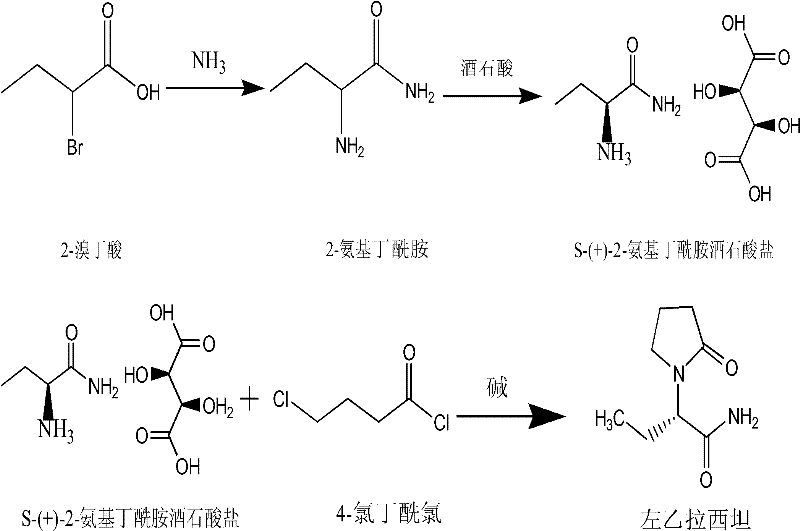
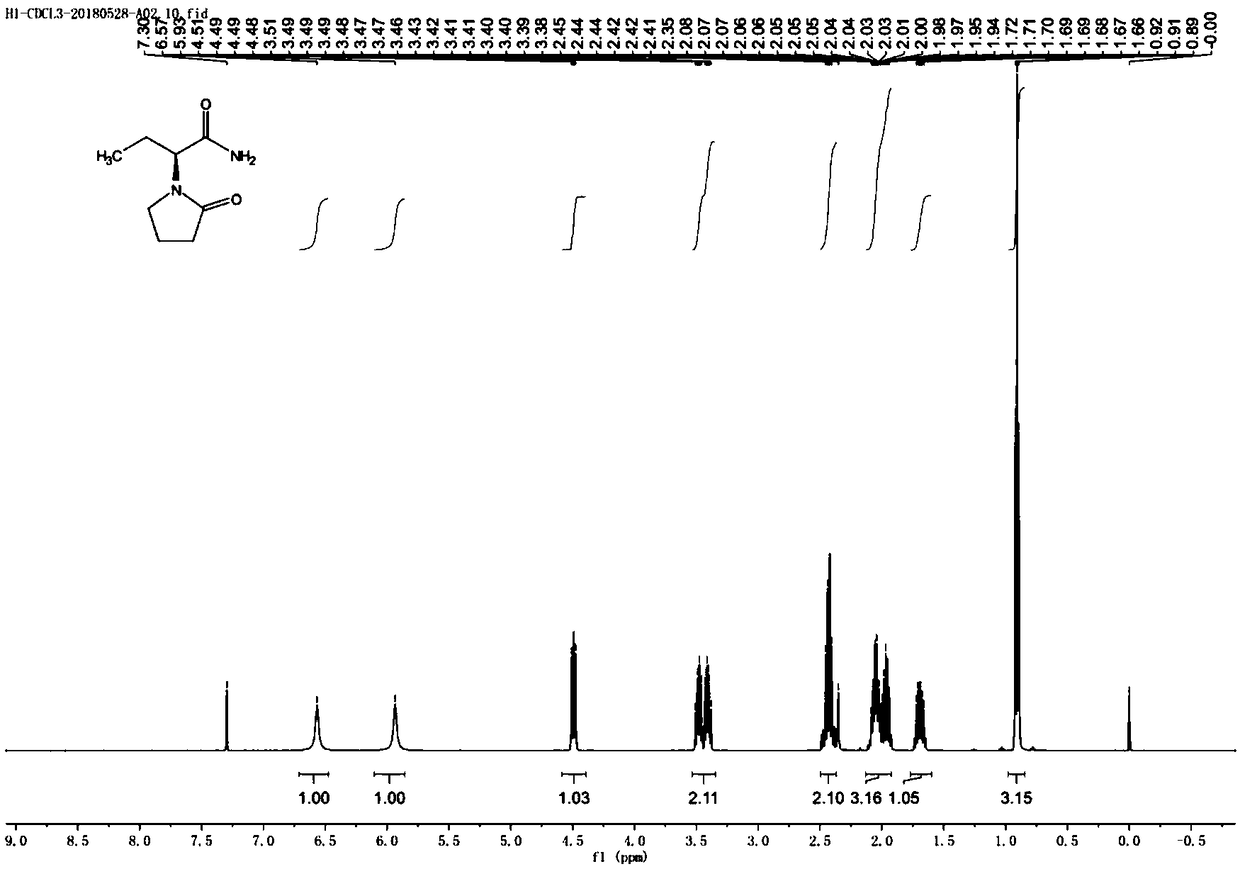
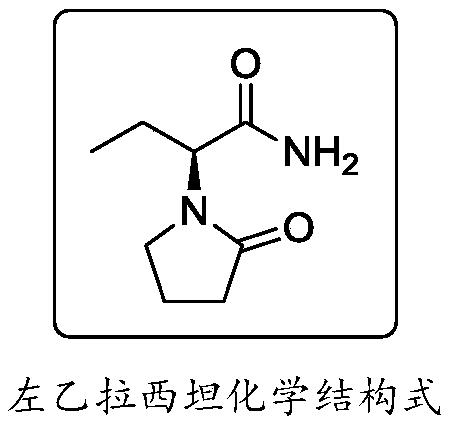
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
Electrorheological fluids
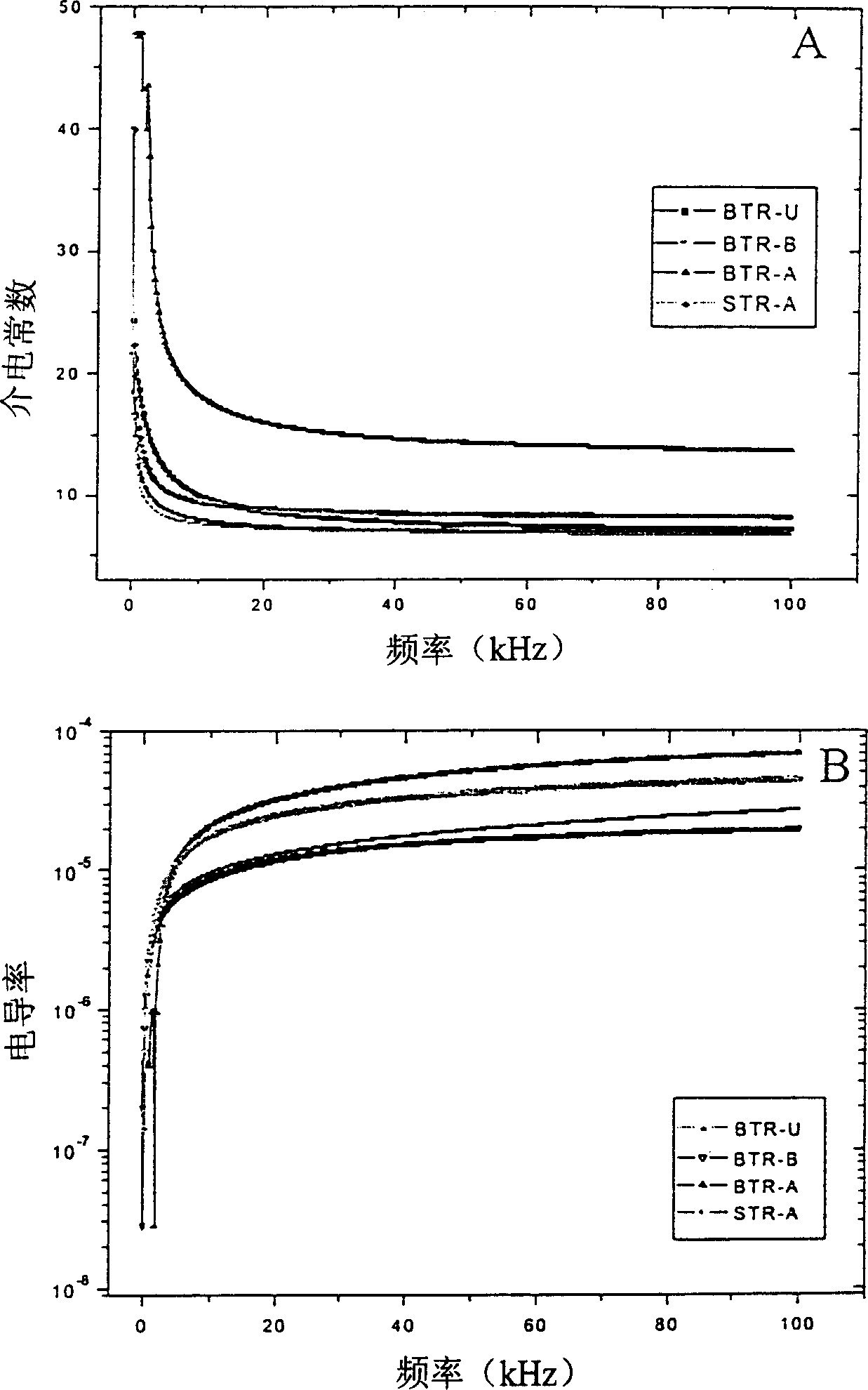
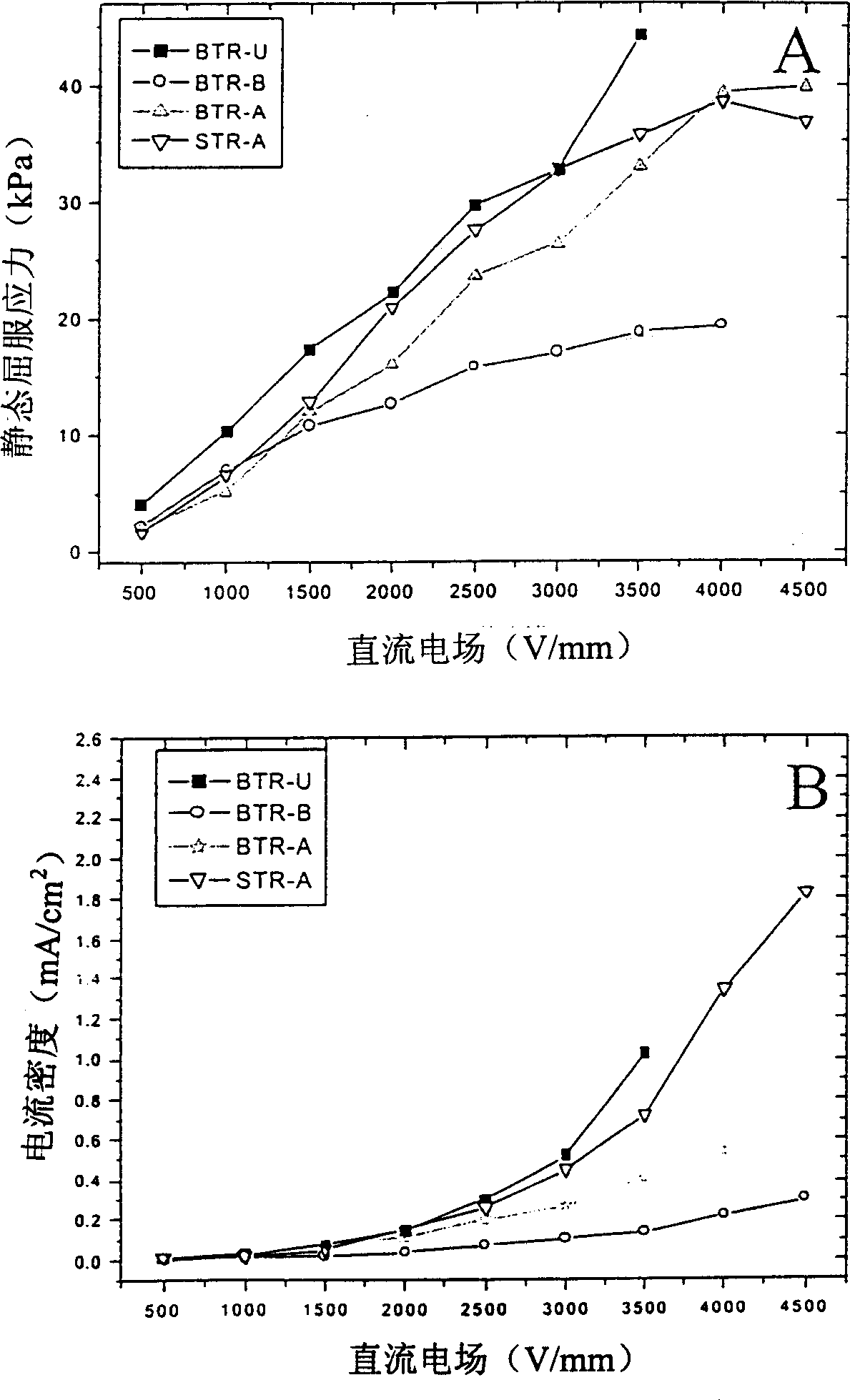
There is described an electrorheological fluid comprising particles of a composite material suspended in an electrically insulating hydrophobic liquid. The composite particles are metal salts of the form M1xM22-2xTiO(C2O4)2 where M1 is selected from the group consisting of Ba, Sr and Ca and wherein M2 is selected from the group consisting of Rb, Li, Na and K, and the composite particles further include a promoter selected from the group consisting of urea, butyramide and acetamide.
Owner:THE HONG KONG UNIV OF SCI & TECH
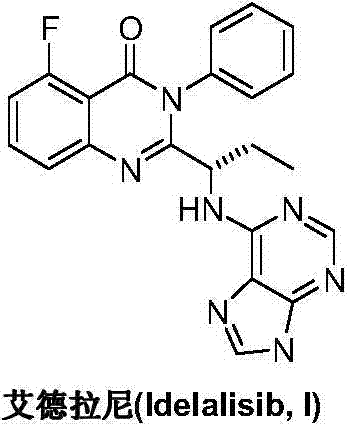
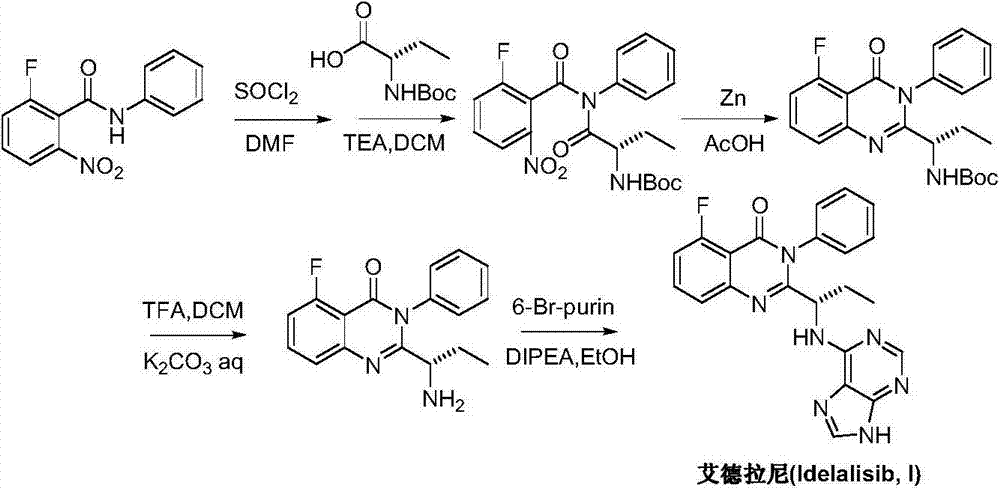
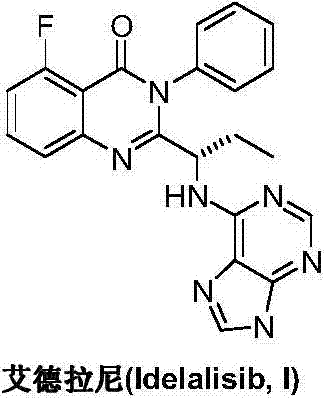
A preparing method of Idelalisib
ActiveCN104262344AEase of industrial productionRaw materials are easy to getOrganic chemistryAcetic anhydrideAminobutyrate
A preparing method of Idelalisib (I) is disclosed. The preparing method includes following steps of: subjecting R-2-hydroxybutyrate (II) and 6-amino-9H-purine to nucleophilic substitution under actions of a leaving agent and an acid-binding agent to produce an intermediate S-2-(N-9H-purin-6-yl)aminobutyrate (III); subjecting the intermediate (III) and 2-amino-6-fluorobenzoic acid to amidation under actions of a catalyst to produce S-2-(N-9H-purin-6-yl)amino-N-(2-carboxyl-3-fluorophenyl)butyramide (IV); subjecting the intermediate (IV) to a cyclization reaction in acetic anhydride; and performing a substitution reaction with phenylamine to obtain the Idelalisib (I). The preparing method has characteristics of easily available raw materials, simple and concise process, capability of being economical and environmental friendly, and suitability for industrial production.
