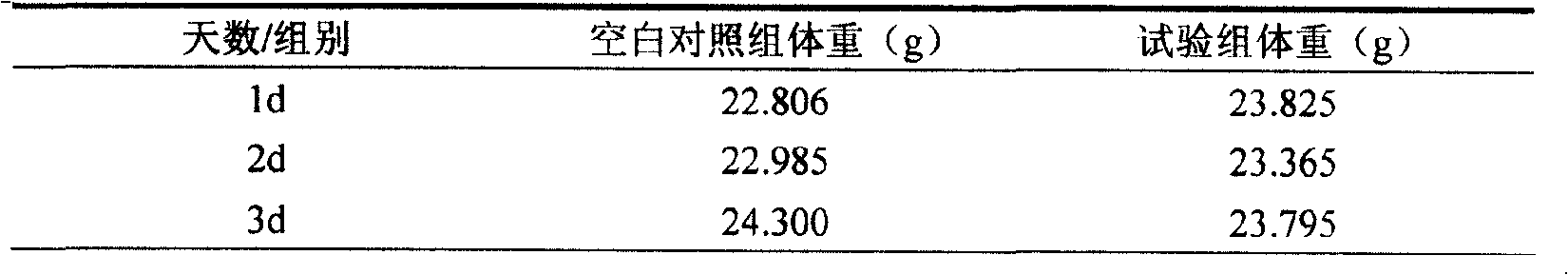
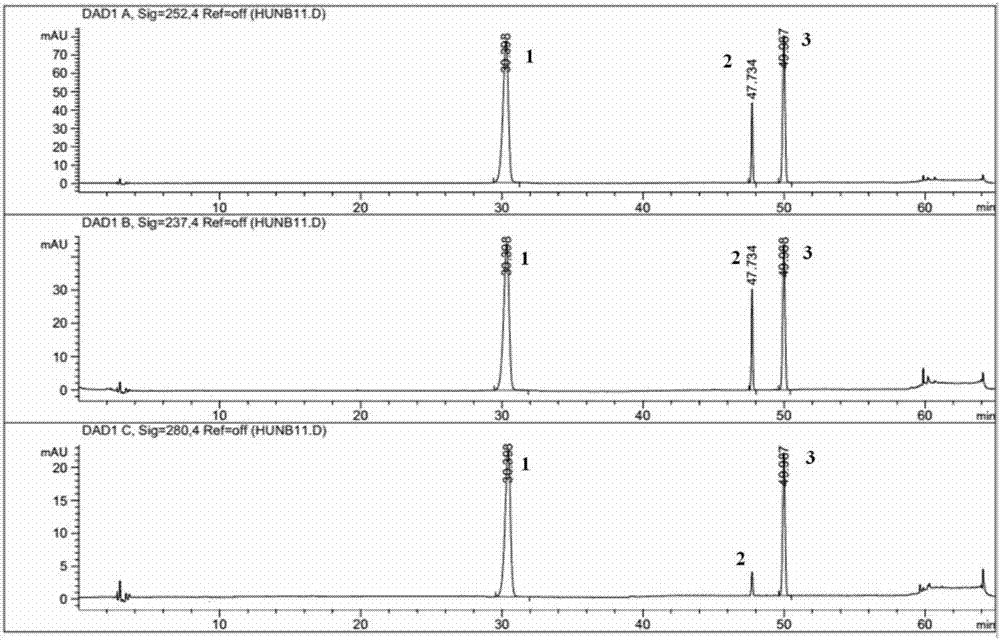
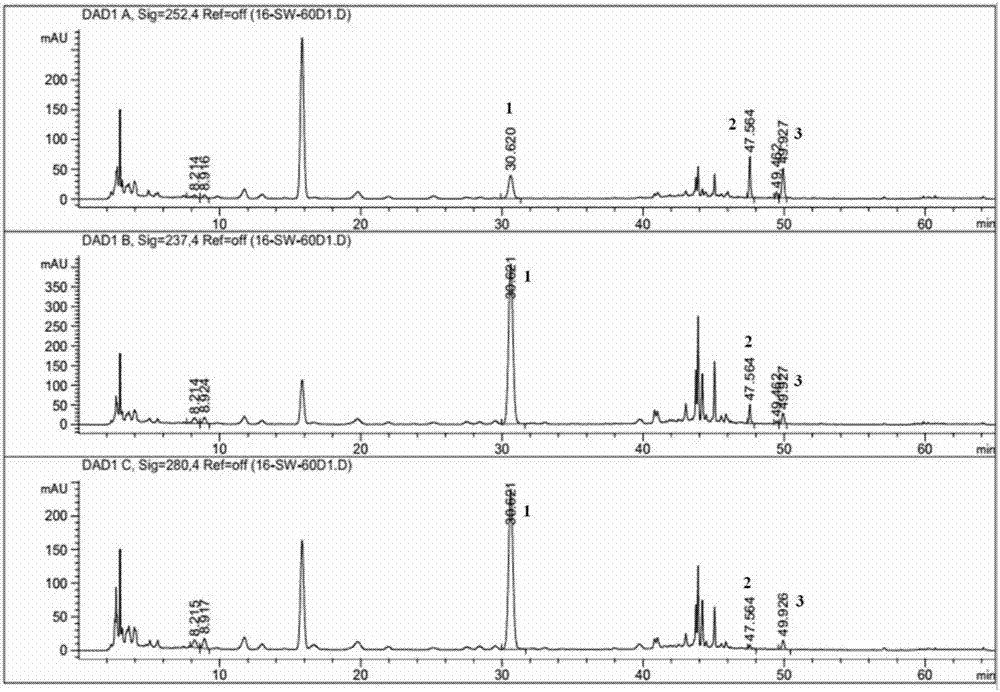
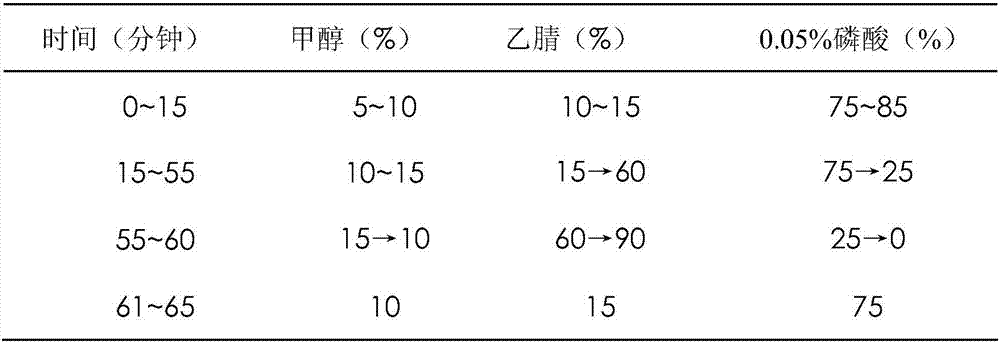
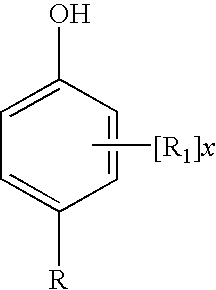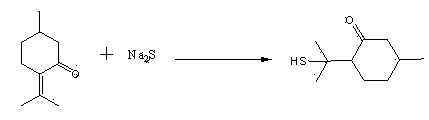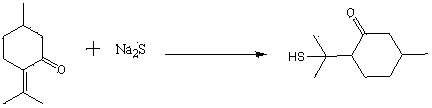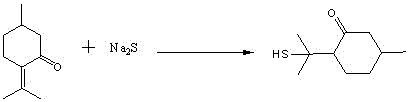Patents
Literature
36 results about "Pulegone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal. It is classified as a monoterpene.
Food products containing a fruit component
InactiveUS20070092623A1Continuous refreshing feelingHigh acceptanceAlcoholic beverage preparationFood preparationMenthoneFruit juice
A fruit juice-containing food product containing following components (a) and (b) in addition to a fruit component and a base having sweetness; and a method for reinforcing a flavor of a fruit juice-containing food product. (a) one or more refreshing feeling substances selected from the group consisting of menthol, menthone, camphor, pulegol, isopulegol, pulegone, cineol, mint oil, peppermint oil, spearmint oil, eucalyptus oil, and fractions there of. (b) one or more cool feeling substances selected from the group consisting of 3-1-menthoxypropane-1,2-diol, N-ethyl-p-menthane-3-carboxamide, 3-1-menthoxy-2-methylpropane-1,2-diol, p-menthane-3,8-diol, 2-1-menthoxyethane-1-ol, 3-1-menthoxypropane-1-ol, 4-1-menthoxybutane-1-ol, cyclic carboxamides, acyclic carboxamides,N,2,3-trimethyl-2-isopropyl butanamide, a menthoxy alkanol (alkyl group having 2 to 6 carbons), a menthoxy alkyl ether (alkyl group having 1 to 6 carbons), and a menthoxy alkanediol (alkyl group having 3 to 6 carbons).
Owner:TAKASAGO INTERNATIONAL CORPORATION
Liquid detergent composition
The present invention provides a liquid detergent composition, comprising: (a) a cleaning effective amount of an enzyme preferably selected from a proteolytic enzyme, an amylolytic enzyme, a lipolytic enzyme, a cellulolytic enzyme and mixtures thereof; (b) from 0.001 to 3% by weight of a perfume composition, not containing a perfume component selected from the group consisting of saturated and unsaturated linear aldehydes, lilial, cyclal c, vanillin, citral, cinnamic aldehyde, pulegone, terpinolene, gamma terpinene, alpha methylionone; (c) from 0.002 to 1% by weight of an antioxidant; and (d) a fatty acid soap in an amount of at most 4% by weight. Said detergent composition was found to have favourable storage stability characteristics.
