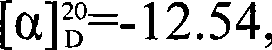Preparation of optically-active pure ibutilide fumarate
A technology of ibutilide fumarate and ethyl, which is applied in the fields of sulfonamide preparation, cardiovascular system diseases, organic chemistry, etc., can solve the problems of relatively expensive price and high industrialization cost, and achieve good industrialized production prospects and operation Simple, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
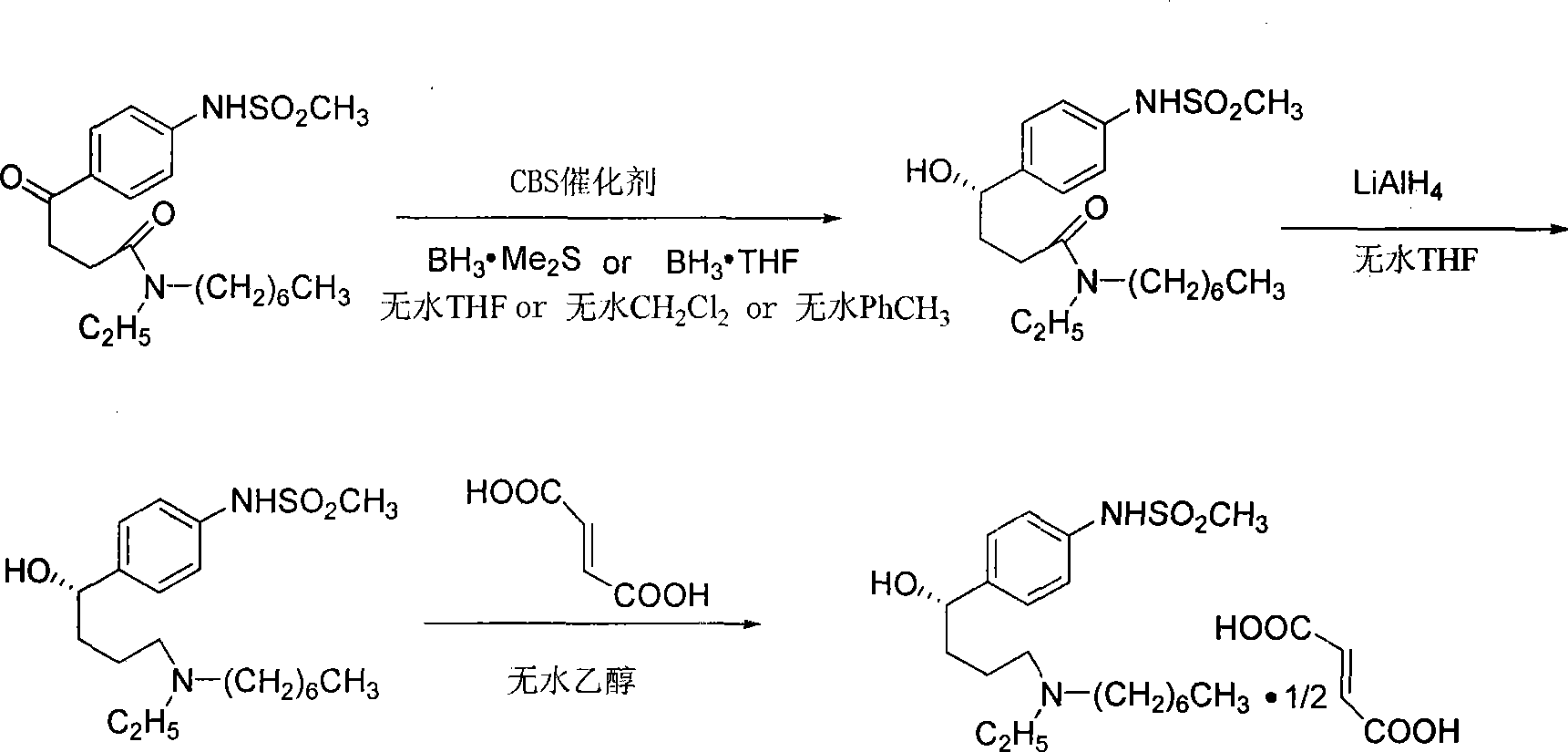
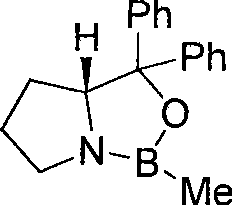
[0024] Under nitrogen protection, at room temperature 25°C, dissolve 0.25mmol of CBS catalyst in 5mL of anhydrous THF, and then add 1.25mL of BH with a concentration of 2mol / L 3 · Me 2 S, after stirring for 10min, dissolve 2.5mmol N-ethyl-N-heptyl-4-oxo-4-(4-methanesulfonylaminophenyl)butanamide in 15mL anhydrous THF, and add dropwise to the above solution , stirred at room temperature 25° C. for 0.5 h, and followed the reaction by TLC. After the reaction was completed, cool in an ice bath, add MeOH and water in sequence to terminate the reaction, extract the aqueous phase with 60 mL of ethyl acetate, combine the ethyl acetate and the organic phase, and wash with water and saturated NaCl in sequence, followed by anhydrous NaCl 2 SO 4 After drying, the solvent (containing ethyl acetate and THF) was evaporated under reduced pressure. Carry out silica gel column chromatography separation with eluent (the volume ratio of methanol and dichloromethane is 1:25) to obtain white sol...
Embodiment 2
