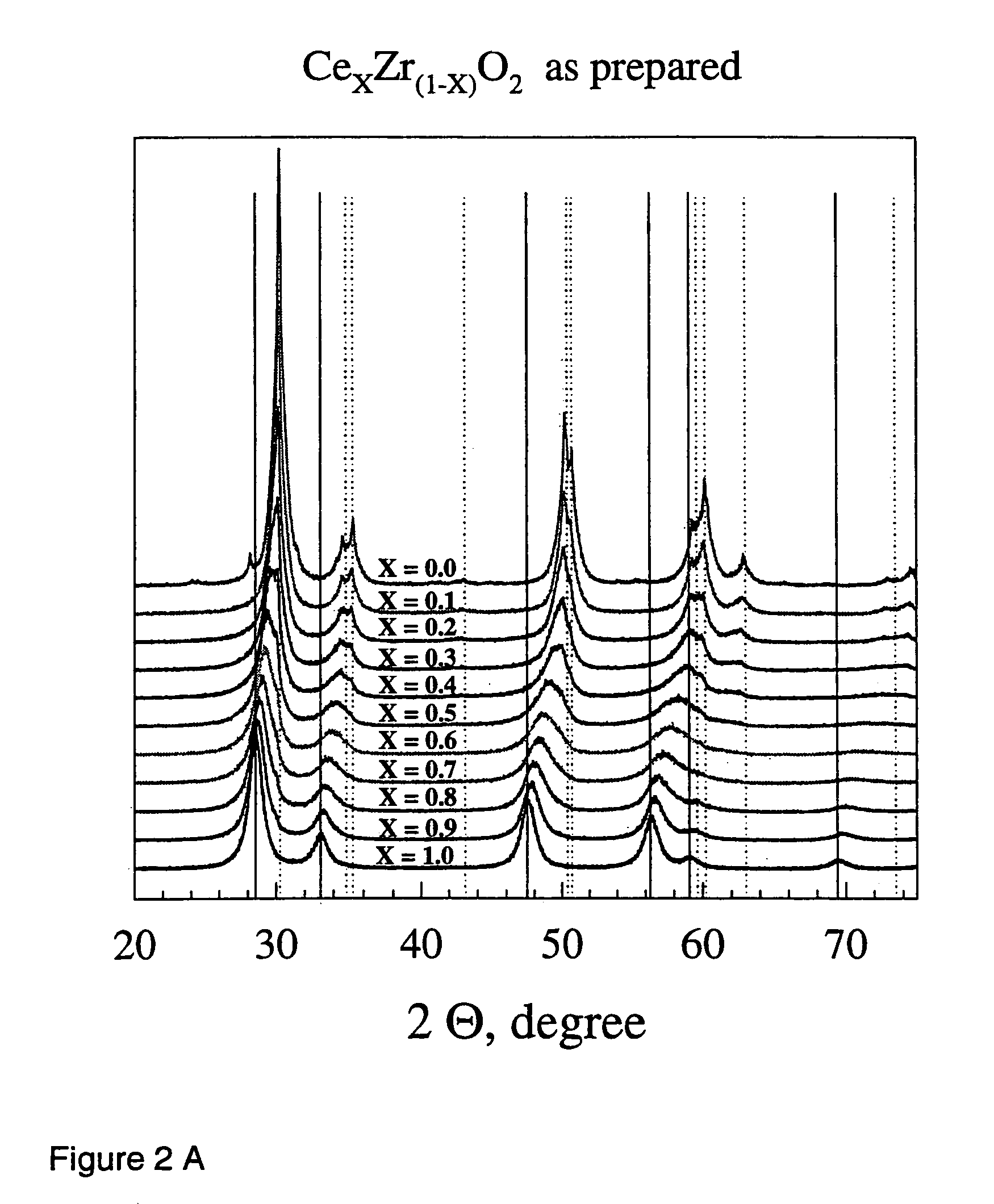
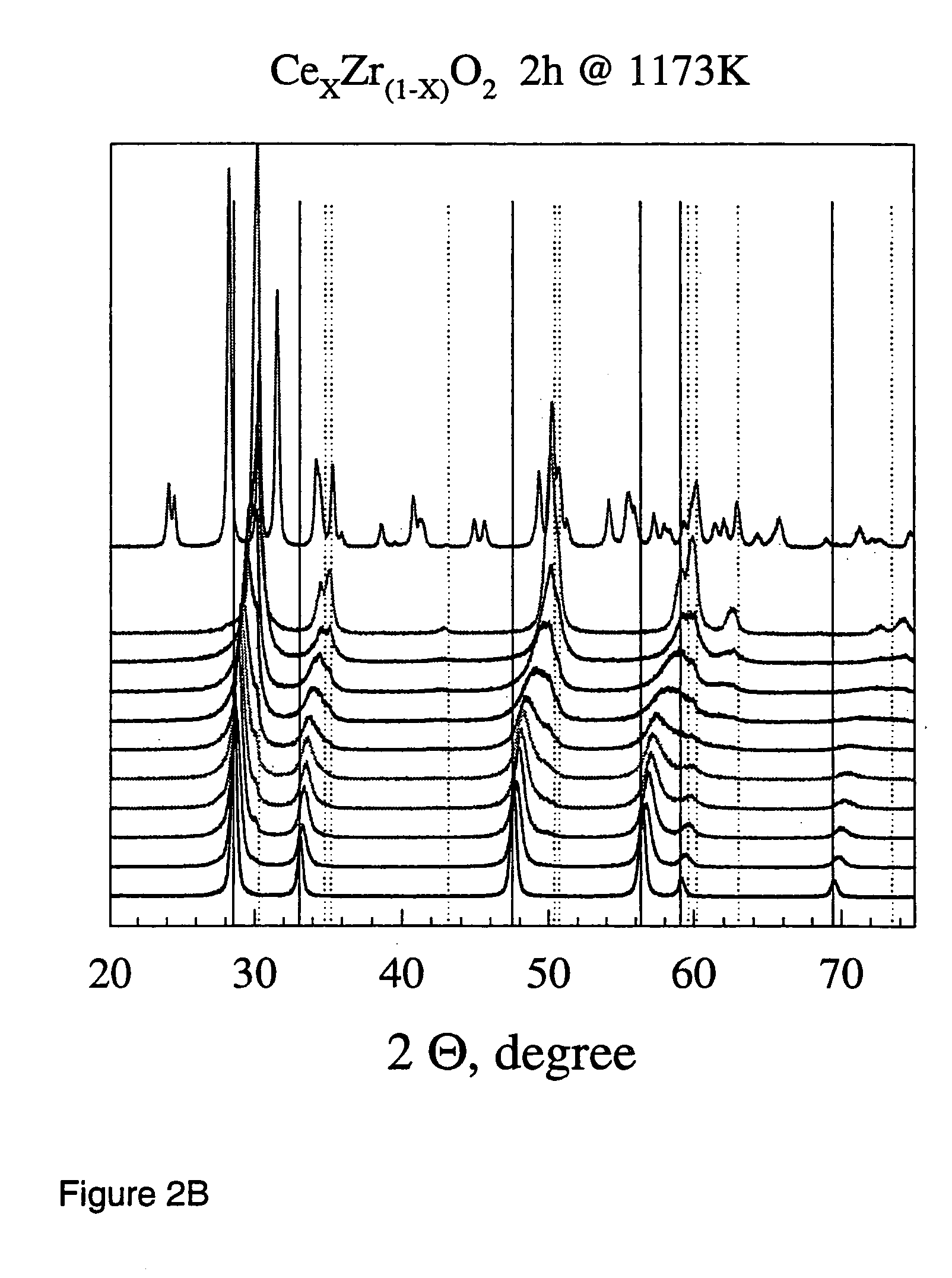
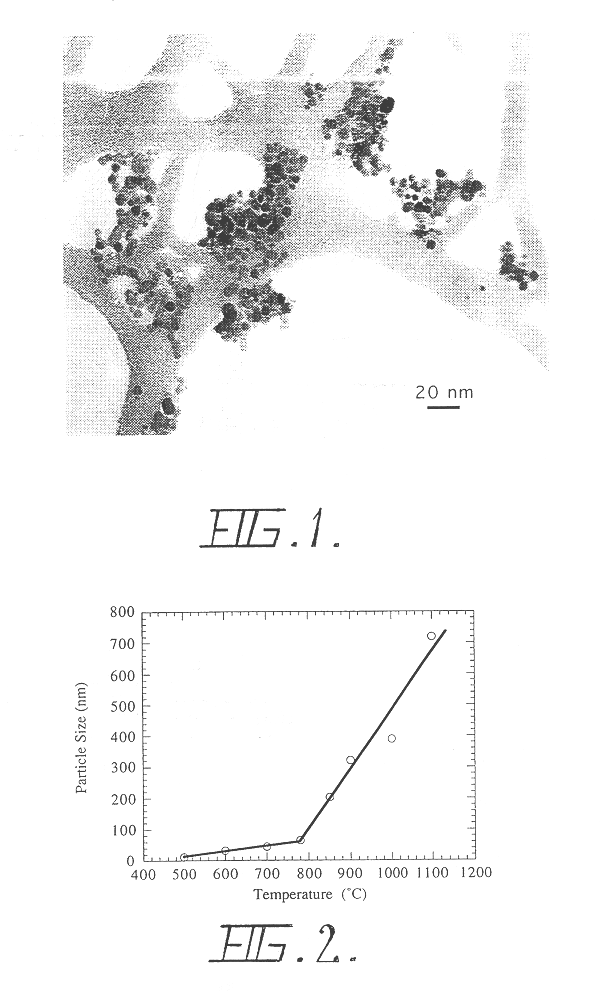
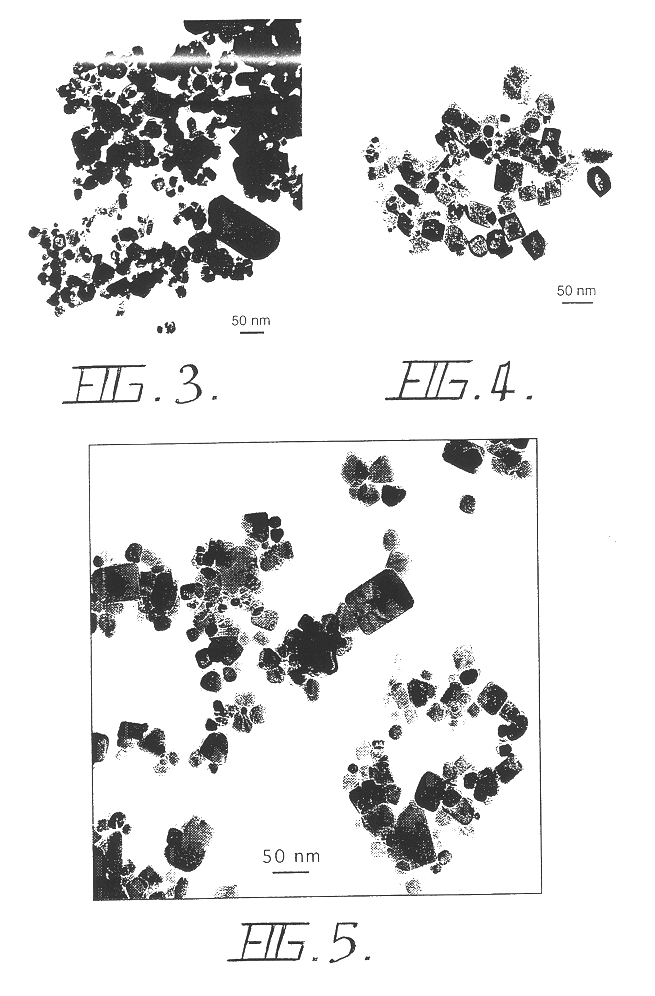
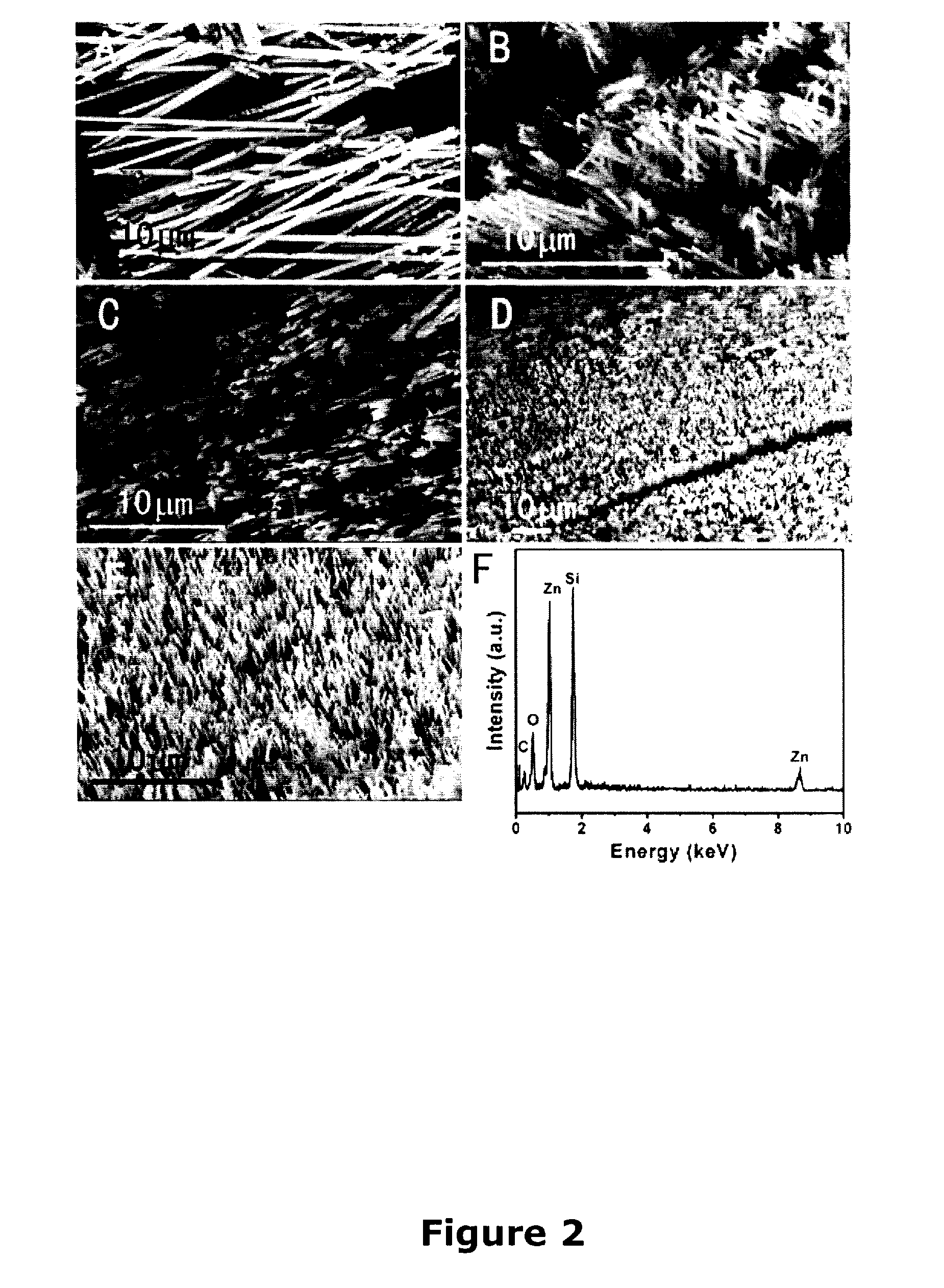
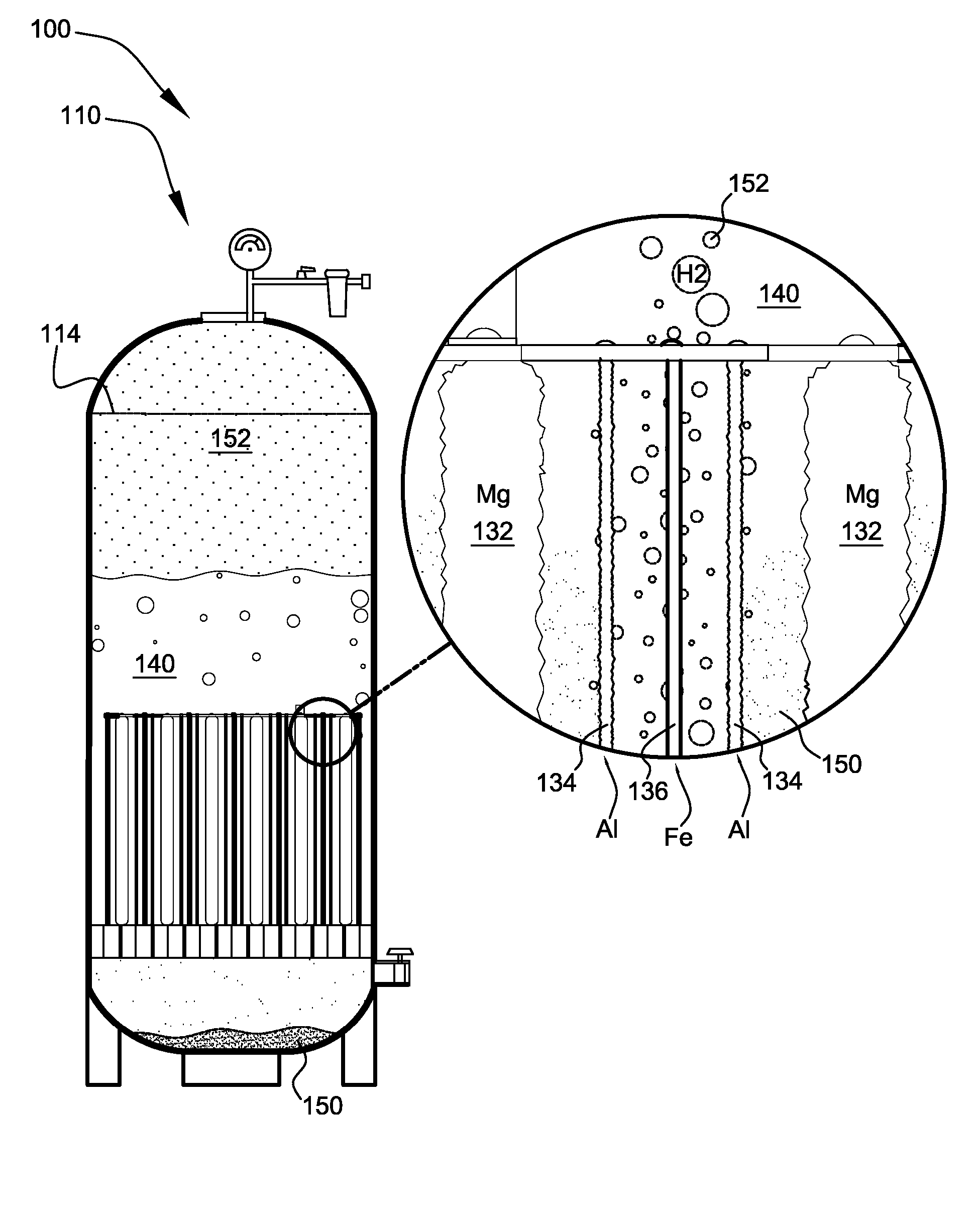
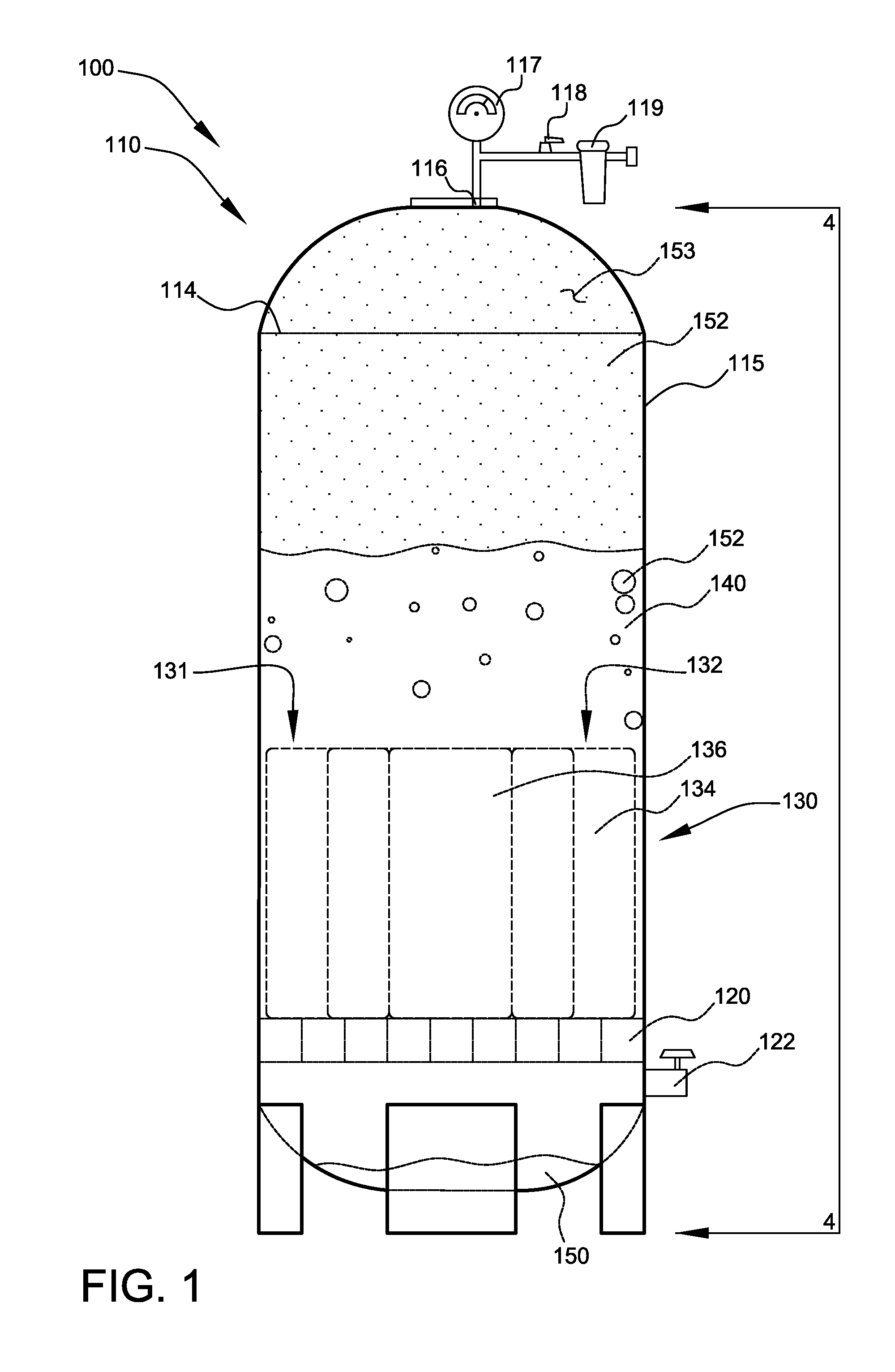
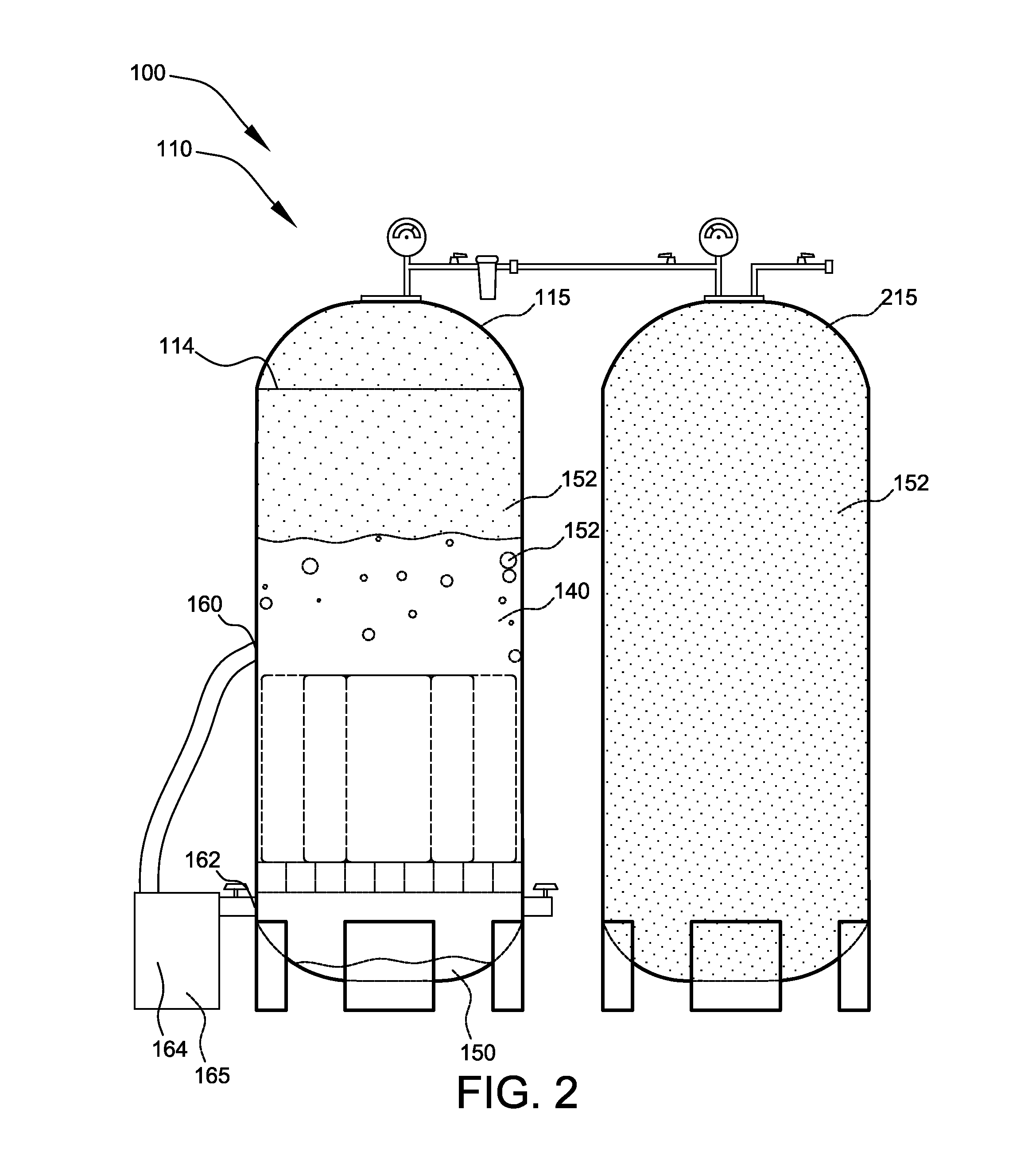
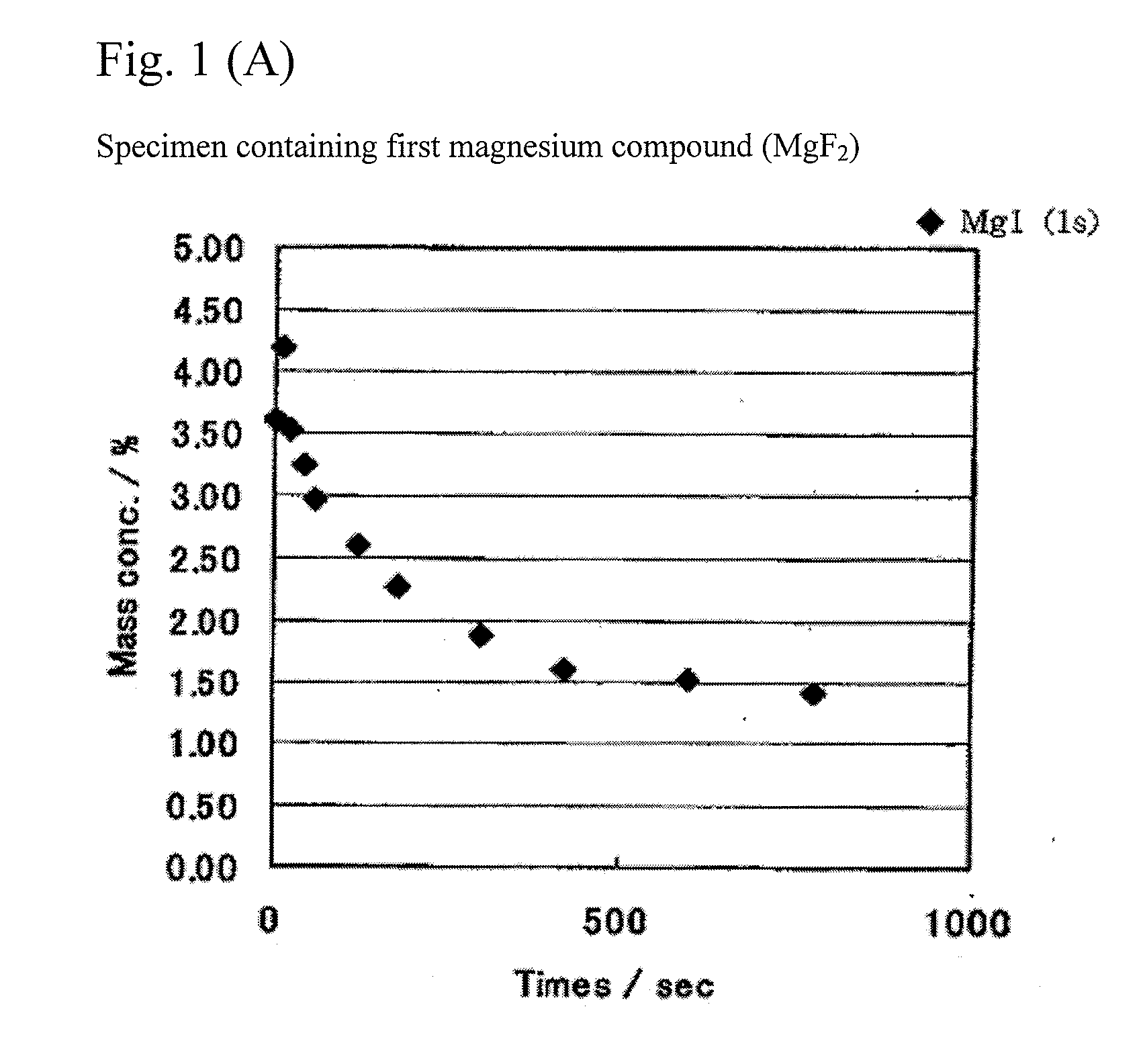
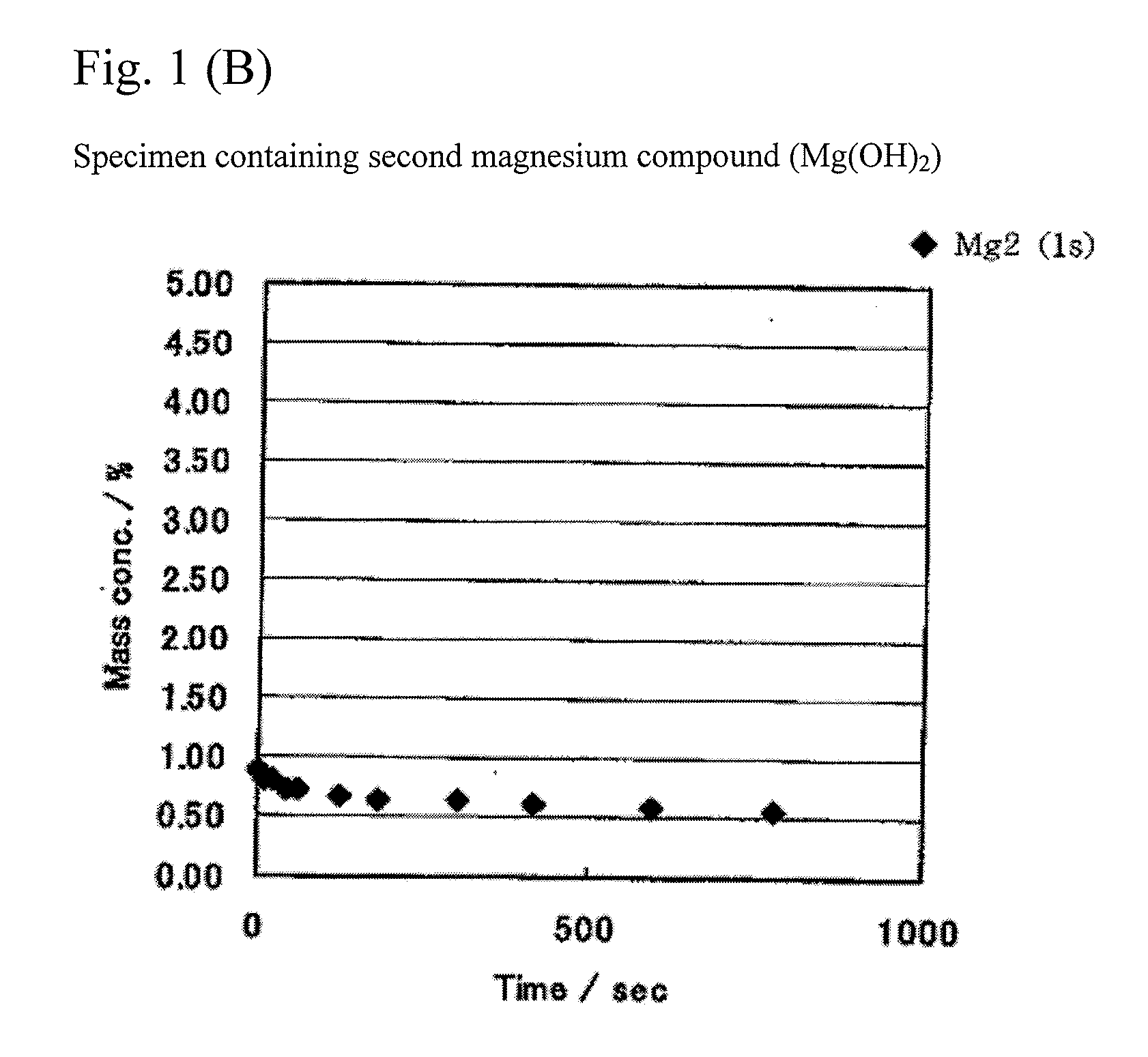
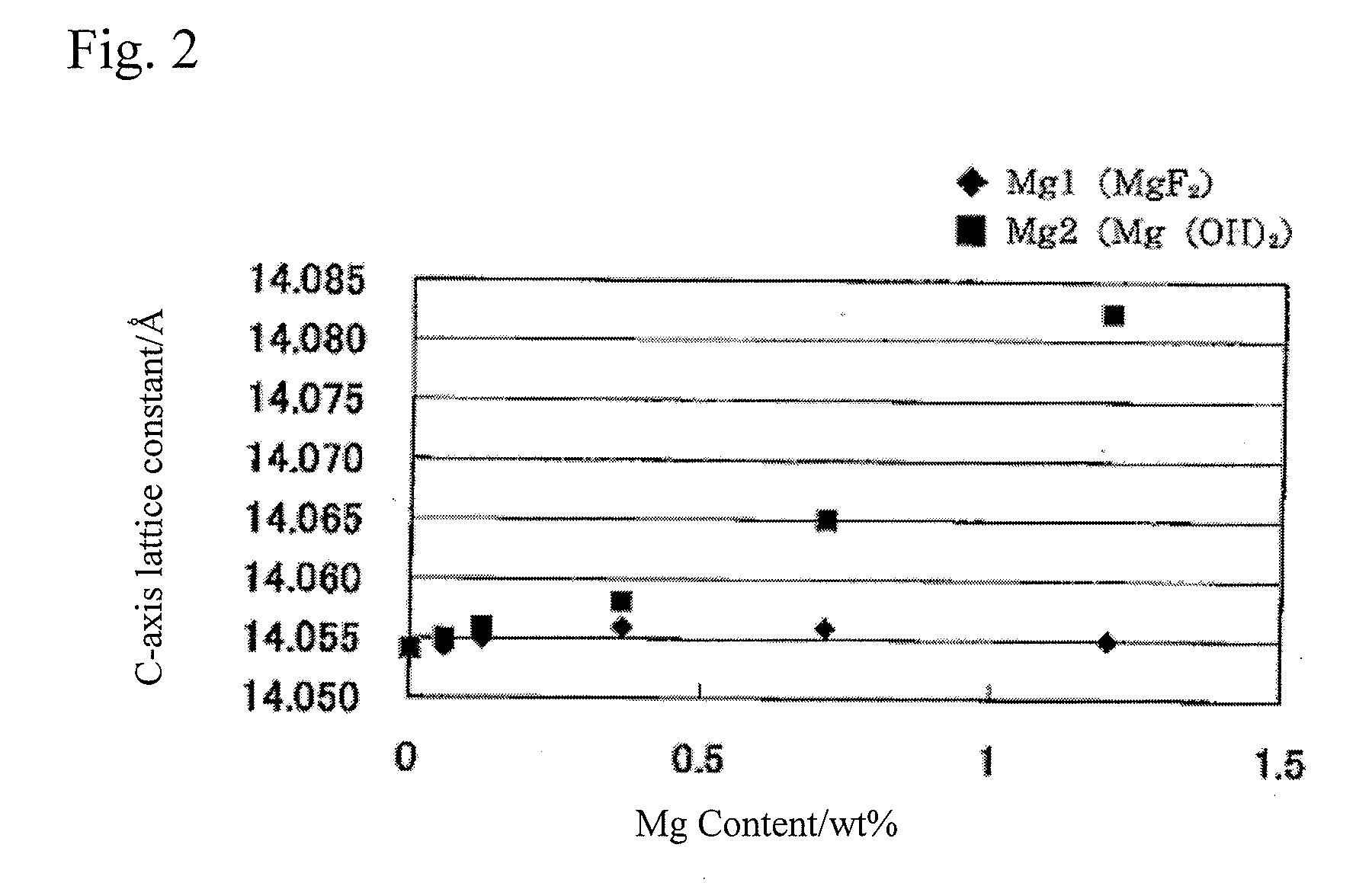
Patents
Literature
1237results about "Magnesium hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