Owner:优标易站(苏州)电子商务有限公司
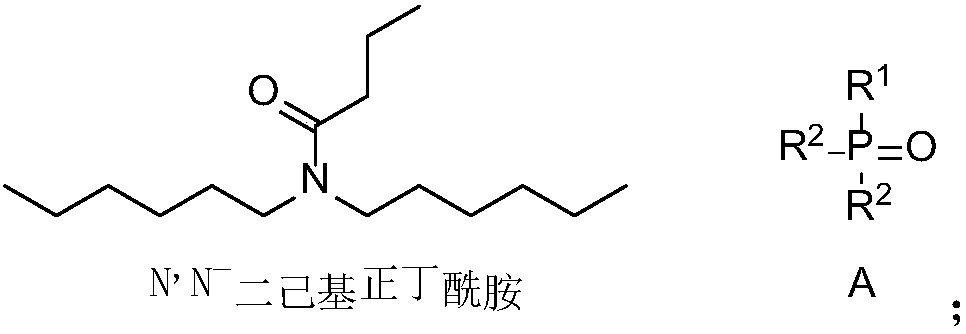
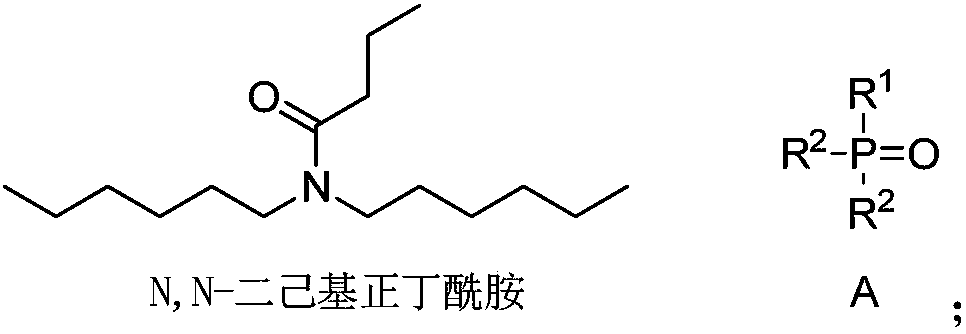
Application of amide compound, extraction composition containing amide compound and extraction system
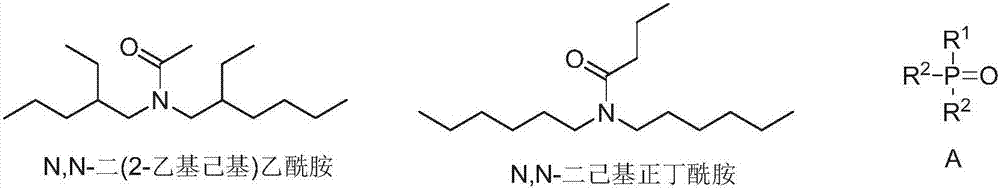
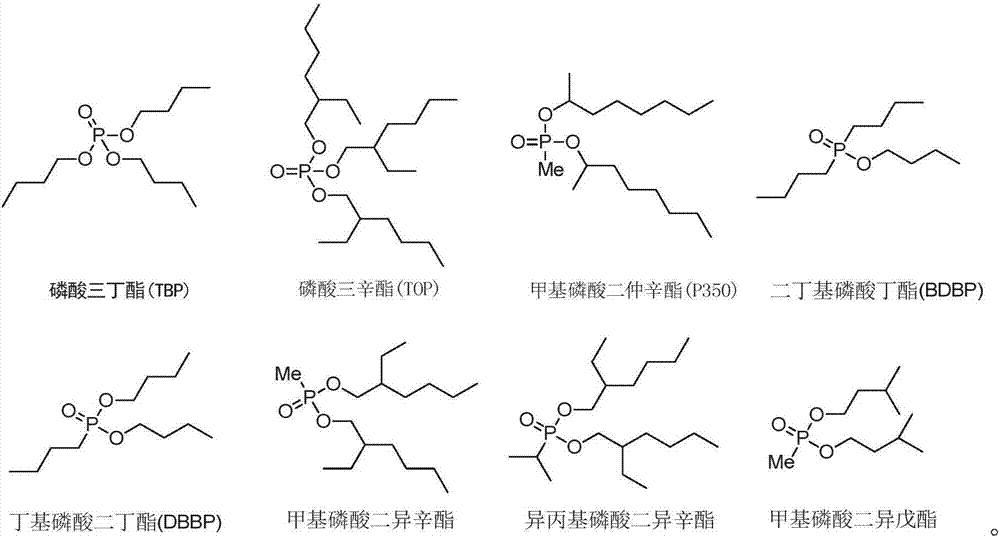
ActiveCN107619929ALow water solubilityReduce corrosionProcess efficiency improvementLithiumButyramide
The invention discloses application of an amide compound, an extraction composition containing the amide compound and an extraction system. The extraction composition contains N,N-dicyclohexyl-butyramide and a diluents, but does not contain neutral phosphorus oxide as shown in the formula A. According to the amide compound or the extraction composition containing the amide compound, the extractionand back-extraction of lithium from Lithium-containing brine can be performed, the Lithium extraction rate of Lithium-containing brine is above 83.89%, and the distribution coefficient of Lithium-Magnesium is above 521; and when back-extraction of lithium is performed by the adoption of HCl, the back-extraction rate is above 91.74%, the corrosion to equipment is small, and the application of theamide compound, the extraction composition and the extraction system are suitable for industrial operation requirements (Please see the formula A in the specification.).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Food products containing a fruit component
InactiveCN1874693ASuppresses decline in refreshing feelingHigh taste acceptabilityAlcoholic beverage preparationFood preparationMenthoneEthyl group
Owner:TAKASAGO INTERNATIONAL CORPORATION
Industrial process for the production of L-carnitine
InactiveUS6566552B2Easily appilcable on an industrial scaleReduce productionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureSubstrate concentration
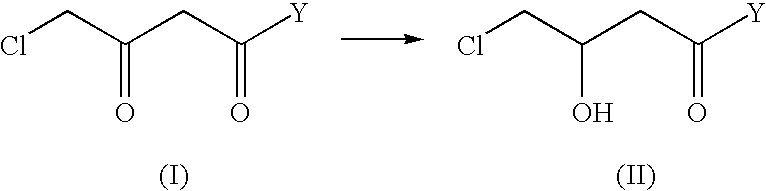
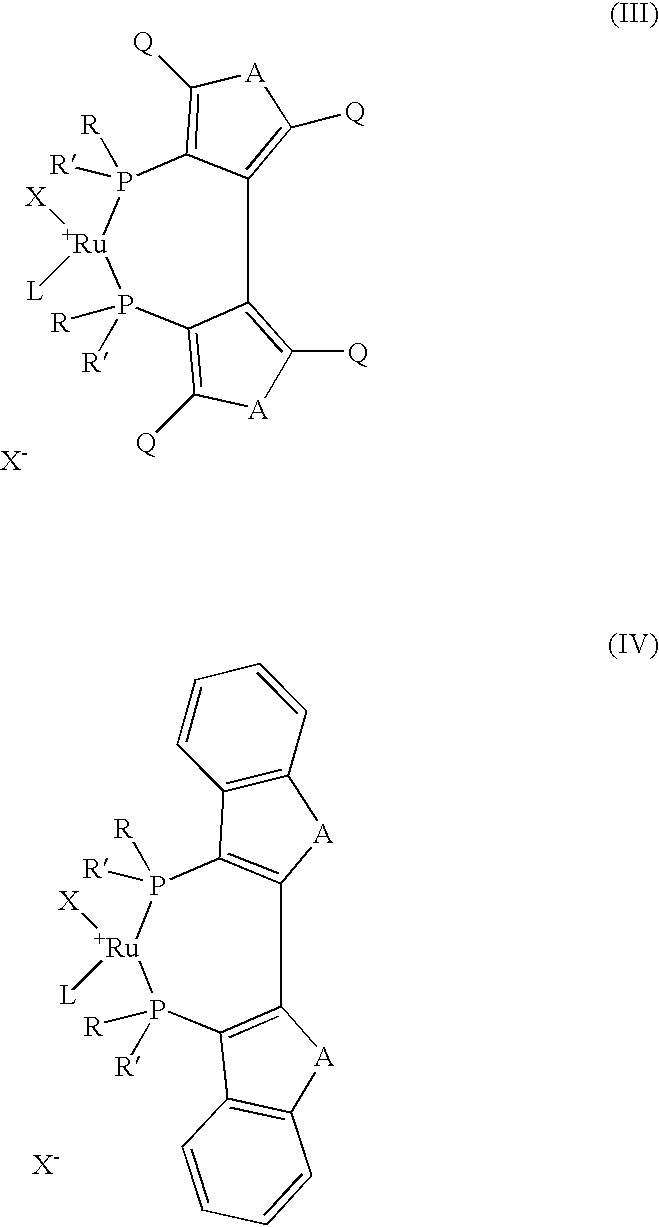
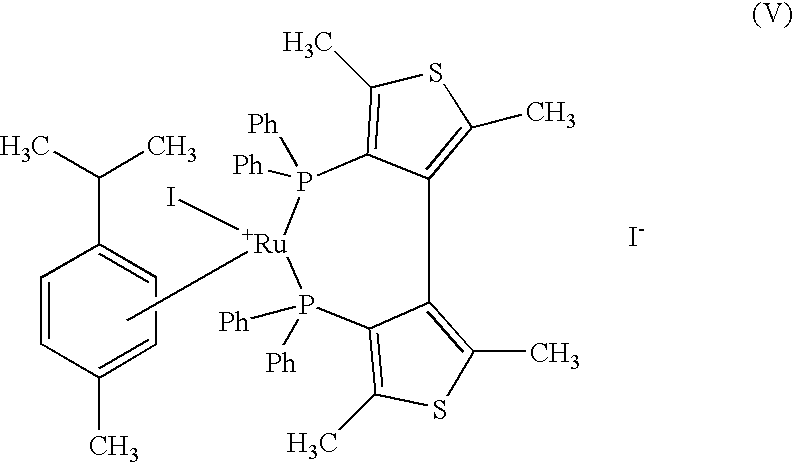
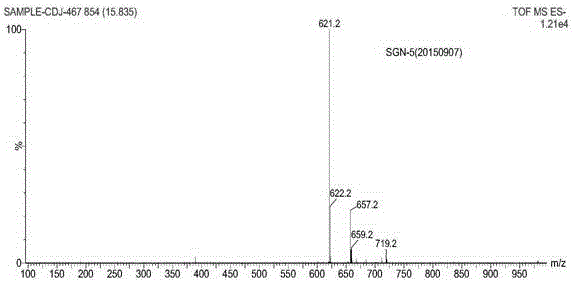
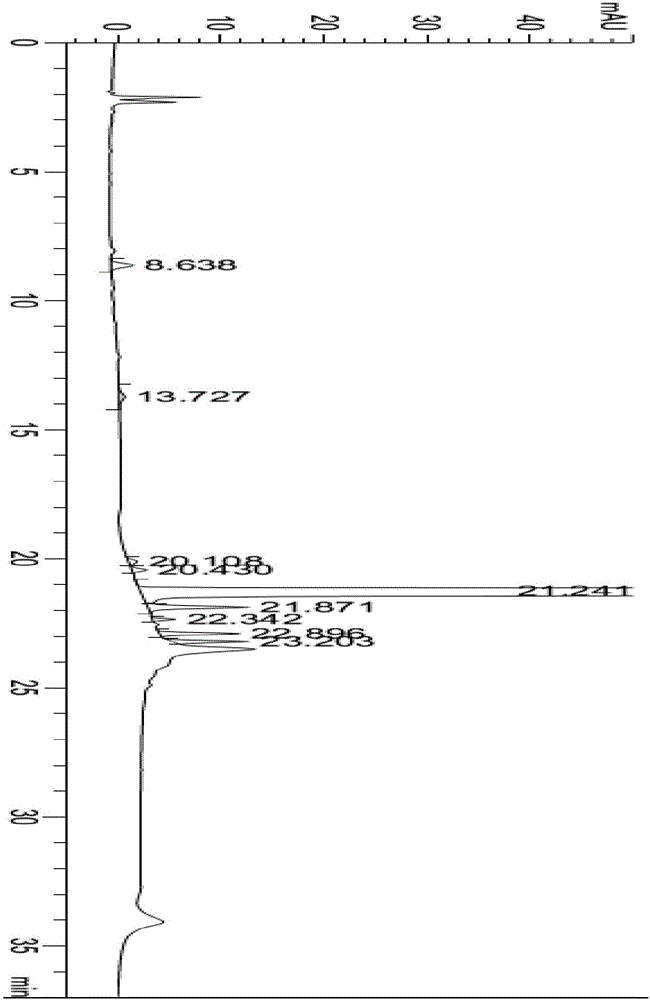
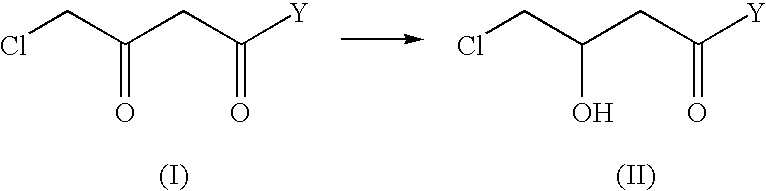
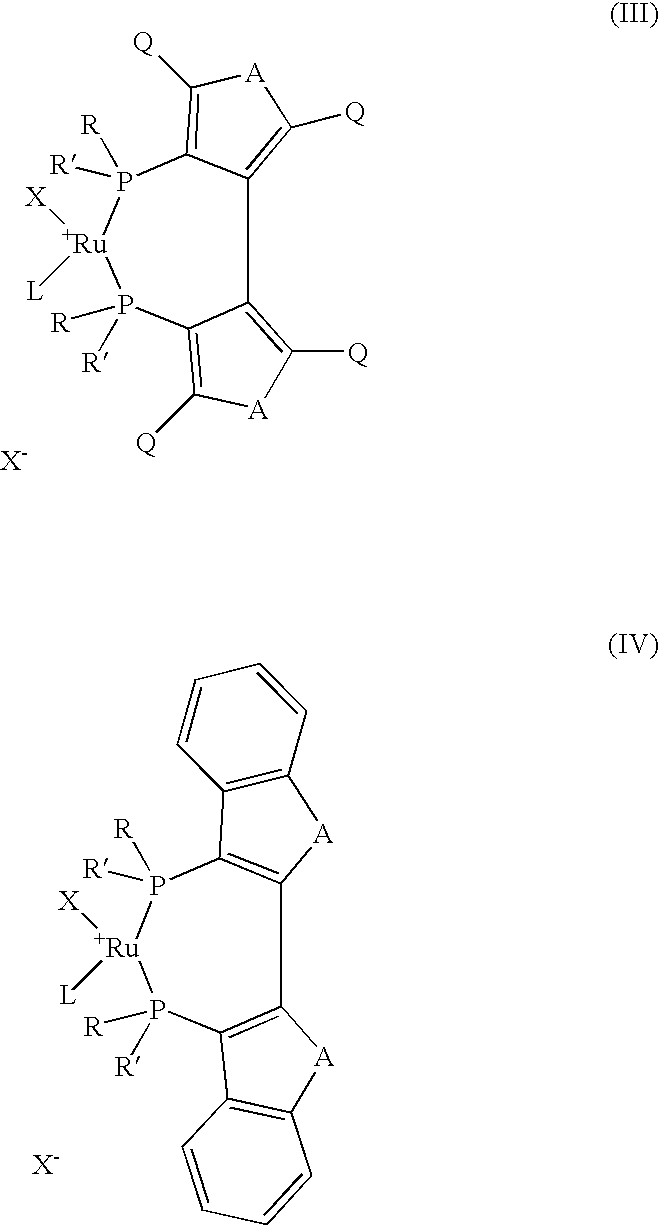
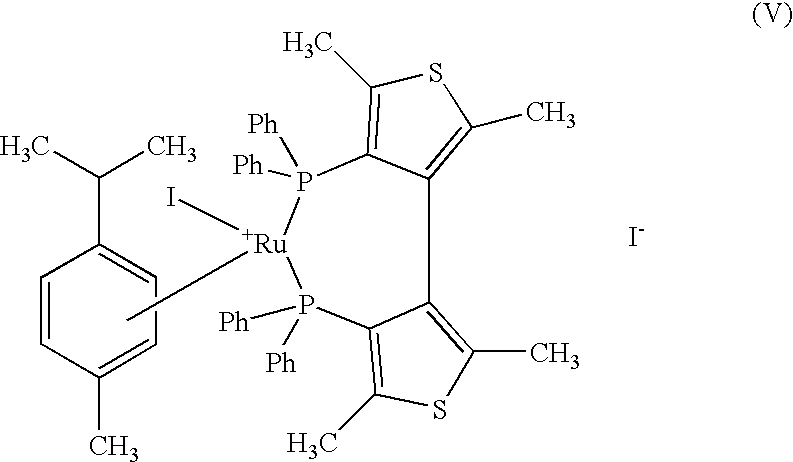
The present invention describes a process for the industrial production of L-carnitine, comprising the enantioselective reduction of an alkyl 4-chloro-3-oxobutyrate or 4-chloro-3-oxobutyramide. The optically active 3-hydroxy derivative thus obtained is reacted with trimethylamine, obtaining crude L-carnitine, which is then finally purified. The catalyst used for the reduction is a complex of ruthenium bound to a penta-atomic bis-heteroaromatic system. The reduction reaction, performed in controlled conditions of hydrogen pressure, substrate concentration, temperature, and substrate: catalyst molar ratio, enables 4-chloro-3-hydoxybutyrate or 4-chloro-hydroxybutyamide to be obtained in a high yield. The process described, which leads to L-carnitine being obtained, is easily applicable on an industrial scale.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Oxiracetam compound and new method thereof
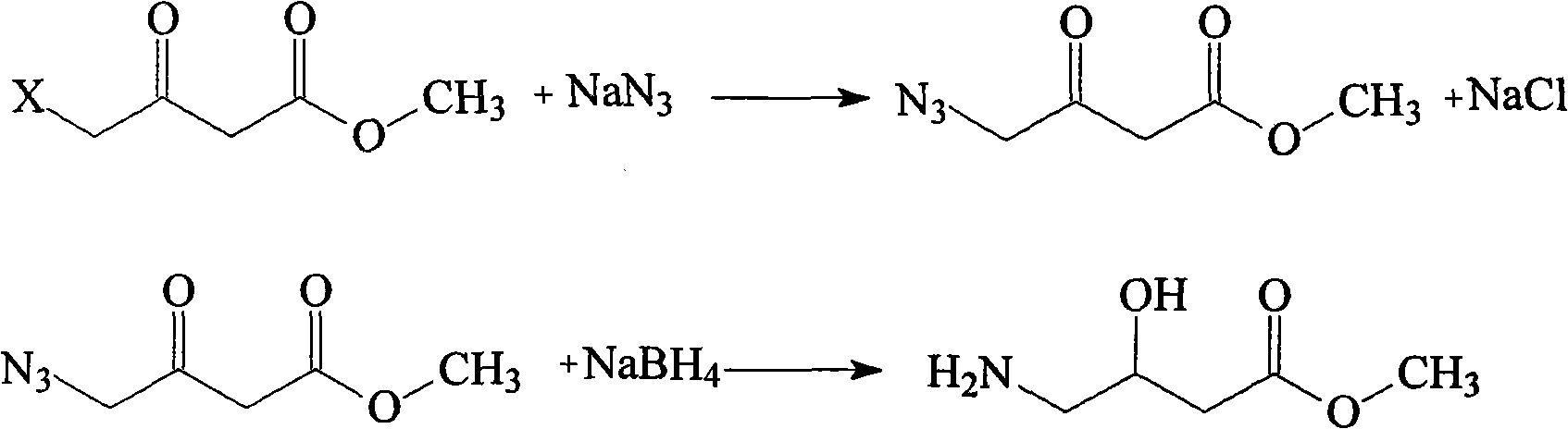
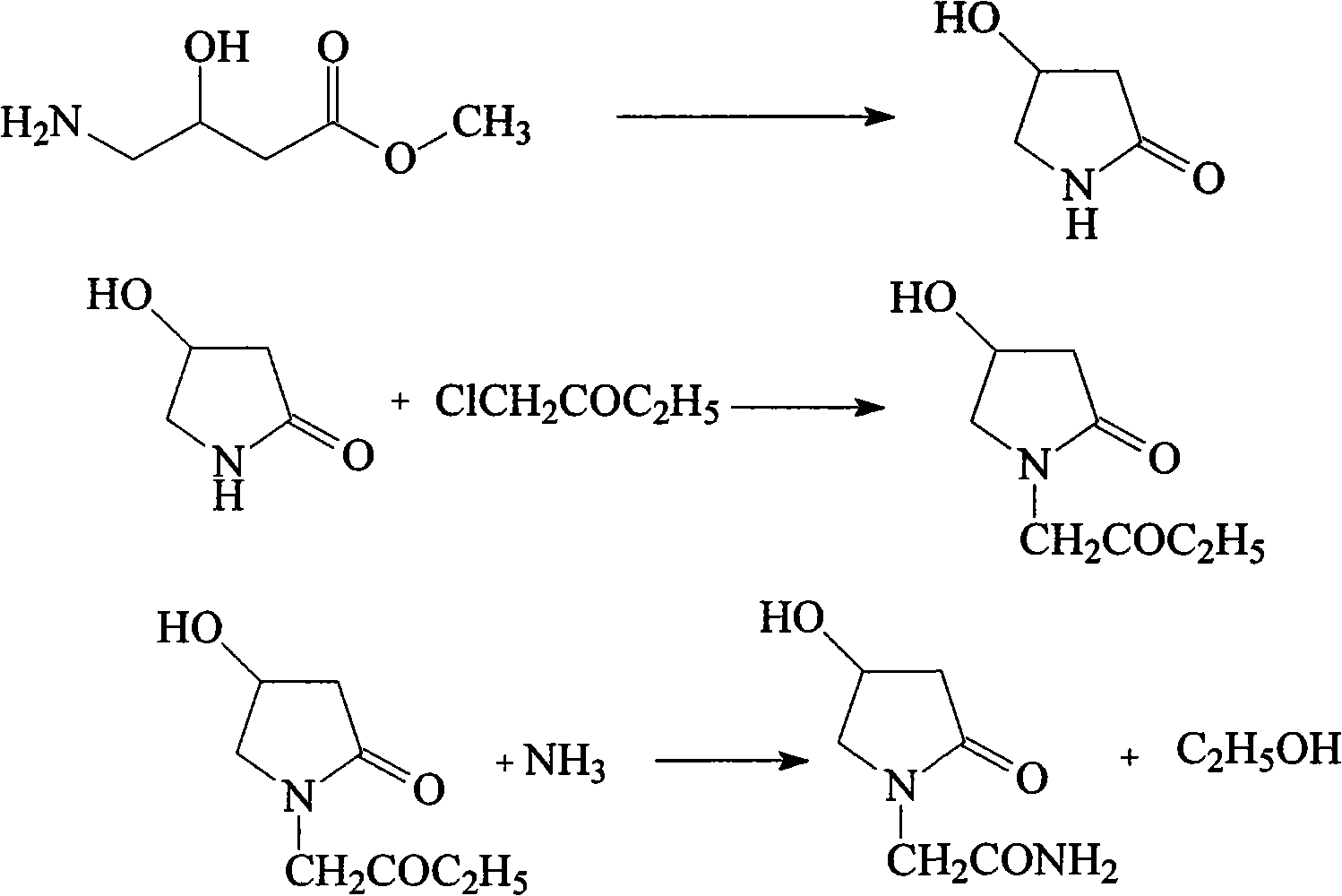
The invention relates to an oxiracetam compound and a new method thereof. In the method, 3-chloro-2-hydroxy propionitrile is used as an initial raw material. The method comprises the following steps of: synthesizing 4-hydroxyl-2-pyrrolidone through 4-chloro-3-hydroxyl-butylamide; and then synthesizing oxiracetam. The invention overcomes the defects of complicated preparation process, trouble steps, high cost, low product purity and difficult purification of the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Electric rheological liquid
Owner:THE HONG KONG UNIV OF SCI & TECH
Processes for the Preparation of Zolpidem and its Hemitartrate
The invention relates to the preparation of a non-hygroscopic polymorphic form of zolpidem hemitartrate, designated as Form I, and pharmaceutical compositions including it. The invention also relates to use of the compositions for treating anxiety, sleep disorders and convulsions. The invention also relates to a process for the preparation of zolpidem or pharmaceutically acceptable salts thereof by condensing 3-bromo-N,N-dimethyl-4-oxo-4-p-tolyl-butyramide with 2-amino-5-methylpyridine in a polar aprotic solvent.
Owner:RANBAXY LAB LTD
Preparation method and application of ionic liquid for absorbing SO2
InactiveCN103071367AImprove gas efficiencyLow costDispersed particle separationN dimethylformamideLithium chloride