Owner:CONOPCO INC D B A UNILEVER
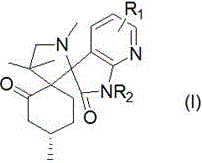
Whole Mouth Malador Control By A Combination Of Antibacterial And Deodorizing Agents
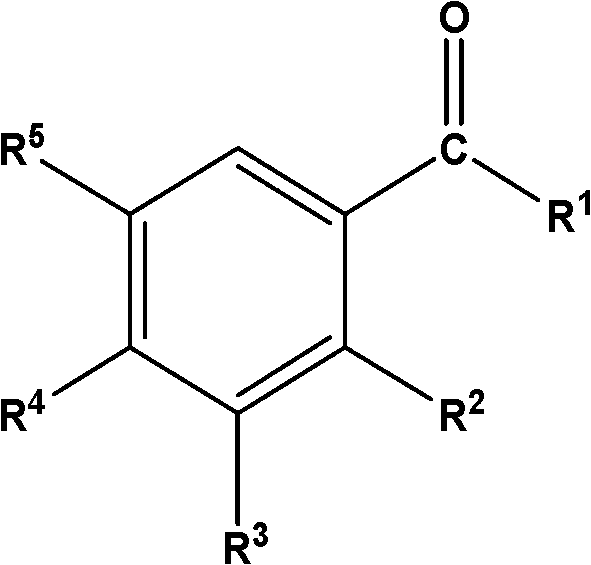
InactiveUS20110239736A1Effective controlCosmetic preparationsToilet preparationsTriclosanAmmonium compounds
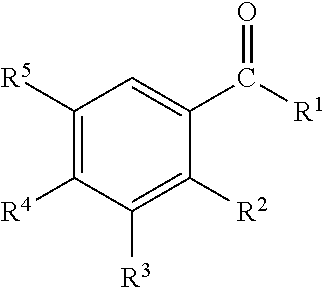
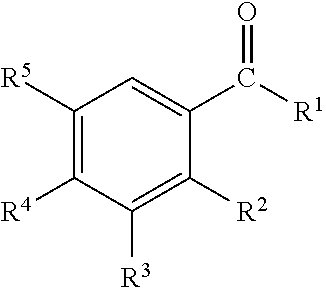
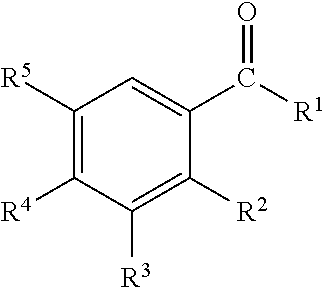
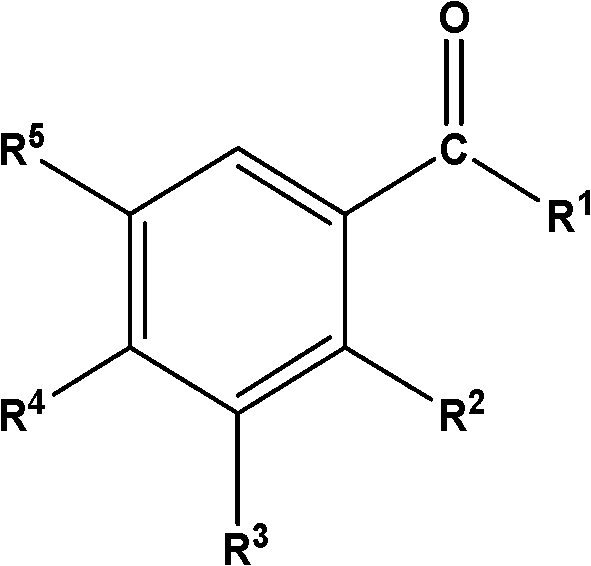
Disclosed are oral care compositions effective to control mouth and breath malodour comprising in a pharmaceutically acceptable carrier, a combination of an antibacterial agent and a deodorizing or odor-neutralizing agent comprising a compound having the structurewherein R1, R2, R3, and R5 may be identical or different, each representing H, a linear or branched C1-C6 alkyl or alkenyl, phenyl, —OH or —ORa; R4 is —OH, —ORa, phenyl, or a linear or branched C1-C6 alkyl or alkenyl; and Ra is phenyl or a linear or branched C1-C6 alkyl or alkenyl. The odor-neutralizing agent may further comprise one or more of an additional odor-neutralizing compound selected from α-damascenone, α-isomethylionone, α-ionone, β-ionone, pulegone, piperitone, carvone, coenzyme Q10 or cinnamaldehyde.The antibacterial agent may comprise one or a mixture of a quaternary ammonium compound selected from cetylpyridinium chloride, tetradecylpyridinium chloride, N-tetradecyl-4-ethyl pyridinium chloride or domiphen bromide; metal ions such as stannous, zinc or copper; chlorhexidine; triclosan; triclosan monophosphate; or selected essential oils.
Owner:THE PROCTER & GAMBLE COMPANY