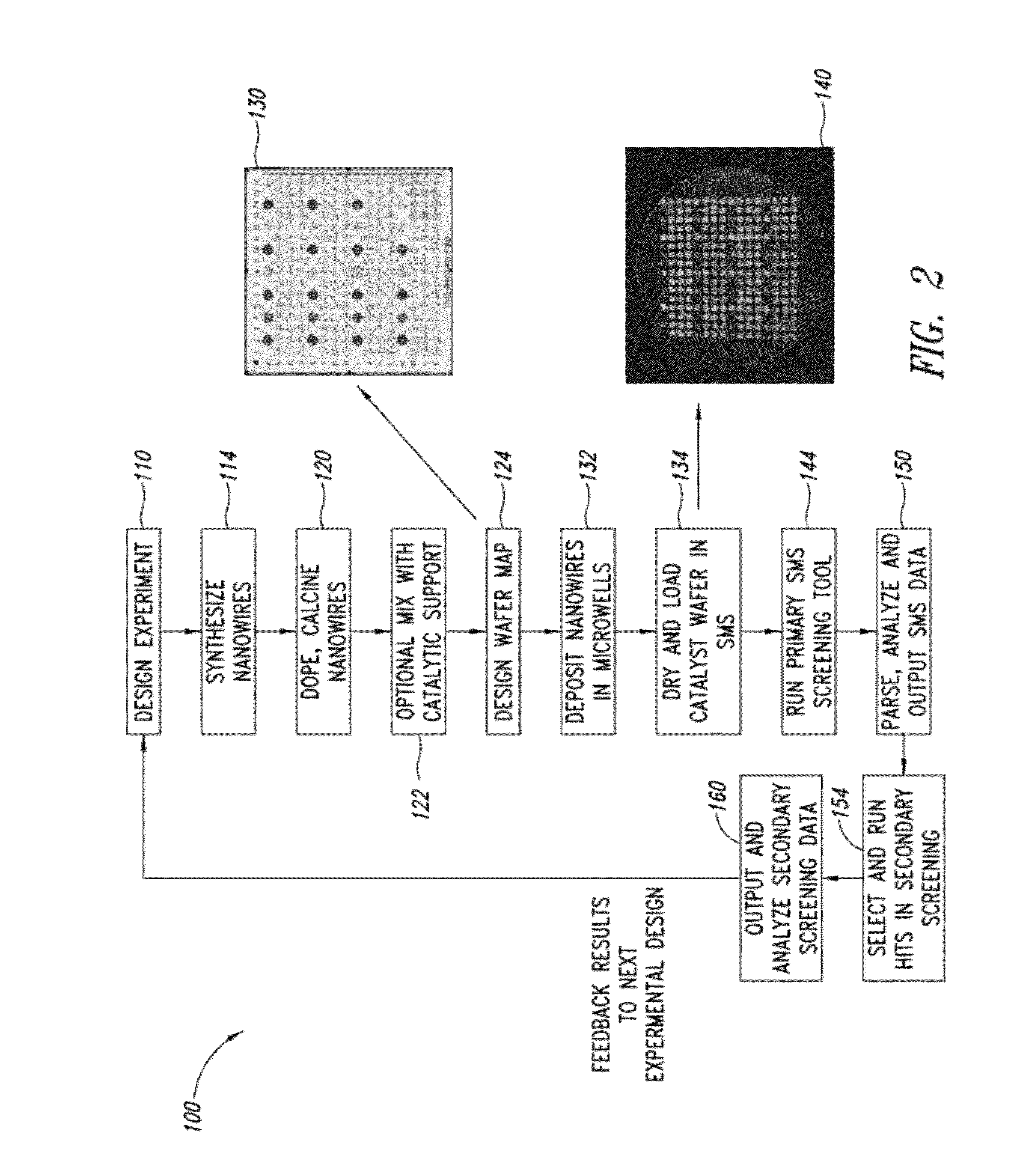
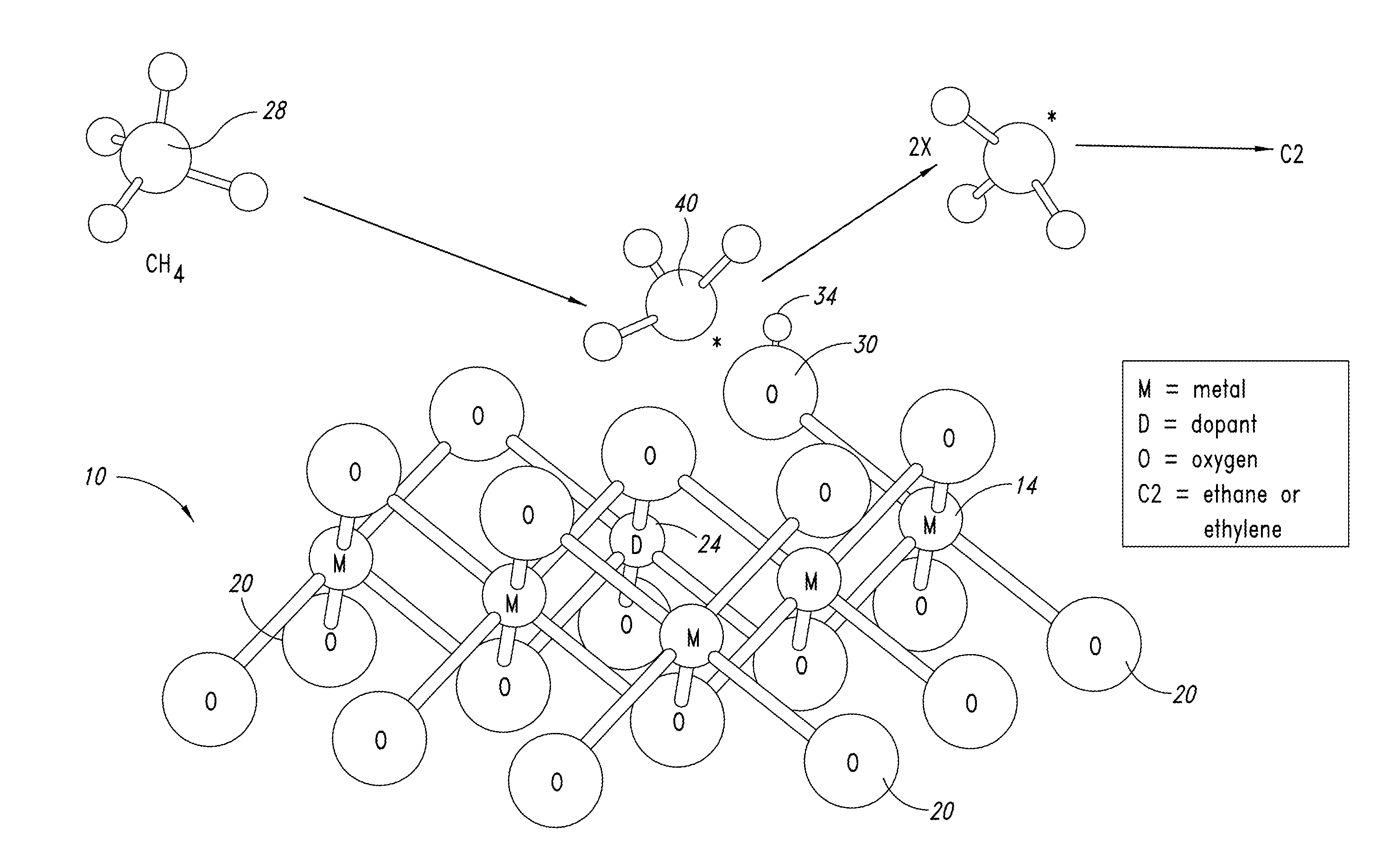
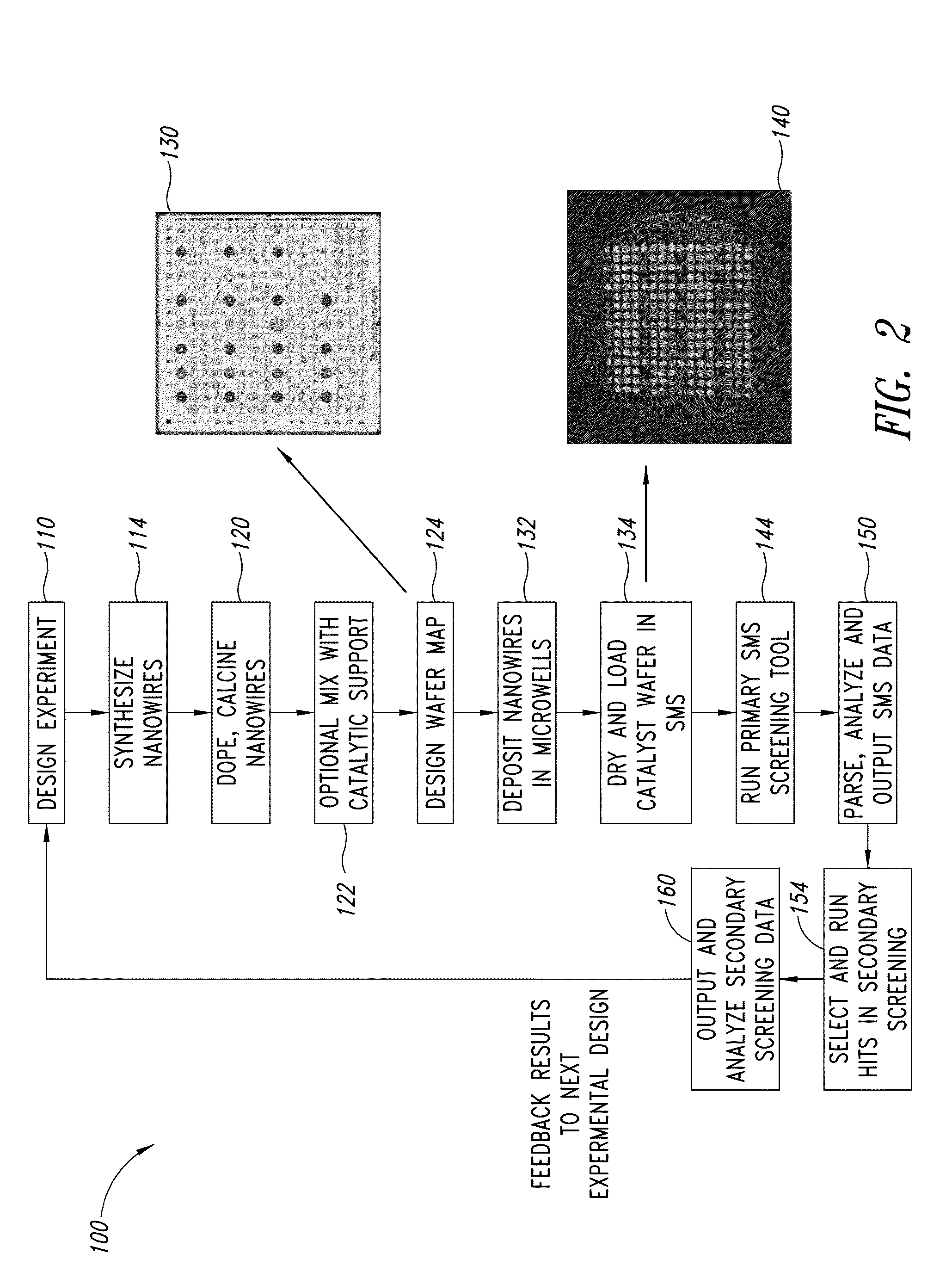
Nanowire catalysts
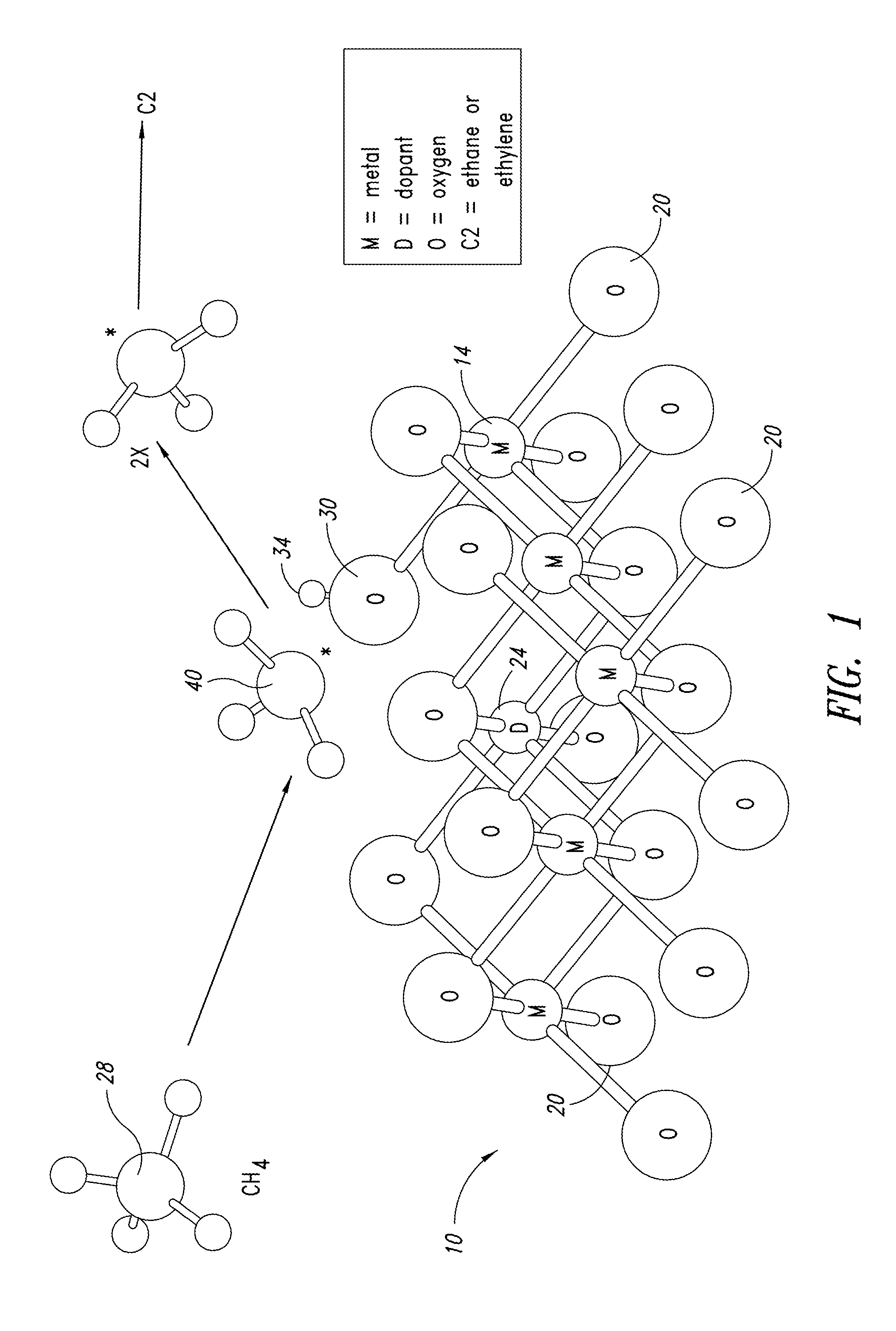
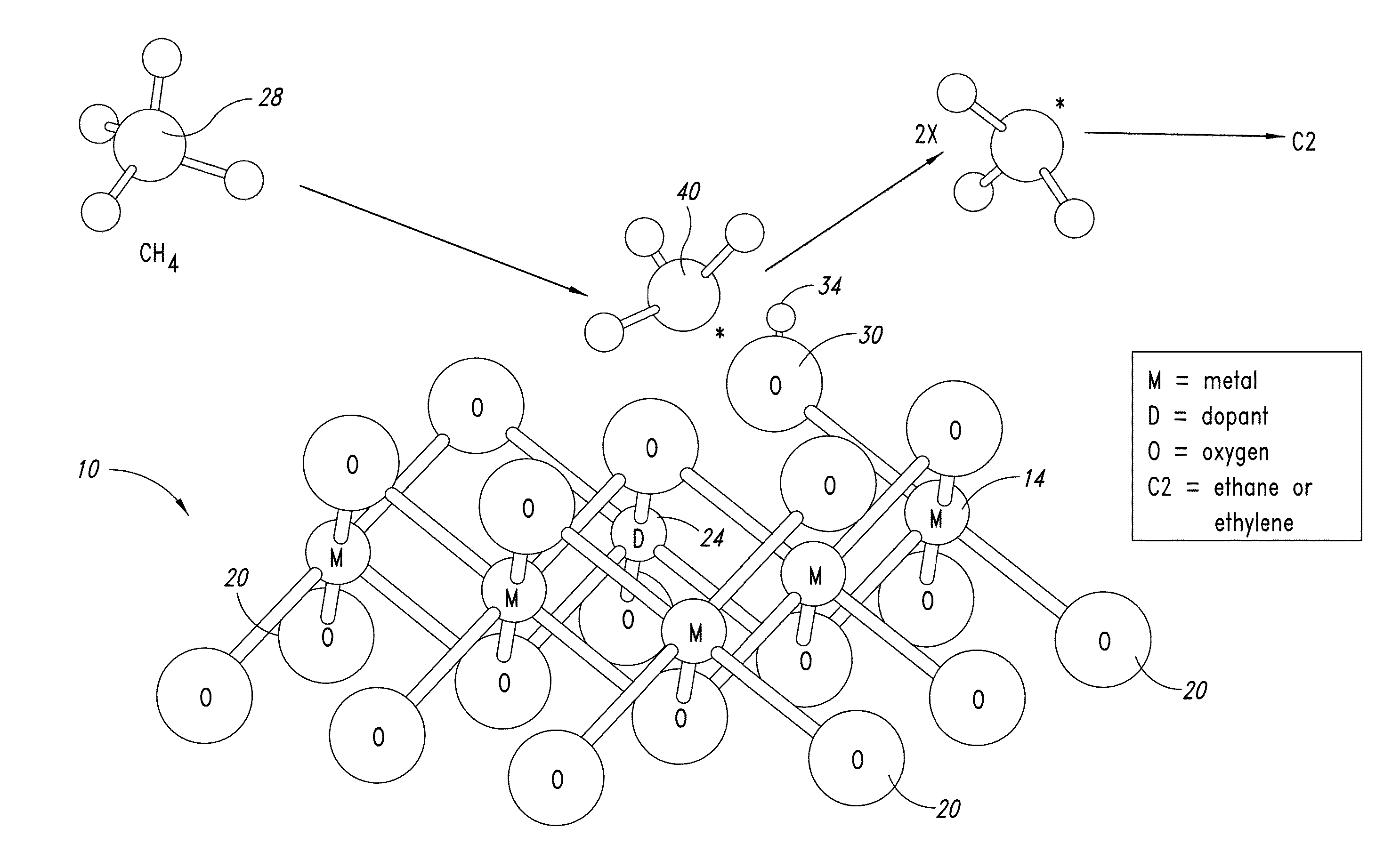
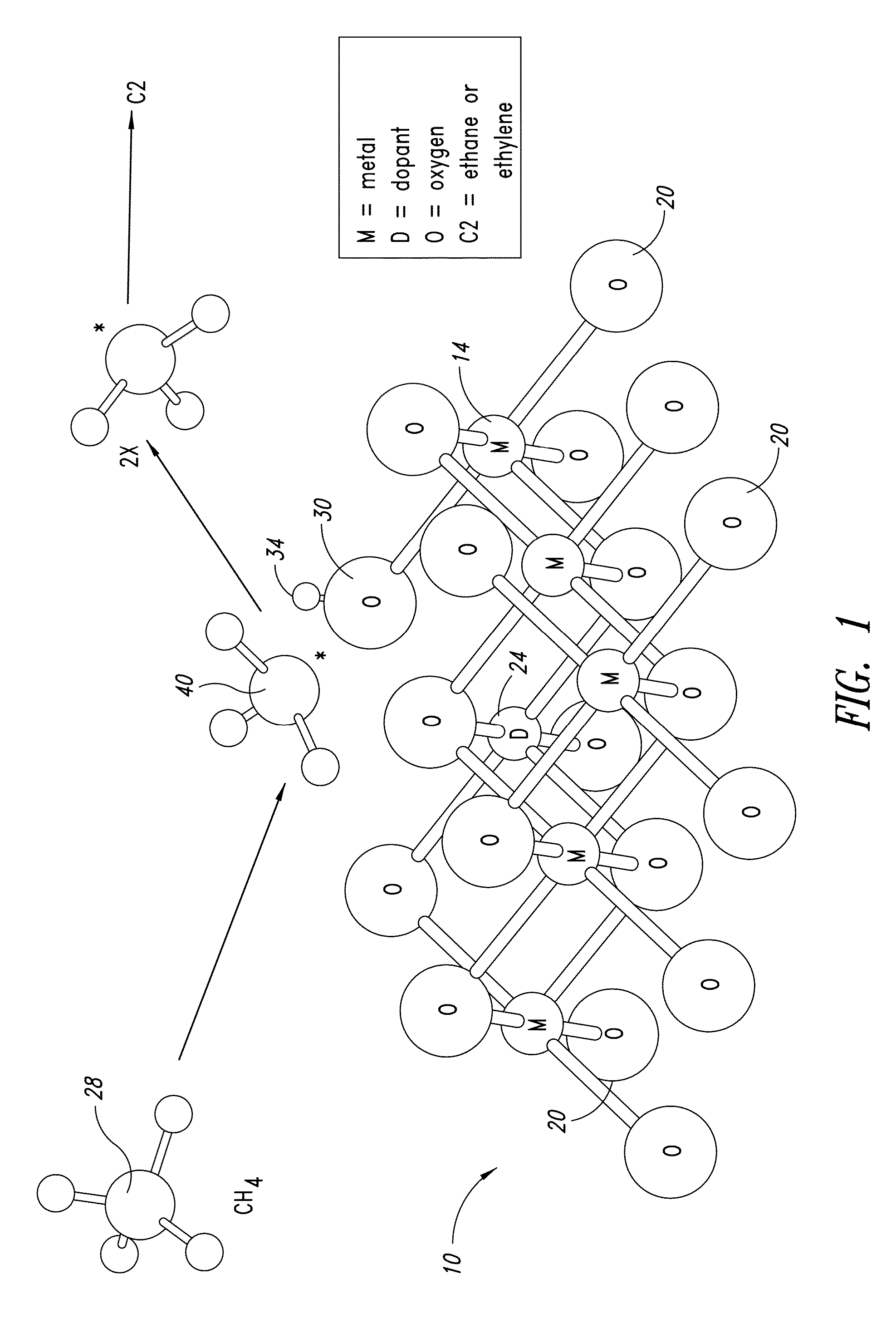
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
Flame made metal oxides
ActiveUS7211236B2Add featureWell mixedMaterial nanotechnologyZirconium oxidesSpray pyrolysisCarboxylic acid
Described is a method for the production of metal oxides by flame spray pyrolysis, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Process for the production of ultrafine powders of metal oxides
InactiveUS6503475B1Low costHigh yield rateAlkaline earth titanatesMaterial nanotechnologyDiluentBiological activation
A process for the production of ultrafine powders that includes subjecting a mixture of precursor metal compound and a non-reactant diluent phase to mechanical milling whereby the process of mechanical activation reduces the microstructure of the mixture to the form of nano-sized grains of the metal compound uniformly dispersed in the diluent phase. The process also includes heat treating the mixture of nano-sized grains of the metal compound uniformly dispersed in the diluent phase to convert the nano-sized grains of the metal compound into a metal oxide phase. The process further includes removing the diluent phase such that the nano-sized grains of the metal oxide phase are left behind in the form of an ultrafine powder.