The invention provides a preparation method of an ionic liquid for absorbing SO2, and an application of the ionic liquid in absorbing SO2. The preparation method of the ionic liquid comprises the steps of uniformly mixing one of aluminium chloride, zinc chloride, lithium chloride, aluminum sulfate, zinc sulfate, lithium sulfate, aluminium nitrate, zinc nitrate and lithium nitrate with one or two of urea, propanamide, butyramide, caprolactam, N,N-dimethylformamide and N,N-dimethylacetamide at a molar ratio of 1:(1-10) or 1:(0.5-3):(1-7), conducting reaction for 1-10h at 70-150 DEG C, conducting vacuum drying on an obtained solution at 40-60 DEG C, and obtaining the ionic liquid. The ionic liquid is mainly used for quickly and efficiently absorbing SO2. With the adoption of the ionic liquid as an SO2 absorbent, requirements on equipment required for absorbing SO2 are simple, the operational conditions are mild, the absorption efficiency is high, the cost is low, the ionic liquid can be recycled, and waste liquid and waste water discharge and other problems do not exist, and the ionic liquid is suitable for industrial production, meets the requirements of green chemical development of the contemporary society, and has good economic and social benefits.
Owner:SHIJIAZHUANG UNIVERSITY
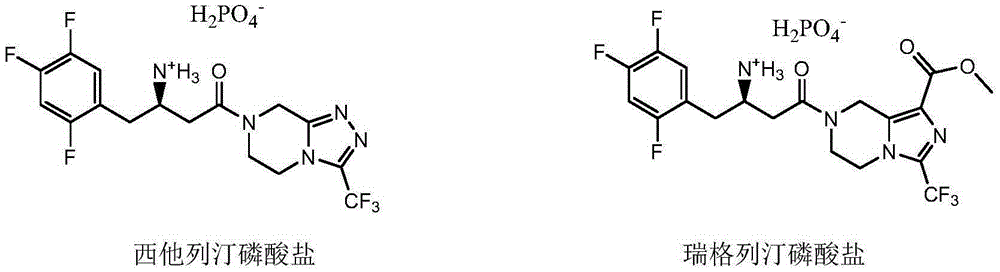
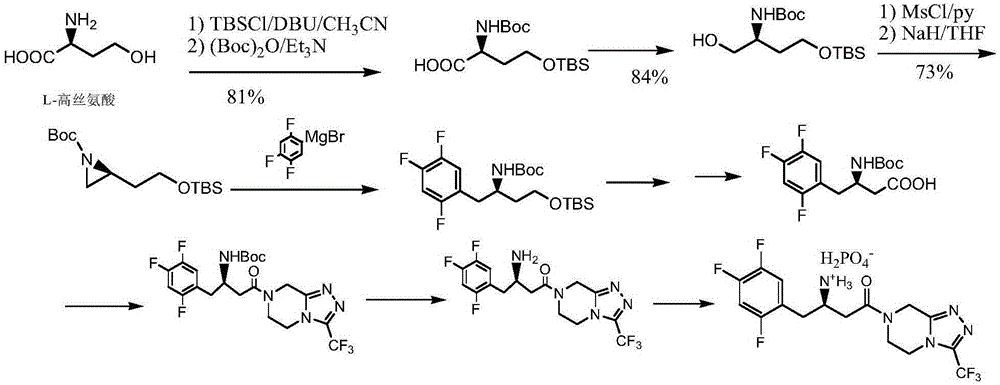
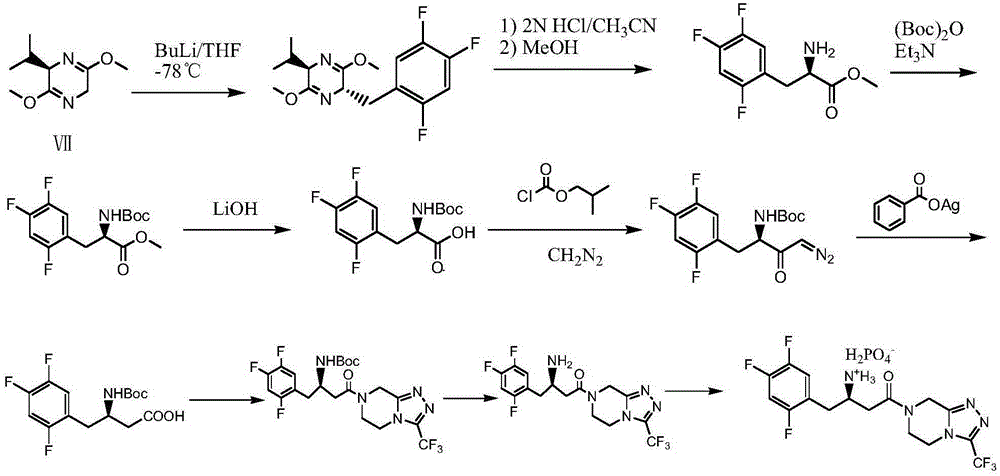
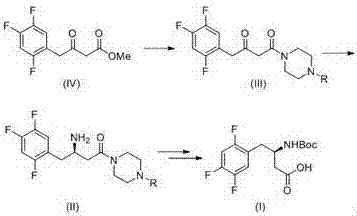
Sitagliptin impurity and preparation and detection method thereof
ActiveCN106478637AEnsuring Safety and ReliabilityHigh yieldOrganic chemistryComponent separationSitagliptinPyrazine
The present invention provides sitagliptin impurity compound I and a preparation method and application thereof in the quality control study of sitagliptin. The sitagliptin impurity compound I is as follows: (R)-3-amino-N-[(R)-4-oxy-4-(3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazole[ 4, 3-a] pyrazine-7(8H)-yl)-1-(2, 4, 5-trifluorophenyl) butyl-2-yl)-4-(2, 4, 5-trifluorophenyl) butyramide.
Owner:NANJING CHIA TAI TIANQING PHARMA
Industrial process for the production of L-carnitine
InactiveUS20020165408A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureEthyl Chloride
The present invention describes a process for the industrial production of L-carnitine, comprising the enantioselective reduction of an alkyl 4-chloro-3-oxobutyrate or 4-chloro-3-oxobutyramide. The optically active 3-hydroxy derivative thus obtained is reacted with trimethylamine, obtaining crude L-carnitine, which is then finally purified. The catalyst used for the reduction is a complex of ruthenium bound to a penta-atomic bis-heteroaromatic system. The reduction reaction, performed in controlled conditions of hydrogen pressure, substrate concentration, temperature, and substrate: catalyst molar ratio, enables 4-chloro-3-hydoxybutyrate or 4-chloro-hydroxybutyamide to be obtained in a high yield. The process described, which leads to L-carnitine being obtained, is easily applicable on an industrial scale.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Preparation process of atorvastatin calcium
The invention provides a preparation process of atorvastatin calcium, which is characterized by being divided into two steps when preparing a condensation compound (4R-cis)-1,1-dimethyl ethyl-6-[2-[2-(4-fluorophenyl)-5-(1-methyl ethyl)-3-phenyl-4-[(phenyl amino)-carbonyl]-1H-pyrrole-1-yl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate: 1), adding (4R-cis)-6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tertbutyl acetate (called ATS-9 for short) into a mixed solvent of n-heptane, tetrahydrofuran and toluene to react with pivalic acid; and 2), adding 4-fluorine-alpha-[2-methyl-1-oxygen-propyl]-gamma-oxo-N,beta-diphenyl phenyl butyramide (called M-4 for short) and rising the temperature for reaction. The invention has the advantage of capability of greatly improving the yield of the intermediate condensation compound and is more in favor of industrialized production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Preparation method of 3R-amino substituted butyrylamide derivative
ActiveCN105294479ASimple methodGood reaction selectivityOrganic compound preparationCarboxylic acid amides optical isomer preparationEnamineButyramide
The invention relates to a preparation method of a 3R-amino substituted butyrylamide derivative. The preparation method of the 3R-amino substituted butyrylamide derivative comprises the following steps: carrying out hydrogenation reduction on enamine II for preparing 3R,3S-amino substituted butyrylamide racemate III; carrying out resolution, and filtering, so as to obtain 3R-amino substituted butyrylamide R(-) mandelate with high ee% value, alkalifying to obtain the 3R-amino substituted butyrylamide derivative; and oxidizing the obtained mother liquor, so as to obtain enamine II in a recycling manner, and repeating oxidation-reduction cycle for multiple times until products at specified yield are obtained. The preparation method of the 3R-amino substituted butyrylamide derivative has the advantages that a cyclic method is utilized for converting 3S-amino substituted butyrylamide racemate or 3R,3S-amino substituted butyrylamide mixture in any ratio into the 3R-amino substituted butyrylamide derivative with high ee% value, so that the mother liquor is effectively utilized; meanwhile, an expensive catalyst or asymmetric reductive amination with high demand condition does not need to be adopted, thereby being beneficial to reduction of cost and clean production.
