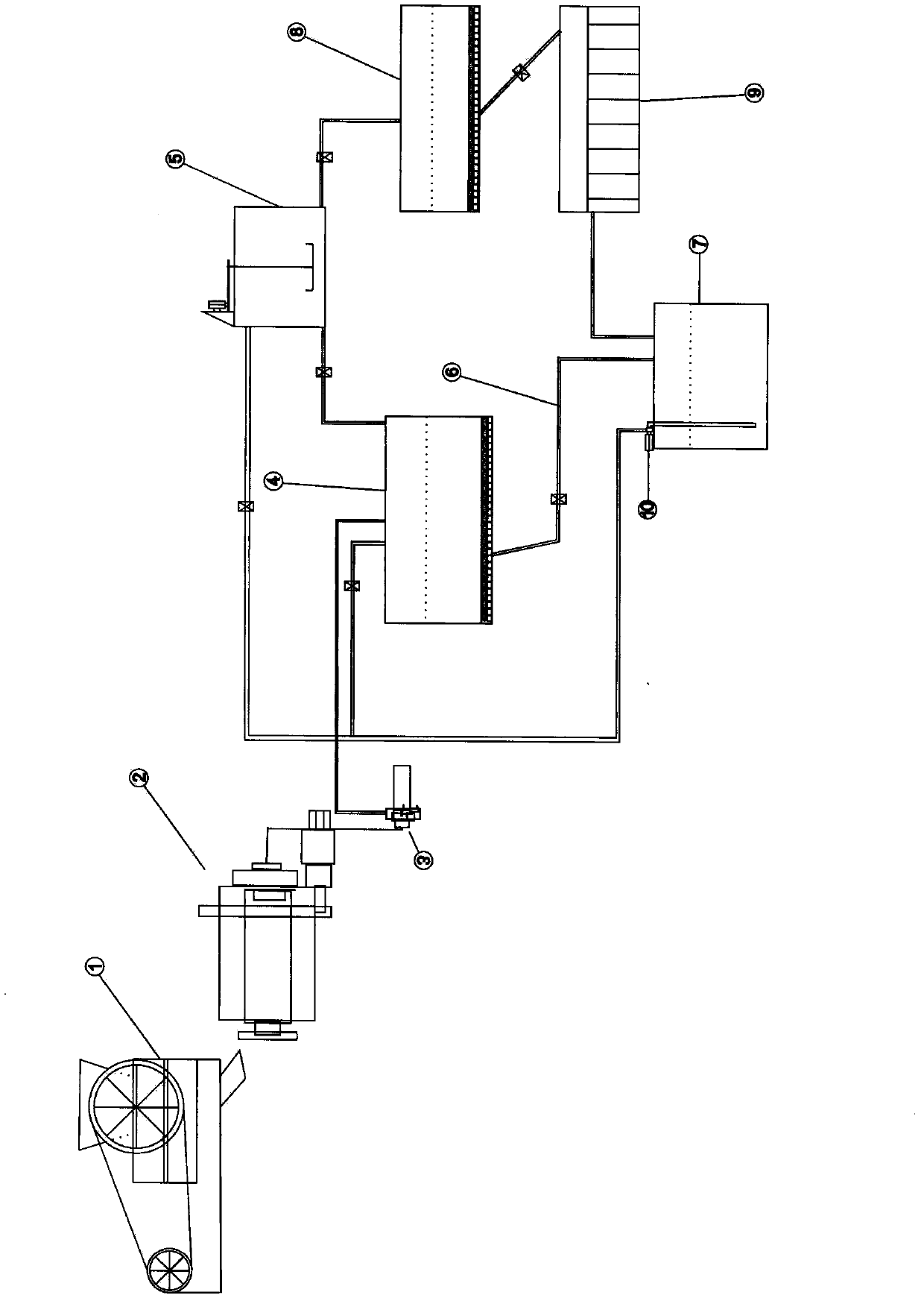
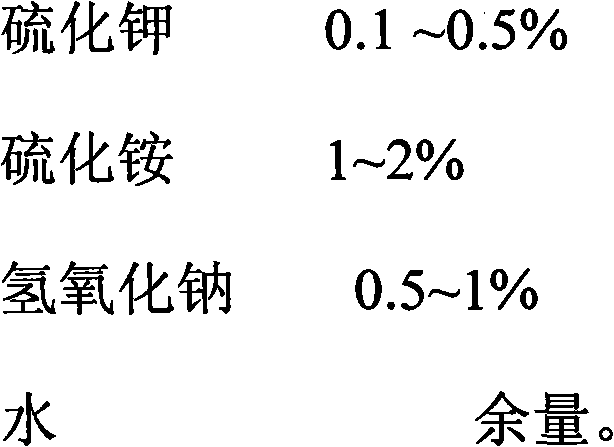
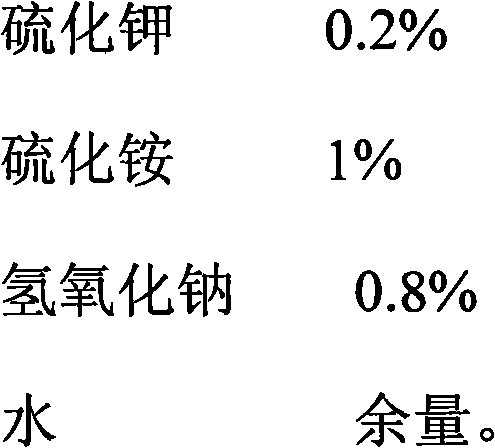
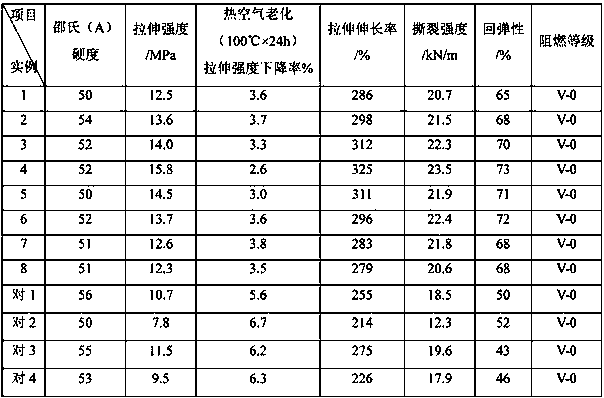
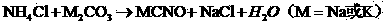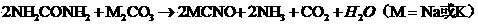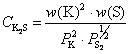Patents
Literature
108 results about "Potassium sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium sulfide is the inorganic compound with the formula K₂S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords potassium hydrosulfide (KSH) and potassium hydroxide (KOH). Most commonly, the term potassium sulfide refers loosely to this mixture, not the anhydrous solid.
Method for preparing alkali sulphide by using sulfur dye waste gas
InactiveCN101654226AReduce emission concentrationSolve processing problemsAlkali metal sulfides/polysulfidesSodium bicarbonateReaction temperature
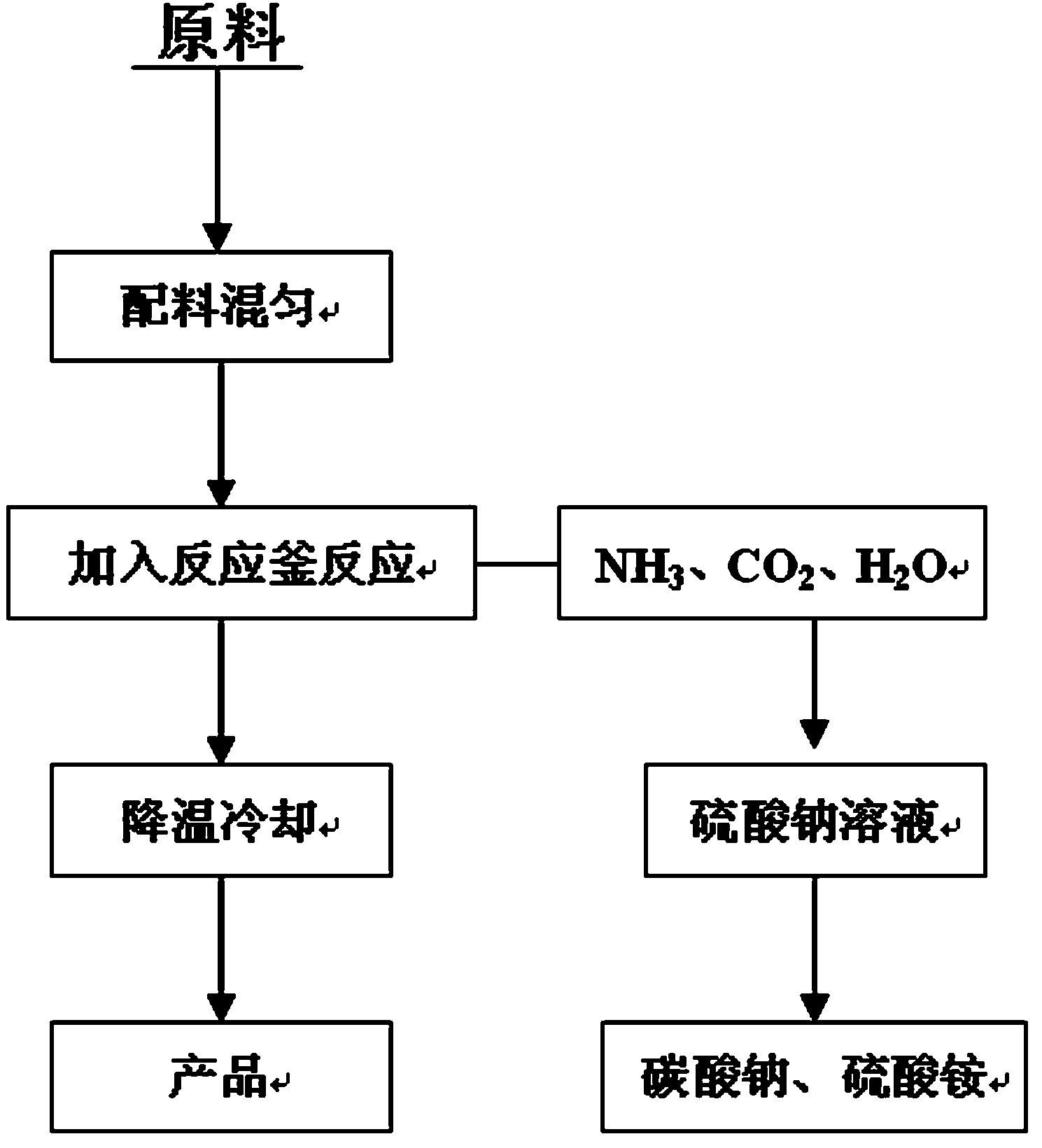
The invention discloses a method for preparing alkali sulphide by using sulfur dye waste gas, which is characterized in that the sulfur dye waste gas containing hydrogen sulfide is used as a raw material and added with alkali to perform reaction at the reaction temperature of between 60 and 85 DEG C, the reaction products are cooled to between 30 and 50 DEG C after the reaction reaches a final point, and the reaction products are filtered to form the alkali sulphide. The alkali added into the sulfur dye waste gas containing the hydrogen sulfide may be one or a mixture of more than two of sodium hydroxide, potassium hydroxide, sodium carbonate, sodium bicarbonate and potassium carbonate, and the ratio of the adding amount of the alkali to the weight of the waste gas is (0.8-1.4): 1. The pressure in the reaction process is -0.12 to -0.06MPa. The alkali sulphide is sodium sulphide and potassium sulfide. The method changes waste into valuable, effectively solves the problem that the sulfurdye waste gas is difficult to treat, reduces the discharge concentration of hydrogen sulfide in the waste gas, achieves the aims of environmental protection, energy conservation and discharge reduction, substance recycle and clean production, has large economic benefit and social benefit, and has the advantages of effectively reducing environment pollution and having less energy consumption.