Owner:SAMSUNG CORNING PRECISION MATERIALS CO LTD +1
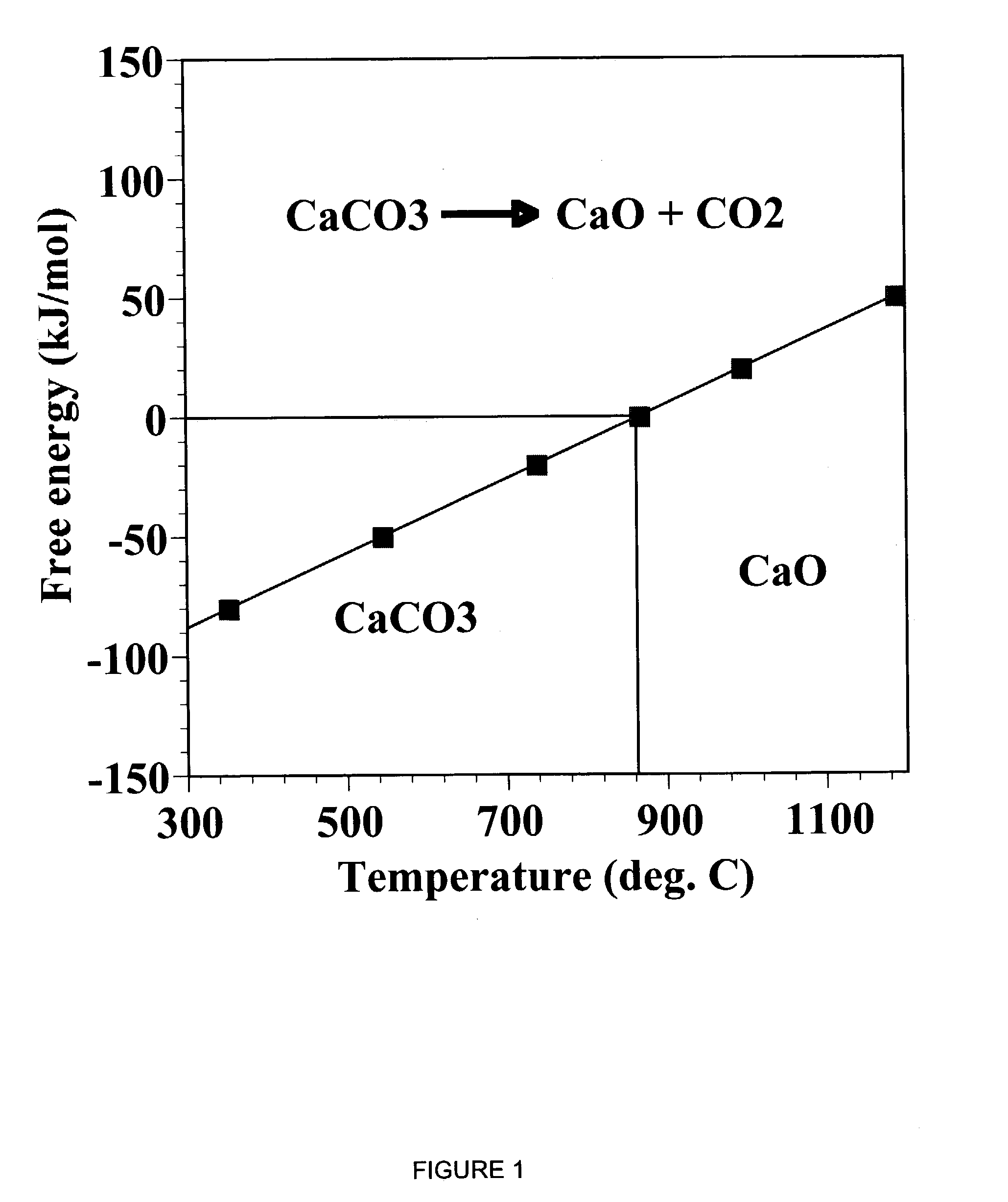
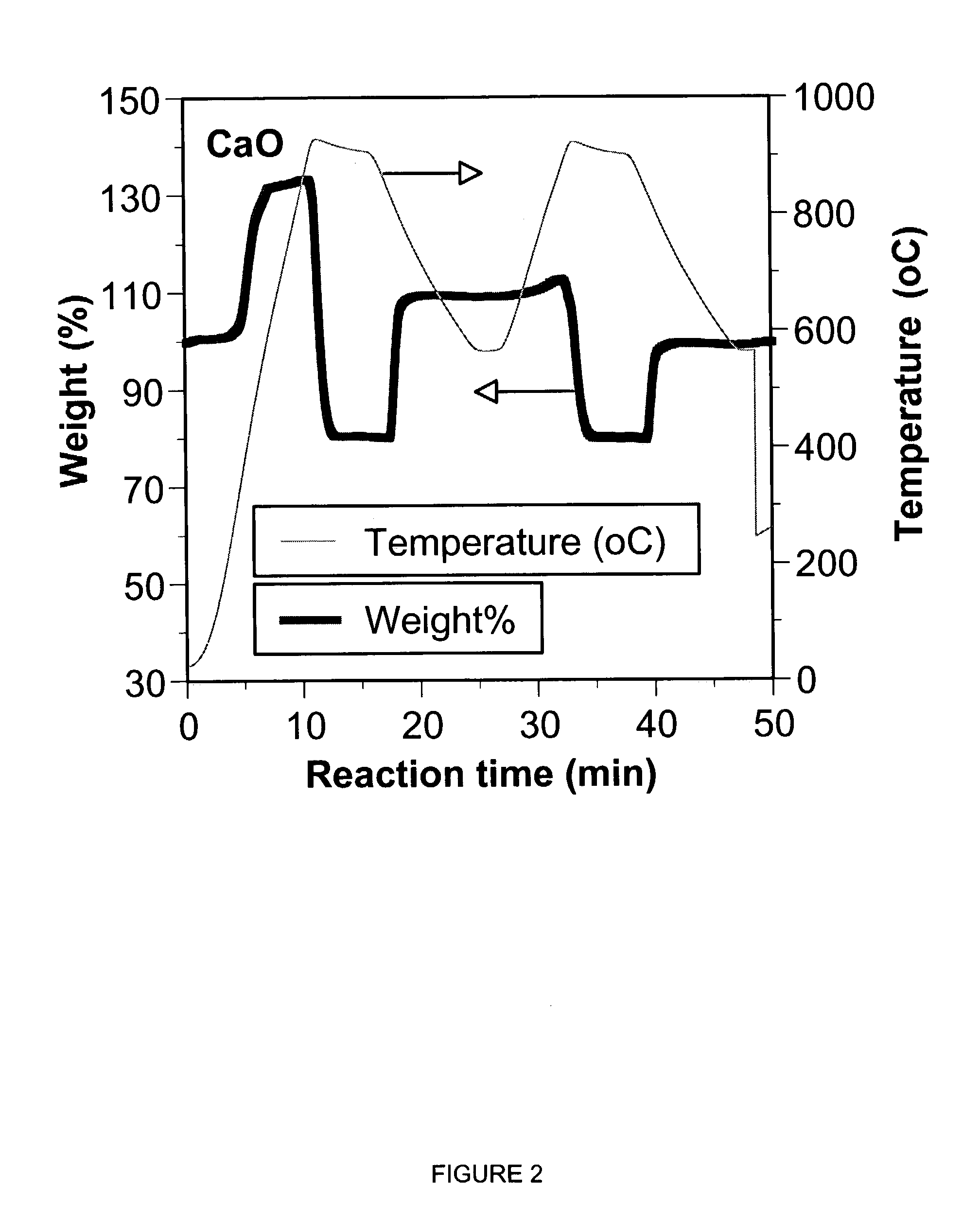
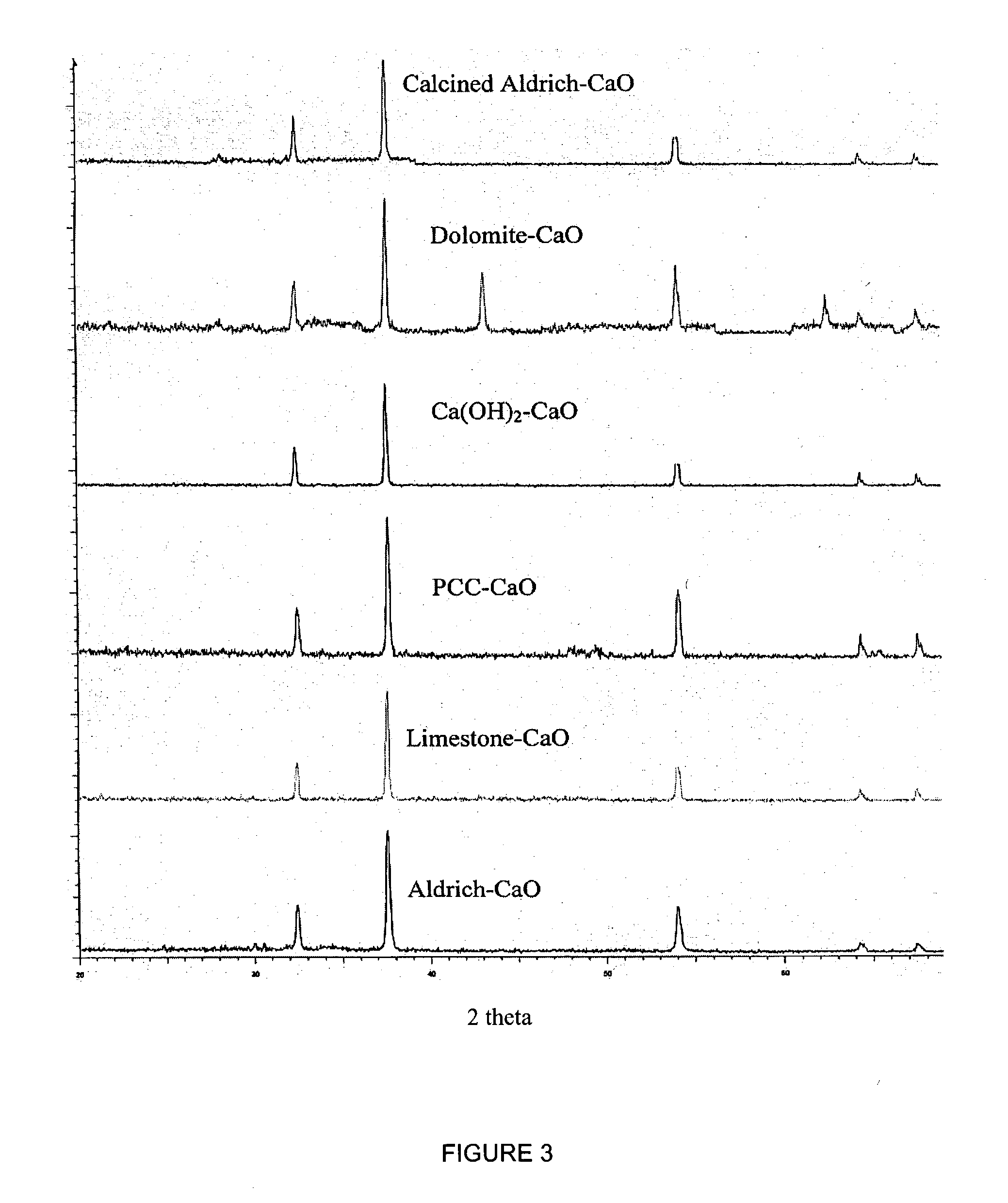
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
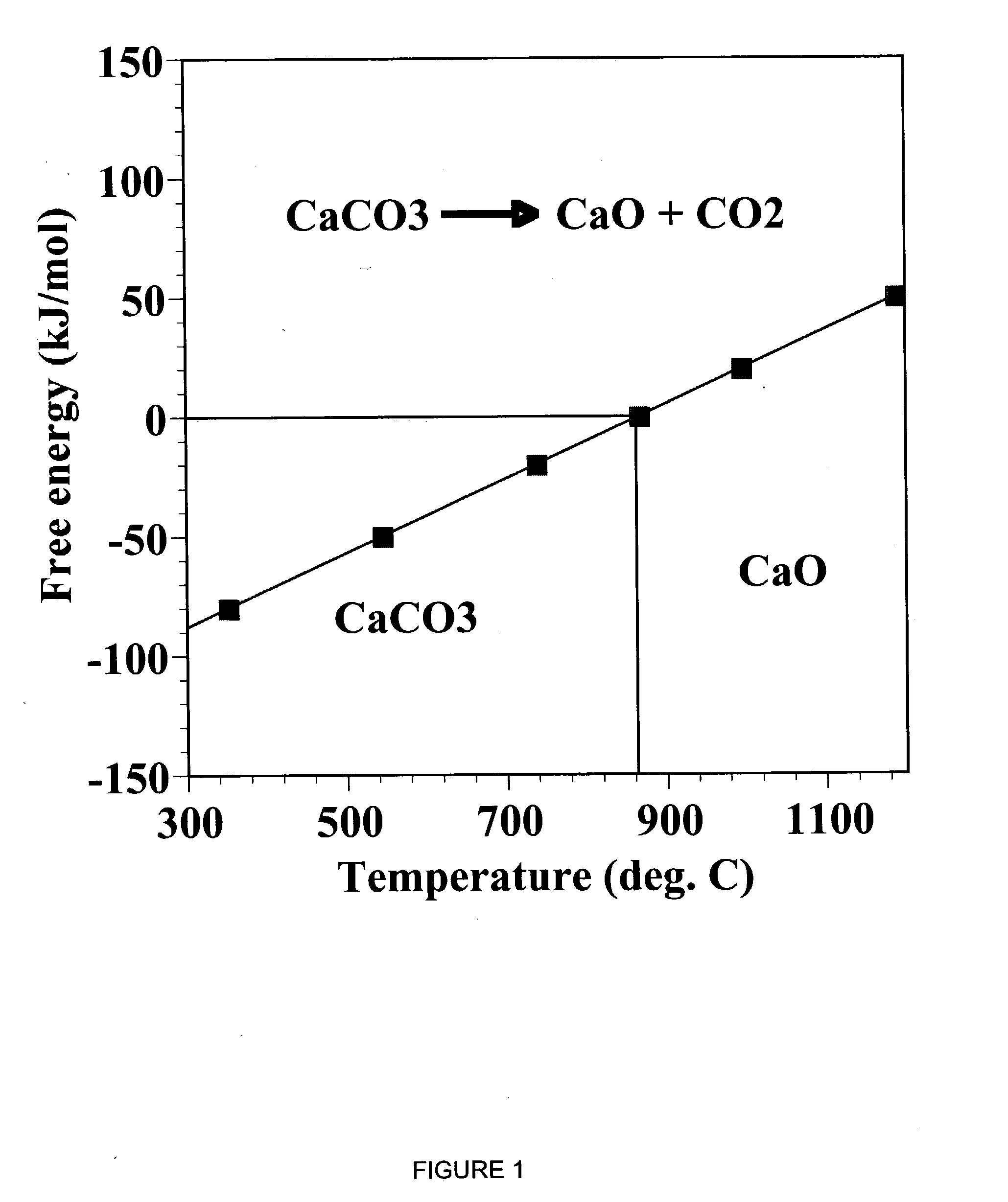
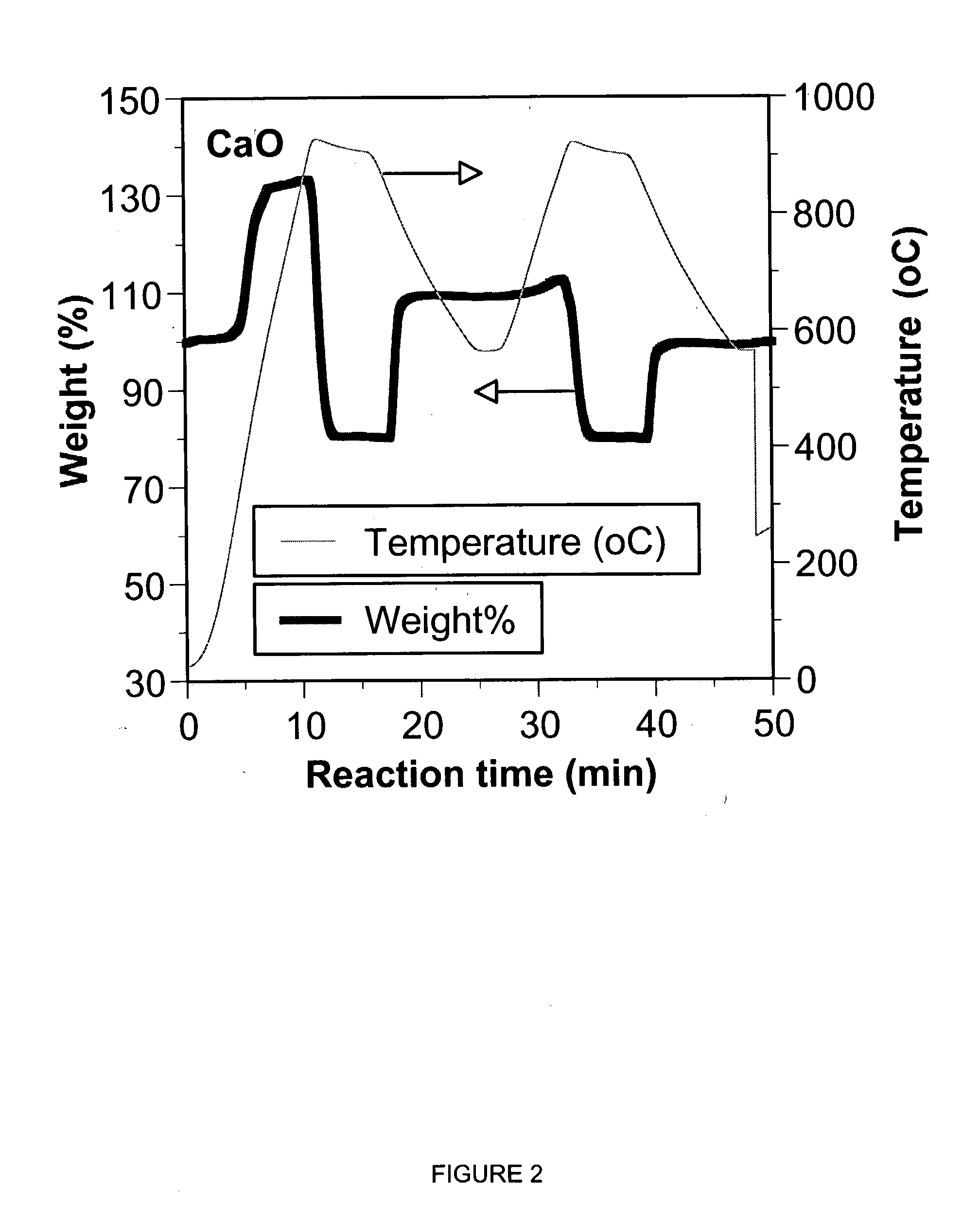
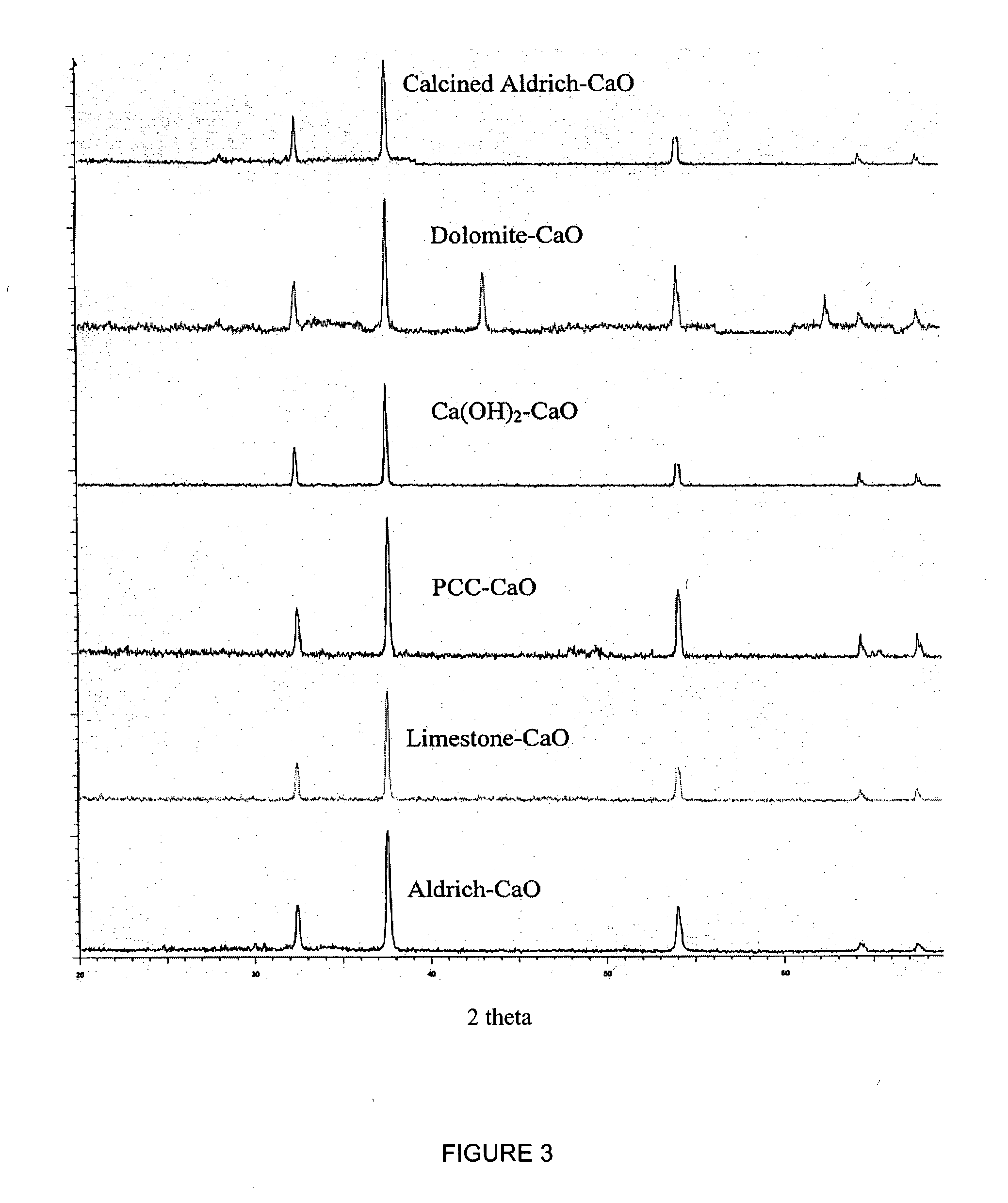
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