Owner:XINFA PHARMA
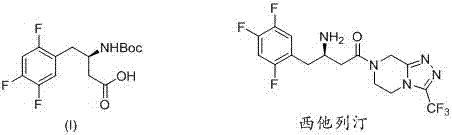
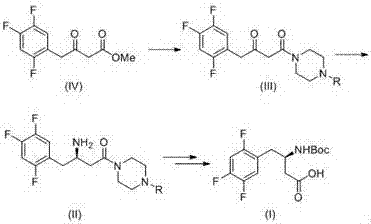
Method for synthetizing (R)-N-BOC-3-amino-4-(2,4,5-trifluorophenyl) butyric acid by adopting transaminase method
InactiveCN107365809AIngenious designThe synthetic route is simpleFermentationButyramideCarboxybenzyl
The present invention provides a method for synthesizing (R)-N-BOC-3-amino-4-(2,4,5-trifluorophenyl) butyric acid (I) by transaminase method, in which 3-oxo-4- (2,4,5-trifluorophenyl) methyl butyrate (IV) is a starting material, reacted with nitrogen-protected piperazine to obtain 3-oxo-4-(2,4,5-trifluorophenyl) butane Amide intermediate (III); (III) reacts in the presence of amino donor and transaminase, and is converted into 3-amino-4-(2,4,5-trifluorophenyl) butyramide intermediate (II); with ( The amide of II) is hydrolyzed into an acid, and the amino group is protected by BOC to obtain the target intermediate (I); wherein the protecting group of piperazine in formula (III) and formula (II) is C1-4 alkoxycarbonyl, benzyloxycarbonyl or methyl. The invention has the advantages of ingenious design, simple and efficient synthesis route, simple and easy process flow, and environmental protection, and provides a new method for industrial scale production of formula (III).
Owner:SHANGHAI PUYI CHEM CO LTD
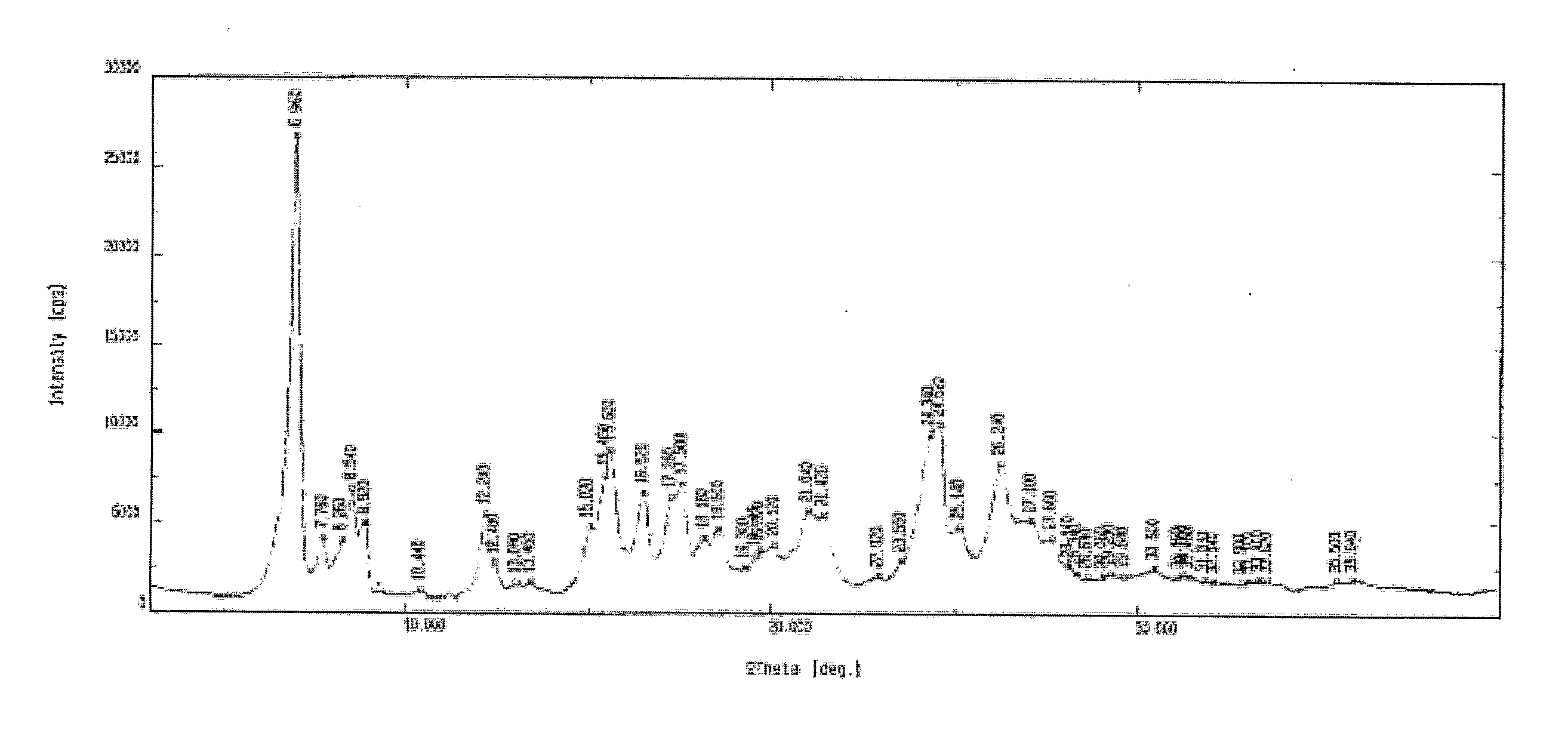
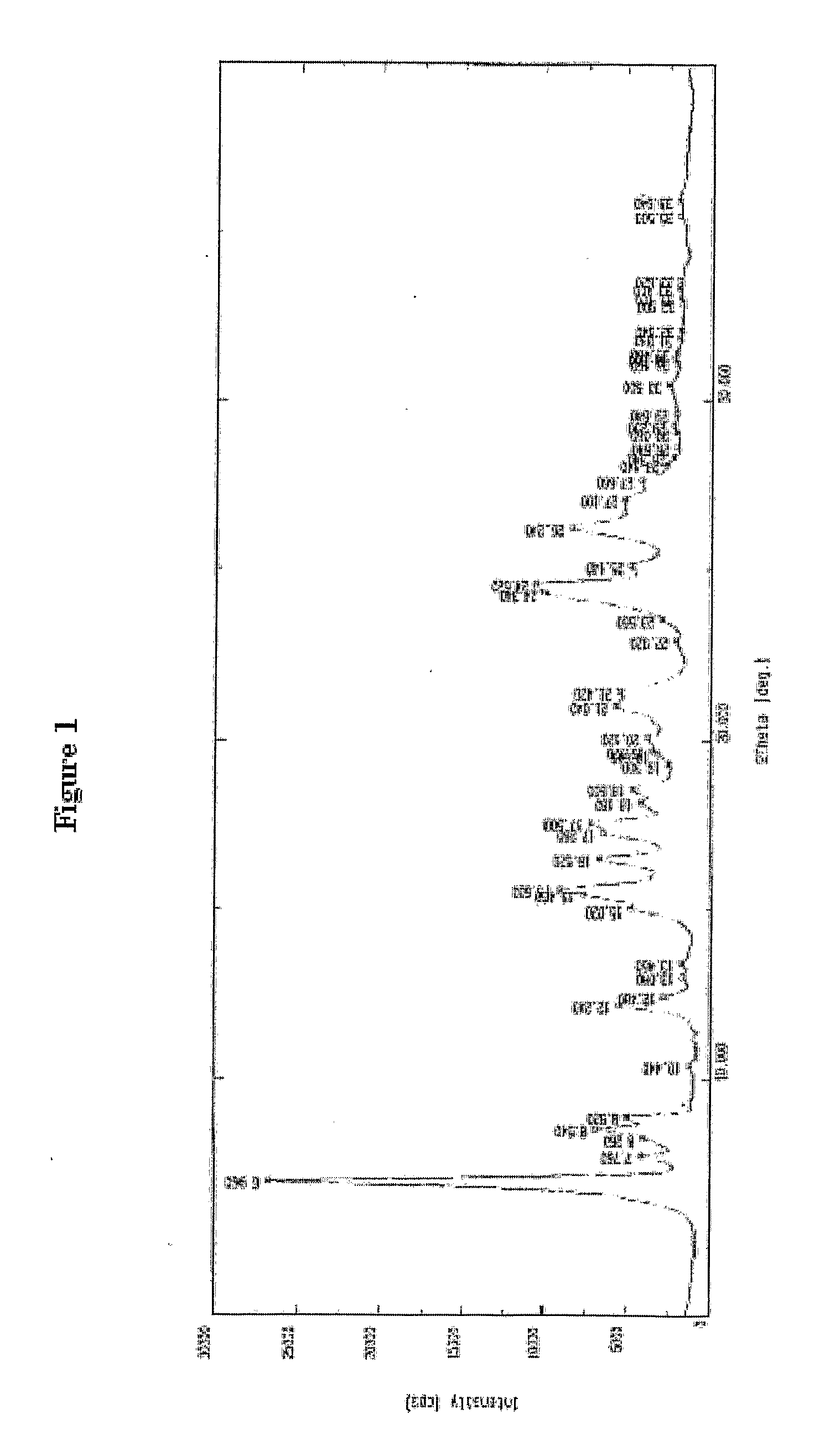