Owner:蔡瑞琳 +2
Preparation method of Cu-Zn-Sn-S thin film
InactiveCN102251235AReduce usageSolve unmanageable problemsFinal product manufactureSolid/suspension decomposition chemical coatingAmmonium sulphidePotassium sulfide
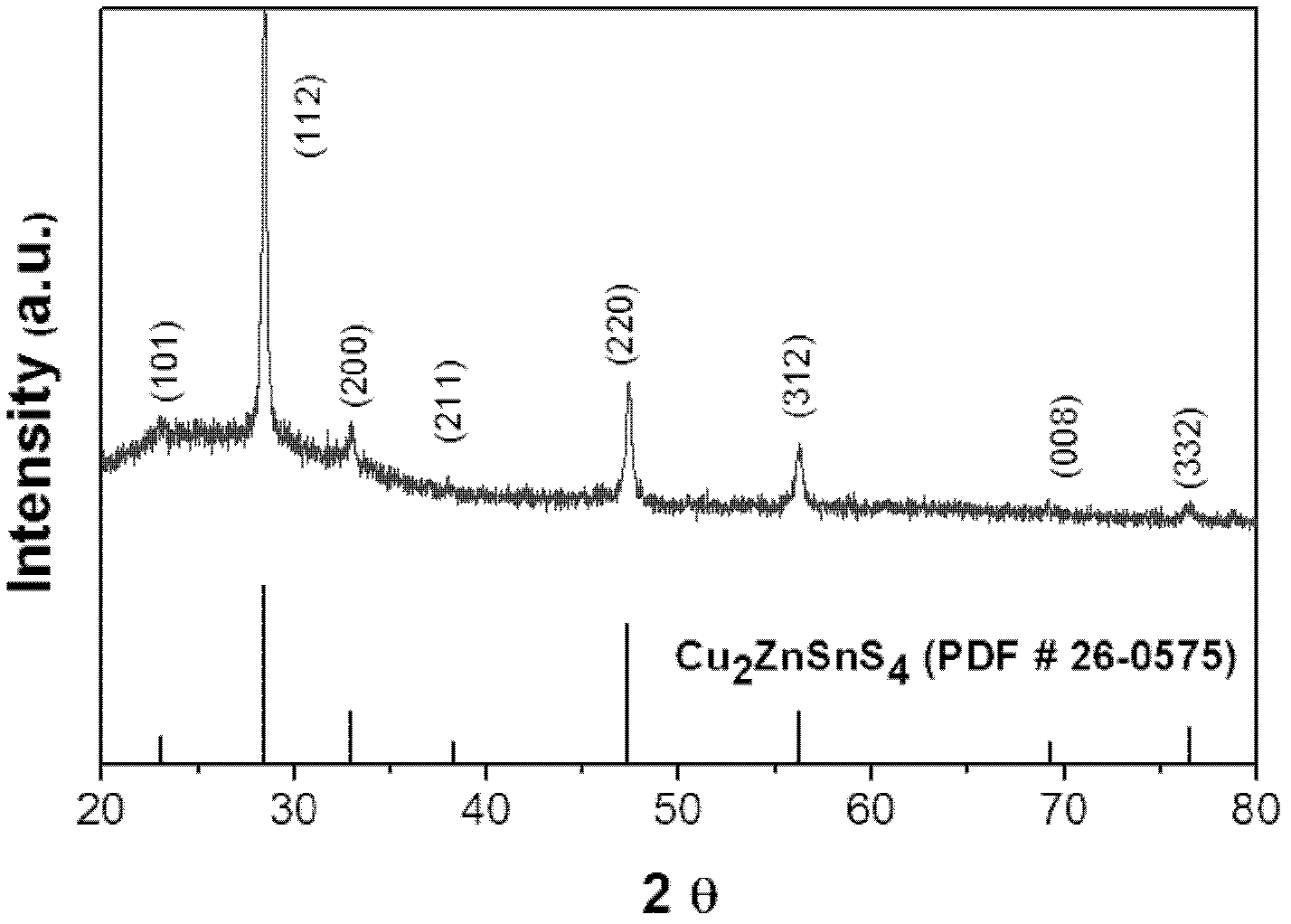
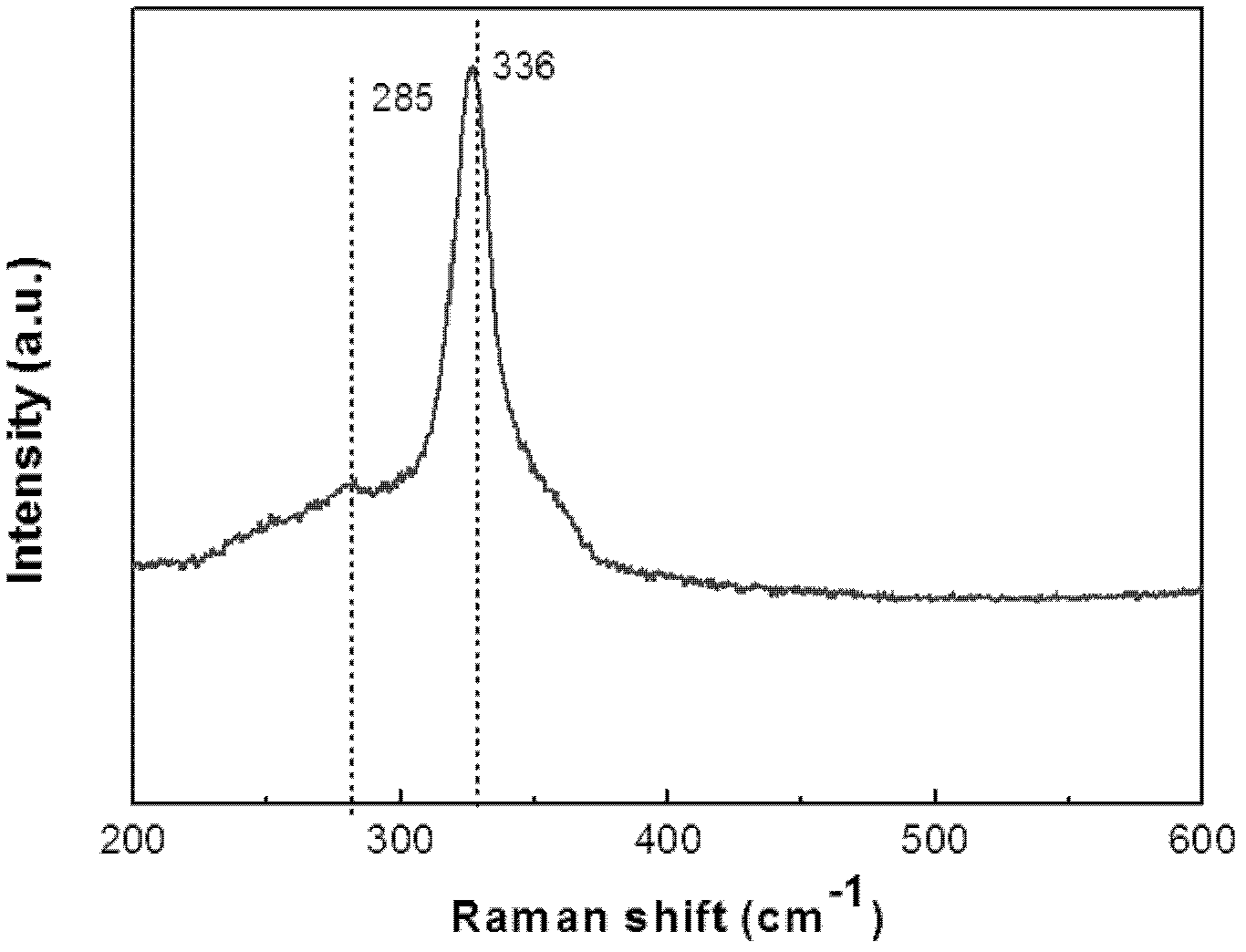
The invention discloses a preparation method of a Cu-Zn-Sn-S thin film, comprising the steps of: by adopting a successive ionic layer adsorption reaction method, sequentially or alternatively soaking one substrate in a cation precursor solution and an anion precursor solution to prepare a Cu2SnSx film and ZnS film laminated precast layer structure or a Cu2S film and ZnSnSx film laminated precast layer structure, and then performing heat treatment to obtain the Cu-Zn-Sn-S thin film; the cation precursor solution comprises at least one of copper ions, stannum ions and zinc ions, and the anion precursor solution is selected from at least one of a sodium sulfide solution, a potassium sulfide solution and an ammonium sulfide solution; the substrate is selected from one of glass, PI (polyimide), a stainless steel plate, a molybdenum plate and a titanium plate. The preparation method of Cu-Zn-Sn-S thin film not only solves the problem that the metal components are difficult to control, but also prevents the copper ions from moving to the surface of the film to form a sulfur copper phase. The preparation method is simple and applicable, low in cost and suitable for industrialization production.