Analgesic and refreshing herbal composition and a process for preparing the same
The invention provides an analgesic and refreshing herbal composition useful as dentrifrices, said composition comprising 50-60% Wt. of betle extract (from Piper betle leaves); 40-50% Wt. of one or more group I essential oil selected from Levender officinal, Dementholised oil (ex-Mentha arvensis), Fennel oil and Ocimum gratissimum; 3.5-6% Wt. of one or more group II essential oils and their isolates selected from Ocimum Sanctum, Pulegone (ex Mentha pulegonium), Carvone (ex. Dill seed) and Menthol (ex. Mentha arvensis); 1-5% Wt. of one or more group III essential oils selected from Camphor, turpentine oil, Cedarwood oil and Safrole oil, along with 0.5-2% Wt. of Thymol and 0.25-1% Wt. of preservative / antioxidant, and a process for preparing the composition.
Owner:COUNCIL OF SCI & IND RES
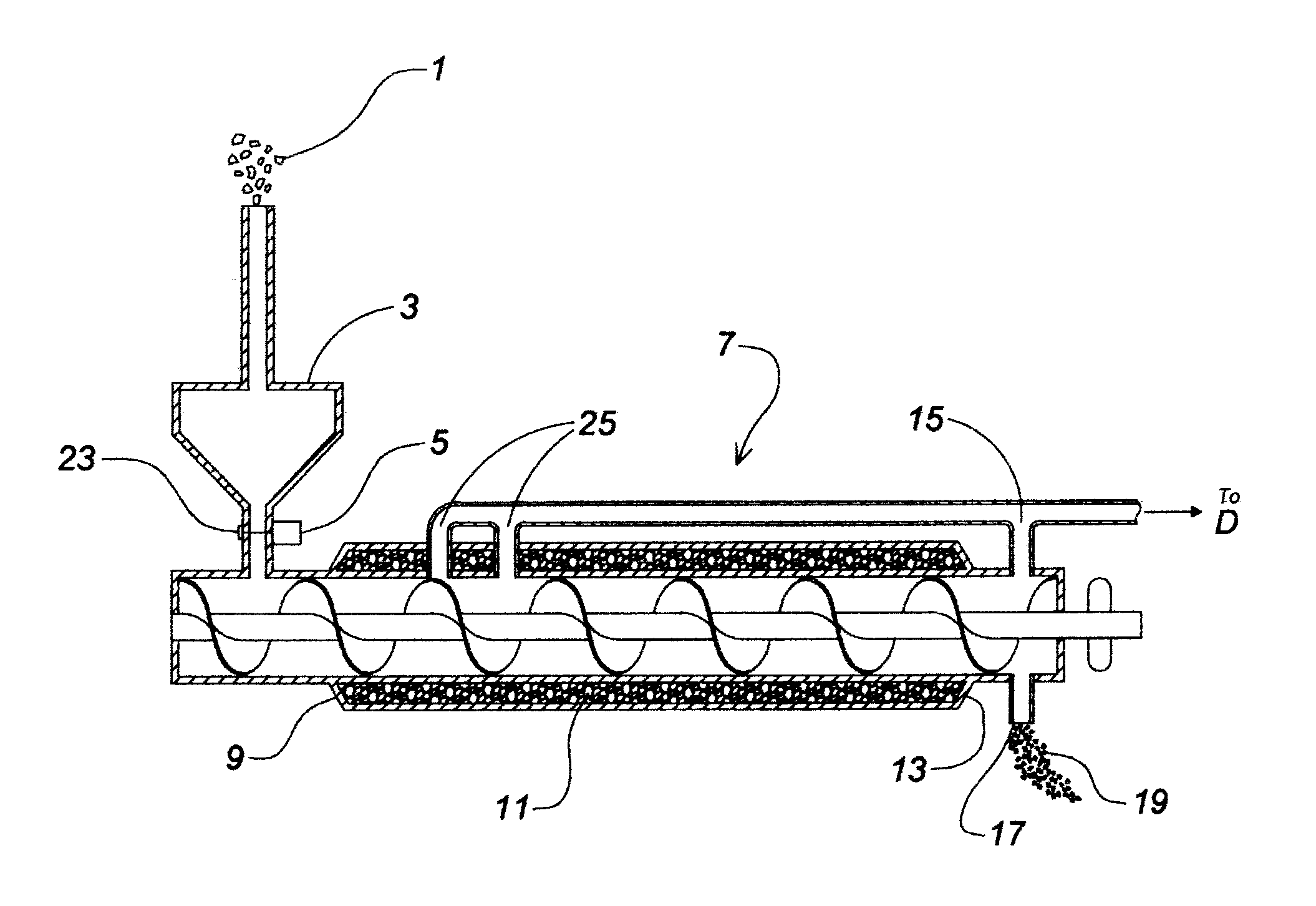
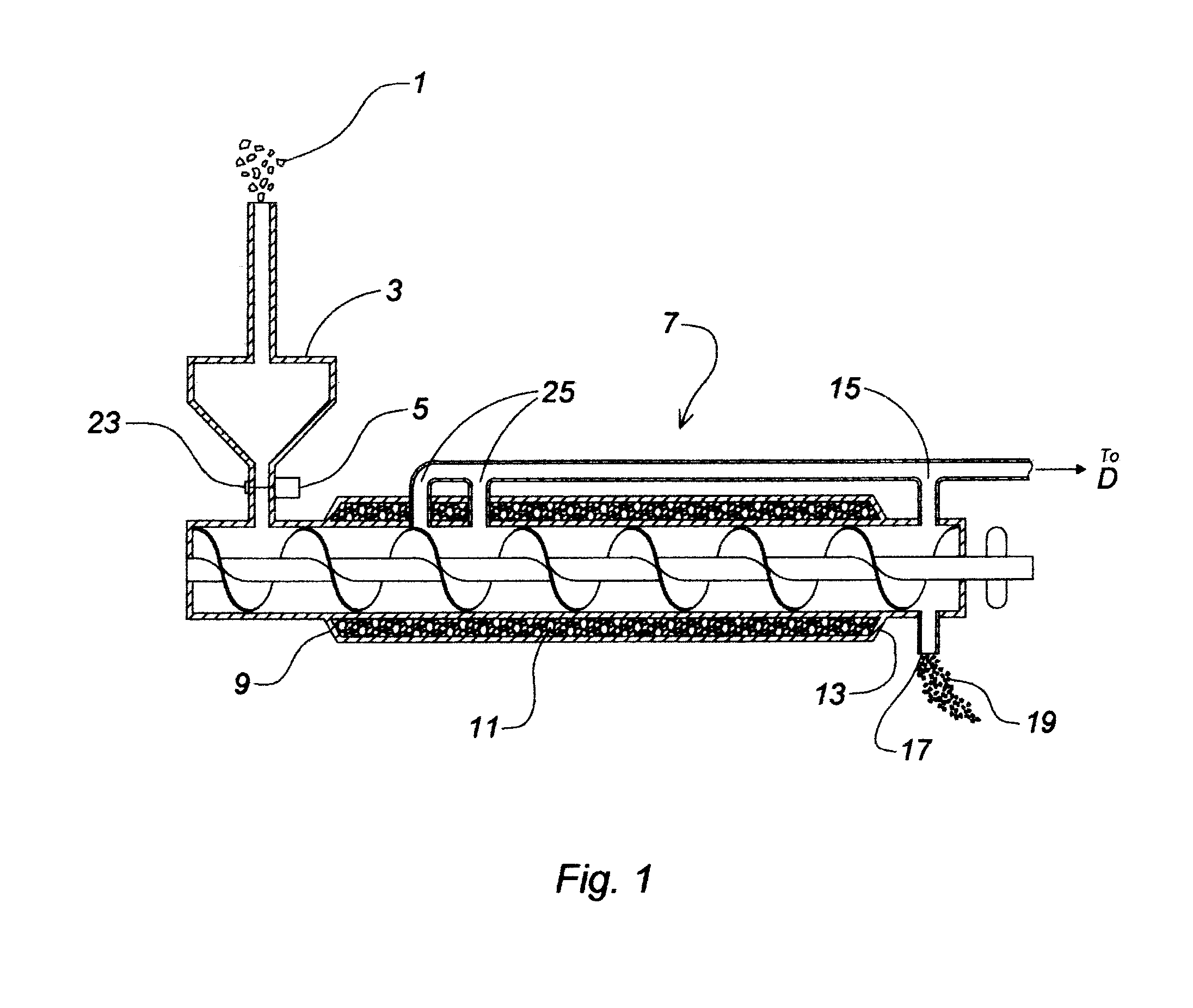
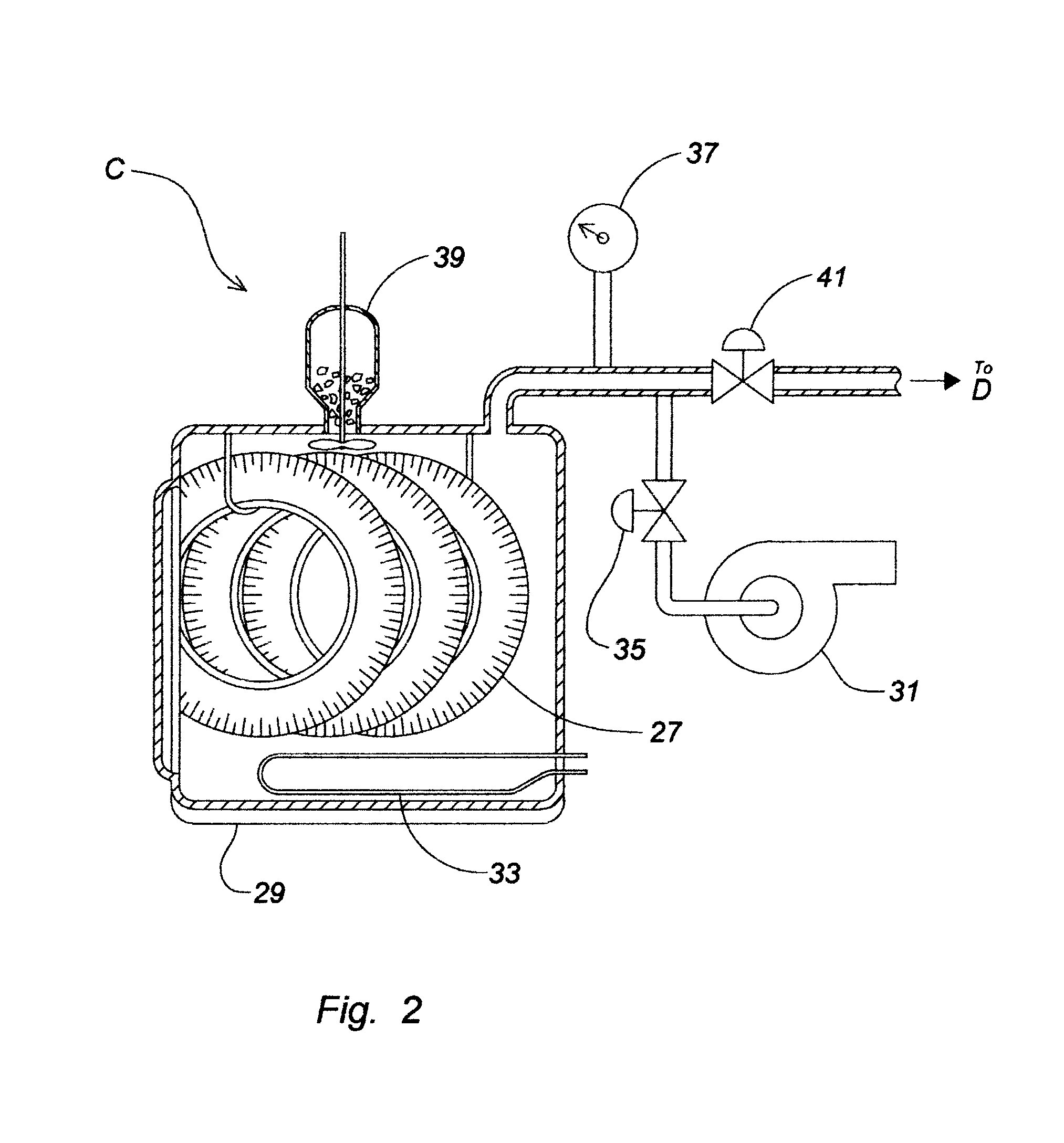
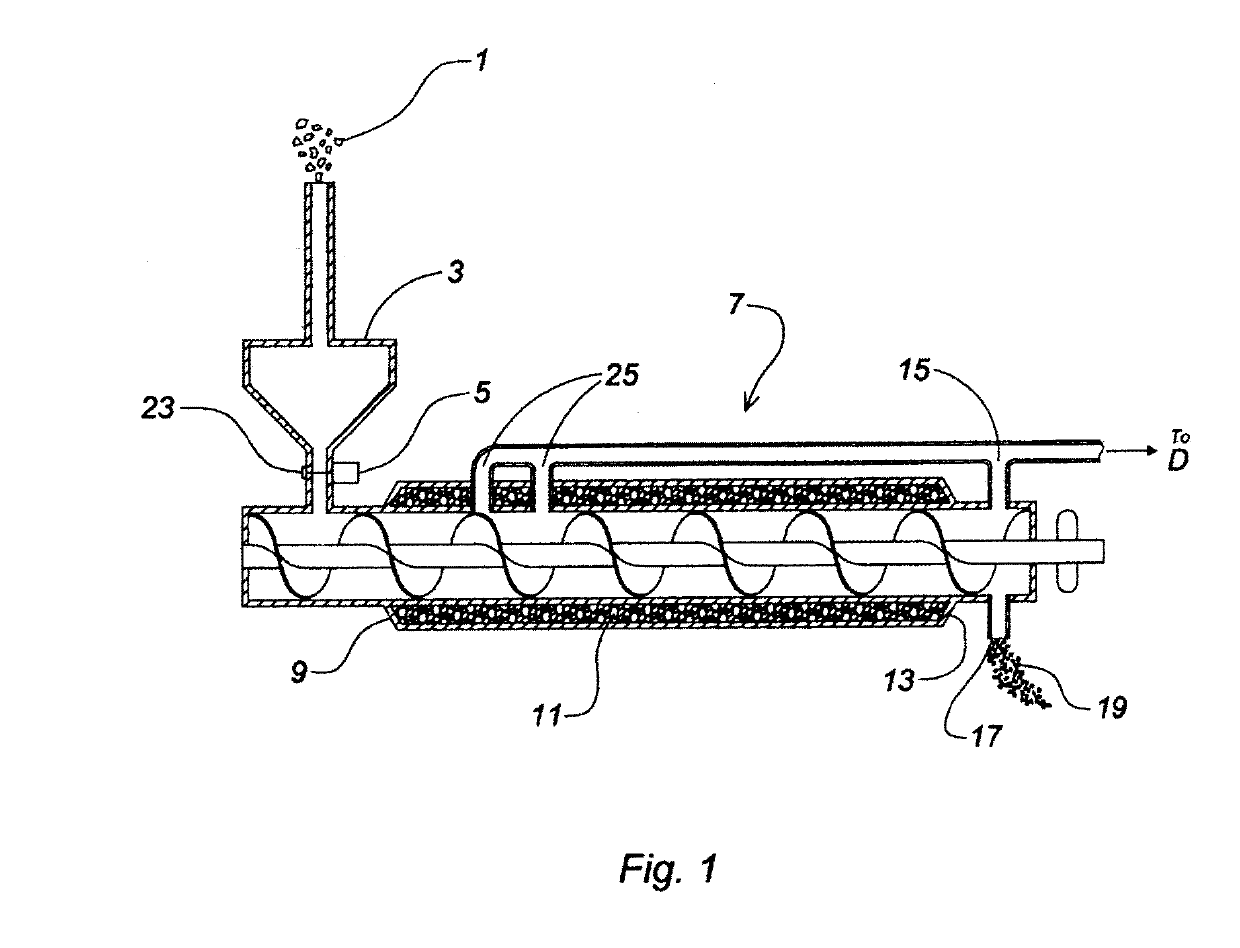
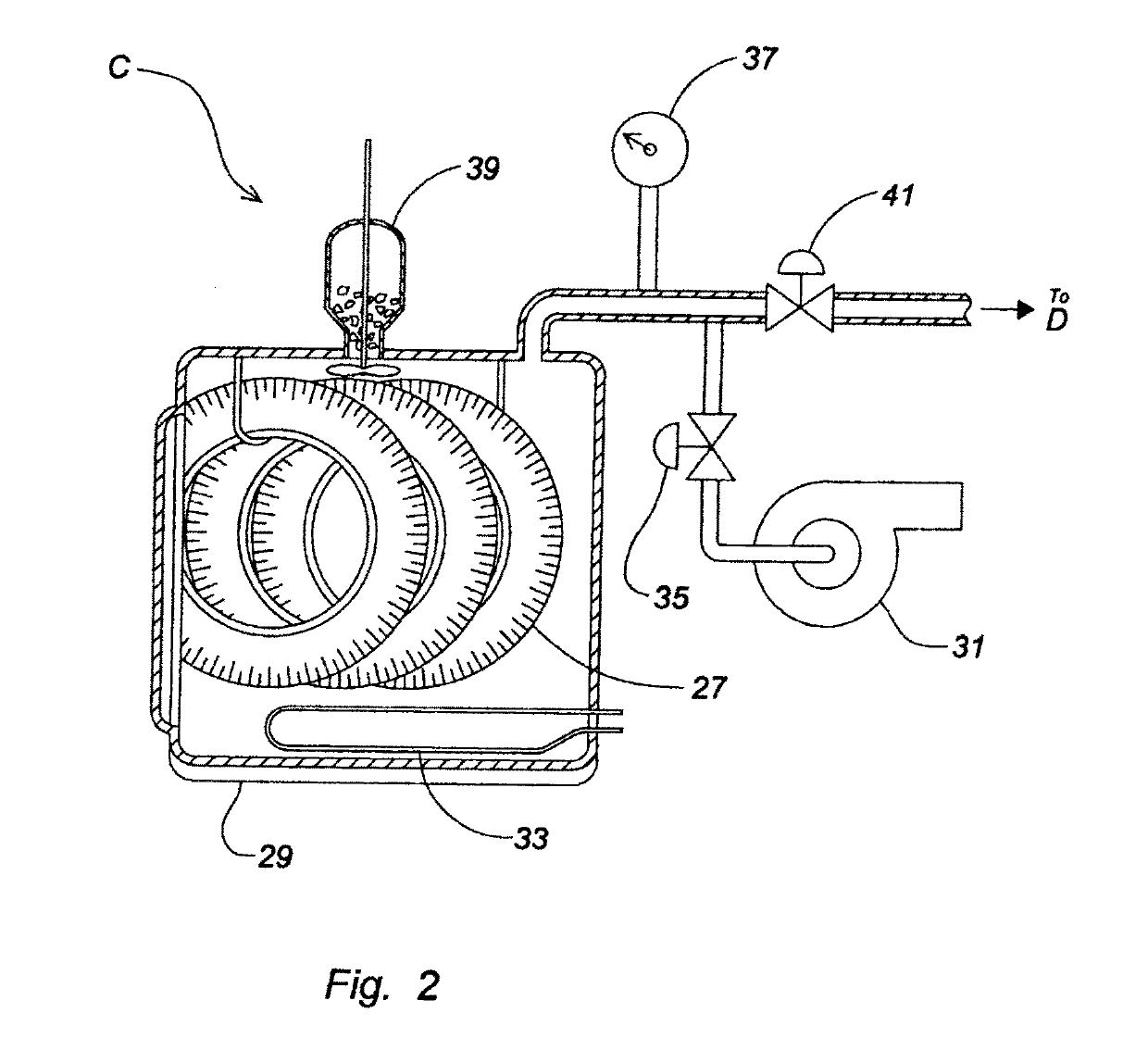
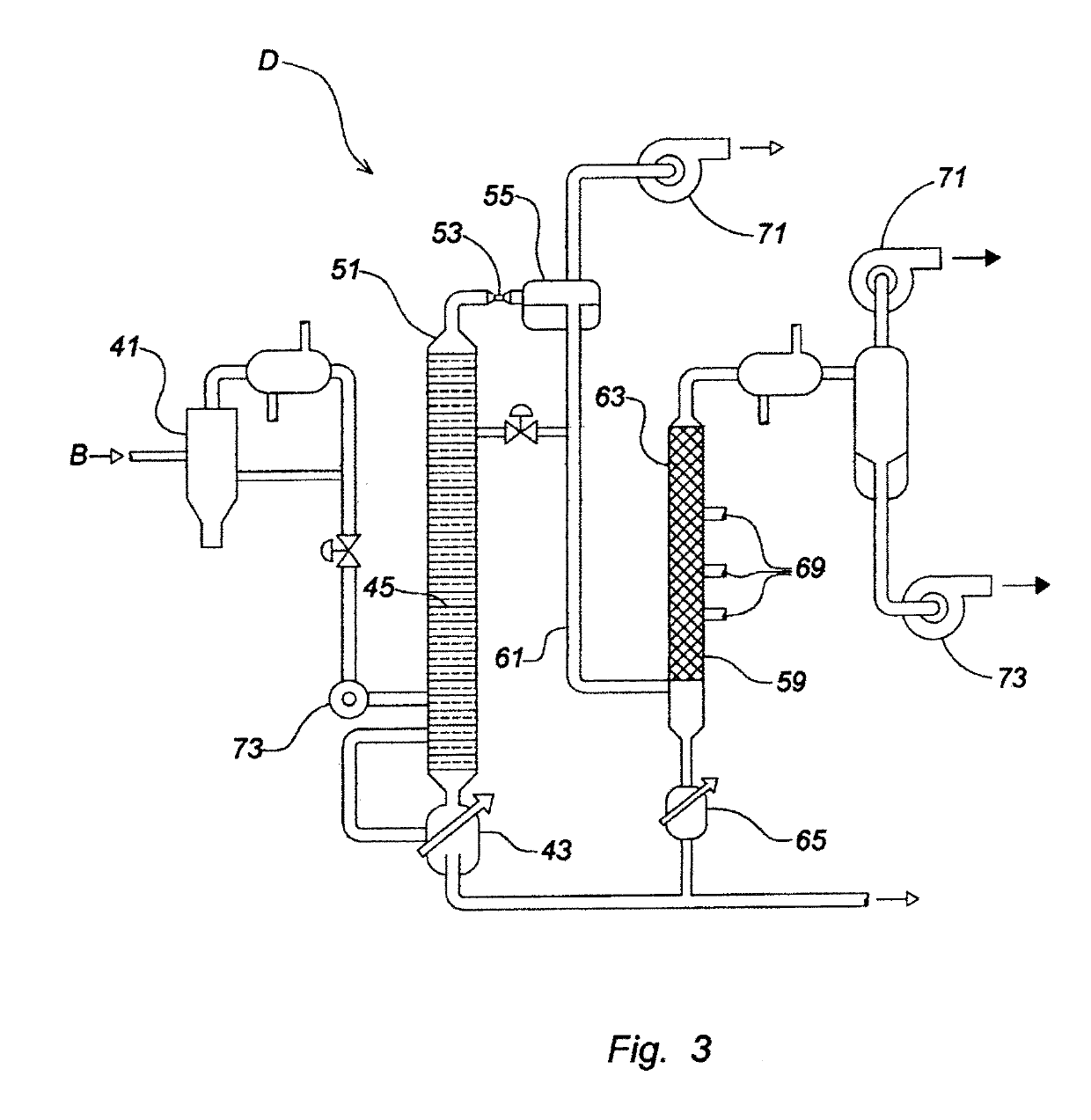
Device and process for the recovery of increased volumes of pure terpenes and terpenoids from scrap polymers and elastomers
Owner:BEAVER EARL R +1
Food products containing a fruit component
InactiveCN1874693ASuppresses decline in refreshing feelingHigh taste acceptabilityAlcoholic beverage preparationFood preparationMenthoneEthyl group
Owner:TAKASAGO INTERNATIONAL CORPORATION
Pelargonium roseum extract and application of extract in cigarette