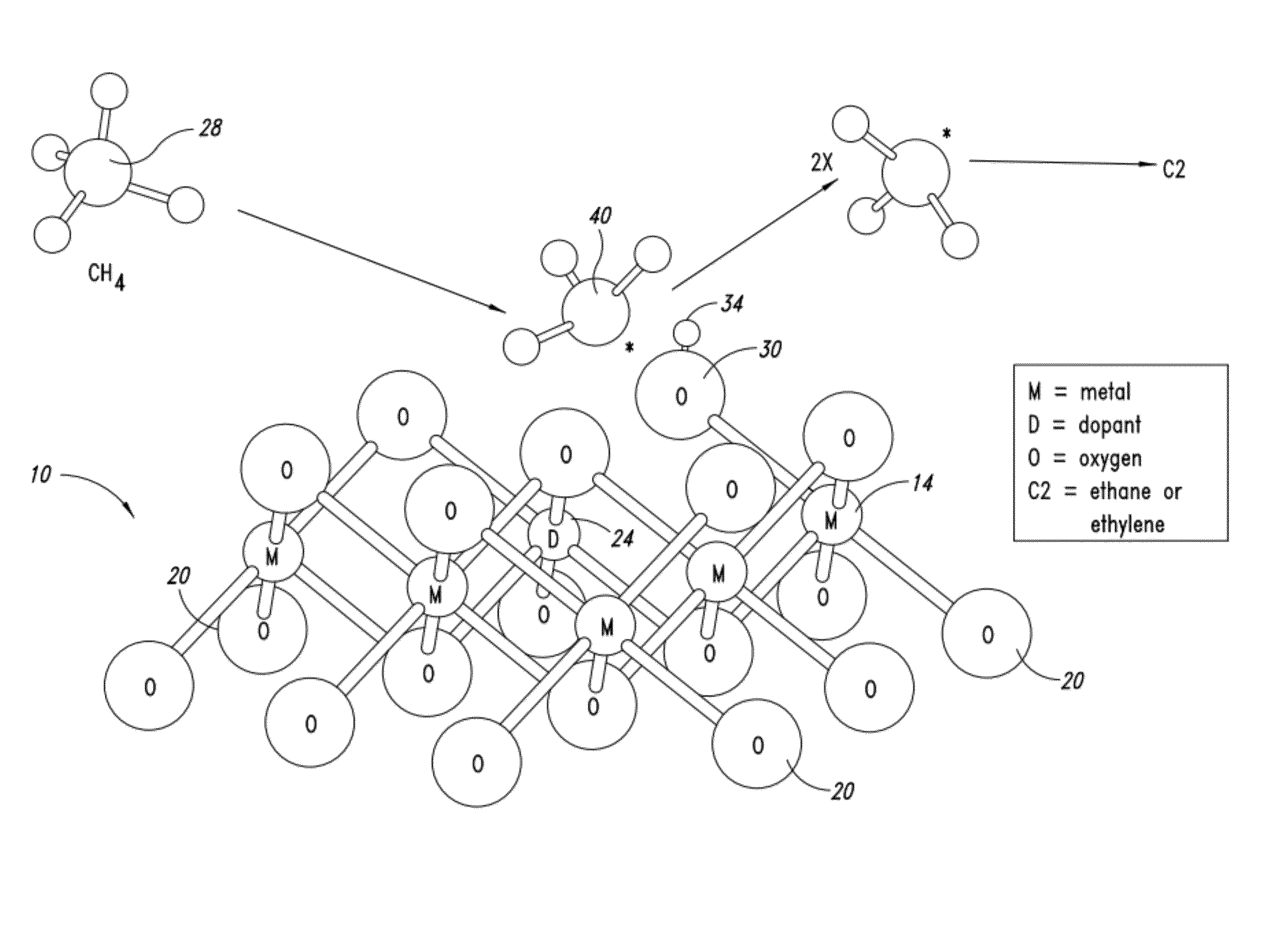
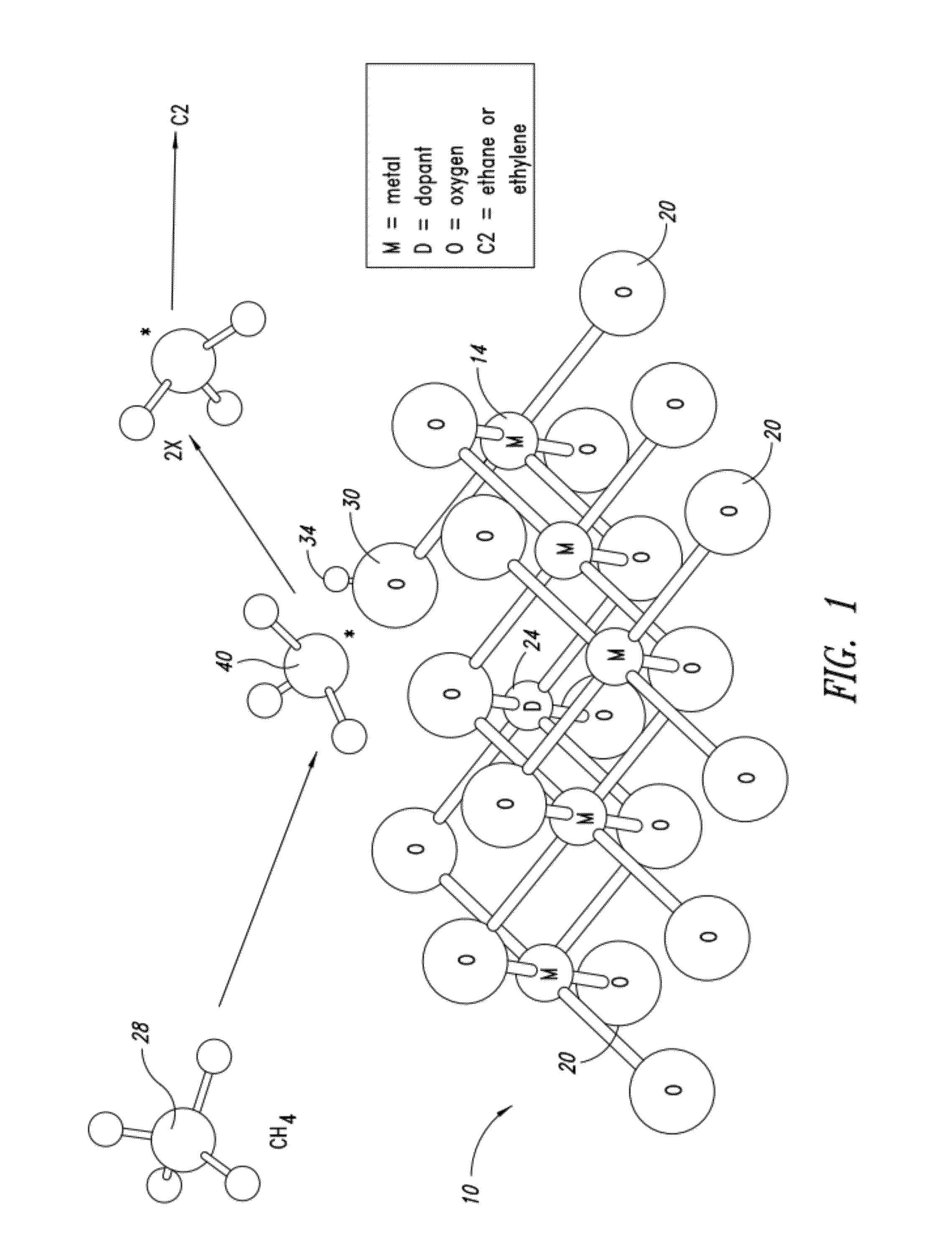
Nanowire catalysts and methods for their use and preparation
ActiveUS20130165728A1Material nanotechnologyManganese oxides/hydroxidesNanowireOxidative coupling of methane
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons. Related methods for use and manufacture of the same are also disclosed.