Owner:CENT SOUTH UNIV
Beneficiation reagent, synthesis method and method for comprehensively utilizing generated waste gases
The invention discloses a beneficiation reagent, a synthesis method and a method for comprehensively utilizing generated waste gases. The beneficiation reagent is formed by solid-phase synthesis of inorganic or organic raw materials and comprises the following raw materials in parts by weight: 300 to 700 parts of a nitrogen-containing compound, 500 to 800 parts of chlorine salt, 400 to 650 parts of carbonate, 80 to 160 parts of strong alkali, 50 to 500 parts of cyanide complex double salt, 0 to 300 parts of cyanate, 5 to 20 parts of potassium sulfide, 50 to 200 parts of carbon and 0 to 100 parts of lime nitrogen. The beneficiation reagent is prepared by the following steps: mixing the raw materials uniformly; adding the mixed raw materials into a reaction kettle; quickly heating to 500 to 1,200 DEG C; maintaining high temperature for 1 to 2 hours; and cooling and crushing to obtain the product. Ammonia gas and carbon dioxide generated in a synthesis process are absorbed by a sodium sulfate solution to generate byproducts sodium carbonate and ammonium sulfate. The invention has the advantages of low production cost, simple operation, low toxicity and easy control. The product can be widely applied to leaching of noble metals such as gold oxide ores and silver oxide ores, floatation inhibitors and metal heat treatment and electroplating industries.
Owner:YUNNAN KEENLY NEW MATERIAL
Use of sulfide-containing liquors for removing mercury from flue gases
InactiveUS7037474B2Inherent safety advantageEfficient removalGas treatmentExhaust apparatusPotassium sulfideIndustrial gas
A method and apparatus for reducing and removing mercury in industrial gases, such as a flue gas, produced by the combustion of fossil fuels, such as coal, adds sulfide ions to the flue gas as it passes through a scrubber. Ideally, the source of these sulfide ions may include at least one of: sulfidic waste water, kraft caustic liquor, kraft carbonate liquor, potassium sulfide, sodium sulfide, and thioacetamide. The sulfide ion source is introduced into the scrubbing liquor as an aqueous sulfide species. The scrubber may be either a wet or dry scrubber for flue gas desulfurization systems.
Owner:THE BABCOCK & WILCOX CO
Preparation method of metal sulfide semiconductor nanocrystalline
InactiveCN1522953ASufficient supplyEfficient synthesisNanostructure manufactureSulfur compoundsAmmonium sulfideMetallic sulfide
The method for preparing metal sulfide semiconductor nano crystal by using template includes the following steps: firstly, using collodion cotton liquor and making it into the semi-transparent film which has uniform thickness and can meet designed thickness, size and form as artificial active film template, then using metal ion solution of zinc, cadmium copper, mercury, silver, lead, iron, cobalt and nickel whose concentration is 0.05-0.20 mol / L and ionic mole number ratio is correspondent to product chemical formula and providing the solution of sodium sulfide, ammonium sulfide, potassium sulfide and hydrogen sulfide of sulfur ion, respectively placing them at two sides of artificial active film, reacting for 24-48 hr, under the condition of room temp. and stirring, then centrifugal separating reaction solution, removing clear liquor, successively using acetone and deionized water to wash remained product, naturally-drying so as to obtain the metal sulfide semiconductor nano crystal whose grain size (or diameter) is 20-200 nm.
Owner:TONGJI UNIV
Synthesis method of magnesium hydroxide nano pipe
InactiveCN1556034ASimple processProduction conditions are easy to controlMagnesium hydroxideReaction temperatureSolvent
A process for synthesizing the magnesium hydroxide nanotubes includes adding aqueous solution of ammonia to the solution of magnesium nitrate (or chloride), stirring to generate deposit, centrifugal washing until its pH value is neutral, dispersing the deposit in the solution of methanol or ethanol and water, adding inorganic salt chosen from potassium chloride, sodium chloride, sodium sulfate, potassium sulfide, potassium nitrate, sodium nitrate and their mixture, stirring, reacting at 200-250 deg.C for 15-24 hr in a sealed reactor, cooling, washing and drying.
Owner:SHANDONG NORMAL UNIV
Manufacturing method of antiqued silver
ActiveCN104924827AGood collection valueNo chemical residueSpecial ornamental structuresMetallic material coating processesPotassium sulfideAcid washing
The invention relates to the technical field of processing of silver products, in particular to a manufacturing method of antiqued silver. The manufacturing method comprises silver molding and burning steps, and further comprises acid washing and blackening treatment for silverwares, wherein the blackening treatment comprises the following steps: (1) 1-3 parts of potassium carbonate, 2-5 parts of sodium hydroxide, 25-35 parts of potassium sulfide and 60-70 parts of purified water are formed to mixed liquid; and the acid-washed silverwares are put in the mixed liquid for dipping 5-10 min; and (2) the surfaces of the dipped silverwares are polished. The silver products obtained by the method take an antiqued color; and the antiqued color of the antiqued silver products is pure, so that the silver products have another special fashion sense and excellent collection value; and the silver products obtained by the method have no any chemical residues, are healthy and environment-friendly, are more beneficial to the human health, and are better in health maintenance value.