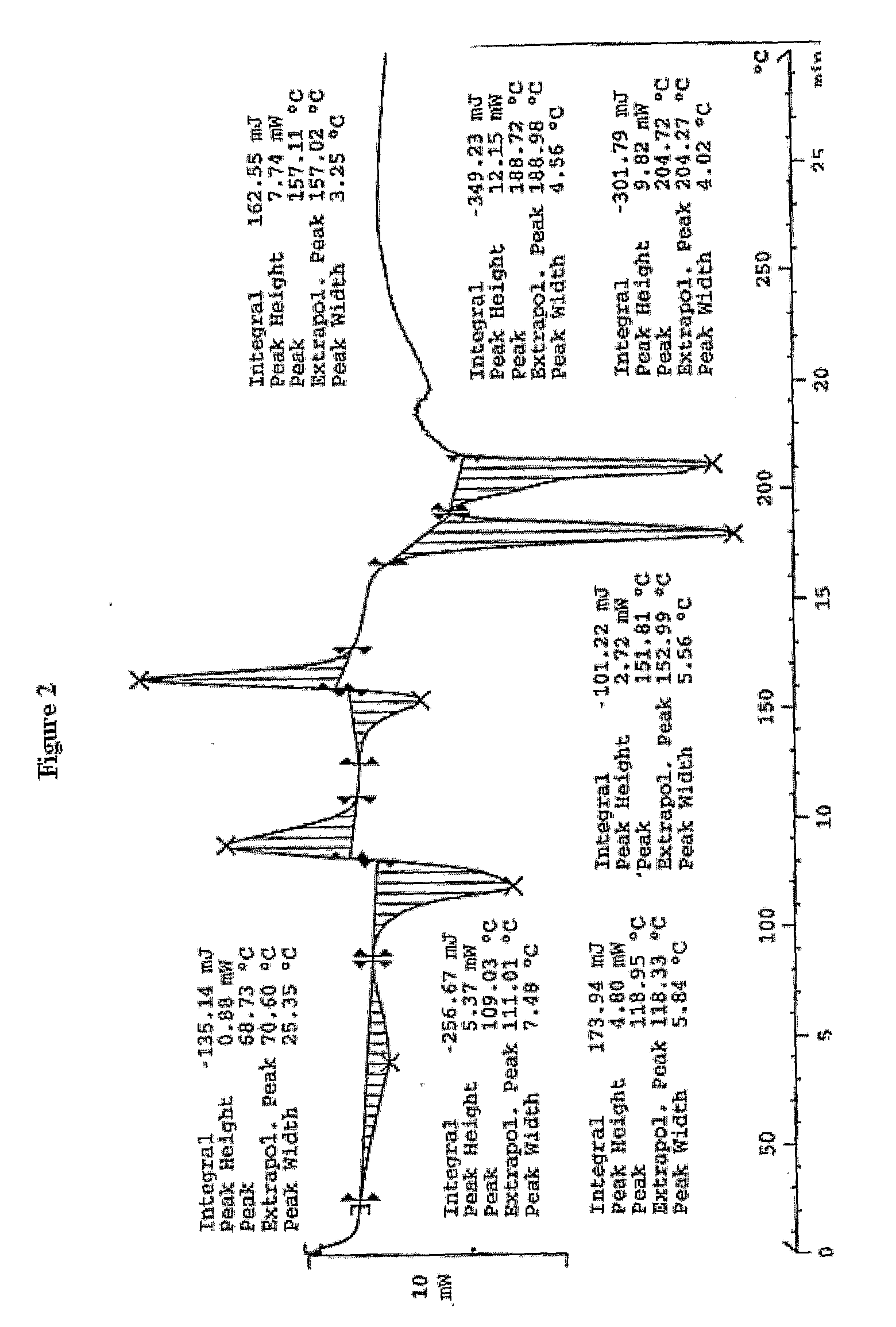
PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE
InactiveUS20130184493A1Minimize formationOrganic compound preparationCarboxylic acid amide separation/purificationDiketoneKetone
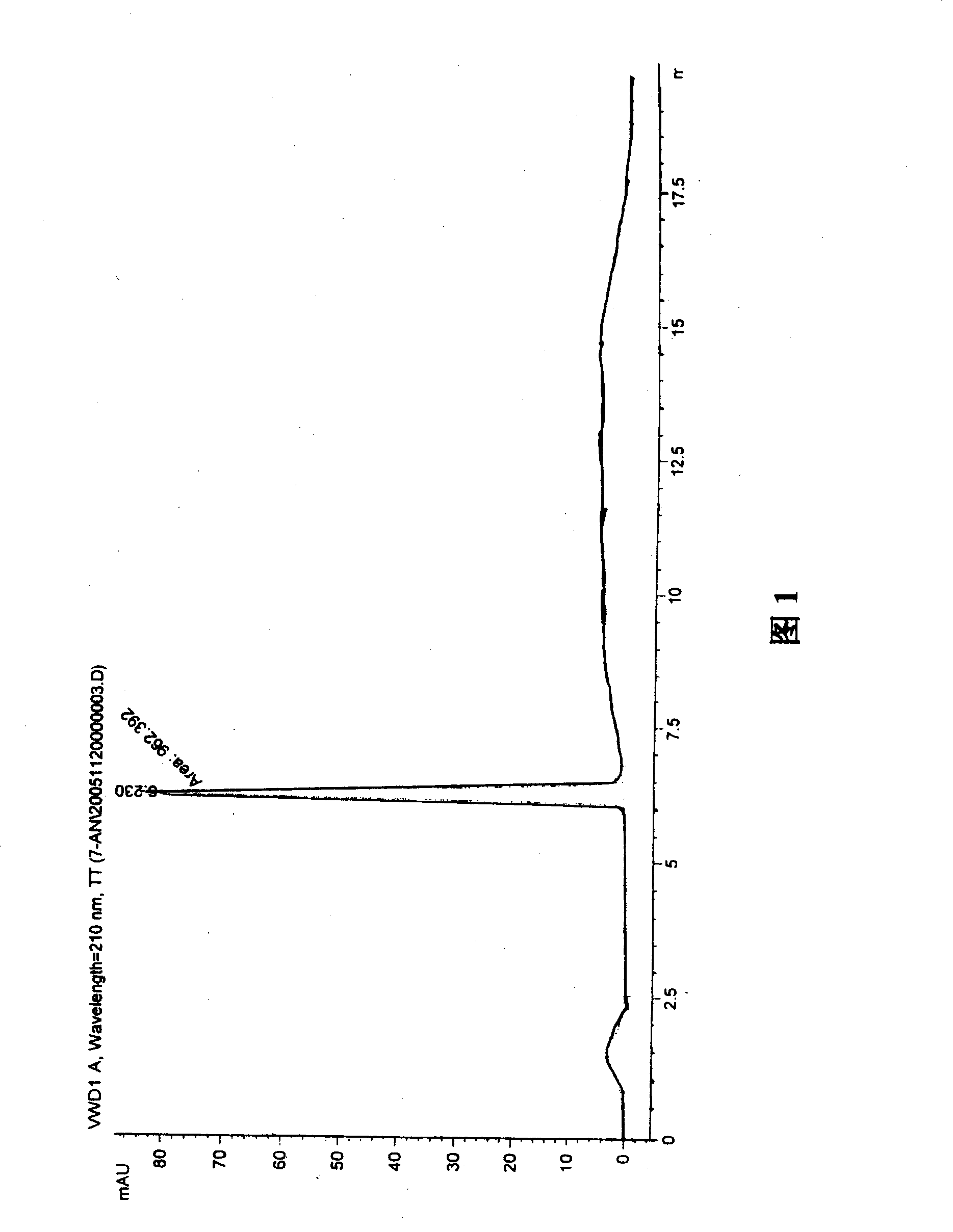
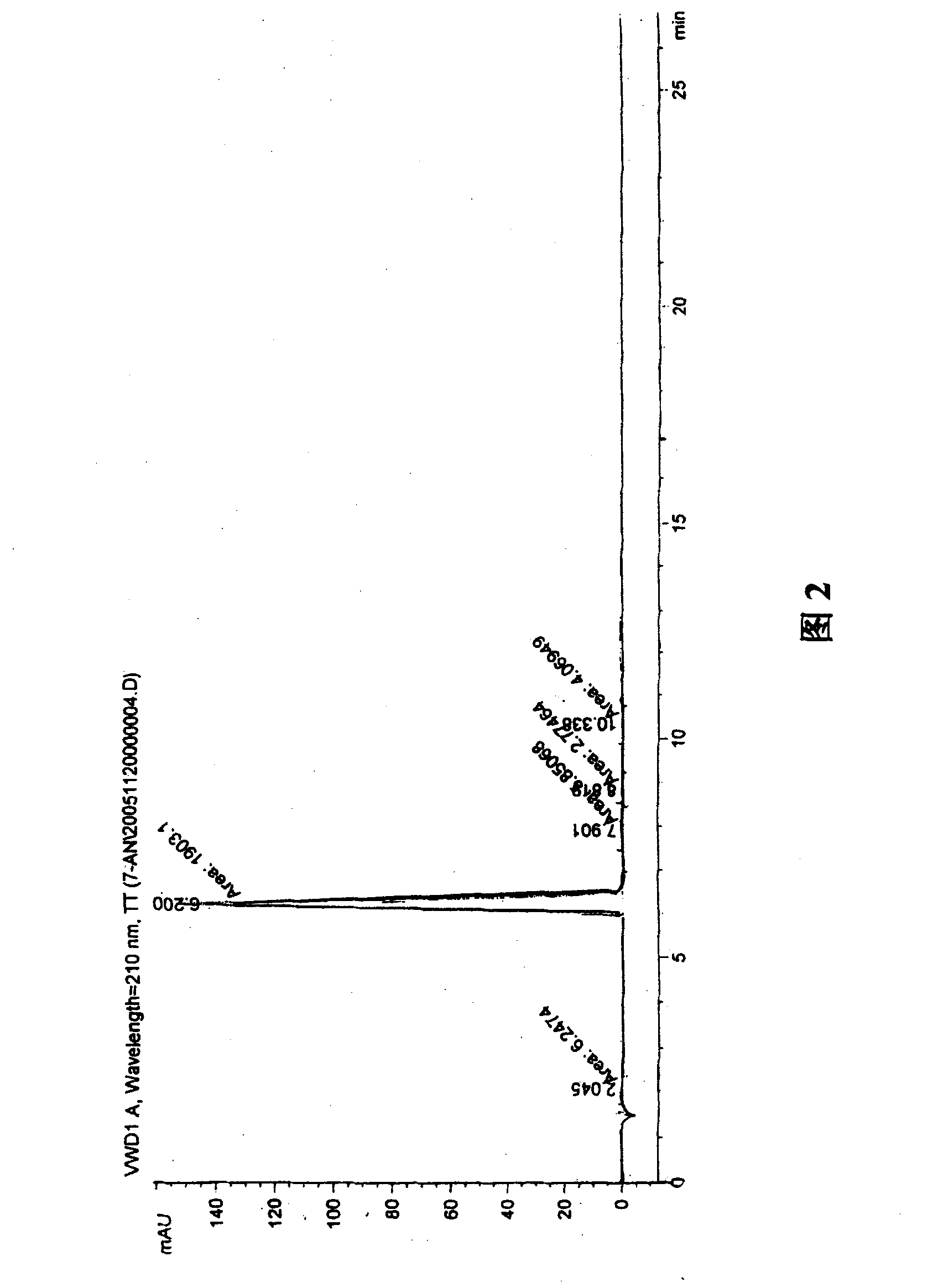
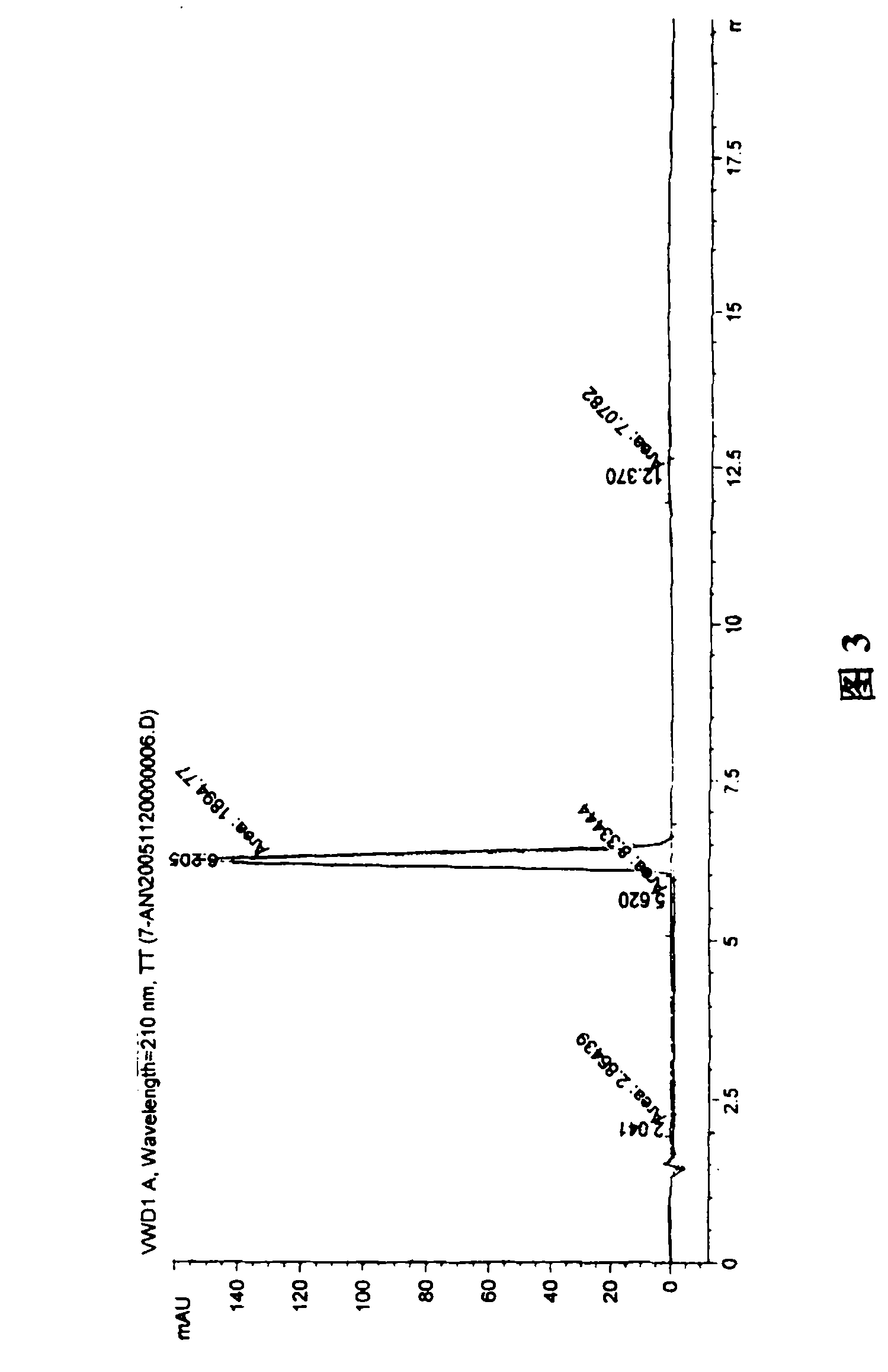
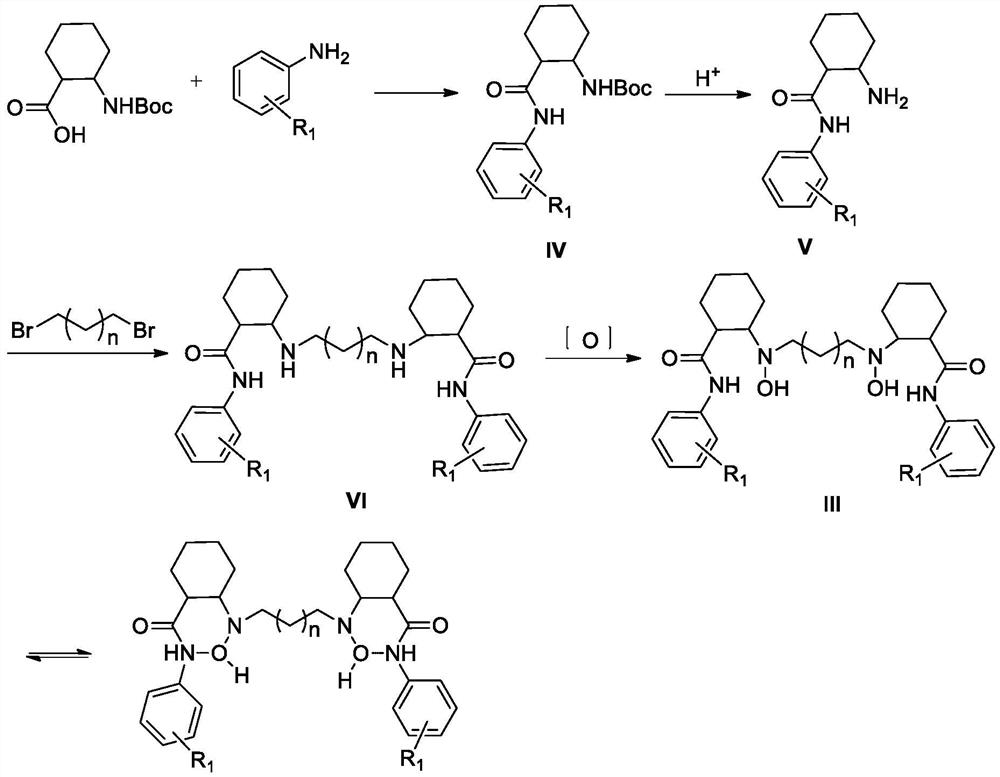
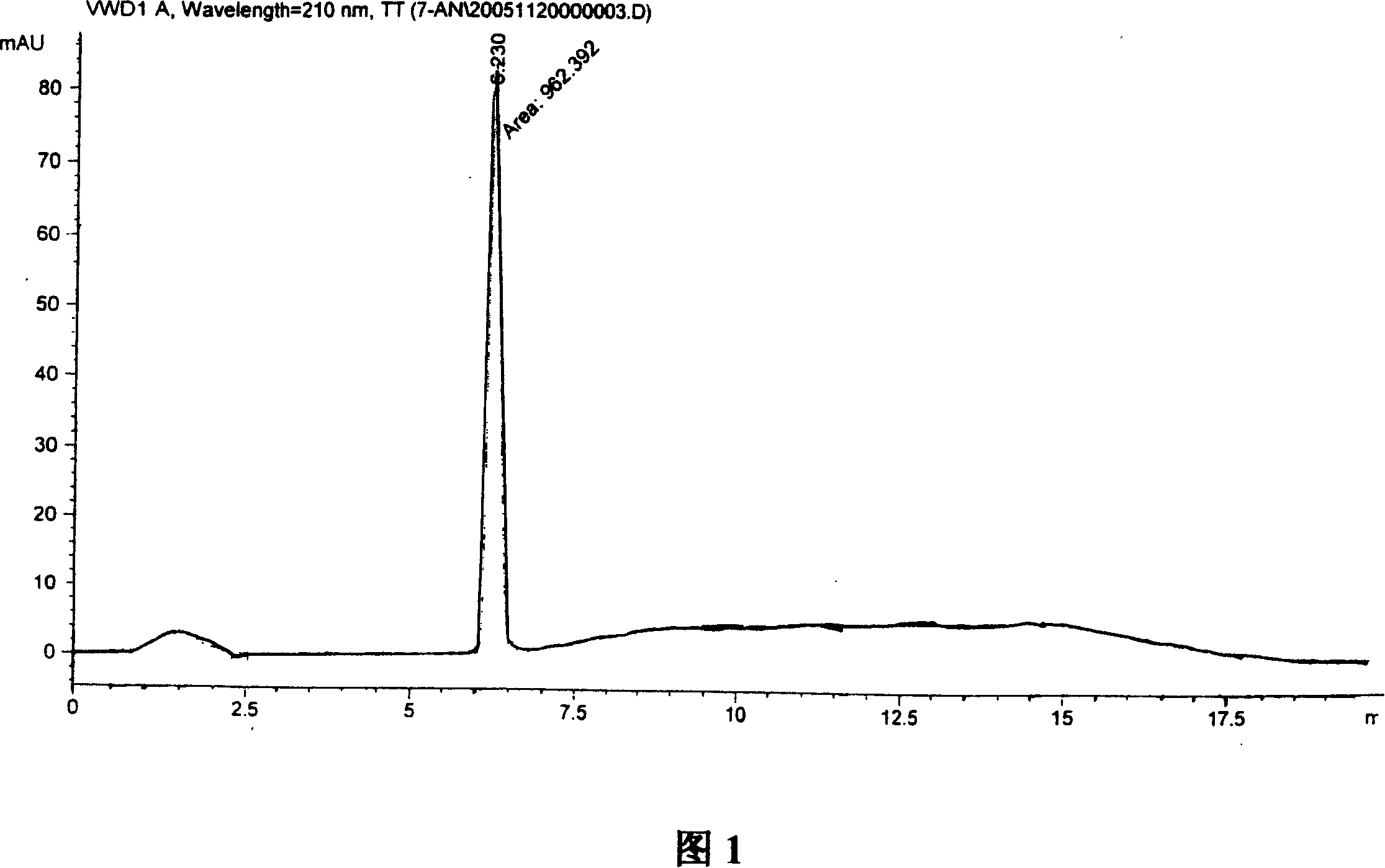
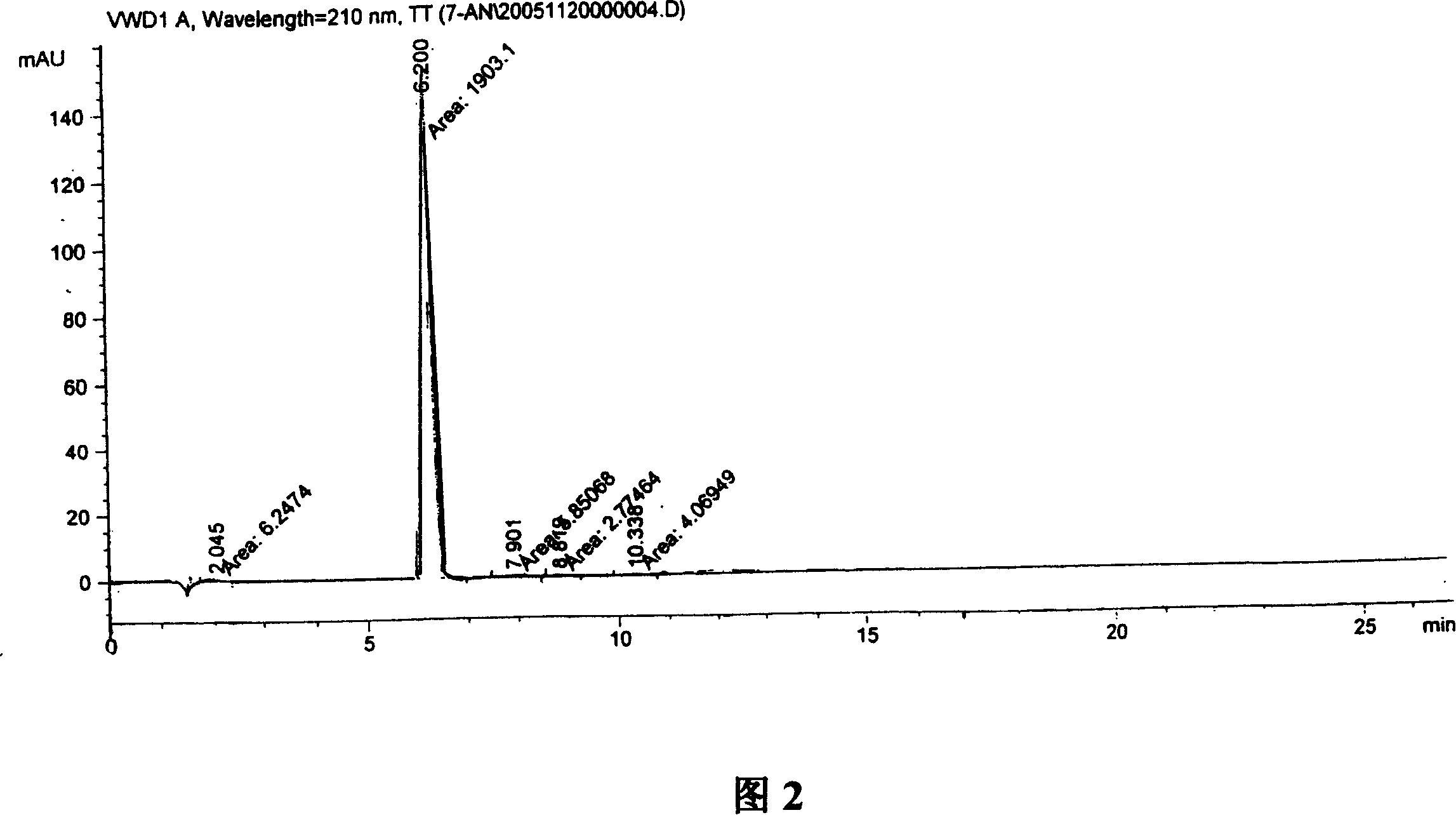
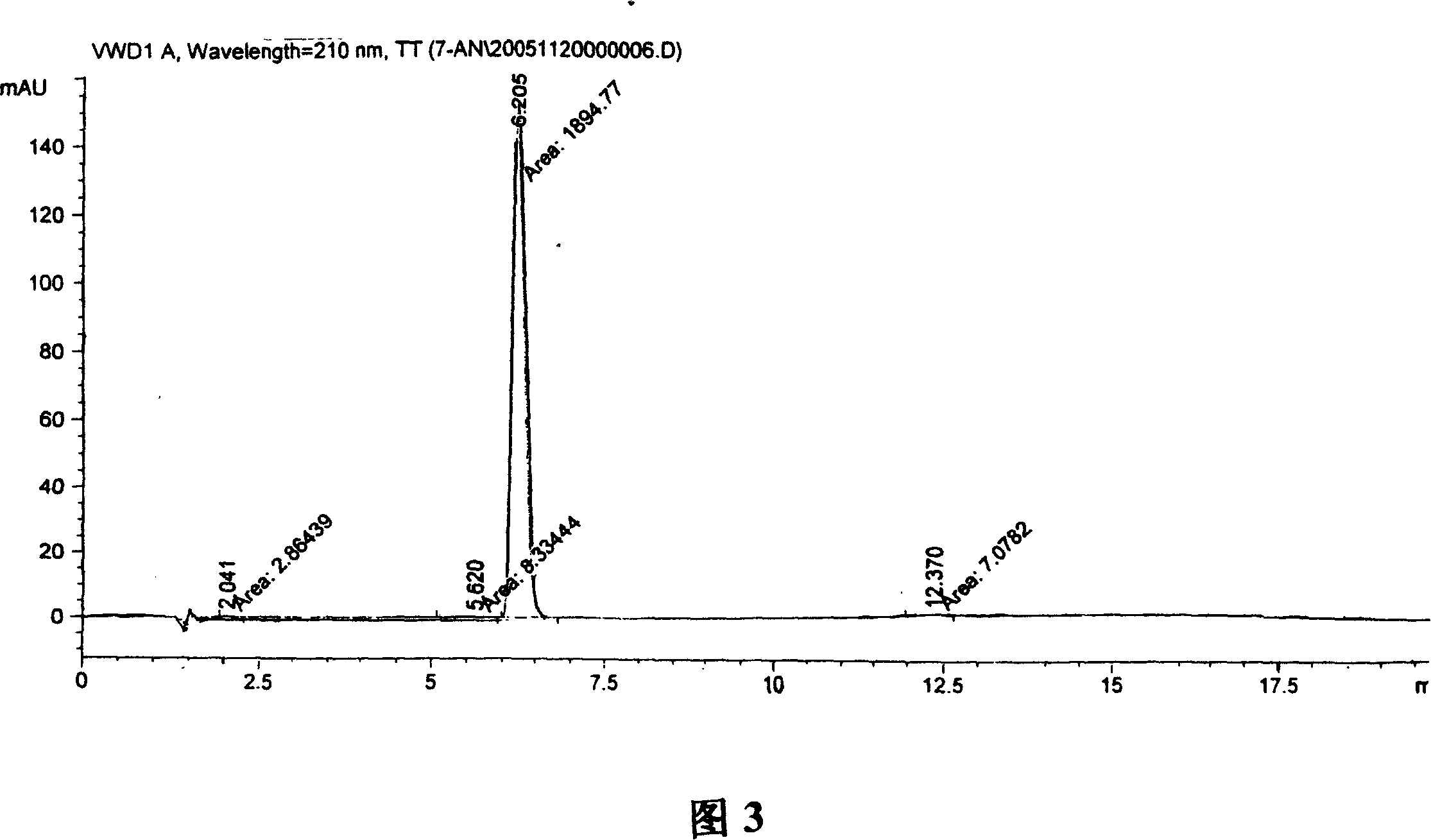
A process for preparation of 4-fluoro-α-[2-methyl-1-oxopropyl]-γ-oxo-N-β-diphenylbenzene butane amide also known as a diketone intermediate of atorvastatin, completely devoid of impurities 3,4-difluoro-α-[2-methyl-1-oxopropyl]-γ-oxo-n-β-diphenylbenzene butane amide; methyl, 2{-2[-(4-fluorophenyl)-2-oxo-1-phenylethyl)]}-4-methyl-3-oxo pentanoate; 1,4-bis(4-fluorophenyl)-2,3-diphenylbutane-1,4-dione, 1-(4-fluorophenyl)-2-phenyl ethanone; 1-(4-fluorophenyl)-2-phenyl ethanone and containing about 0.05% or less of 2-methyl-1-oxopropyl]-γ-oxo-N-β-diphenylbenzene butane amide. In that process the said diketone intermediate of formula 1 is obtained by maintaining temperature −25° C. to 50° C. during Friedel-Crafts acylation, in situ halogenation of formula II in presence of a solvent and nucleophilic substitution from a compound of formula III with formula IV in presence of a base.