InactiveCN103146483AHigh economic valueIncrease sweetnessTobacco preparationEssential-oils/perfumesPulegoneDistillation
The invention discloses a pelargonium roseum extract and application of extract in a cigarette, and belongs to the field of a tobacco additive. The aromatic plant pelargonium roseum (pelargonium graveolens L'Herit) is taken as a material and extracted by a supercritical CO2 extraction technology combined with a molecular distillation technology to prepare the tobacco additive. The natural plant extract comprises the main ingredients such as citronellol, geraniol, linalool, citronellyl formate and pulegone. The extract has the effects of increasing fresh and sweet flavor and fineness, reducing thrill, covering offensive odor and improving the comfort after the extract is added to the cigarette.
Owner:HONGYUN HONGHE TOBACCO (GRP) CO LTD
Process for simultaneously abstracting essential oil, chromocor and triterpenes components from agastache
InactiveCN101285021AAchieve separationHigh extraction rateEssential-oils/perfumesSteroidsHerpes zoster virusEssence oil
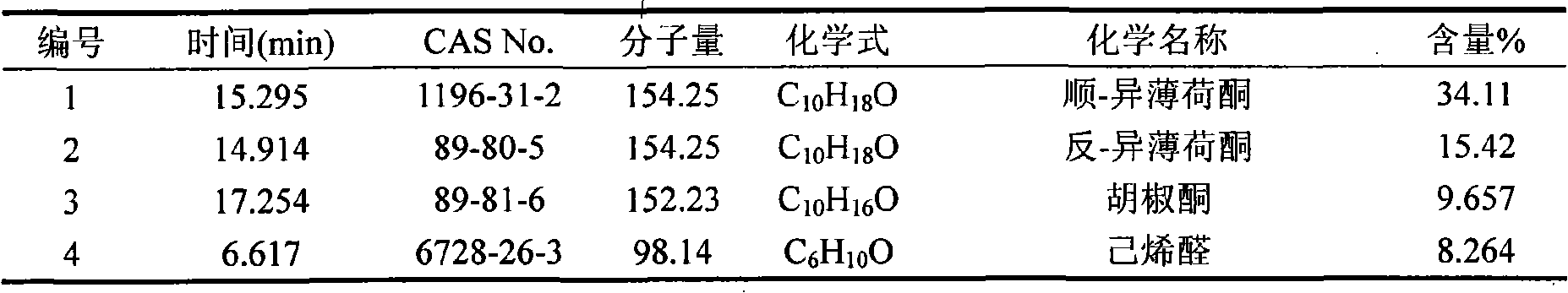
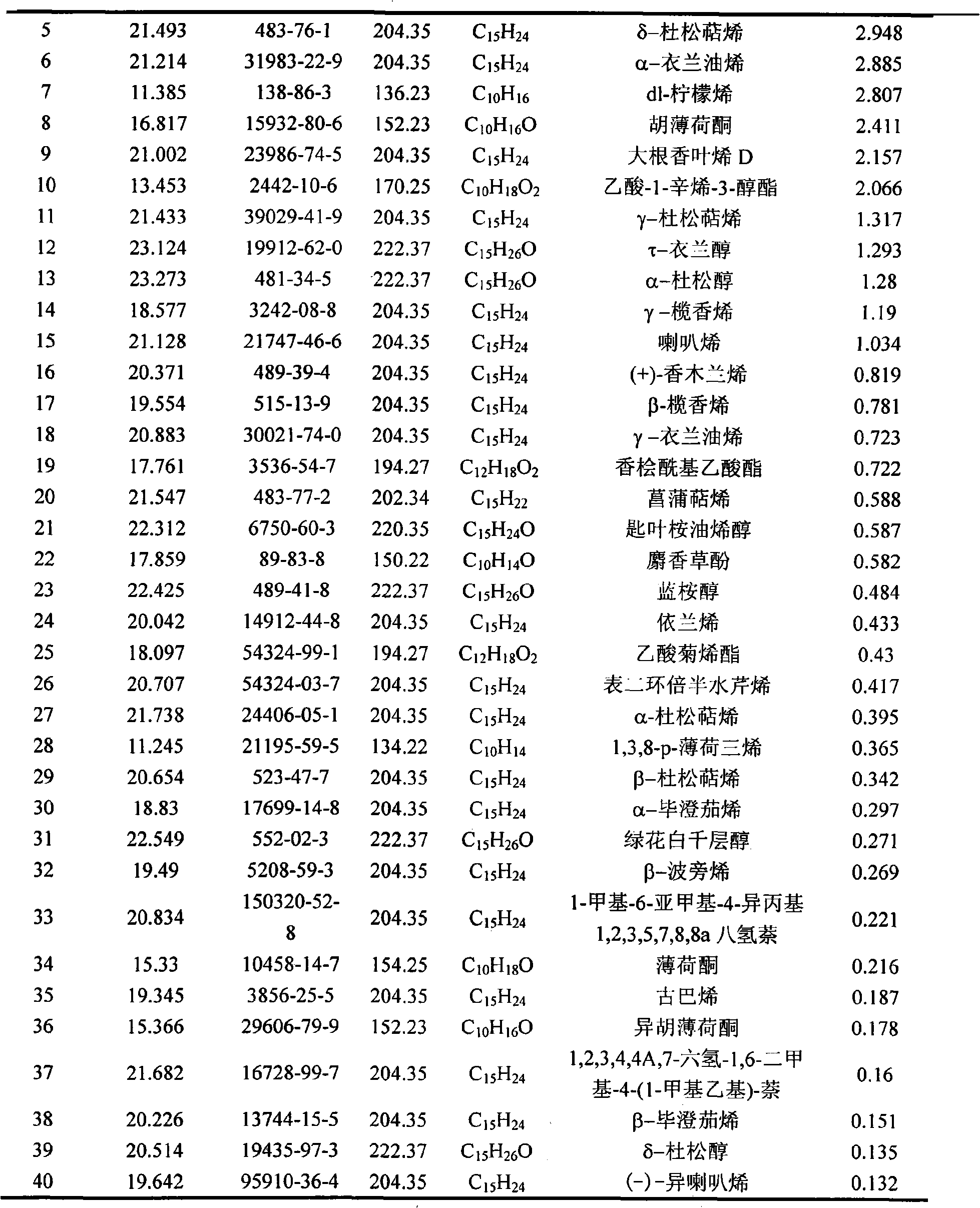
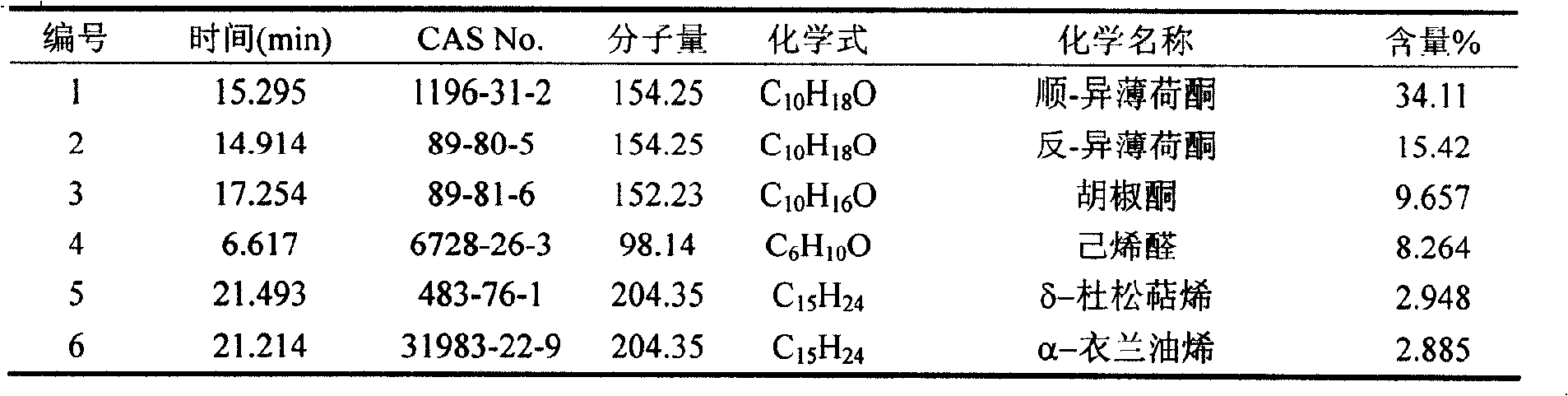
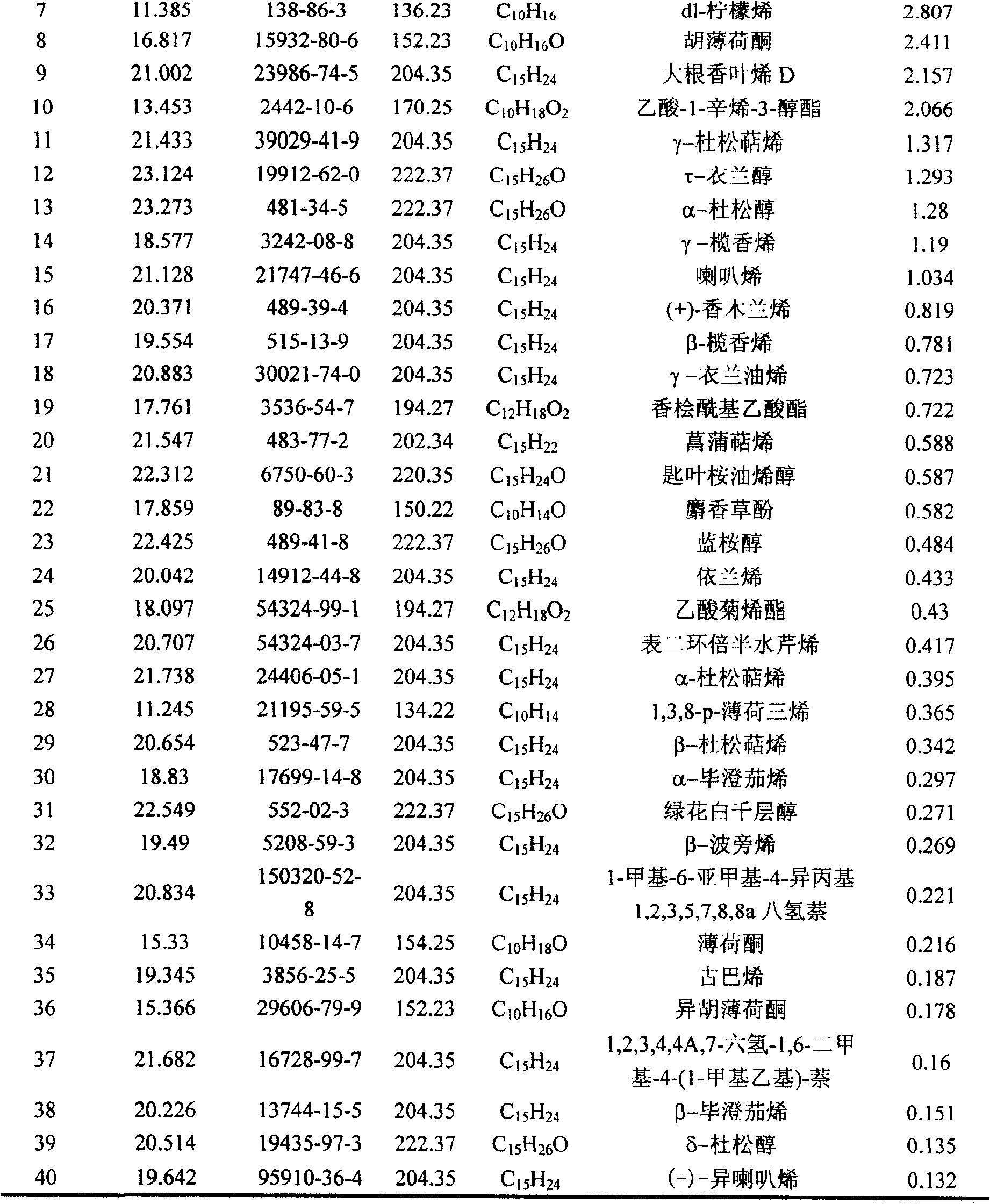
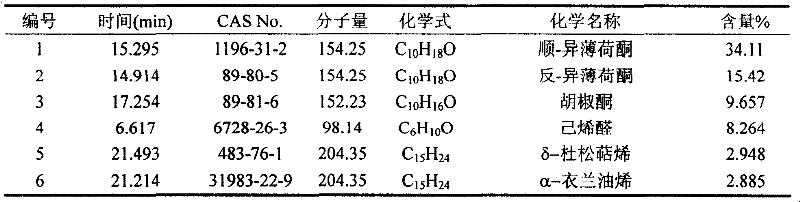
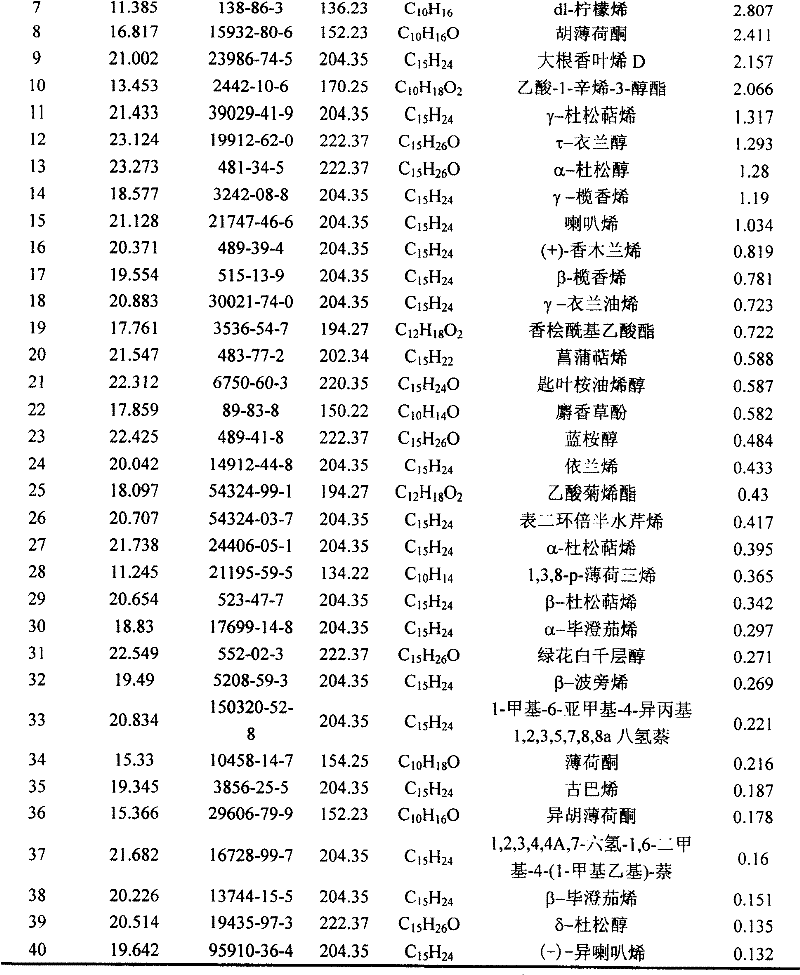
The invention belongs to natural products separating and extracting technical field, particularly relating to a process for simultaneously extracting essential oil, flavone and triterpene out of wrinkled giant hyssop. The invention provides a novel separating and extracting process, which can simultaneously separate and extract essential oil, flavone