Owner:河南梦祥纯银制品有限公司
Method for preparing silver/silver sulfide nanowire with core shell structure
The invention relates to a method for preparing a silver / silver sulfide nanowire with a core shell structure. The method for preparing the material comprises the first step of scattering a silver nanowire regular in shape in a water solution containing a macromolecule protective agent, adding ferric chloride water solution to oxidize the surface of a silver wire to obtain a silver / silver chloride nanowire with a core shell structure; the second step of scattering the generated silver / silver chloride nanowire in the water solution, adding a water solution containing a sulphur source (thioacetamide, sodium sulfide or potassium sulfide), obtaining the silver / silver sulfide nanowire with the core shell structure; and the third step of performing hydrothermal crystallization on the obtained silver / silver sulfide nanowire. The method for preparing the silver / silver sulfide nanowire with the core shell structure has the advantages of being simple in preparation process and controllable in shape. The nanowire with the silver / silver sulfide core shell structure can be used for the fields of photoelectrodes and photocatalysis and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
High-stability water-base silver-plating protective agent and preparation method thereof
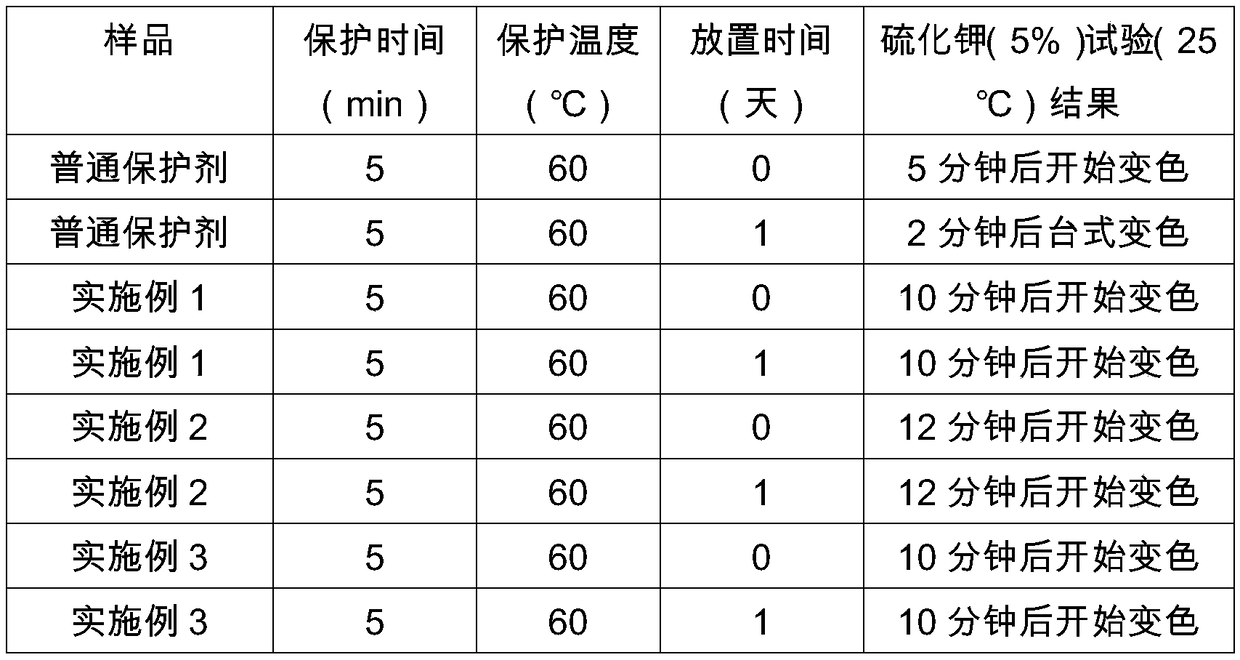
The invention provides a high-stability water-based silver-plating protective agent. Every liter of the high-stability water-based silver-plating protective agent comprises 40-100 g of octadecanethiol, 4-10 g of trimethyl mercapto phosphate, 100 mg of reduced graphene oxide, 40-100 g of an emulsifier, 10 g of a stabilizer and the balance deionized water. The high-stability water-based silver-plating protective agent adopts the stabilizer and graphene, so that the service life of the protective agent is prolonged, and the corrosion resistance and color change resistance of protected metal are improved; and the surface appearance and performance of the silver-plating layer protected by the protective agent are not affected, a potassium sulfide resistance test of electronic products can be maintained for more than 12 minutes, and the protection time is greatly prolonged.
Owner:SUZHOU HIYIE CHEM CO LTD
ECF bleaching method for reducing AOX formation amount
The present invention relates to an ECF bleaching method for reducing the AOX formation amount. The method is mainly characterized by comprising: (1) carrying out hemicellulase pretreatment on an unbleached pulp, and (2) in the presence of chlorine dioxide, sodium hydroxide, hydrogen peroxide and potassium sulfide, carrying out ECF bleaching on the bagasse pulp. According to the present invention, the hemicellulase pretreatment is performed on the unbleached bagasse pulp so as to remove hexenuronic acid capable of reacting with chlorine dioxide and concurrently remove hemicelluloses, such that the more residual lignin can be exposed; with the addition of potassium sulfide, the sulfur ions can be provided for the alkali extraction stage, the alkali extraction stage can be strengthened, the dissolution of the lignin is easily achieved, it can be ensured that the chlorine dioxide bleaching stage uses the less chlorine dioxide, and the AOX generated during the bleaching process can be reduced; and compared with the traditional ECF bleaching, the bleaching method of the bagasse pulp in the present invention has the following characteristics that: the chlorine dioxide consumption can be saved by 15-20%, and the AOX formation amount can be reduced by 20-30%.
Owner:GUANGXI UNIV
Method for producing heavy metal passivators for farmland soil and application of heavy metal passivators
InactiveCN108410473AEliminate destructionEliminate hazardsAgriculture tools and machinesContaminated soil reclamationSodium BentonitePhosphate
The invention discloses a method for producing heavy metal passivators for farmland soil and application of the heavy metal passivators, and belongs to the field of environmental protection. The method includes steps of adding calcium polysulfide, potassium sulfide, potassium dihydrogen phosphate, sodium lignosulfonate, potassium humate, bentonite and quicklime into a suspending machine, stirringthe calcium polysulfide, the potassium sulfide, the potassium dihydrogen phosphate, the sodium lignosulfonate, the potassium humate, the bentonite and the quicklime and uniformly mixing the calcium polysulfide, the potassium sulfide, the potassium dihydrogen phosphate, the sodium lignosulfonate, the potassium humate, the bentonite and the quicklime with one another to obtain first mixtures; transferring the first mixtures into a ball mill and smashing the first mixtures; adding sodium silicate and EDTA (ethylene diamine tetraacetic acid) into the first mixtures and uniformly stirring the sodium silicate, the EDTA and the first mixtures. The method and the application have the advantages that the heavy metal passivators which are mixtures can be added into the soil contaminated by differenttypes of heavy metal, accordingly, the different types of heavy metal in the soil can be immobilized and passivated and can be turned into insoluble metal salt or alkali, and the activity, the mobility and the bioavailability of the heavy metal in the soil can be deteriorated to a great extent; contamination of the soil due to the different types of heavy metal can be abated, accordingly, the soil productivity and underground water can be protected, and the food safety can be guaranteed.
Owner:吴洪生
Process for metal recovery in flotation operations
InactiveUS20190256950A1Significant positive effectReduce activationFlotationPotassium sulfideAmmonium sulfide
The present invention is related to a process for increasing copper or metal recovery in flotation processes, specially of minerals that are dissolved during the grinding stage, by the use of any sulfidizing agent or ionizing sulfide such as, but not limited to, sodium hydrogen sulfide, sodium sulfide, potassium hydrogen sulfide, potassium sulfide, ammonium hydrogen sulfide or ammonium sulfide, hydrogen sulfide (H2S), polysulfides of potassium, calcium, magnesium or ammonium to precipitate during the grinding stage or immediately after the grinding stage, metals that have been dissolved prior or during the milling or grinding stage prior to normal flotation.
Owner:PASSAU SA
Sprayable agricultural compositions and method
InactiveUS7666309B1Reduce and eliminate precipitationPrecipitation is prevented and reducedBiocidePlantingPotassium sulfidePolysulfide
A composition including an aqueous solution of calcium polysulfide and an amount of a precipitation preventing compound effective to reduce or eliminate precipitation of the calcium polysulfide at low concentrations. The precipitation preventing compound is selected from the group consisting of sodium hydrosulfide, potassium hydrosulfide, sodium sulfide, and potassium sulfide. A method of reducing or preventing precipitation of calcium polysulfide added to the water in an irrigation system by first injecting the precipitation preventing compound into the water in an amount effective to reduce or eliminate precipitation of the calcium polysulfide at low concentrations. Sodium methyldithiocarbamate can be added to the calcium polysulfide / precipitation preventing compound solution without any substantial precipitation.
Owner:BAKER GEORGE B
Method for producing copper concentrate by using copper oxide ore or copper slag
A method for producing copper concentrate by using copper oxide ore or copper slag employs a process comprising ammonia leaching of ore powder, filtering and copper precipitation, and is especially suitable for processing low grade copper oxide or copper slag with copper content of 0.5-5%. First, the ore is ground into an ore powder; then a mixed solution of ammonia and ammonium hydrogen carbonate solution or a mixed solution of sodium hydroxide and ammonium hydrogen carbonate as a leaching agent reacts with the ore powder, so that the copper in the ore enters into the solution in the form of a copper ammonia complex and impurities including aluminum, cadmium, manganese, calcium and silicon are kept in the slag, thereby realizing the separation of copper and impurities; and then one or more selected from sulfide ammonia, sodium sulfide and potassium sulfide are used as a precipitator for copper precipitation to produce copper concentrate, the copper concentrate is filtered to obtain the copper concentrate product after drying, and the filtrate returns to a leaching ore tank for recycling. The method has the advantages of normal temperature operation, low energy consumption, good quality, short flow and simple clean liquid operation, and can effectively use the copper resources to be developed.
Owner:谷亮
Repairing agent for simultaneously repairing As and Cr polluted soil, as well as preparation and application thereof
InactiveCN106957655AShort repair cycleLow costContaminated soil reclamationOrganic fertilisersIron saltsGoethite
The invention discloses a repairing agent for simultaneously repairing As and Cr polluted soil. The repairing agent is prepared from the following components in parts by weight: 25-85 parts of inorganic iron salt, 15-75 parts of clay inorganic minerals, 10-30 parts of silicon dioxide crystallizing agent, 10-30 parts of a magnesium compound and 25-80 parts of sulfide, wherein the inorganic iron salt is one or an arbitrary combination of more than two of ferric sulfate, goethite, ferrous chloride and ferrous sulfide; the magnesium compound is one or an arbitrary combination of more than two of magnesium oxide, dolomite and magnesite; and the sulfide is one or an arbitrary combination of more than two of calcium polysulfide, sodium sulfide and potassium sulfide.