Owner:LUMMUS TECH LLC
Polymer templated nanowire catalysts
InactiveUS20130158322A1Improve drawing legibilityMaterial nanotechnologyManganese oxides/hydroxidesNanowirePolymer science
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are prepared by polymer templated methods and are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethane and / or ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
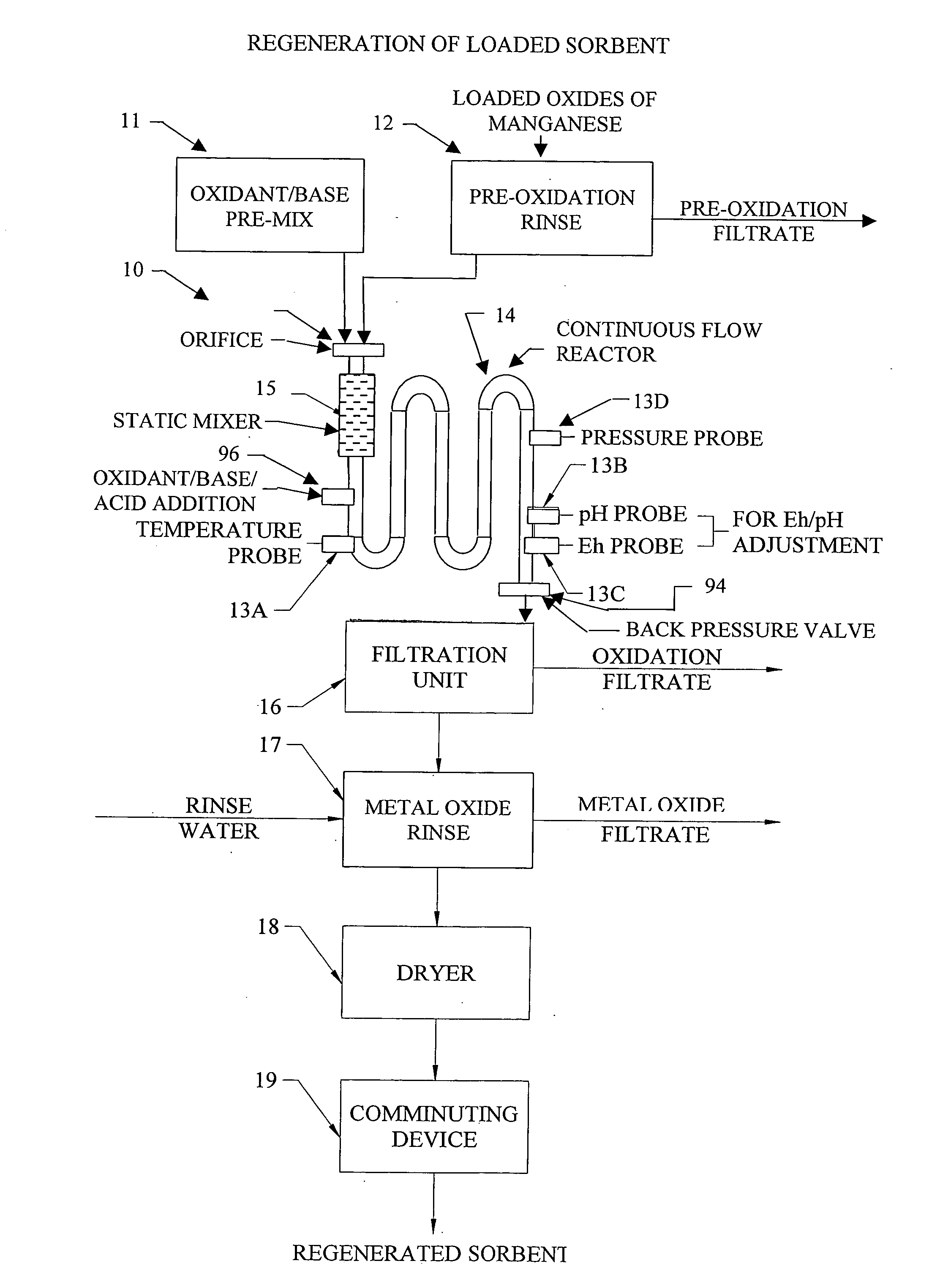
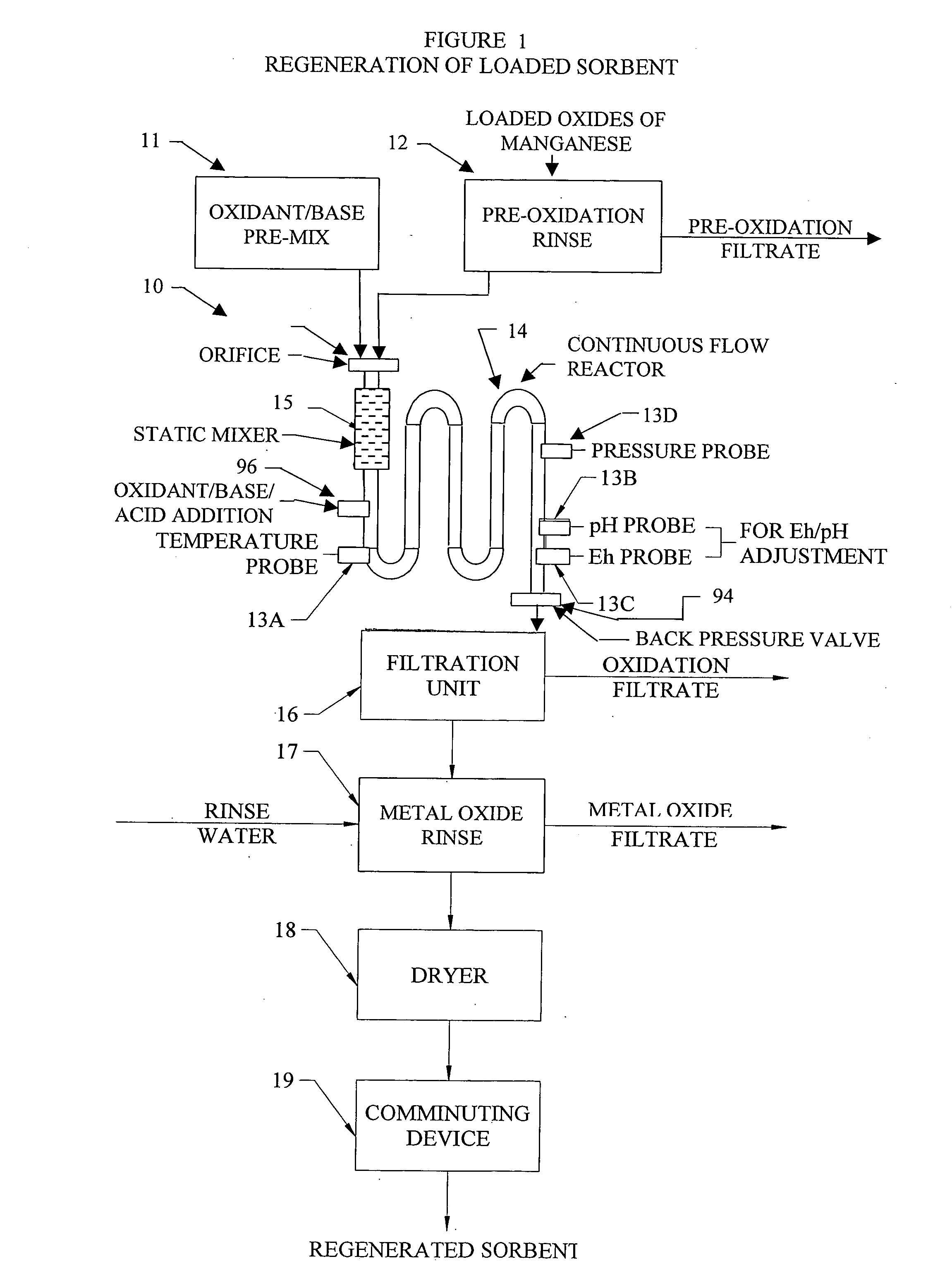
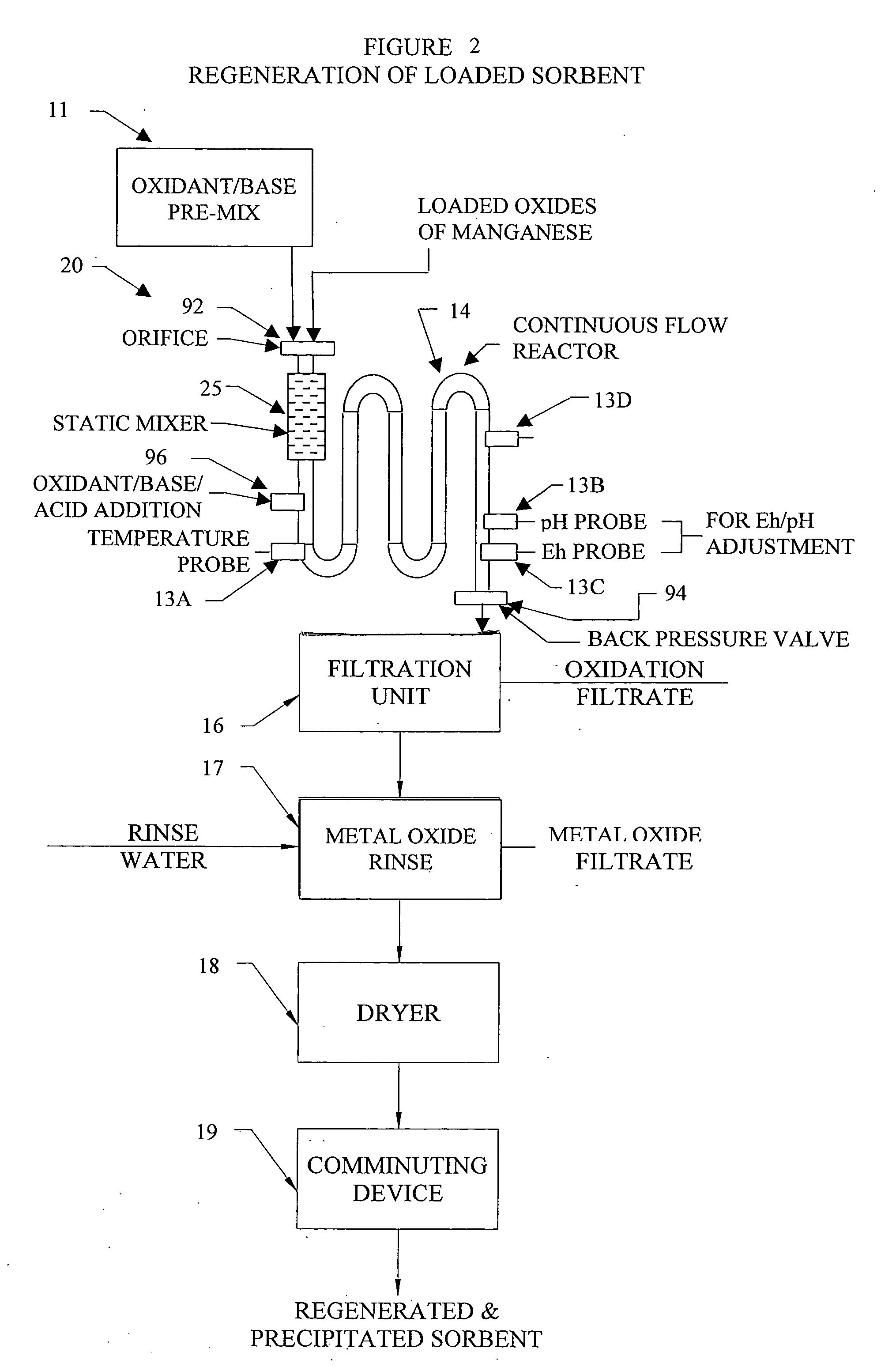
Metal oxide processing methods and systems
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Process of making hydrophobic metal oxide nanoparticles
InactiveUS7081234B1Uniform coatingSulfur compoundsCopper oxides/halidesMetal oxide nanoparticlesTransport layer
A process of treating metal oxide nanoparticles that includes mixing metal oxide nanoparticles, a solvent, and a surface treatment agent that is preferably a silane or siloxane is described. The treated metal oxide nanoparticles are rendered hydrophobic by the surface treatment agent being surface attached thereto, and are preferably dispersed in a hydrophobic aromatic polymer binder of a charge transport layer of a photoreceptor, whereby π—π interactions can be formed between the organic moieties on the surface of the nanoparticles and the aromatic components of the binder polymer to achieve a stable dispersion of the nanoparticles in the polymer that is substantially free of large sized agglomerations.
Owner:XEROX CORP
Flame-Retardant Magnesium Hydroxide Compositions and Associated Methods of Manufacture and Use
InactiveUS20070176155A1Improve resistance performanceSuitable for usePigmenting treatmentGlass/slag layered productsParticulatesPolymer resin
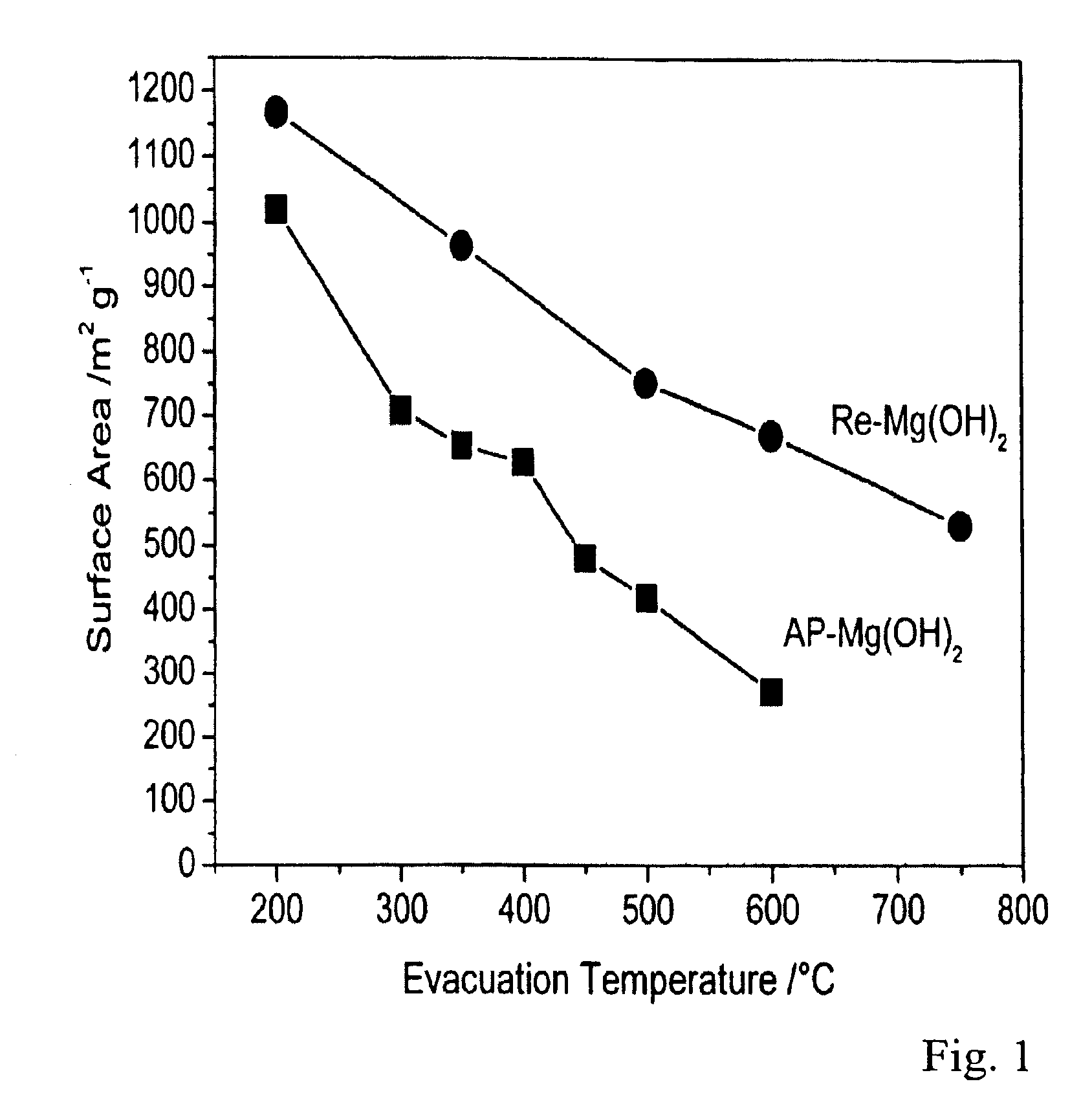
The invention provides a submicron magnesium hydroxide particulate composition comprising a first distribution of magnesium hydroxide particles having a D50 of no more than about 0.30 μm, a D90 of no more than about 1.5 μm, and a BET surface area of at least about 35 m2 / g, which can be used as a flame-retardant additive for synthetic polymers, optionally in combination with other flame-retardant additives such as nanoclays and larger-sized magnesium hydroxide particulate compositions. Polymeric resins comprising the submicron magnesium hydroxide particles and methods of manufacturing submicron magnesium hydroxide particles are also provided.
Owner:MARTIN MARIETTA MATERIALS
Carbon-coated metal oxide nanoparticles
InactiveUS6843919B2Effective airborne decontaminationProvide protectionMaterial nanotechnologySolid sorbent liquid separationMetal oxide nanoparticlesToxin
Composites for destroying chemical and biological agents such as toxins and bacteria, and methods of preparing and using those composites are provided. According to the invention, the substance to be destroyed is contacted with the inventive composites which comprise finely divided metal oxide nanoparticles at least partially coated with carbon. Advantageously, the composites exclude water while not excluding the target compound or adsorbates. The desired metal oxide nanoparticles can be pressed into pellets for use when a powder is not feasible. Preferred metal oxide nanoparticles include MgO, SrO, BaO, CaO, TiO2, ZrO2, FeO, V2O3, V2O5, Mn2O3, Fe2O3, NiO, CuO, Al2O3, SiO2, ZnO, Ag2O, and mixtures thereof.
Owner:KANSAS STATE UNIV RES FOUND
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Flame-retardant magnesium hydroxide compositions and associated methods of manufacture and use
InactiveUS7514489B2Improve resistance performanceSuitable for usePigmenting treatmentGlass/slag layered productsParticulatesPolymer resin
Owner:MARTIN MARIETTA MATERIALS
Hydrogen Energy Systems
InactiveUS20070039815A1Avoid overall overheatingEfficiently safely easily provideCellsAluminium compoundsPetroleumDiesel engine
A hydrogen energy system comprising galvanic hydrogen generators and hydrogen input manifolds for vehicle engines. The galvanic hydrogen generators generate hydrogen gas, magnesium hydroxide, and heat by the galvanic reaction of magnesium anodes with steel cathodes in salt water. Heat exchangers channel excess heat to a heat sink such as a thermocouple, Stirling engine, hot water system, etc. The hydrogen input manifold is bolted between the engine block and the air intake manifold of a gasoline or diesel engine. Hydrogen gas is injected into the hydrogen input manifold to provide supplementary fuel to the engine, lowering the amount of petroleum that is used.