and triterpene by taking wrinkled giant hyssop as raw materials; the used raw materials can be wrinkled giant hyssop as well as cablin pacholi, fresh or dried herbs as well as fresh or dried leaves, stems or other parts. When fresh wrinkled giant hyssop leaves are used as the raw materials, the essential oil thereof comprises more than 40 volatile components such as cis form - isomenthone, anti form - isomenthone piperitone, hexenoic aldehyde, delta-cadinene, cadinol, alpha-muurolene, limonene and pulegone, wherein main volatile components are the cis form - isomenthone and the anti form- isomenthone, contents of which are 34.11 percent and 15.42 percent respectively; the total flavone comprises flavonoids such as acacia substance, tilianin, linarin, agastachoside, isoagastachoside, agastachin, apigenin, apigenin-7- glucoside, 6-methoxyl group apigenin, luteolin and luteolin-7- glucoside, etc., wherein main flavone components are the agastachoside and the isoagastachoside , contents of which are 25.8 percent and 8.21 percent respectively; the triterpene materials comprises triterpene compounds such as cralaegolic acid, oleanolic acid, 3-acetyl oleanolic aldehvde, 3- acetyl oleanolic acid, alpha-amyrin, beta- amyrin, campesterol, campestanol, ursolic acid, erythrodiol, erythrodiol-3-acetic ester, wherein the main triterpene component is the cralaegolic acid , the content of which is 34.6 percent. The components have wide biological activities and a plurality of effects such as liver protection, anti-tumor, anti-HIV-1, anti-proteinase activity of HIV-1, anti-herpes zoster virus and immunity improvement, etc.