Owner:BEIJING GEOENVIRON ENG & TECH
Electrochemical desulphurization method and application of intermediate product
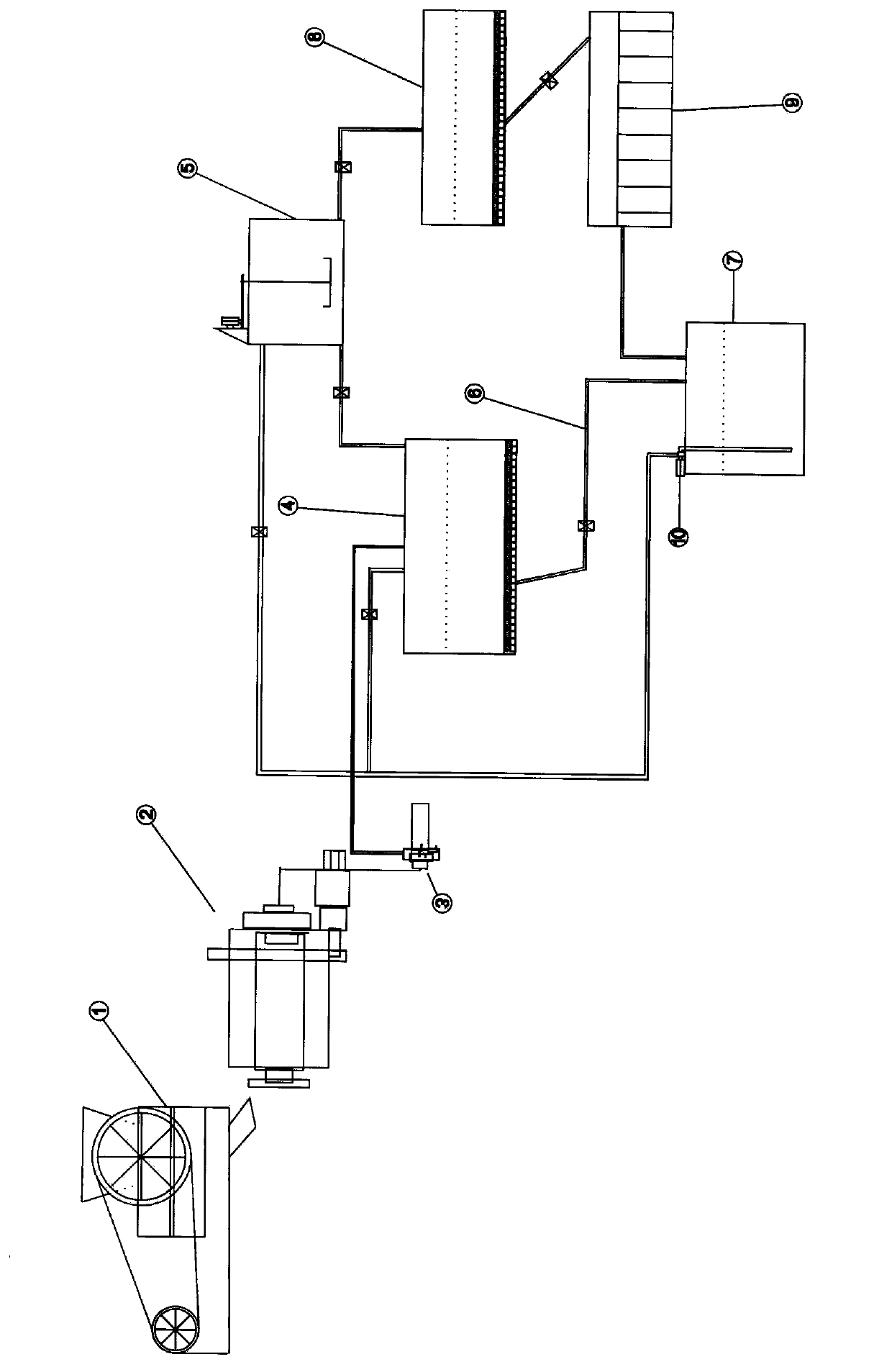
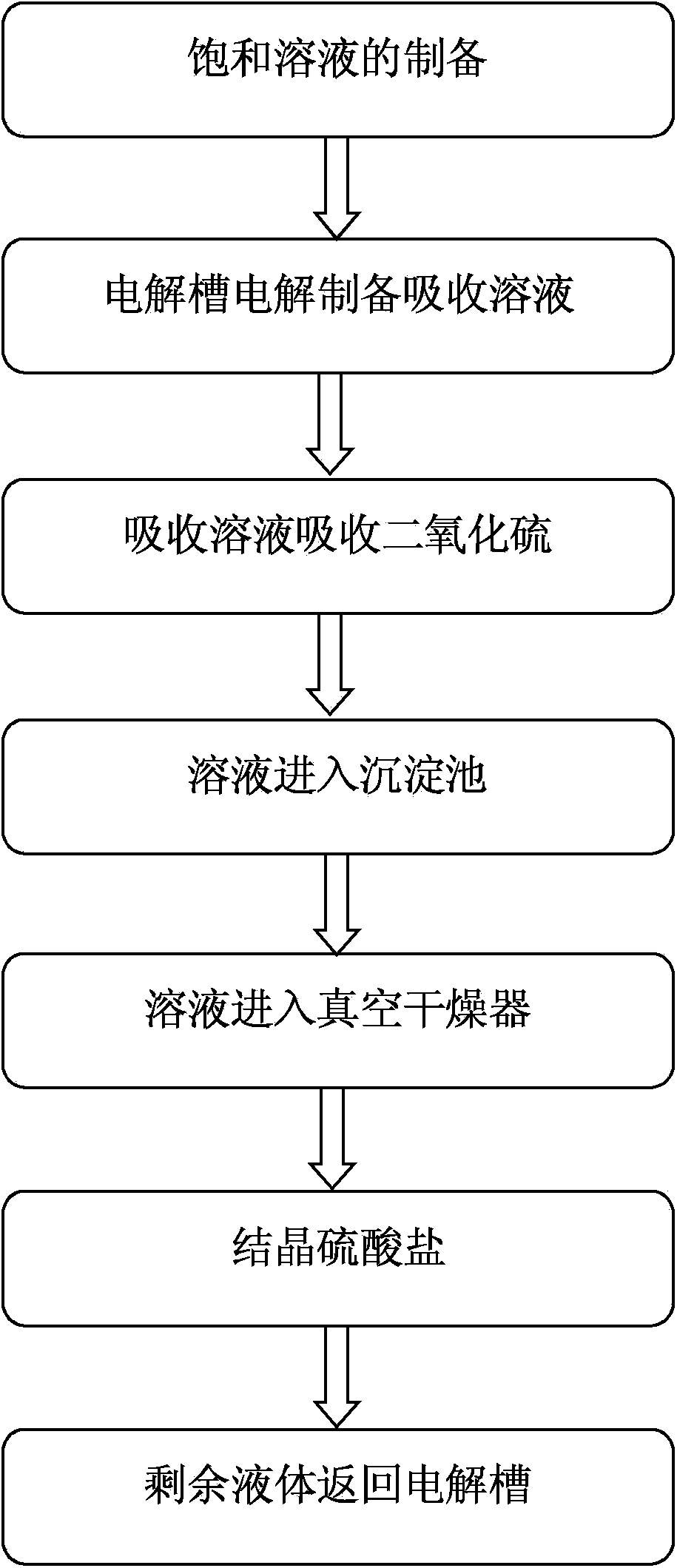
InactiveCN103638804ADispersed particle separationAlkali metal silicatesElectrochemistryEthyl Chloride
The invention discloses an electrochemical desulphurization method. The method comprises the following steps: (1) preparing a saturated solution of sodium salt or kali salt of halogen elements; (2) electrolyzing the saturated solution so as to produce an absorbent solution; (3) spraying the absorbent solution into a sulfur dioxide absorption tower so as to be fully contacted with gas containing sulfur dioxide; (4) recycling the absorbent solution. According to the method, the solution of kali salt or sodium salt of the halogen elements is electrolyzed so as to produce the absorbent solution for absorbing sulfur dioxide gas; as the absorbent solution is alkaline, the absorptivity of sulfur dioxide reaches more than 97%, furthermore, the absorbed sulfite is rapidly oxidized into sulfate by chlorine, bromine or iodine in the solution, no solid waste is generated in the whole desulphurization process, and the intermediate product sodium sulphide or potassium sulphide can be widely applied to industrial production or agricultural production.
Owner:常州和方环保科技有限公司
Aluminum section bar three acid polishing and leveling fog inhibitor for furniture and finishing method thereof
InactiveCN101457362AReduce wear and tearFlat and smooth surface appearancePotassium sulfidePhosphoric acid
The invention discloses a three acid polishing and flattening fog inhibitor used on a furniture aluminum section. The fog inhibitor is made from the following components by weight: 70-85% of phosphoric acid, 6-12% of sulfuric acid, 3-9% of nitric acid, 0.5-3% of boracic acid, 1-5% of potassium sulfide and 1-3% of magnesium oxide. The operation flow of the chemical polishing working procedures comprises the following steps: (1) removing oil stains from surfaces of an aluminum section according to the conventional oil removal working procedures; and (2) chemical polishing: at first, soaking and cleaning the aluminum section with hot water for 4-5min, then polishing in a chemical polishing and flattening fog inhibitor at the temperature of 105-110 DEG C for 4-5min, taking out and cleaning, then soaking and cleaning with 20% of dilute nitric acid by mass percent for 5-8min, and finally oxidizing and sealing holes according to the conventional procedures to obtain a finished product. The fog inhibitor avoids complex mechanical polishing working procedures, and is employed to directly perform three-acid chemical polishing on the aluminum section, which obtains the same process effect as that obtained by the mechanical polishing working procedures, reduces the aluminum section waste and environmental pollution and can significantly lower the production cost.