Owner:VIJAYASRI ORGANICS
Synthesis, split and racemization method for preparing chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504BReduce dosageHigh purityOrganic compound preparationCarboxylic acid amides optical isomer preparationEthyl groupRacemization
Owner:ABA CHEM CORP
Method for industrially improving production efficiency of N,2,3-trimethyl-2-isopropyl butyramide
InactiveCN106220524AImprove reaction speedImprove conversion efficiencyOrganic compound preparationCarboxylic acid amide separation/purificationAcetic acidAqueous sodium hydroxide
The invention provides a method for industrially improving the production efficiency of N,2,3-trimethyl-2-isopropyl butyramide. The method comprises the following steps: (1) adding 2,3-dimethyl-2-isopropyl-butyronitrile and polyphosphoric acid, which are reaction materials, into a reaction kettle, slowly adding dimethyl carbonate dropwise under the conditions of heating and stirring, adding water dropwise in batches after finishing dropwise adding, continuing to preserve the heat at the temperature of 110-180 DEG C for 12 hours, stopping heating; (2) reducing the temperature of the reaction kettle to lower than 90 DEG C after finishing the reaction, slowly adding a sodium hydroxide aqueous solution of 30% dropwise until the PH value of a system reaches 7, adding ethyl acetate, fully stirring, removing an aqueous phase, cooling, crystalizing to obtain a finished product of the N,2,3-trimethyl-2-isopropyl butyramide. The adding mode of the dimethyl carbonate is changed to slowly adding dropwise, proper amount of water is added in batches after finishing adding the dimethyl carbonate dropwise, so that the reaction speed and the conversion efficiency of the 2,3-dimethyl-2-isopropyl-butyronitrile are both greatly improved.
Owner:ANHUI FENGLE PERFUME
Efficient cleanup additive for gas well and preparing method of efficient cleanup additive
InactiveCN103820096AReduce surface tensionIncrease contact angleDrilling compositionFatty acidBackflow
The invention discloses an efficient cleanup additive for a gas well and a preparing method of the efficient cleanup additive. The method comprises the following steps: adding 2-5% by volume of a base agent namely perfluoro alkyl butyrylamide-propyl dimethylamine ammonia chloride-sodium sulfonate into a reactor; uniformly mixing, heating, keeping the temperature in the reactor between 80 DEG C and 100 DEG C, and then adding 5-8% by volume of ethanol, 84-91% by volume of water and 2-3% by volume of methyl propyl dibutyl fatty acid ammonium; reacting for 3 to 5 hours while agitating so as to obtain colorless transparent liquid. The preparing technology is simple, the materials are easy to obtain, and the effect of reducing the damage of aggressive water to a hyposmosis gas reservoir is obvious; through the adoption of the cleanup additive for gas well fracturing, the surface tension and extrusion pressure are reduced, and the backflow of operational liquid is facilitated.
Owner:PETROCHINA CO LTD
Method for preparing levetiracetam
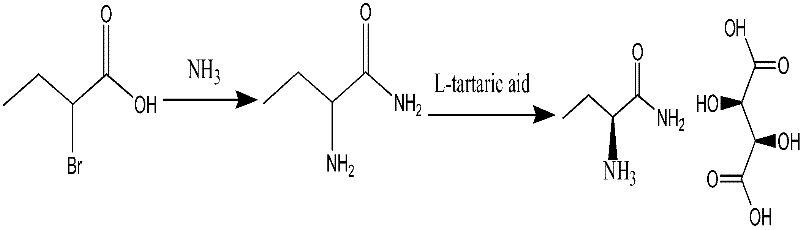
The invention provides a method for preparing levetiracetam, comprising the following steps of: taking 2-bromine butyric acid as a raw material, subjecting to ammonolysis reaction, concentrating till drying, adding carbinol, crystallizing and drying so as to obtain 2-amino butyramide solid; dissolving the 2-amino butyramide solid in the carbinol, resolving and salifying by utilizing L-(+) - tartaric acid under the existence of a catalyst so as to obtain S-(+)-2- amino butyramide tartrate; and putting the S-(+)-2- amino butyramide tartrate into an aprotic solvent, adding anhydrous sodium sulfate and tetrabutyl amonium bromide, reacting with 4-chlorobutyryl chloride under an alkaline condition, filtering, decompressing filtrate, recovering the aprotic solvent, adding acetone into the surplus filtrate, recrystallizing and drying so as to obtain levetiracetam solid. The preparation method provided by the invention has the advantages of short reaction step, mild reaction condition, low cost and high yield.
Owner:ZHEJIANG JIANGBEI PHARMA
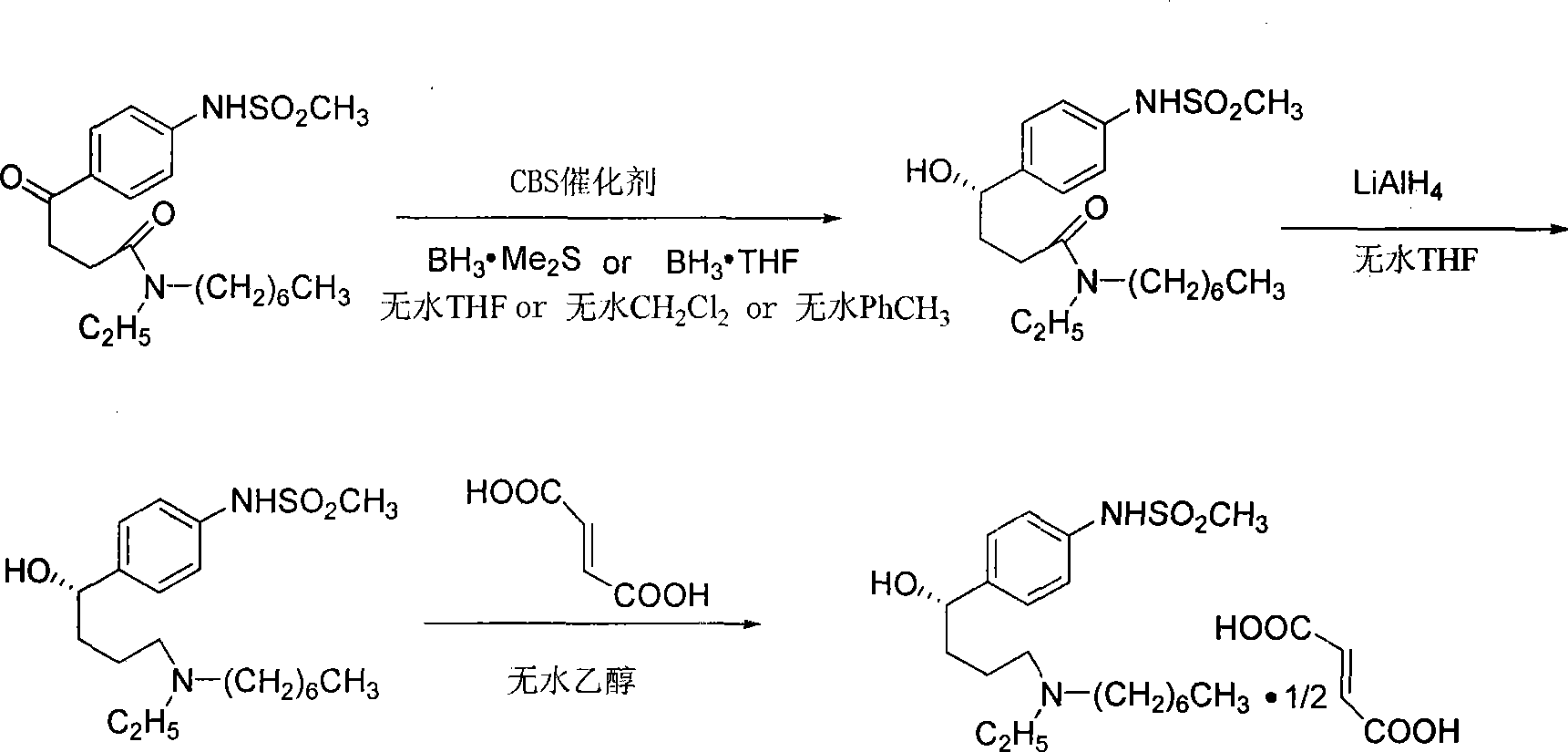
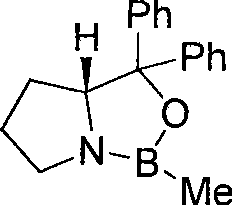
Preparation of optically-active pure ibutilide fumarate
InactiveCN101412687ASimple and fast operationHigh yieldSulfonic acid amide preparationCardiovascular disorderButyramideCBS catalyst
The invention provides a method for preparing optical rotation pure ibutilide fumarate. The method is characterized by comprising the following steps: N-ethyl-N-heptyl-4-oxo-4-(4-mesylamino phenyl) butyrylamide is subjected to asymmetric catalytic reduction by using CBS catalyst to obtain (S)-N-ethyl-N-heptyl-4-hydroxide radical-4-(4-(mesylamino)phenyl) butyrylamide; the (S)-N-ethyl-N-heptyl-4-hydroxide radical-4-(4-(mesylamino)phenyl) butyrylamide is reduced by LiAlH4 to be salified with fumaric acid to finally obtain the optical rotation pure ibutilide fumarate. The preparation method has the advantages of simple and convenient operation, mild reacting condition, good enantioselectivitiy, environment al friendliness, low cost and high yield.
Owner:ZHEJIANG NORMAL UNIVERSITY
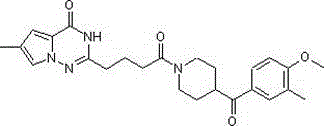
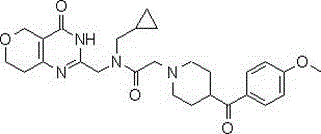
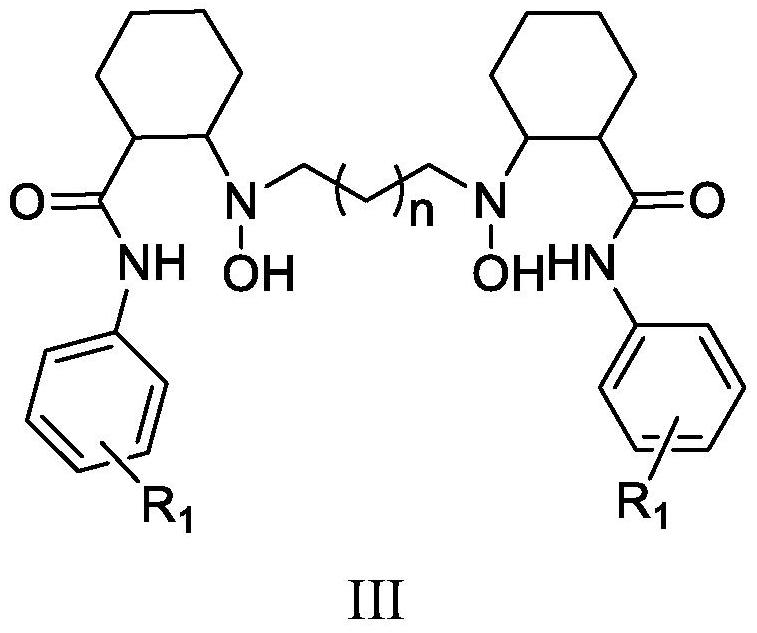
Heterocyclyl-butanamide derivatives
Compounds of the formula I, in which W, X and Y have the meanings indicated in Claim 1, are inhibitors of Tankyrase, and can be employed, inter alia, for the treatment of diseases such as cancer, cardiovascular diseases, central nervous system injury and different forms of inflammation.