[0036] Under nitrogen protection, 0.125 mmol of CBS catalyst was dissolved in 10 mL of anhydrous CH 2 Cl 2 , then add 2.5 mL of BH with a concentration of 1 mol / L 3 THF, after stirring for 10min, cooled to -30°C, dissolved 2.5mmol N-ethyl-N-heptyl-4-oxo-4-(4-methanesulfonylaminophenyl)butyramide in 15mL of anhydrous CH 2 Cl 2 , was added dropwise to the above solution, stirred at -30°C for 1 h, and followed the reaction by TLC. After the reaction was completed, the temperature was raised to 0°C, MeOH was added sequentially, water was used to terminate the reaction, and 60mL CH 2 Cl 2 Extract the aqueous phase, CH 2 Cl 2 After combining with the organic phase, wash with water, saturated NaCl, anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated under reduced pressure (CH 2 Cl 2 ). Silica gel column chromatography with eluent (volume ratio of methanol and dichloromethane is 1:25) to obtain a white solid: (S)-N-ethyl-N-heptyl-4-hydroxyl-4-(4 -(methylsulfona...
Embodiment 3
[0048] Under nitrogen protection, 0.25 mmol of CBS catalyst was dissolved in 10 mL of anhydrous PhCH 3 , then add 0.75mL concentration of 2mol / LBH 3 · Me 2 S, after stirring for 10min, heated to 45°C, dissolved 3.75mmol N-ethyl-N-heptyl-4-oxo-4-(4-methanesulfonylaminophenyl)butyramide in 15mL anhydrous PhCH 3 , was added dropwise to the reaction flask, stirred at 45°C for 1.5h, and followed by TLC. After the reaction was completed, MeOH was added sequentially, water was used to stop the reaction, and 60 mL of ethyl acetate was used for extraction. 2 SO 4 After drying, the solvent (containing ethyl acetate and PhCH 3 ). Silica gel column chromatography with eluent (volume ratio of methanol and dichloromethane is 1:25) to obtain white solid (S)-N-ethyl-N-heptyl-4-hydroxyl-4-(4- (Methylsulfonamide) phenyl) butyramide 0.675g. (S)-N-Ethyl-N-heptyl-4-hydroxy-4-(4-(methylsulfonamide)phenyl)butanamide: white solid, 45% yield, [ α ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com