Owner:HUNAN XINMEIGE INNOVATORY DECORATIVE MATERIAL
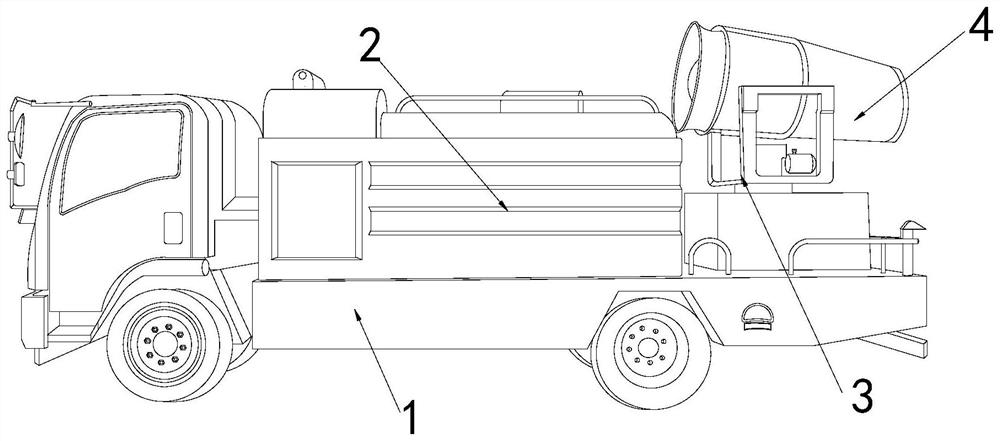
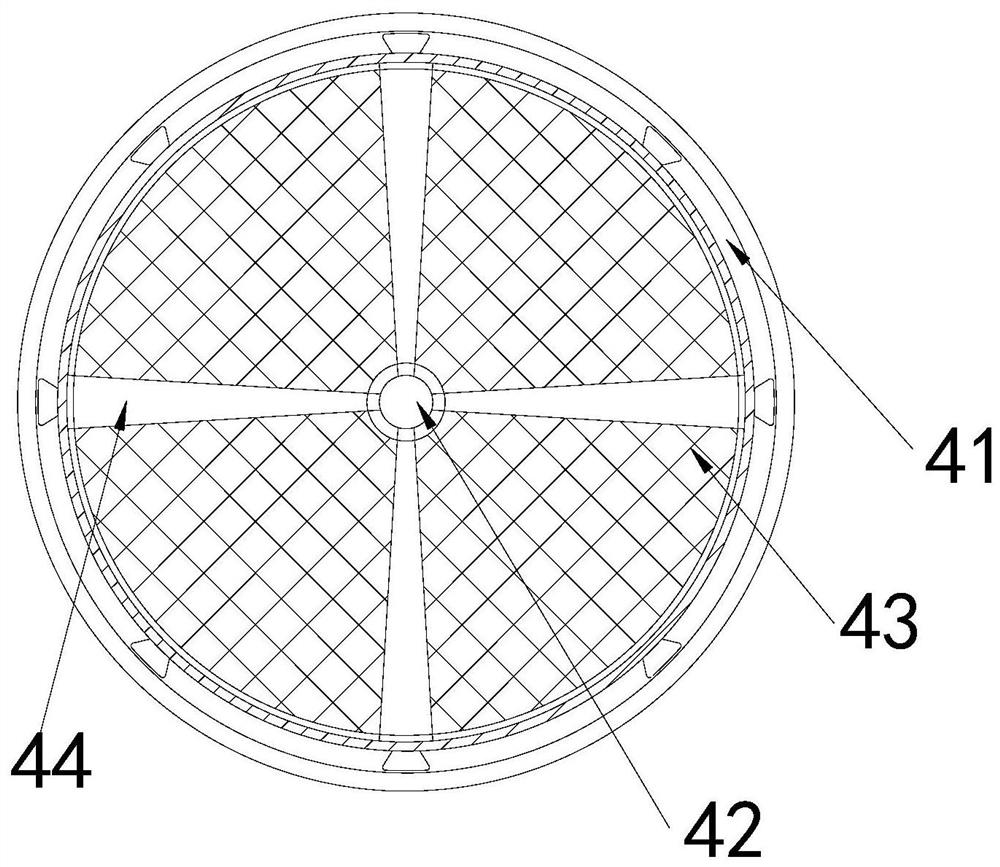
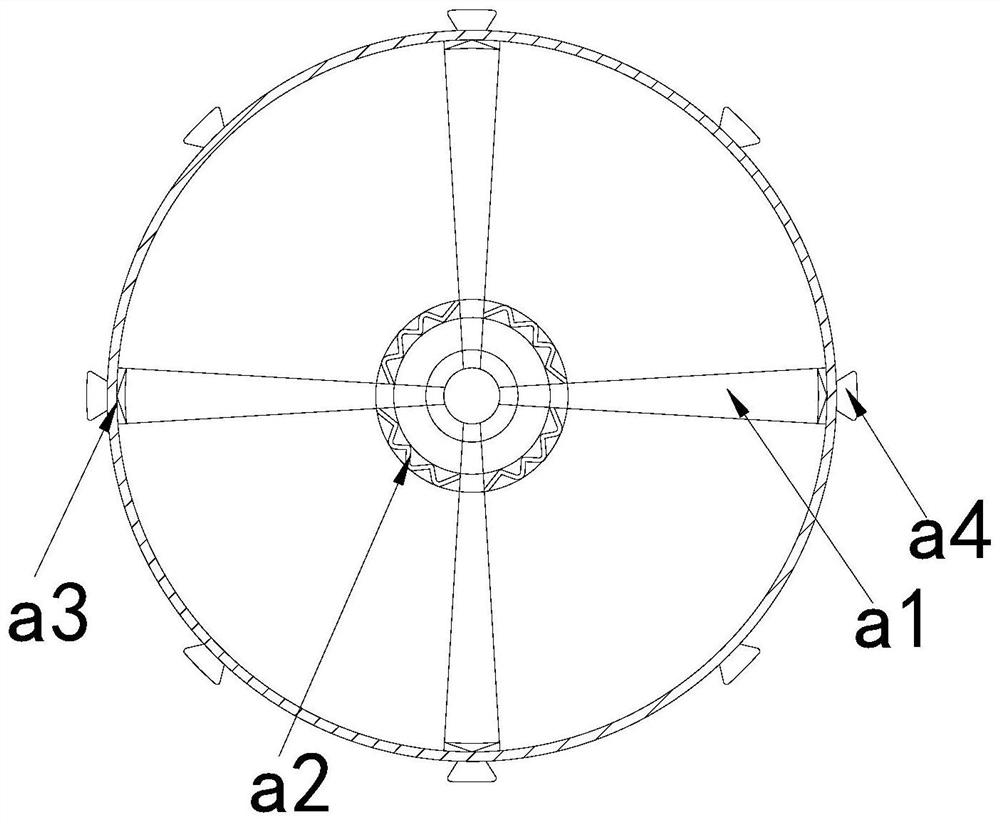
Dust removal vehicle for tunnel construction
ActiveCN111779532APrevent from dissolvingHighly corrosiveSpraying apparatusDust removalWater storage tankMechanical engineering
The invention discloses a dust removal vehicle for tunnel construction. The dust removal vehicle structurally comprises a transport vehicle, a water storage tank, a supporting table and an atomizing spray head, wherein the water storage tank is fixed at the top of the right side of the transport vehicle, the supporting table is mounted on the top of the right side of the transport vehicle and located on the right side of the water storage tank, and the atomizing spray head is movably clamped with the top of the support table. A dissolved potassium sulfide solution is scraped by collection heads of a solution collection device, and the situations that potassium sulfide emitted by blasting is dissolved in water, substances with strong corrosivity are generated, the generated substances are accumulated in gaps between an atomizing opening and edges of an atomizing net, so that the deterioration of the atomizing opening is accelerated, normal atomization of the dust removal vehicle to thewater solution is affected, and the solution scraped by the collection heads remains in a gap between the atomizing opening and a atomizing ring are prevented. The solution is isolated and collected through the collection heads, the collected solution is prevented from backflow, pollution gas in the solution is filtered, and pollution to the environment caused by direct discharge is prevented.