Owner:JIANGNAN UNIV
Technique for preparing health care drink series using fresh Agastache leaf as raw material
The invention belongs to the field of processing and utilizing natural health food resources, in particular relating to a technology using fresh pogostemon leaves as raw materials for preparing functional drink series, the invention provides a novel preparation technology using fresh pogostemon leaves as raw materials for preparing functional drink series of pogostemon essential oil, pogostemon dew, pogostemon tea and so on. Intermediate products can be obtained by the technology, such as distillate, essential oil, decoction, concentration liquid and so on, wherein the distillate consists of forty volatile ingredients, such as cis-isomenthone, trans-isomenthone piperitone, hexenal, delta-juniper terpene, cadinol, alpha-muurolene, limonene, pulegone and so on, wherein the main volatile ingredients include 34.11% of cis-isomenthone and 15.42% of trans-isomenthone. The intermediate products are mixed by different proportion to obtain pogostemon tea drinks with the same effect as the infusion of fresh pogostemon leaves by a folk method, as well as other improved drinks, such as pogostemon essential oil and pogostemon dew. The drinks have the health care effects of refreshing and enlivening the brain, promoting digestion, no toxicity and side effects.
Owner:JIANGNAN UNIV
Method for simultaneously measuring contents of arctiin, glycyrrhizic acid and pulegone
InactiveCN107085065ARapid determinationAccurate measurementComponent separationPulegoneLicorice roots
The invention discloses a method for simultaneously measuring contents of arctiin, glycyrrhizic acid and pulegone in traditional Chinese medicine. The method comprises the steps that firstly, arctigenin, glycyrrhizinate and pulegone standard substances and a sample containing fructus arctii, licorice root and schizonepeta spikes (or schizonepeta) are taken respectively to be dissolved in a methanol aqueous solution, and a standard solution and a sample solution are prepared respectively; secondly, the standard solution and the sample solution which are identical in volume are taken to be injected into an efficient liquid chromatograph, then the peak area of the standard solution and the test solution is measured, and the contents of arctiin, glycyrrhizic acid and pulegone in the sample are obtained. The quick and accurate method is provided for measuring the contents of arctiin, glycyrrhizic acid and pulegone in traditional Chinese medicine containing fructus arctii, licorice root and schizonepeta spikes (or schizonepeta) and in extracts or preparations of the traditional Chinese medicine.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
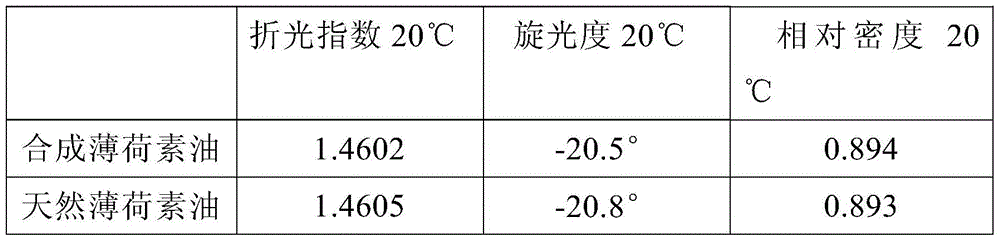
Synthesized dementholized peppermint oil
The invention discloses a synthesized dementholized peppermint oil. The synthesized dementholized peppermint oil is prepared from L-limonene, isopulegol, peppermint-oil head oil, octanol-3, menthone, racementhol, neomenthol, synthesized levogyration menthol, pulegone, piperitone, carvone, a-terpilenol, 4-terpene alcohol, B-caryophyllene, cis-3-hexenyl isovalerate, linalool, peppermint tail oil and menthyl acetate. The GC main indexes and the physical and chemical indexes of the synthesized dementholized peppermint oil are basically consistent with the GC main indexes and the physical and chemical indexes of natural peppermint oil; in the fragrance aspect, the bottom fragrance of the natural dementholized peppermint oil is thick and solid, the bottom fragrance of the synthesized dementholized peppermint oil is slightly thin, and the synthesized dementholized peppermint oil is purer than the natural dementholized peppermint oil in fragrance; in the taste aspect, the synthesized dementholized peppermint oil is fuller than the natural dementholized peppermint oil in cooling feeling, and the synthesized dementholized peppermint oil is basically consistent with the natural dementholized peppermint oil in specific peppermint peppery feeling degree.
Owner:SHANGHAI WANXIANG FLAVORS & FRAGRANCES
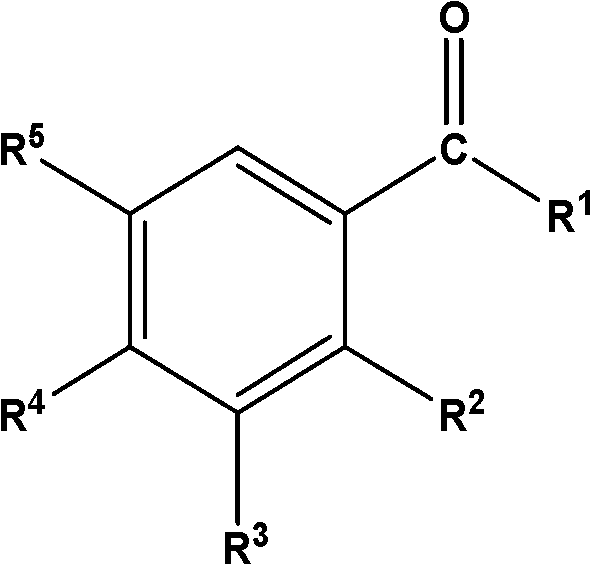
Whole mouth malodor control by a combination of antibacterial and deodorizing agents
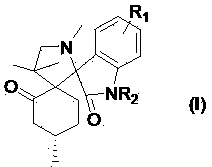
Disclosed are oral care compositions effective to control mouth and breath malodour comprising in a pharmaceutically acceptable carrier, a combination of an antibacterial agent and a deodorizing or odor-neutralizing agent comprising a compound having the structure (I) wherein R1, R2, R3, and R5 may be identical or different, each representing H, a linear or branched C1-C6 alkyl or alkenyl, phenyl, -OH or -ORa; R4 is -OH, -ORa, phenyl, or a linear or branched C1-C6 alkyl or alkenyl; and Ra is phenyl or a linear or branched C1-C6 alkyl or alkenyl. The odor-neutralizing agent may further comprise one or more of an additional odor-neutralizing compound selected from a-damascenone, a-isomethylionone, a-ionone, ss-ionone, pulegone, piperitone, carvone, coenzyme Q10 or cinnamaldehyde. The antibacterial agent may comprise one or a mixture of a quaternary ammonium compound selected from cetylpyridinium chloride, tetradecylpyridinium chloride, N-tetradecyl-4-ethyl pyridinium chloride or domiphen bromide; metal ions such as stannous, zinc or copper; chlorhexidine; triclosan; triclosan monophosphate; or selected essential oils.
Owner:PROCTER & GAMBLE CO
Harmful-insect-eliminating formed resin article and animal collar using the same
InactiveUS7311923B2Eliminating a harmful insectEliminate effectiveBiocideDead animal preservationPulegoneMedicine
Disclosed is a harmful-insect-eliminating formed resin article, wherein the article contains a 1,2-polybutadiene resin and a volatile harmful-insect-eliminating component, e.g. eucalyptol and pulegone, in an amount effective for eliminating a harmful insect. A typical use of the formed article is an animal collar.
Owner:SUMITOMO CHEM CO LTD
Application of agastache rugosus volatile oil to suppression of growth of drug-resistant escherichia coli
ActiveCN106138197AGrowth inhibitionMitigate or resolve drug-resistant infectionsAntibacterial agentsOrganic active ingredientsEscherichia coliBiofilm
The invention discloses application of agastache rugosus volatile oil to suppression of growth of drug-resistant escherichia coli. A single chemical component, i.e. pulegone, is obtained from an agastache rugosus volatile oil to achieve a better in vitro suppression effect on the growth of human-derived drug-resistant escherichia coli, i.e. human-derived ESBLs-producing escherichia coli. The application has the beneficial effects that the first research on the effect of the agastache rugosus volatile oil on the drug-resistant escherichia coli finds that the growth of the drug-resistant escherichia coli can be suppressed; a main component serving as a drug-resistant suppressing agent is screened from the agastache rugosus volatile oil and then researched; data show that the main monomer component, i.e. the pulegone, of the agastache rugosus volatile oil can suppress the growth of the ESBLs-producing escherichia coli and affect the formation of a biofilm so as to relieve or avoid the drug-resistant infection of the escherichia coli and reduce the fatality rate; a new idea is brought up to solve and treat the human-derived ESBLs-producing escherichia coli; and the application has a great research significance.
Owner:XINJIANG MEDICAL UNIV
Schizonepeta extractive and application thereof
InactiveCN104719351AGood insect resistanceImprove insect resistanceBiocideAnimal repellantsPulegonePiperitone
The invention provides a schizonepeta extractive which is characterized in that the schizonepeta extractive is obtained in a manner that the total content of piperitone and pulegone is lowered on the basis of schizonepeta volatile oil. In addition, the invention further provides a pesticide composition of the schizonepeta extractive and a preparation method of the pesticide composition. The schizonepeta extractive has lasting insect resistant effect.
Owner:CHENGDU NEWSUN CROPSCI
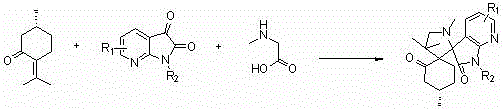
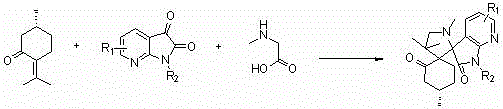
Double-spiro compound containing isatin mother nucleus with antineoplastic activity and synthesis method thereof
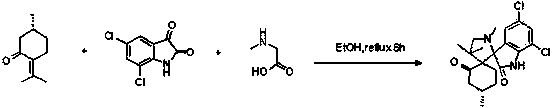
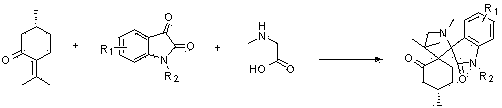
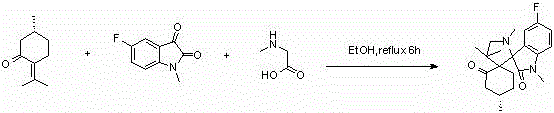
ActiveCN104193749AGood antitumor activityRaw materials are easy to getOrganic chemistryAntineoplastic agentsPulegoneChemical compound
The invention discloses a compound shown in a structural general formula (I) in the Specification and a synthesis method thereof. The double-spiro compound containing the isatin mother nucleus uses pulegone, isatin and derivatives thereof, and sarcosine as raw materials, and the synthesis process uses a one-pot method, so that complicated multi-step operations are avoided, the operation is simple and convenient, the reaction conditions are mild, and the yield is better. The bioactivity tests show that the double-spiro compound containing the isatin mother nucleus has better antineoplastic activity.