Owner:MERCK PATENT GMBH
Application of amide compounds to preparation of quorum sensing activity inhibitors
InactiveCN107473980ASimple preparation processControllable fermentation conditionsBiological material analysisMicroorganism based processesOrganismVirulence factor
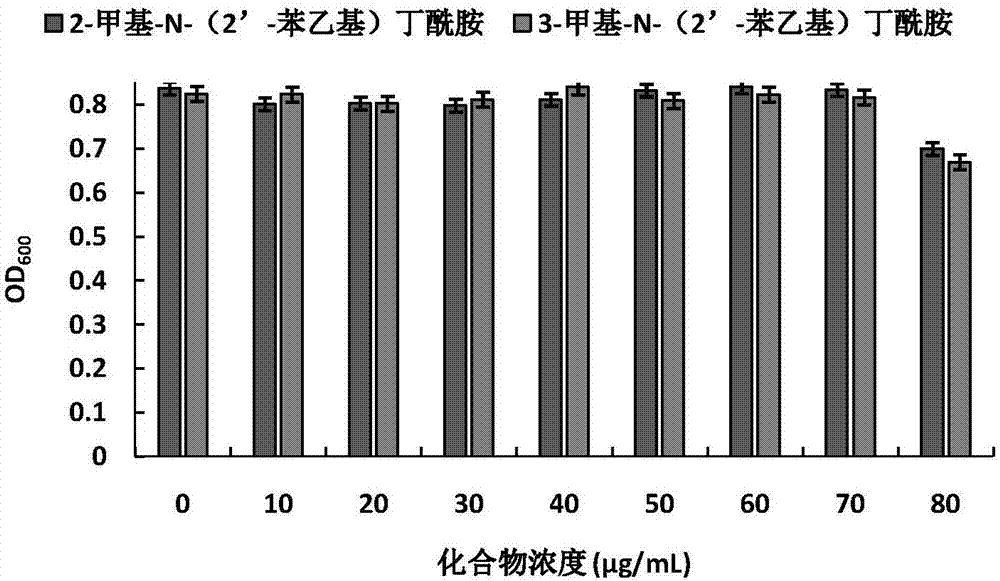
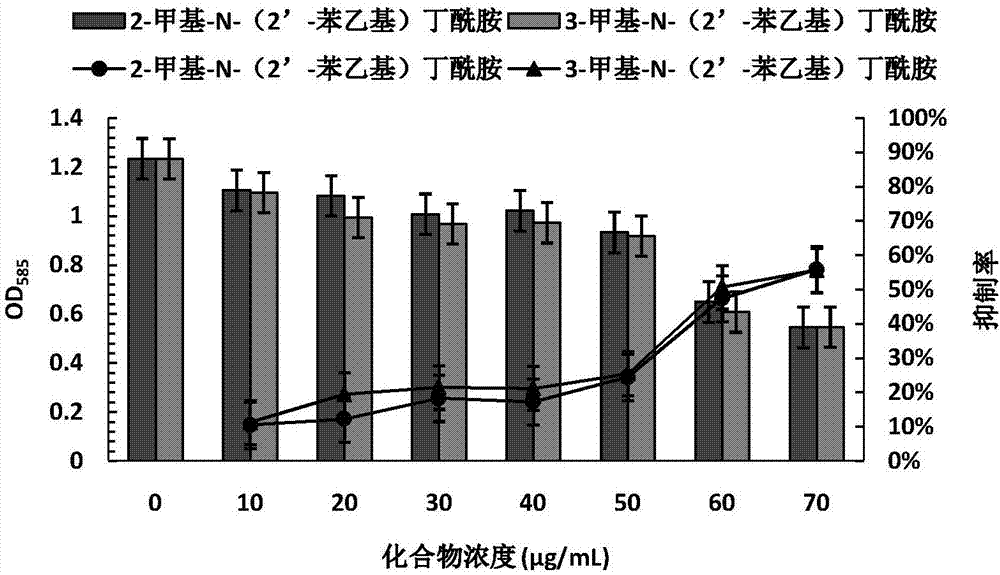
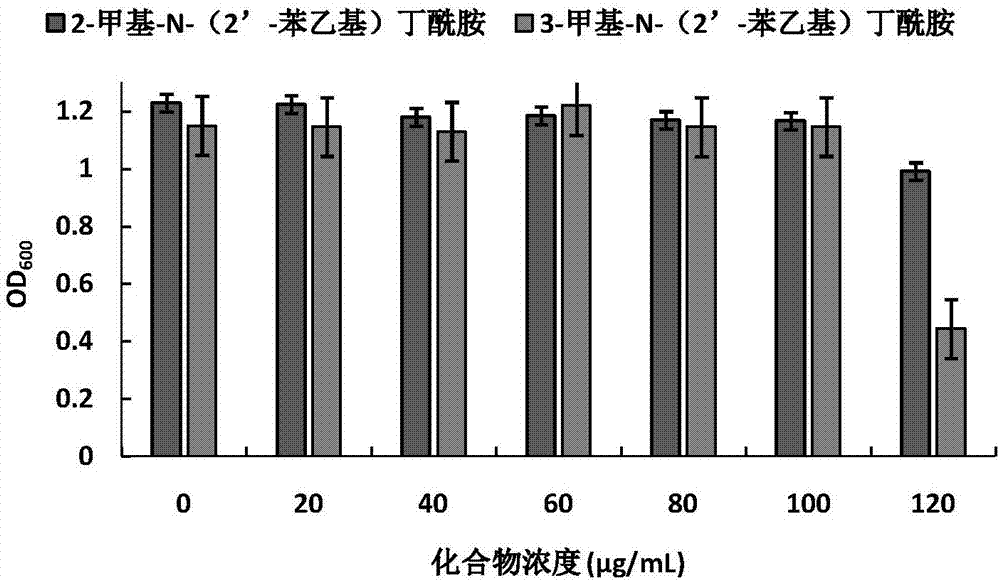
The invention discloses application of amide compounds to the preparation of quorum sensing activity inhibitors. Oceanobacillus sp. XC22919 fermentation broth is utilized for separation and purification to prepare compounds 2-methyl-N-(2'-phenethyl)butyramide and 3-methyl-N-(2'-phenethyl)butyramide, which do not inhibit the growth of chromobacterium violaceum CV026 within a concentration range from 0Mu g / mL to 70Mu g / mL, but can remarkably reduce the generation of chromobacterium violaceum purpurin; moreover, as concentration is gradually increased, the effect of inhibiting the generation of purpurin is higher; within a concentration range from 0Mu g / mL to 100Mu g / mL, the compounds do not affect the growth of pseudomonas aeruginosa PAO1, but can remarkably decrease the expression of the virulence factor of pseudomonas aeruginosa, and can remarkably decrease the expressions of virulence factors such as pyocyanin, elastase, proteolytic enzyme and organism membranes, and the effect of the compounds in inhibiting quorum sensing is concentration-dependence.
Owner:ZHEJIANG UNIV OF TECH
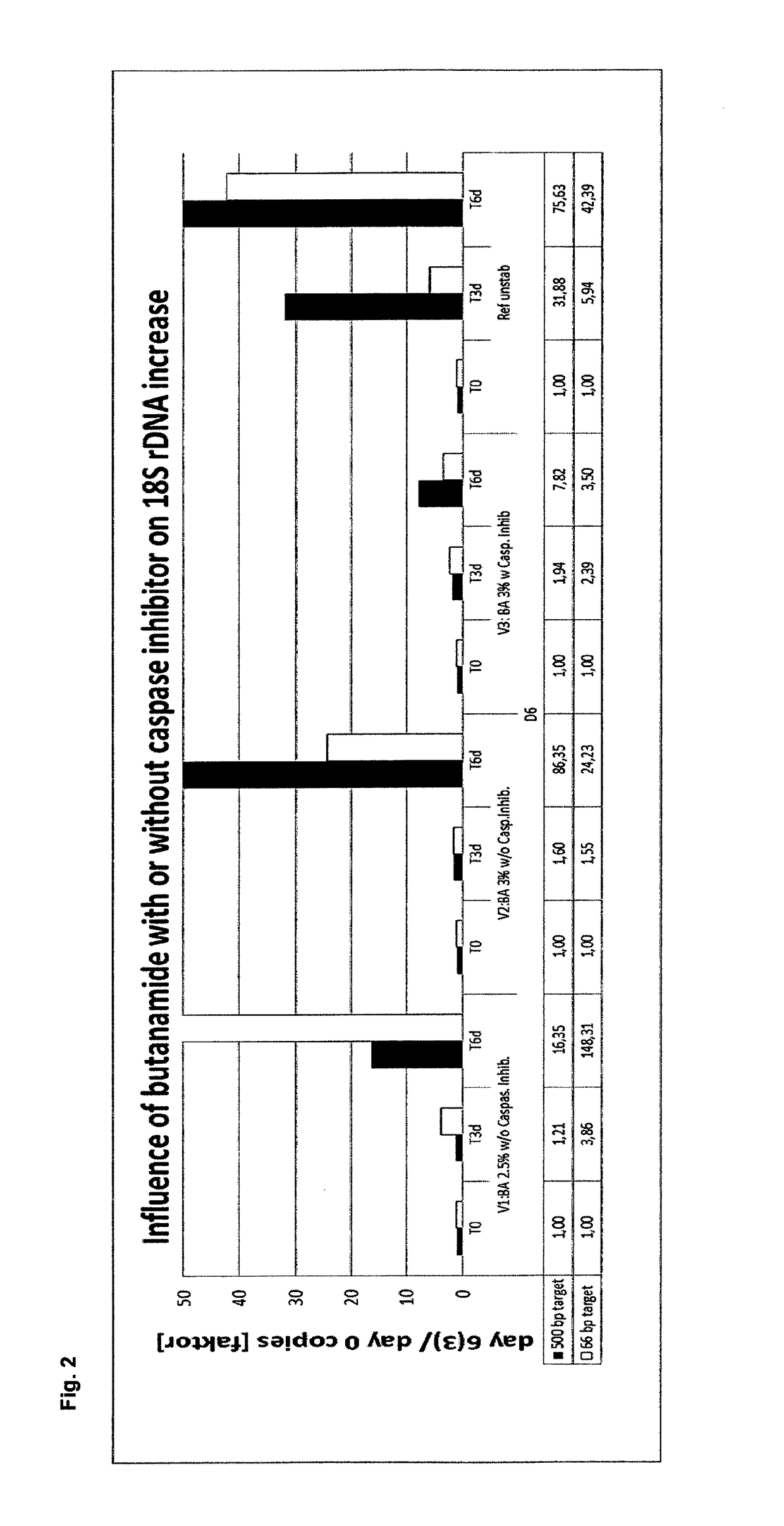
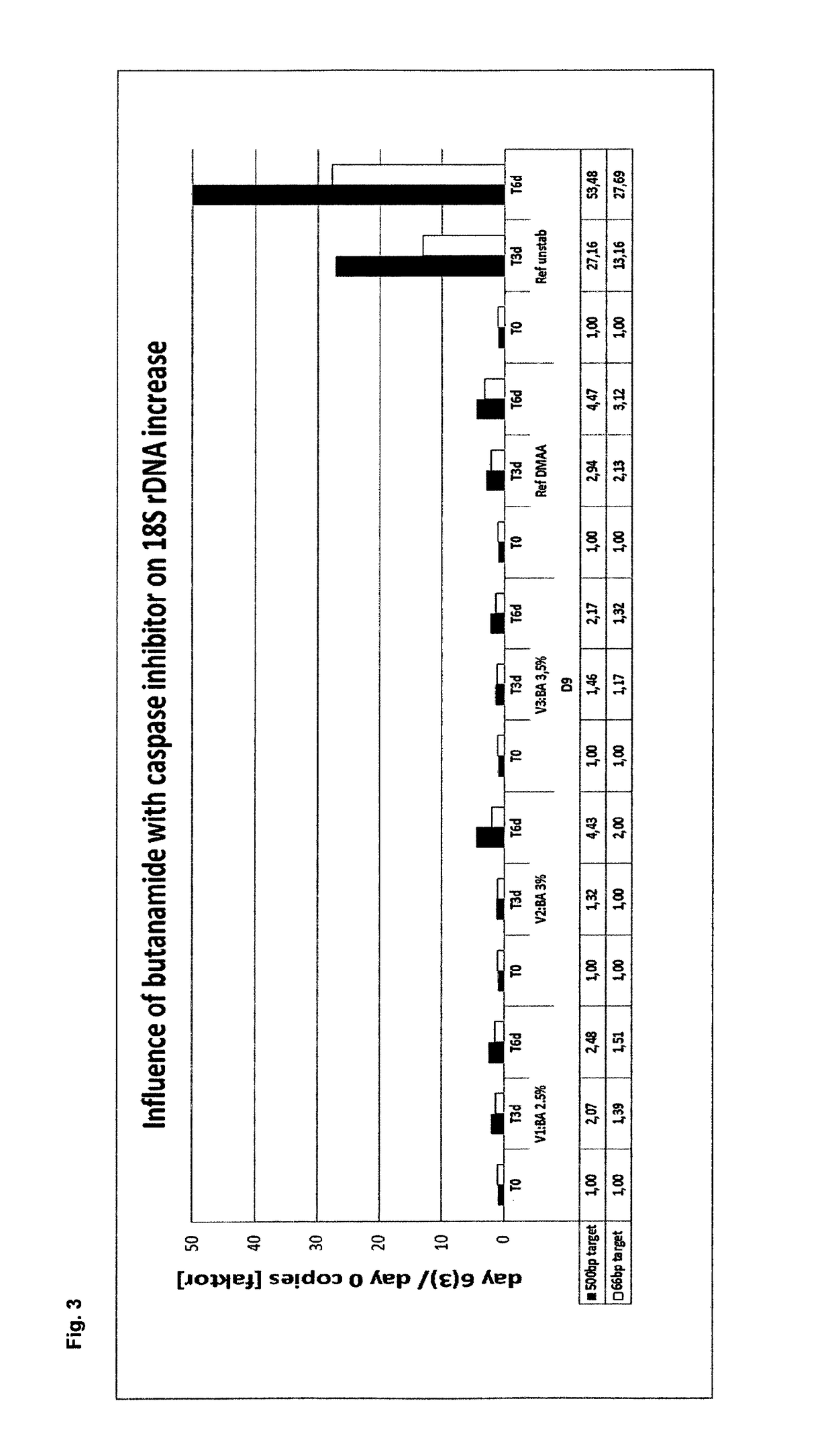
Stabilization and isolation of extracellular nucleic acids
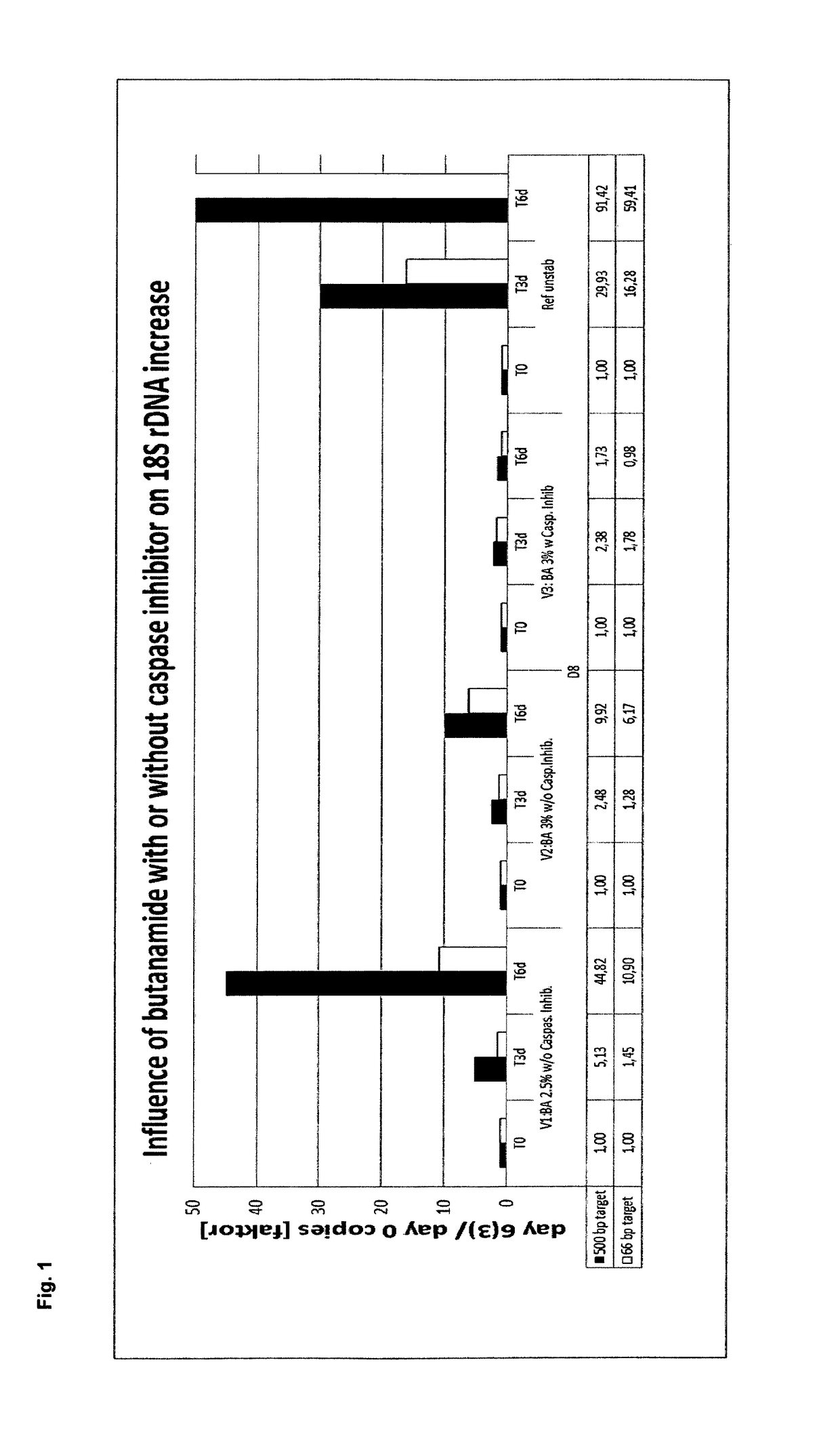
ActiveUS10144952B2Efficient preservation and isolationReduce degradationMicrobiological testing/measurementDNA preparationExtracellularButyramide
The present invention provides methods, compositions and devices for stabilizing the extracellular nucleic acid population in a cell-containing biological sample using butanamide.