Owner:济南中鲁特种汽车有限公司
A Method for Evaluating the Comprehensive Alkali Removal and Desulfurization Ability of Blast Furnace Slag
InactiveCN102279203AEliminate hazardsSimple methodChemical analysis using combustionColor/spectral properties measurementsPotassium sulfideSlag
A method for evaluating a comprehensive alkali-removing and desulfuration capacity of blast furnace slag. The method is utilized for solving a problem that a comprehensive alkali-removing and desulfuration capacity of blast furnace slag is evaluated through a potassium sulfide capability. The method comprises the following steps of preparing a slag sample, setting positions of a potassium carbonate reagent and the slag sample in a corundum tube of a high temperature furnace, carrying out a slag sample process, determining a potassium sulfide capability and evaluating a comprehensive alkali-removing and desulfuration capacity of blast furnace slag. Aiming at solving a problem that thermodynamic conditions of blast furnace desulfuration are conflicting with thermodynamic conditions of blastfurnace alkali-removing, the method brings forward a way for determining a potassium sulfide capability of blast furnace slag by a gas-slag equilibrium technology and can evaluate a comprehensive alkali-removing and desulfuration capacity of blast furnace slag according to a potassium sulfide capability, and thus appropriate thermodynamic conditions satisfying both alkali-removing and desulfuration are obtained. The method is simple and convenient, has good repeatability and can provide basic data for formulating of a reasonable operation system suitable for a blast furnace. Through the method, a blast furnace can produce eligible molten iron and keep the greatest alkali-removing capacity simultaneously, and under the conditions of high alkali load and low-slag smelting, damages of alkalimetals on the blast furnace are reduced.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Special bio-organic and inorganic compound fertilizer for nuisanceless agricultural products
InactiveCN103351252AAct quicklyUrine enzyme activity can be controlledFertilizer mixturesPhosphateDecomposition
The invention discloses a special bio-organic and inorganic compound fertilizer for nuisanceless agricultural products. The bio-organic and inorganic compound fertilizer comprises the following components in part by weight: 38 parts of biological fermentation dry rabbit dung, 25 parts of urea, 16 parts of monoammonium phosphate, 16 parts of potassium sulphide, 5 parts of dolomite calcium powder, 0.5 part of borax, 0.2 part of copper sulfate, 0.1 part of zinc sulfate, 0.2 part of manganese sulfate and 0.1 part of ammonium molybdate; in addition, the bio-organic and inorganic compound fertilizer further comprises photosynthetic liquid. The bio-organic and inorganic compound fertilizer has the advantages that the content is high, the nutrition is comprehensive, the fertilizer efficiency is long and fast, the rate of absorption and utilization is high, the urease activity of soil can be controlled, the rapid circular decomposition and releasing of nutrient can be accelerated, nitrogen is fixed, phosphorus and potassium are dissolved, the soil is activated, the permeability of the soil is improved, the photosynthesis is promoted, and the rate of absorption and utilization and fertilizer efficiency period are improved greatly.
Owner:SICHUAN HUANGJIA AGRI GRP
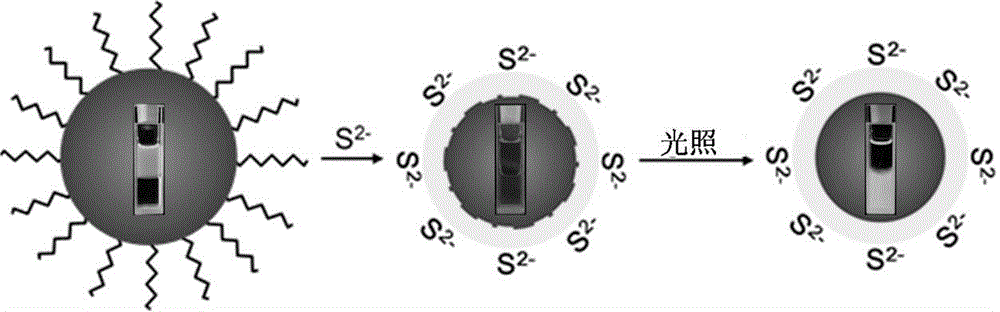
Method for preparing CdSe/CdS nuclear shell semiconductor quantum dots at normal temperature
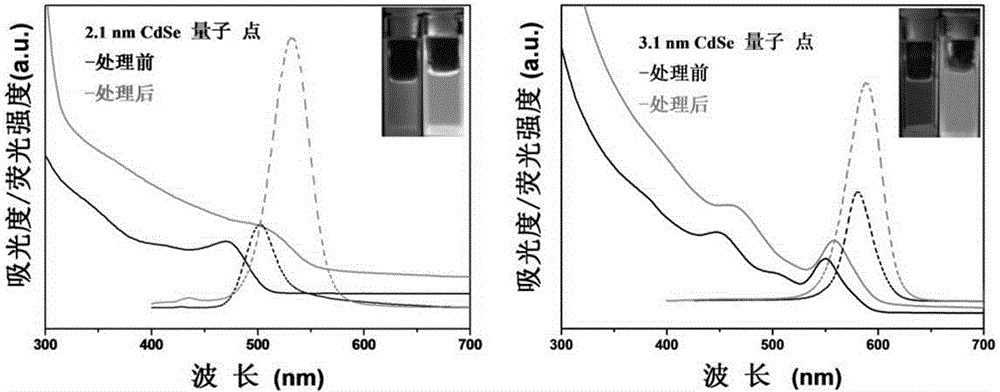
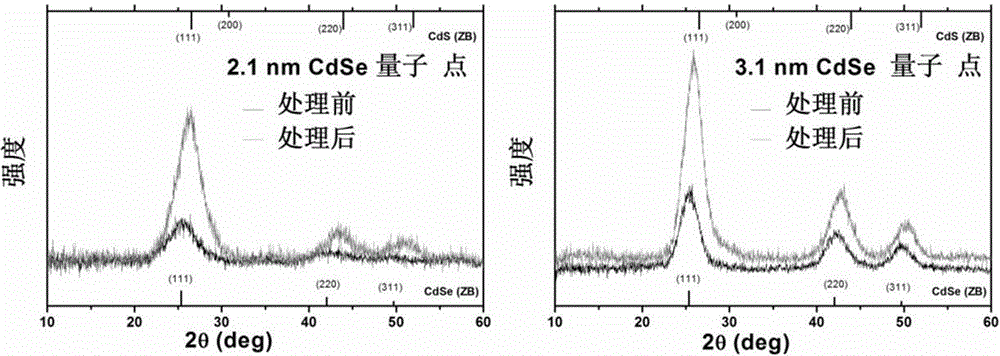
InactiveCN105154086ASimple processMild reaction conditionsLuminescent compositionsQuantum yieldCarbon chain
The invention belongs to the technical field of inorganic materials, and particularly relates to a method for preparing CdSe / CdS nuclear shell semiconductor quantum dots at a normal temperature. According to the method, a formamide solution of ammonium sulfide or potassium sulphide is used for treating CdSe nano particles at the normal temperature, and CdSe / CdS nuclear shell semiconductor nano particles are obtained; meanwhile, S2- replaces organic long carbon chain ligands on the surfaces of the CdSe nano particles, and the nano particles are dissolved in formamide polar solvent; afterwards, illumination is conducted for two days under the air condition, and fluorescence of the semiconductor nano particles is enhanced; (NH4)2S is used for treating CdSe nano particles of 2 nm so that the quantum yield can be increased to 39.8%, and the quantum yield of CdSe nano particles of 3 nm can be increased to 9.4%; K2S is used for treating the CdSe nano particles of 2 nm so that the quantum yield can be increased to 26.1%, and the quantum yield of the CdSe nano particles of 3 nm can be increased to 3.3%. The method is simple, the reaction condition is moderate, the prepared CdSe / CdS nuclear shell semiconductor quantum dots can be dissolved in the polar solvent, and the quantum yield is high. Broad application prospects can be achieved in the fields of photodiodes, fluorescent marks and the like.
Owner:FUDAN UNIV
Di-(2-hydroxyl isopropyl) benzene production method
ActiveCN102911014AReduce consumptionSimple processPreparation by oxygen reductionPublic projectBenzene
The invention relates to a di-(2-hydroxyl isopropyl) benzene production method which mainly solves the problems of long technical process and excessive public project consumption in the prior art. The technical scheme for better solving the problems includes that diisopropylbenzene oxidant solution containing di-(hydrogen peroxide isopropyl) benzene is used as a raw material to obtain the di-(2-hydroxyl isopropyl) benzene by means of reaction of the di-(hydrogen peroxide isopropyl) benzene and reducing agent, wherein the reaction temperature is 40-200 DEG C, the reaction pressure is 0.2-1.0MPa, the reducing agent is selected from at least one of sodium thiosulfate , potassium thiosulfate, potassium sulfide, potassium hydrosulfide, sodium sulfide or sodium hydrosulfide, and the weight ratio of the di-(hydrogen peroxide isopropyl) benzene to the reducing agent is (1-30):(1-20). The di-(2-hydroxyl isopropyl) benzene production method can be used for industrial production of di-(2-hydroxyl isopropyl) benzene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for extracting and separating glutathione from GSCu precipitate
ActiveCN106220708AImprove product qualityReduce usagePeptide preparation methodsPotassium sulfideAmmonium sulfide
The invention provides a method for extracting and separating glutathione from GSCu precipitate. The method comprises the following steps: adding GSCu precipitate and water into a reactor, forming suspension liquid, completely stirring, adding acid to perform acidification, adding sulfide, reacting under the stirring condition, centrifuging or filtering, and removing the generated Cu2S precipitate to prepare a glutathione mother liquid; concentrating and crystallizing to prepare a glutathione crystal pure product, wherein the sulfide is selected from one or more of the following compounds: ammonium sulfide, sodium sulfide and potassium sulfide. By the method, the raw materials are effectively selected, and the proportion and the reaction condition are reasonably controlled and the use of hydrogen sulfide is avoided, so that environmental pollution is reduced, the convenience of production and operation is improved, the prepared glutathione has stable product quality, and the method is quite suitable for large-scale industrialized production. In conclusion, the method for extracting and separating glutathione from the GSCu precipitate, provided by the invention, has good application prospect and market potential.