Owner:SHAANXI UNIV OF SCI & TECH
Pharmaceutical composition for treating or preventing swollen sore throat
InactiveCN104274480AActive ingredients are clearGood treatment effectOrganic active ingredientsRespiratory disorderDiseasePulegone
The invention discloses a pharmaceutical composition for treating or preventing swollen sore throat. The pharmaceutical composition is characterized by comprising the following components in parts by mass: 1-10 parts of liquiritin, 1-10 parts of pulegone and 10-40 parts of arctiin. The pharmaceutical composition is prepared by mixing the components by adopting a conventional method. The pharmacological experiment research proves that the pharmaceutical composition has the pharmacological activities of diminishing inflammation and relieving pain and can be used for treating diseases such as swollen sore throat and pneumonia.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
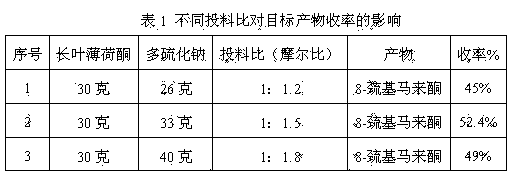
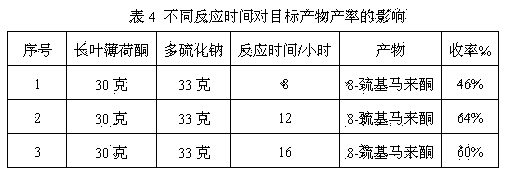
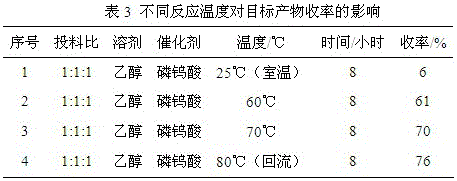
Preparation method of edible essence 8-mercaptomenthone
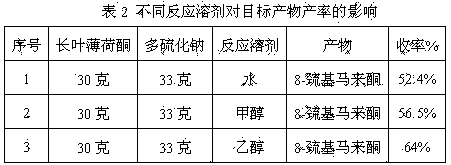
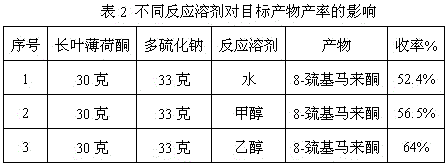
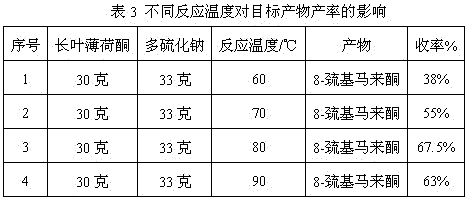
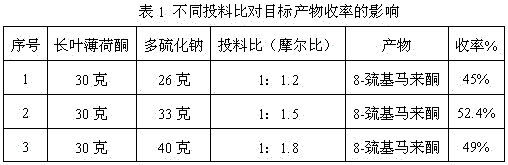
The invention discloses a preparation method of edible essence 8-mercaptomenthone. The preparation method utilizes polysulfides and pulegone as raw materials. Compared with the prior art, the preparation method is free of toxic H2S use and multiple complicated processes and has high operation safety and environmental friendliness. The preparation method has a high 8-mercaptomenthone yield, simple processes and mild reaction conditions and is suitable for industrial production.
Owner:南京誉佳医药科技有限公司
A kind of isatin core double spiro compound with antitumor activity and its synthesis method
ActiveCN104193749BGood antitumor activityRaw materials are easy to getOrganic chemistryAntineoplastic agentsPulegoneSynthesis methods
The invention discloses a compound shown in a structural general formula (I) in the Specification and a synthesis method thereof. The double-spiro compound containing the isatin mother nucleus uses pulegone, isatin and derivatives thereof, and sarcosine as raw materials, and the synthesis process uses a one-pot method, so that complicated multi-step operations are avoided, the operation is simple and convenient, the reaction conditions are mild, and the yield is better. The bioactivity tests show that the double-spiro compound containing the isatin mother nucleus has better antineoplastic activity.
Owner:SHAANXI UNIV OF SCI & TECH
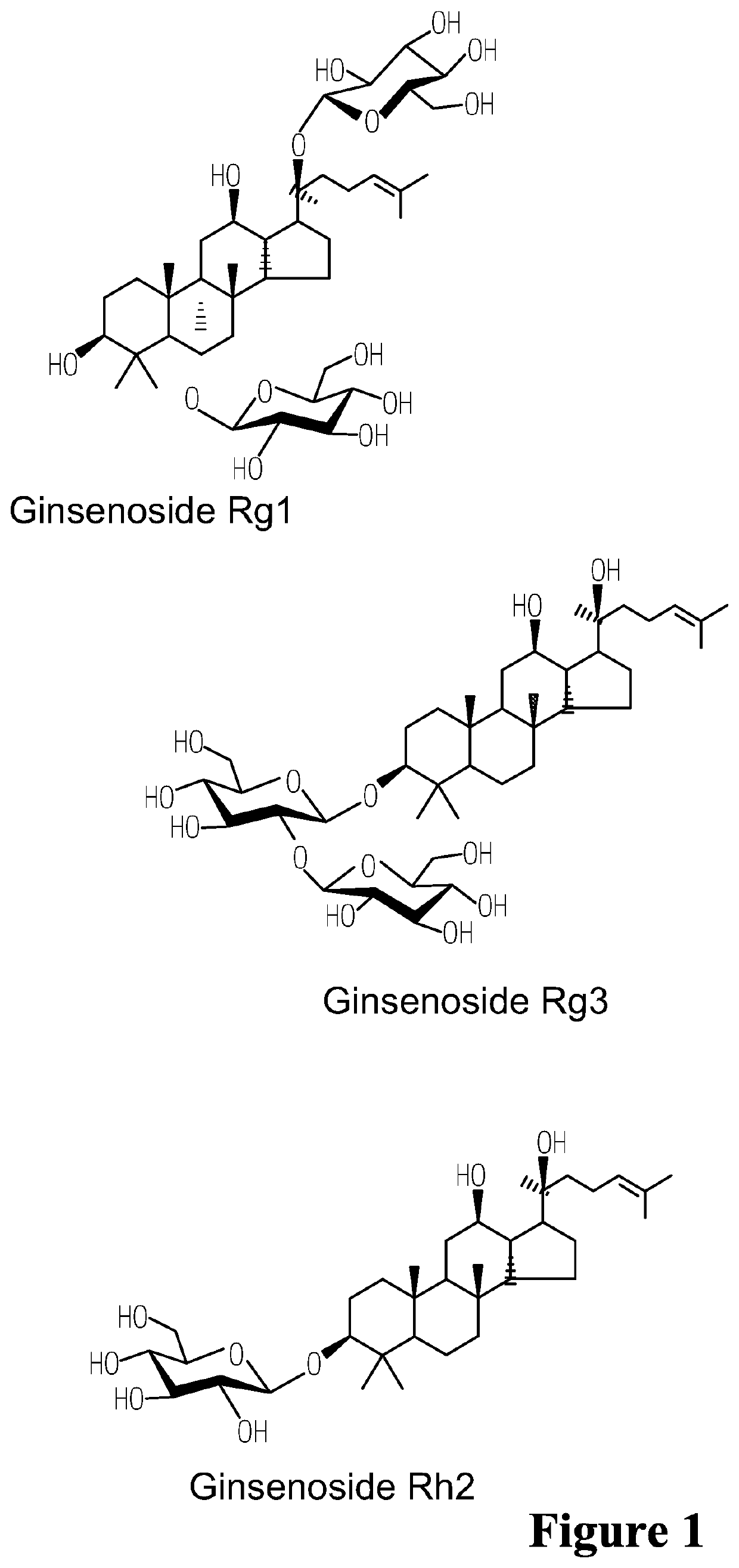
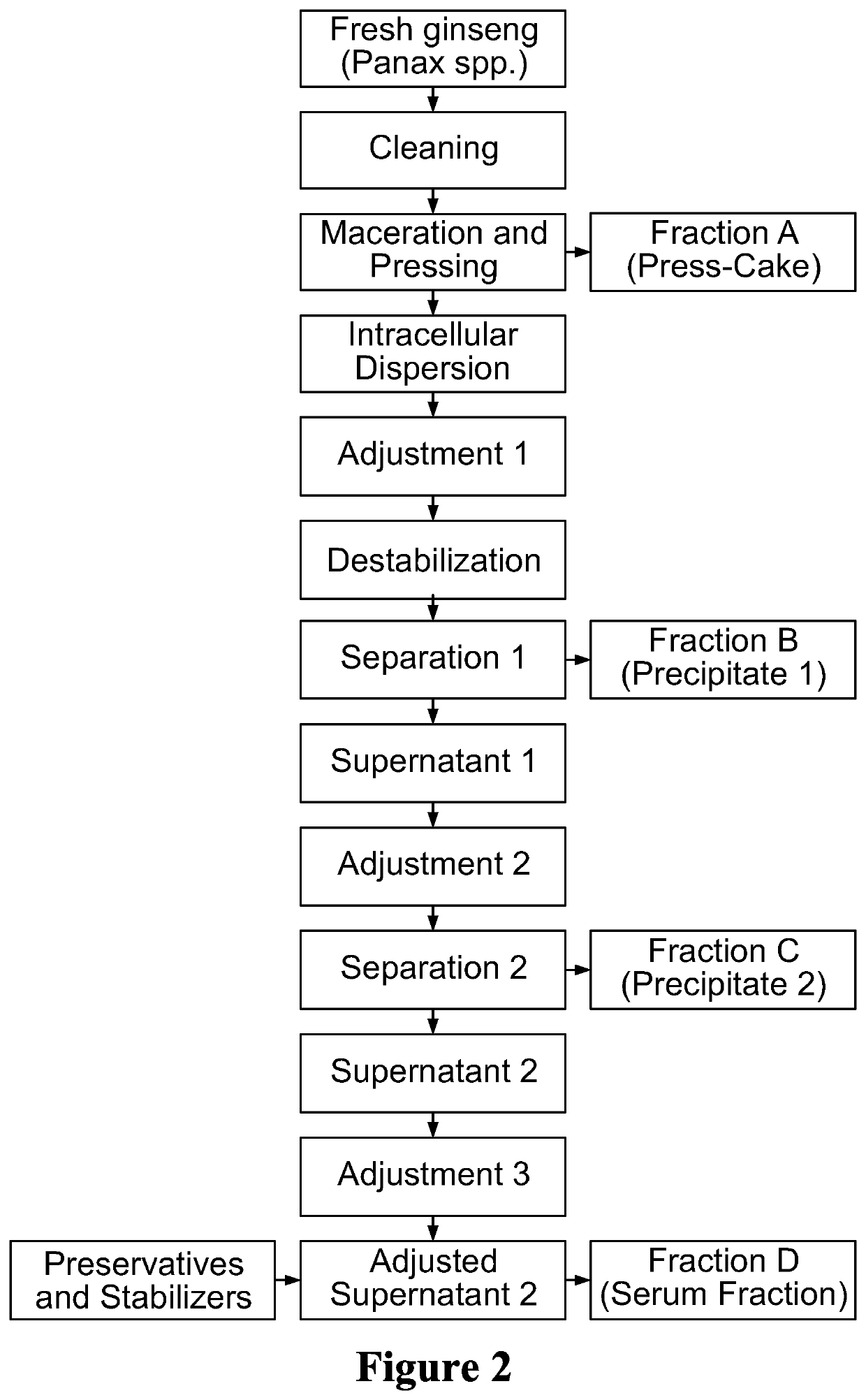
Bioactive compositions from ginseng plant (Panax spp.) and methods for their production and use
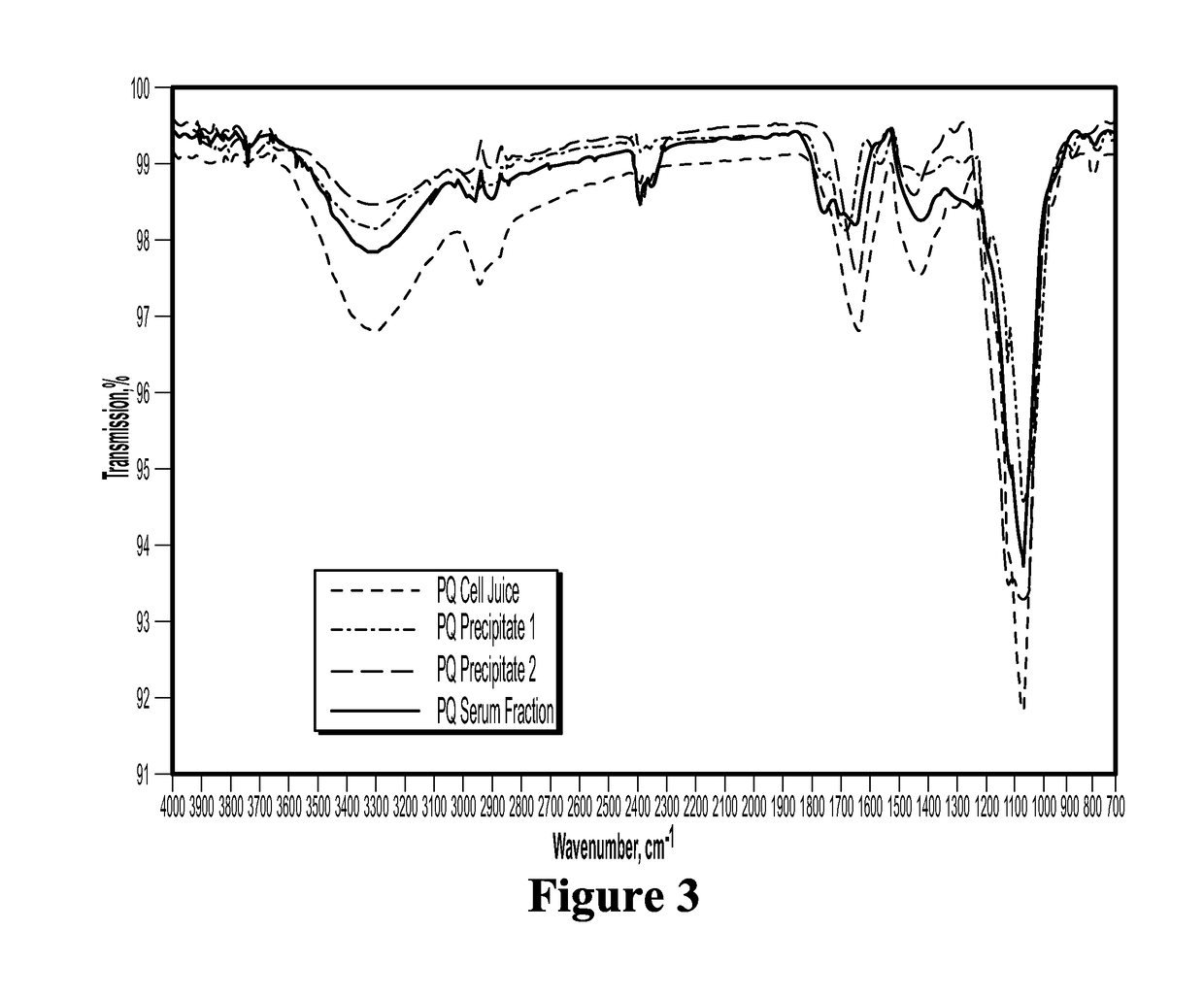
The present invention relates to bioactive compositions (including, for example, bioactive serum fractions and bioactive extracts) derived from ginseng (Panax spp.) fresh whole plant, a particular part of the plant (e.g., rhizome, roots, leaves, stems, flowers, fruit) or any combination of these plant parts. The bioactive serum fractions and bioactive extracts are free of or substantially free of pulegone and / or free of or substantially free of proteins. The present invention also provides products comprising the bioactive serum fractions and / or bioactive extracts. The present invention further provides methods of making and using the bioactive serum fractions and / or bioactive extracts. Further, the bioactive serum fractions and bioactive extracts have anti-inflammatory and / or anti-irritant and / or anti-aging activities.
Owner:ISP INVESTMENTS LLC
A kind of 7-azaisatin double spiro compound with antitumor activity and its synthesis method
ActiveCN104402886BGood antitumor activityRaw materials are easy to getOrganic chemistryAntineoplastic agentsPulegoneSynthesis methods
The invention relates to a 7-aza isatin nuclear parent-containing dispirocyclic compound with antitumor activity and shown as a structural formula (I) and a synthetic method thereof. The dispirocyclic compound is synthesized from raw materials of pulegone, 7-aza isatin and its derivatives, and sarcosine through a one-pot synthesis process; and the process avoids complicated multi-step operation, and has the advantages of mild reaction condition and good yield. Biological activity tests show that the 7-aza isatin nuclear parent-containing dispirocyclic compound has good antitumor activity and broad application prospects in the field of medicine.