Owner:QIAGEN GMBH
Method for synthesizing atorvastatin calcium intermediate by multi-component one-pot method
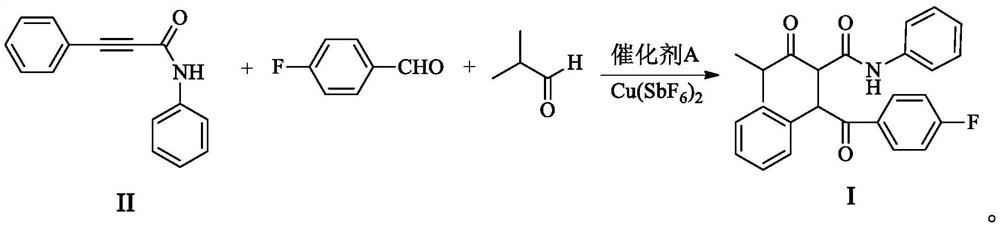
ActiveCN111909048AEmission reductionShort reaction stepsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAcyl group
The invention provides a method for synthesizing an atorvastatin calcium intermediate by a multi-component one-pot method, and particularly provides a one-pot method for synthesizing 4-(4-fluorophenyl)-2-(2methylpropionyl)-3-phenyl 4-oxo-N-phenyl butyramide. The preparation method is characterized in that N-phenyl phenylpropiolamide, 4-fluorobenzaldehyde and isobutyraldehyde are synthesized by a one-pot method under the action of Cu(SbF6)2 and a Pd-ligand catalyst to obtain a target compound. The one-pot method conforms to the characteristics of green chemistry and high atom economy, and emission of three wastes and pollution factors is remarkably reduced; the reaction steps are short, and the yield (about 80-87%) is obviously higher than that of the technical scheme of the existing multi-step synthesis method; raw materials are easily available; the process operation is simple; the EHS risk is low; and the industrialized feasibility is high.
Owner:ZHEJIANG HONGYUAN PHARMA
Key material of modified straw flocculant and technical method for same
InactiveCN106242006ASynchronization of the production processInhibit productionWater/sewage treatment by flocculation/precipitationHydroxyanthraquinoneSuccinic acid
The invention discloses a key material of a modified straw flocculant and a technical method for same. The key material includes, by weight part ratio: ozonized ultrapure water, straw powder, phenyl succinic acid, nonyl phenol sulfosuccinic monoester disodium salt, N-(2-methphenyl)trichloroacetamide, 1-amino-2-bromo-4-[(4-methphenyl)amino]-9,10-anthracenedione, 1-amino-4-bromoanthraquinone, 3,5-dimethoxybromobenzene, berkelium nano particles, 1-amino-2-bromo-4-hydroxyanthraquinone, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2,3-dihydro-2-oxy-1H-benzoimidazole-5-yl)-3-oxo-butanamide, and 2-[[4-cyclohexylamino-9,10-dihydro-9,10-dioxy-1-anthryl]amino]-5-ethoxybenzenesulfonic sodium salt. The key material herein achieves synchronization of an organic flocculant during production and avoids complex steps of producing and purifying a bio-flocculant and compounding the bio-flocculant with the organic flocculant to complete the production of the compound flocculant, thereby reducing synthesize process and reducing cost.
Owner:XUZHOU UNIV OF TECH
Preparation method of zolpidem
ActiveCN110272414ASimple and fast operationLow costOrganic chemistryElimination reactionRaw material
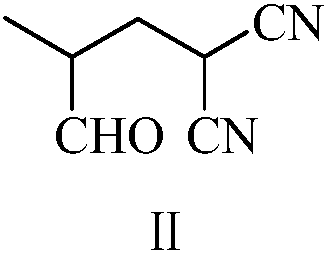
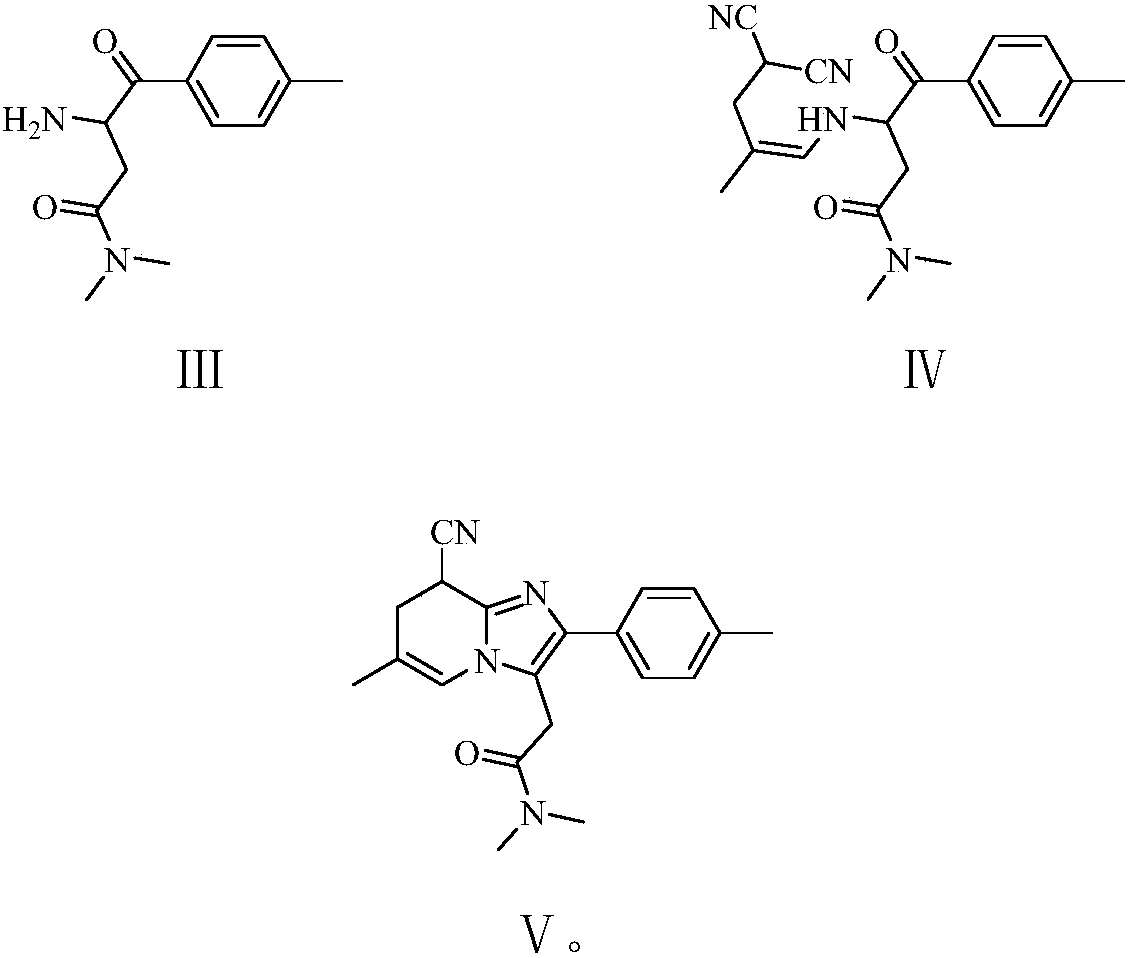
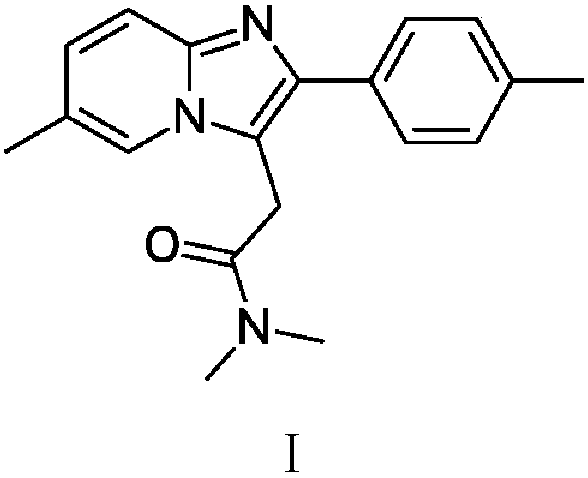
The invention provides a preparation method of zolpidem. The method comprises the following steps: malononitrile and 2-methacrolein which are used as raw materials undergo a 1,4-addition reaction to prepare 2-methyl-4,4-dicyano-n-butyraldehyde, the 2-methyl-4,4-dicyano-n-butyraldehyde and N,N-dimethyl-4-p-methylphenyl-4-oxo-3-amino-n-butyramide undergo a two-stage dehydration condensation reaction, and the obtained reaction product and an acid binding agent undergo an elimination agent to prepare the zolpidem. The method of the invention has the advantages of cheap and easily available raw materials, short process flow, mild reaction conditions, simplicity in operation, low cost, low amount of three wastes, greenness, environmental protection, good selectivity, high yield and high purity of the product, and facilitation of industrial production of the zolpidem.
Owner:XINFA PHARMA
Synthesis process of levetiracetam
The invention relates to the technical field of pharmaceutical preparation, in particular to a preparation method of an antiepileptic drug. The synthesis process of levetiracetam designed by the invention takes (S)-2-(4-chlorobutyramide) butyric acid as an initial raw material, pyridine as an alkali and (Boc)2O as an activating reagent of carboxylic acid, an ammonium salt is added to prepare (S)-2-(4-chlorobutyramide) butyramide, and finally a cyclization reaction is carried out in the presence of alkali to obtain levetiracetam. The process does not need chemical resolution, does not use highly toxic or corrosive chemical reagents, is simple to operate, mild in conditions, environment-friendly and high in finished product quality, and is suitable for industrial production.
Owner:HONGGUAN BIO PHARMA CO LTD
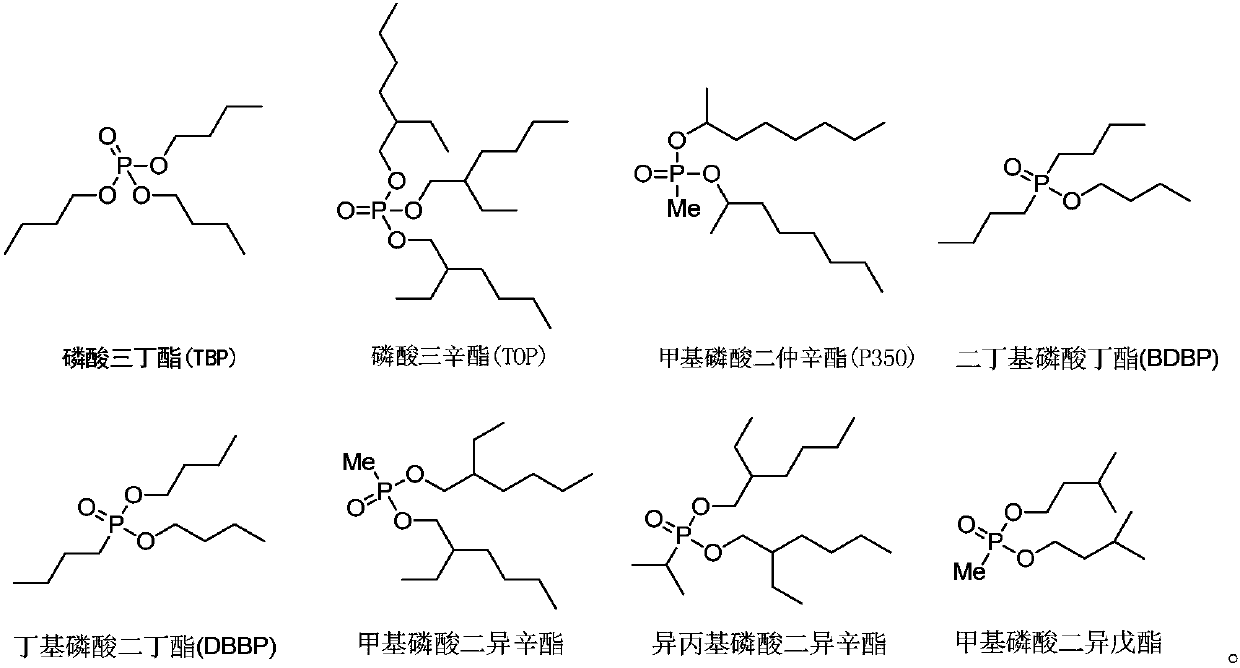
Extraction composition, extraction system and application thereof
ActiveCN107441765ASpeed up extractionImprove stripping performanceLithium compoundsLiquid solutions solvent extractionLithiumButyramide
The invention discloses an extraction composition, an extraction system and application thereof. The extraction composition disclosed by the invention comprises an extraction agent and a neutral phosphorus oxide compound of formula A as shown in the description, wherein the extraction agent comprises N,N-di(2-ethylhexyl) acetamide and N,N-dihexyl butyramide. As a mixture of amide compounds and the neutral phosphorus oxide compound of specific structures is adopted as the extraction agent, the extraction rate for Li of lithium-containing brine is 86% or greater, and can be up to 93.58% at most; the lithium-magnesium allocation coefficient is as high as 500 or greater, and can be up to 773 at most; when lithium is reextracted with HCl, the reextraction rate is 86% or greater, the extraction and reextraction properties for extracting lithium from the lithium-containing brine are greatly improved, the cost is reduced, and industrial production can be relatively well met.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
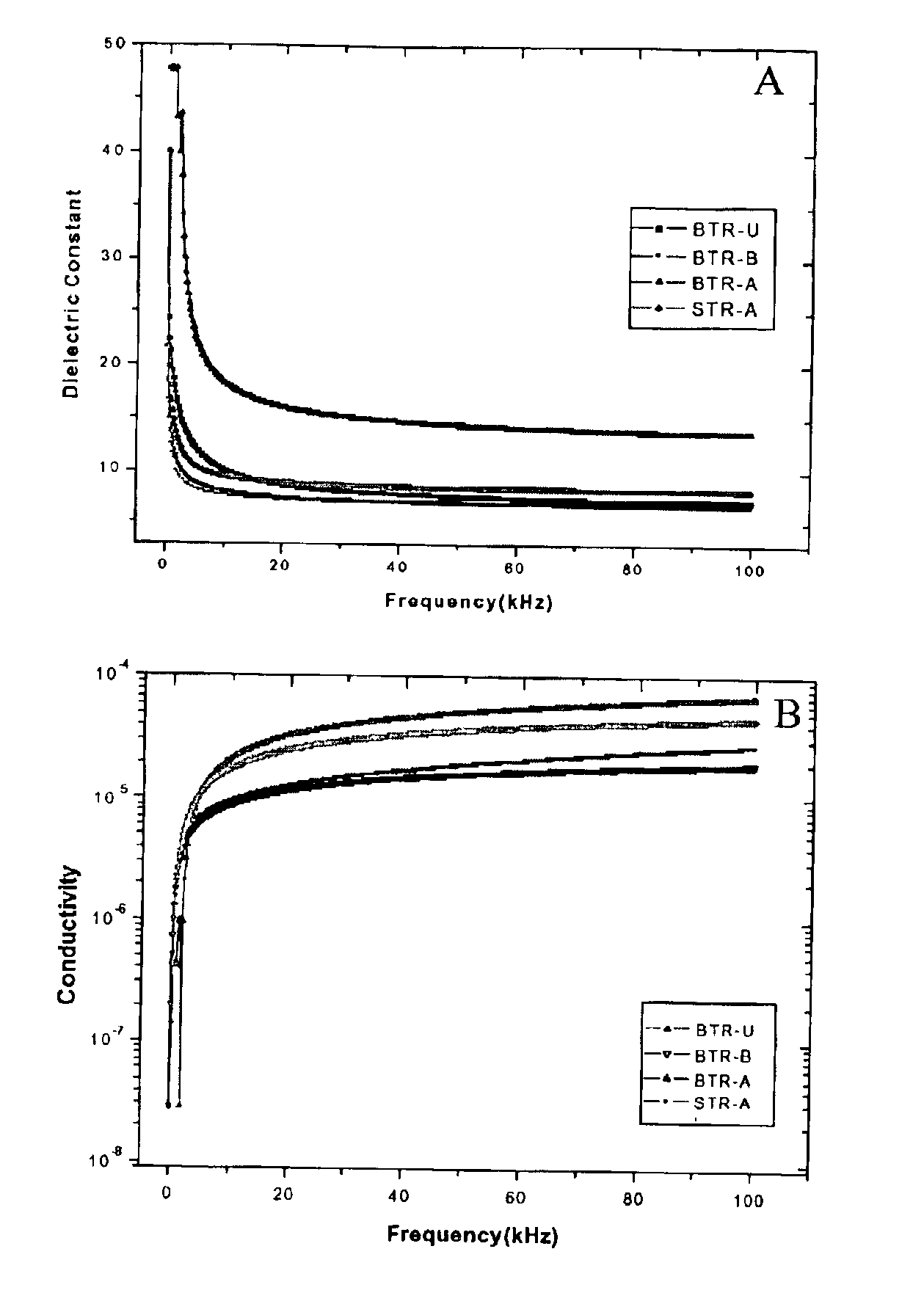


![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka.patsnap.com/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00001.png)
![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka.patsnap.com/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00002.png)
![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka.patsnap.com/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00003.png)