Owner:SHANGHAI QINGPING PHARMA CO LTD
Stabilizer, preparation method thereof and method for repairing heavy-metal-polluted soil
InactiveCN107955626ASafeEnvironmentally friendlyAgriculture tools and machinesContaminated soil reclamationPhosphateStabilizing Agents
The invention relates to the technical field of heavy metal pollution treatment and environment repair, in particular to a stabilizer, a preparation method thereof and a method for repairing heavy-metal-polluted soil. The invention is directed to solve the problems that, for instance, a traditional stabilizer has a single component, is high in cost, and is easily affected by a soil environment, and point source pollution is caused. The stabilizer comprises: by weight, 20-40% of montmorillonite, 20-40% of diatomite, 10-15% of phosphates, 10-15% of potassium sulfide, 10-15% of magnesium oxide and 5-10% of a metal complexing agent. The acting force of chemical bonds between a chemical substance and a pollutant as well as a coagulating agent are used, a stable complexing encapsulation substance is formed, and a good stable effect is achieved.
Owner:北京本农科技发展有限公司
Method for extracting copper, gold and silver from copper oxide ore
A method for extracting copper, gold and silver from copper oxide ore uses a process comprising ore powder leaching, filtering and copper, gold and silver precipitation to produce a copper concentrate containing gold and silver, and is especially suitable for treating low-grade copper oxide containing silver and gold and having copper content of 0.2-5%. The method is as below: first the ore is prepared into an ore powder; then a mixed solution of ammonia, ammonium bicarbonate and sodium thiosulfate or a mixed solution of sodium hydroxide, ammonium bicarbonate and sodium thiosulfate as a leaching agent reacts with the ore powder at 10-80 DEG C, so that the copper, gold and silver in the ore enter into the solution in the form of copper, gold and silver complexes, thereby realizing separation of copper, gold and silver from impurities; and then one or more selected from sulfide ammonia, sodium sulfide and potassium sulfide are used as a precipitating agent for copper, gold and silver precipitation gold, so as to produce the copper concentrate containing gold and silver, the copper concentrate containing gold and silver is filtered and dried to obtain the copper concentrate product containing gold and silver products, and the filtrate returns to a leaching tank for recycling.
Owner:谷亮
Metal antique bronze color colorant
InactiveCN102041498AReasonable formulaEasy to useMetallic material coating processesPotassium sulfideAmmonium sulfide
Owner:石晓春
A kind of rust liquid and its method for making rust on the surface of bronze ware
The method of rusting the bronze-colored surface of the bronze ware is that the original hair texture of the utensil remains unchanged, and a bronze-colored antique rust layer is formed on the surface of the utensil. The texture of the rust layer is clear and remains the same, and it gives the appliance a sense of vicissitudes of a long history. No fading, no sticky hands, clean and neat. Put the cast bronze ware into the potassium sulfide solution, soak it alternately with the sodium sulfate solution several times, then place the bronze ware in the pit, fill the inside and outside of the bronze ware with ancient tomb soil, and use heated The mixture of acetic acid and ammonium chloride is poured on the buried bronze burial soil, compacted and covered with plastic film to form a closed environment. After 3 months, it is taken out and oxidized in the air, and then placed on the ground below 3 meters for storage. , The cellar should be sealed for more than 3 months, and the surface should be cleaned with saponin extract.
Owner:洛阳粤钰青铜器有限公司
Cable blocking material and preparation method thereof
ActiveCN109054396AGood low temperatureHigh mechanical strengthRubber insulatorsFiberTriallyl isocyanurate
The invention discloses a cable blocking material and a preparation method thereof. The cable blocking material is prepared from the following raw materials in parts by weight: 30-45 parts of methyl vinyl silicone rubber, 0.5-1 part of light calcium carbonate, 3-6 parts of white carbon black VN-3, 3-8 parts of refractory fiber, 0.6-1.5 parts of hydroxyl silicone oil, 1-2 parts of naphthenic oil, 10-15 parts of aluminum hydroxide, 0.2-0.5 part of potassium sulfide, 1-3 parts of vulcanizing agent and 0.5-1.0 part of triallyl isocyanurate. The raw materials in the invention have a synergistic effect; the acquired material has moderate hardness, high mechanical strength, excellent ageing resistance, excellent elasticity and excellent flame retardant property; when the cable blocking material is used for blocking gaps between cables and pipelines, walls or cabinets, the cable blocking material is difficult to crack or fall off.
Owner:国网河南省电力公司镇平县供电公司
Sulfide precipitation method for treating wastewater containing heavy metal ions
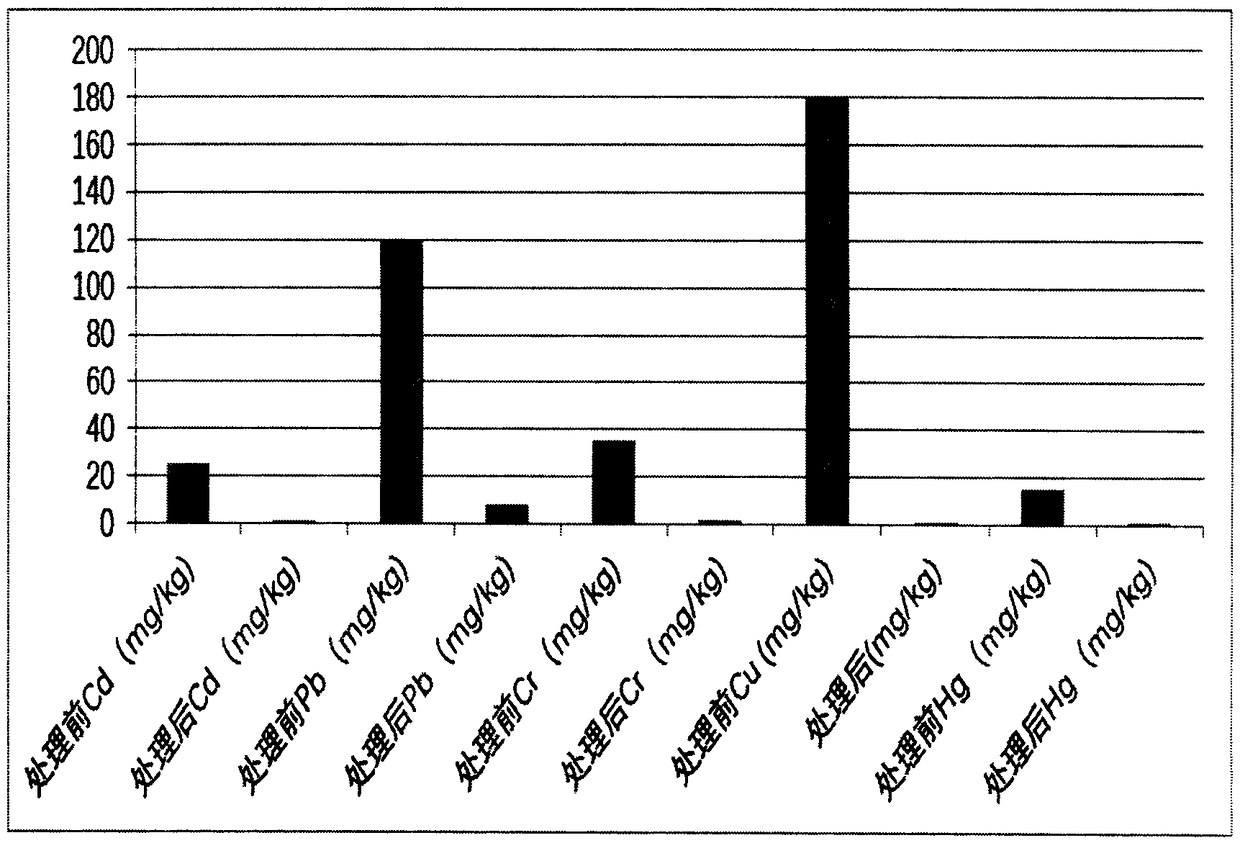
InactiveCN107226567AReduce dosageLow running costWater contaminantsMultistage water/sewage treatmentPotassium sulfideWater insoluble
The invention belongs to the technical field of wastewater treatment, and particularly relates to a sulfide precipitation method for treating wastewater containing heavy metal ions. The method comprises the following steps: heating the wastewater to 30-40 DEG C, and controlling the pH value at 6-8; and adding an inorganic sulfide into the wastewater containing heavy metal ions, reacting the heavy metal ions with the inorganic sulfide to generate water-insoluble metal sulfides, and performing standing stratification to realize wastewater treatment, wherein the inorganic sulfide is one or more of sodium sulfide, potassium sulfide, calcium sulfide, magnesium sulfide and lithium sulfide; and the inorganic matter is added according to the removal amount of the heavy metal ions as per 90-110% of the stoichiometric mol ratio of the heavy metal ions to sulfur in the generated metal sulfides. The sulfide precipitation method provided by the invention has the advantages of cheap and accessible reagents, low addition amount, simple removal process, low investment and operation cost and the like; and the removal rate of the heavy metal ions is up to 99% or above.
Owner:SHANGHAI SECOND POLYTECHNIC UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com