Owner:SHENYANG HONGQI PHARMA
Synthetic peppermint oil composition and preparation method thereof
The invention provides a synthetic peppermint oil composition and a preparation method thereof. The synthetic peppermint oil composition comprises beta-caryophyllene, menthyl acetate, pulegone, piperitone, menthone, isomenthone, neomenthol, L-menthol, D-menthol, L-limonene, octanol-3, isopulegol, 1,8-cineole, linalool and a ferrocene carboxylic acid compound. Preferably, the synthetic peppermint oil composition is obtained by conducting aging under the condition of illumination of a mercury lamp. The composition can emit fragrance similar to that of natural dementholized peppermint oil, is sufficient in cool feeling and has better stability. The synthetic peppermint oil composition can be applied to the fields of cosmetics, daily chemicals, perfume and the like.
Owner:WANHUA CHEM GRP CO LTD
Bioactive compositions from ginseng plant (panax spp.) and methods for their production and use
The present invention relates to bioactive compositions (including, for example, bioactive serum fractions and bioactive extracts) derived from ginseng (Panax spp.) fresh whole plant, a particular part of the plant (e.g., rhizome, roots, leaves, stems, flowers, fruit) or any combination of these plant parts. The bioactive serum fractions and bioactive extracts are free of or substantially free of pulegone and / or free of or substantially free of proteins. The present invention also provides products comprising the bioactive serum fractions and / or bioactive extracts. The present invention further provides methods of making and using the bioactive serum fractions and / or bioactive extracts. Further, the bioactive serum fractions and bioactive extracts have anti-inflammatory and / or anti-irritant and / or anti-aging activities.
Owner:ISP INVESTMENTS LLC
A kind of preparation method of edible essence 8-mercaptomaleone
The invention discloses a preparation method of edible essence 8-mercaptomaleone, which adopts polysulfide and menthone as raw materials, and compared with the prior art, it not only avoids the poisonous gas H 2 The use of S also avoids complicated multi-step operations, which is highly safe and environmentally friendly. The 8-mercaptomaleone produced by the method of the invention has high yield, simple process steps, mild reaction conditions and is suitable for industrial production.
Owner:南京誉佳医药科技有限公司
Technique for preparing health care drink series using fresh Agastache leaf as raw material
Owner:JIANGNAN UNIV
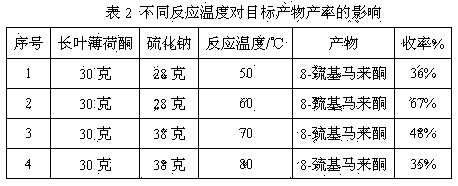
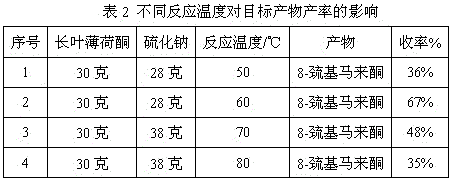
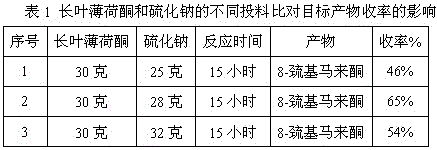
Method for preparing edible essence 8-mercaptomenthone from pulegone and sodium sulfide
Owner:南京誉佳医药科技有限公司
A kind of method utilizing longleaf menthone and sodium sulfide to prepare edible essence 8-mercaptomaleone
The invention relates to a method for preparing edible essence 8-mercaptomenthone from pulegone and sodium sulfide. The method utilizes sodium sulfide, potassium sulfide or ammonium sulfide as a raw material. Compared with the prior art, the method does not use toxic gas H2S, is free of multiple complicated processes, has high operation safety and is environmentally friendly. The method has a high 8-mercaptomenthone yield, simple processes and mild reaction conditions, is suitable for industrial production and solves the problems of a low yield of the prior art.
Owner:南京誉佳医药科技有限公司
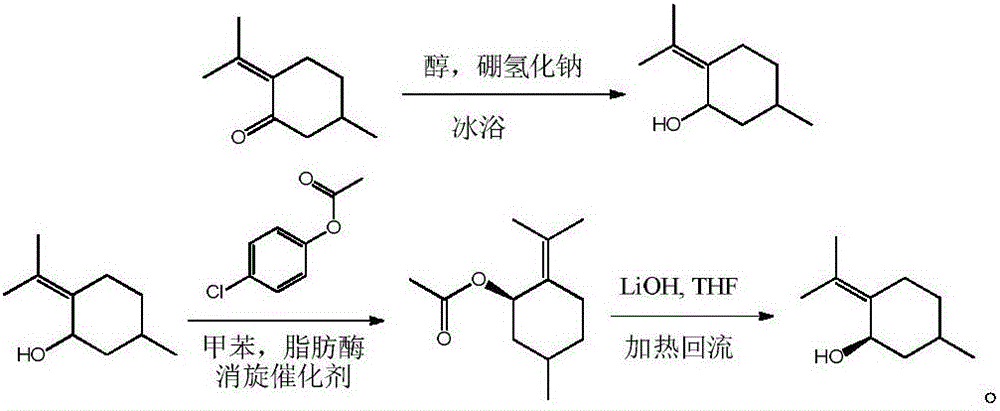
Preparation method of pulegone derivative
The invention discloses a method for deriving an optical purity hydroxy compound by taking pulegone as a raw material. The pulegone is reduced by a hydrogenating reagent to obtain 5-methyl-2-(1-methylethylene) cyclohexanol, and the 5-methyl-2-(1-methylethylene) cyclohexanol is split to obtain (1R)-5-methyl-2-(1-methylethylene) cyclohexanol. Prochiral ketone in the pulegone is basically changed into a chiral center and further split. The method has the advantages of simplicity in operation, high product yield, fine optical purity and the like.
Owner:安徽爱有澄生物科技有限公司
Ziziphora clinopodioides Lam fingerprint and establishment method thereof
InactiveCN102662019BMonitor qualityQuality improvementComponent separationHplc fingerprintFiltration
Owner:XINJIANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com