Owner:BARTEL BRIAN G
Process for the Production of Carbon Graphenes and other Nanomaterials
InactiveUS20120068124A1Low costAvoid insufficient purityMaterial nanotechnologyNon-metal conductorsMultiple layerOxidation reduction
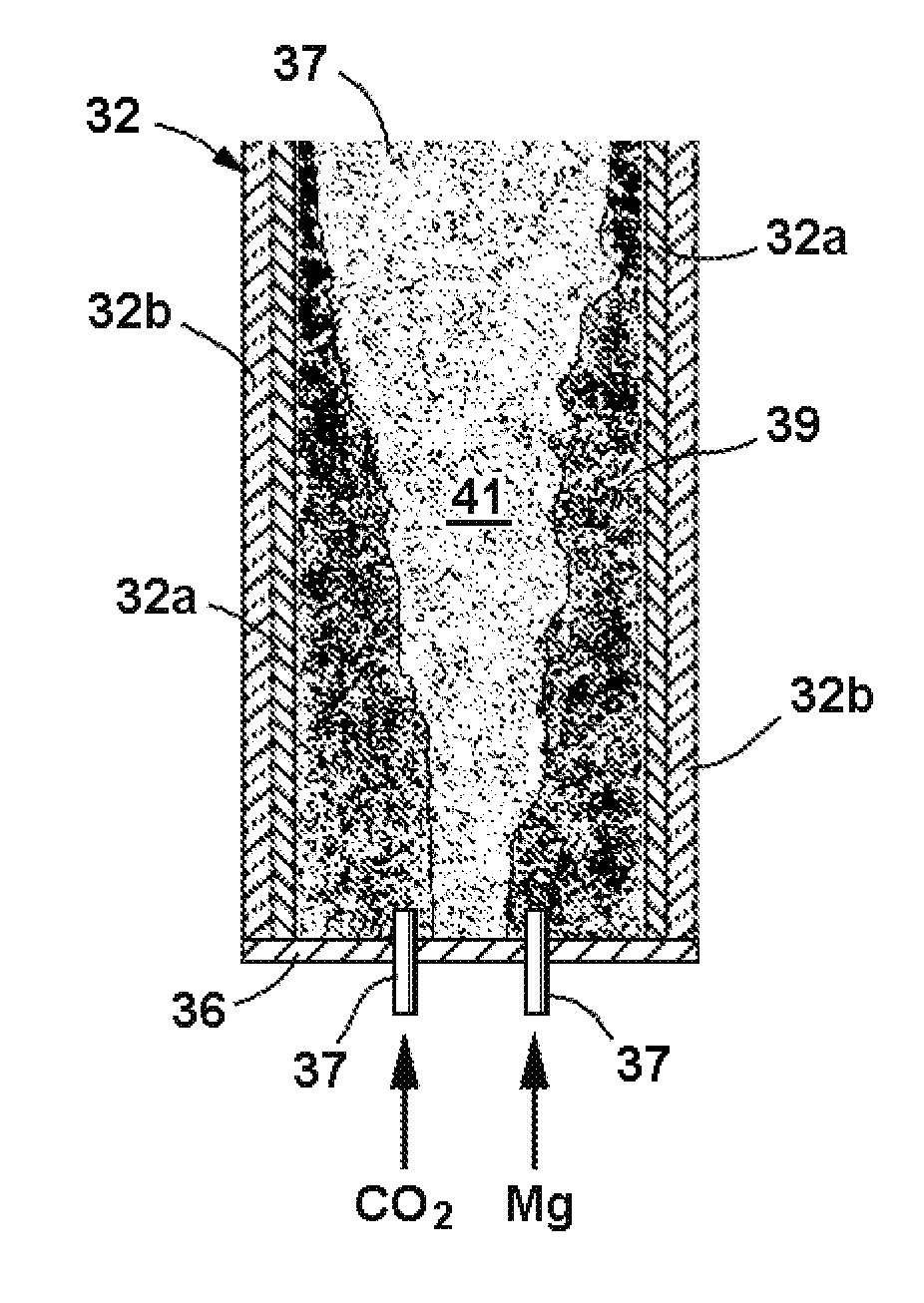
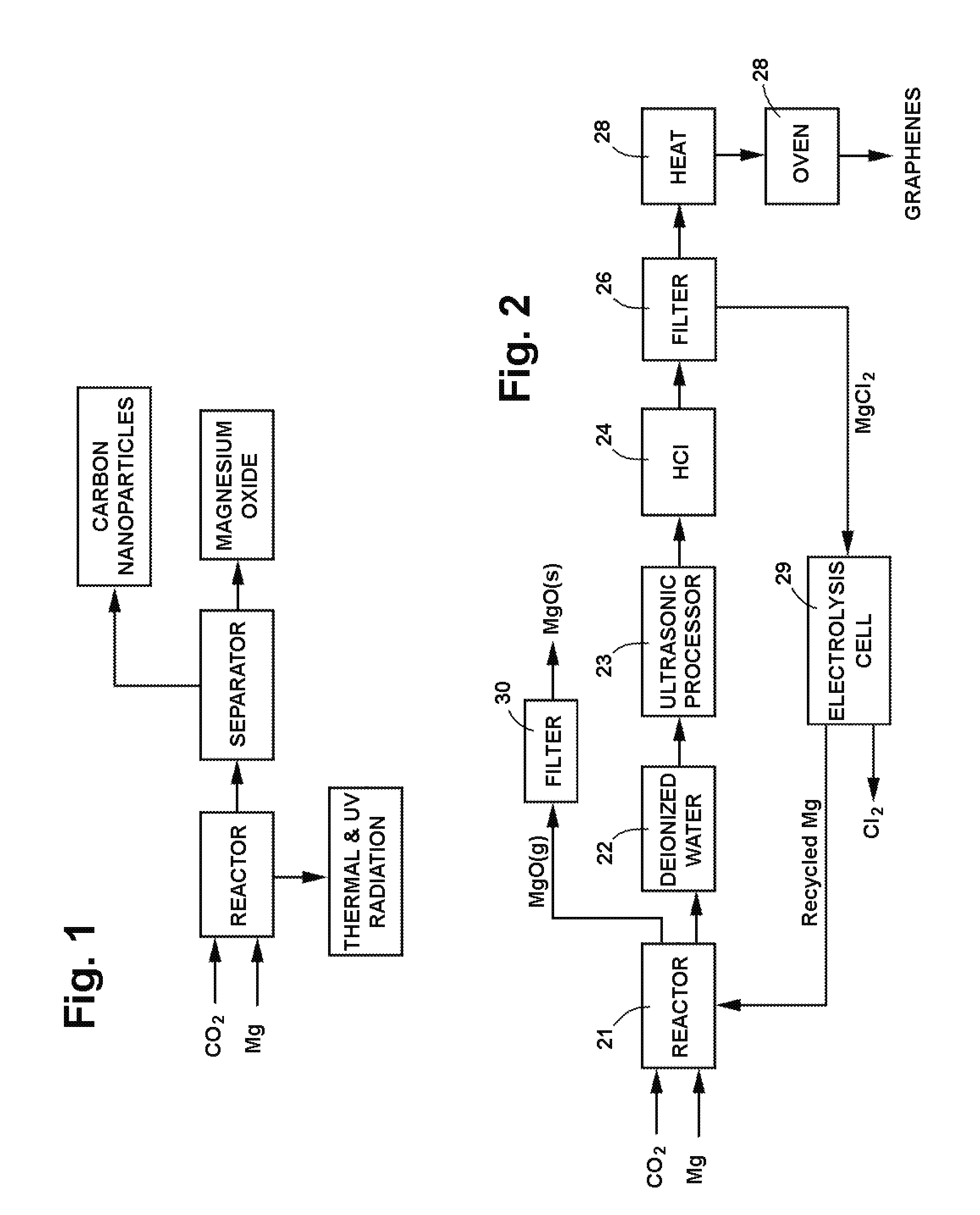
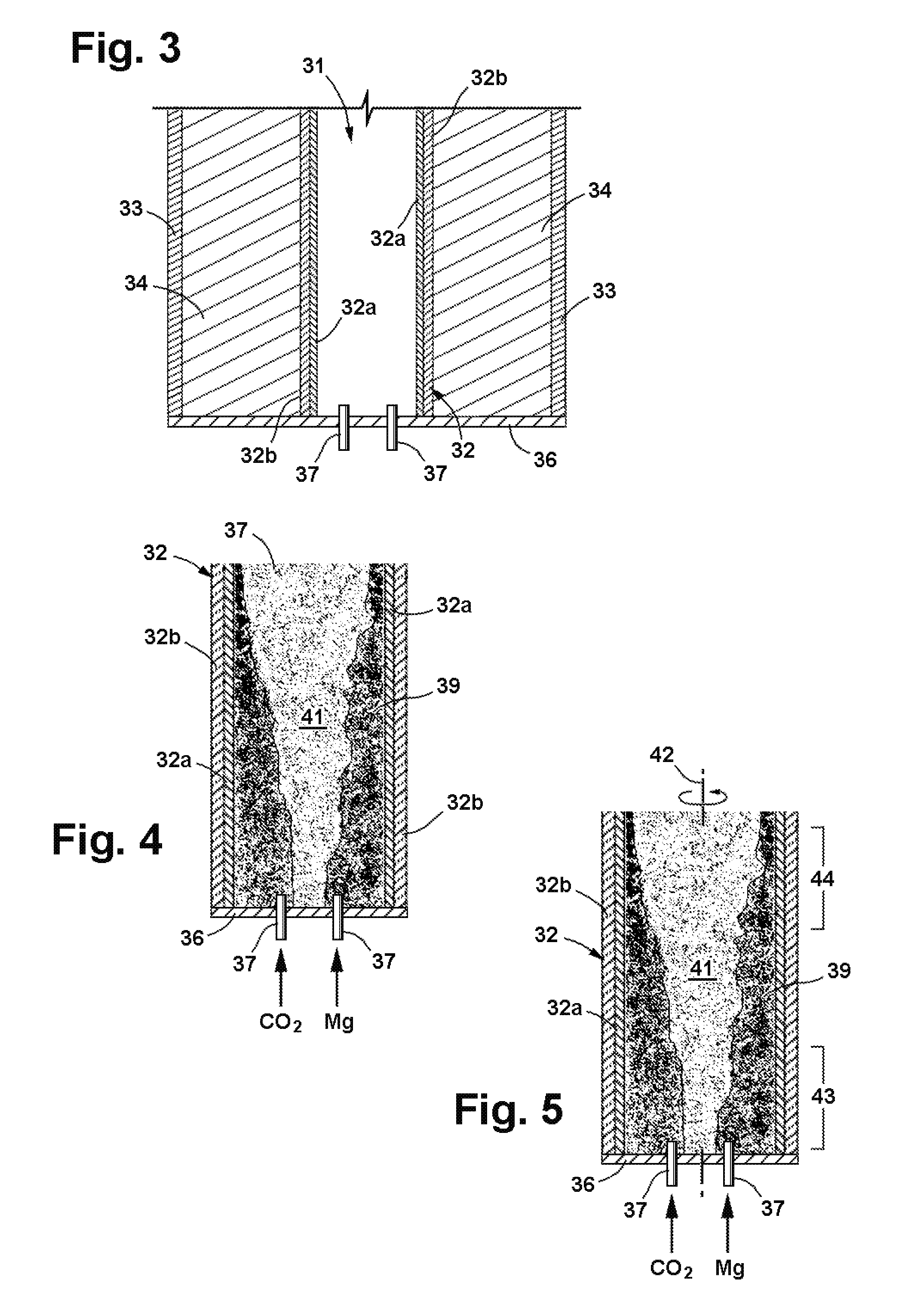
Process for producing nanomaterials such as graphenes, graphene composites, magnesium oxide, magnesium hydroxides and other nanomaterials by high heat vaporization and rapid cooling. In some of the preferred embodiments, the high heat is produced by an oxidation-reduction reaction of carbon dioxide and magnesium as the primary reactants, although additional materials such as reaction catalysts, control agents, or composite materials can be included in the reaction, if desired. The reaction also produces nanomaterials from a variety of other input materials, and by varying the process parameters, the type and morphology of the carbon nanoproducts and other nanoproducts can be controlled. The reaction products include novel nanocrystals of MgO (percilase) and MgAl2O4 (spinels) as well as composites of these nanocrystals with multiple layers of graphene deposited on or intercalated with them.
Owner:GRAPHENE TECH
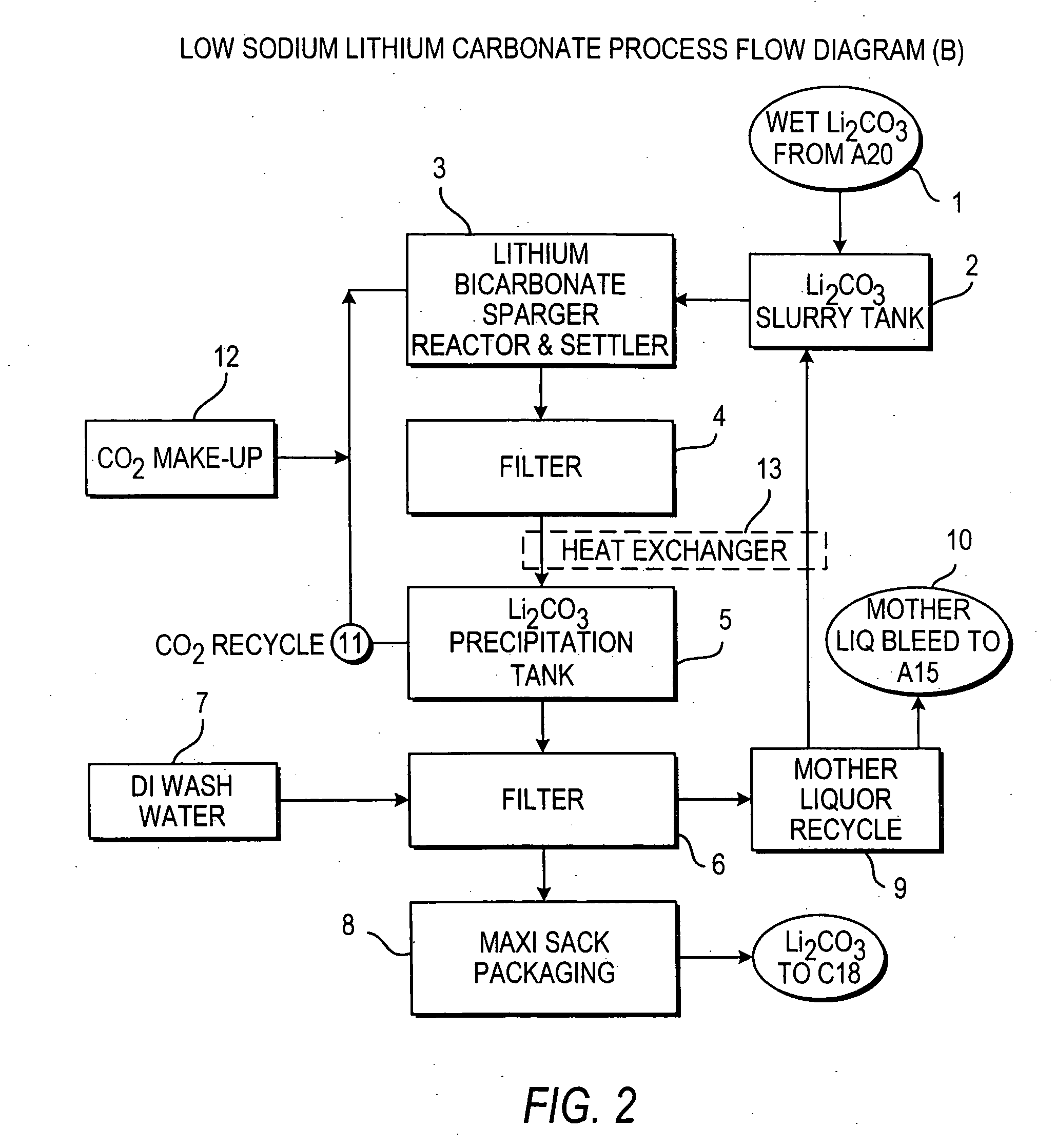
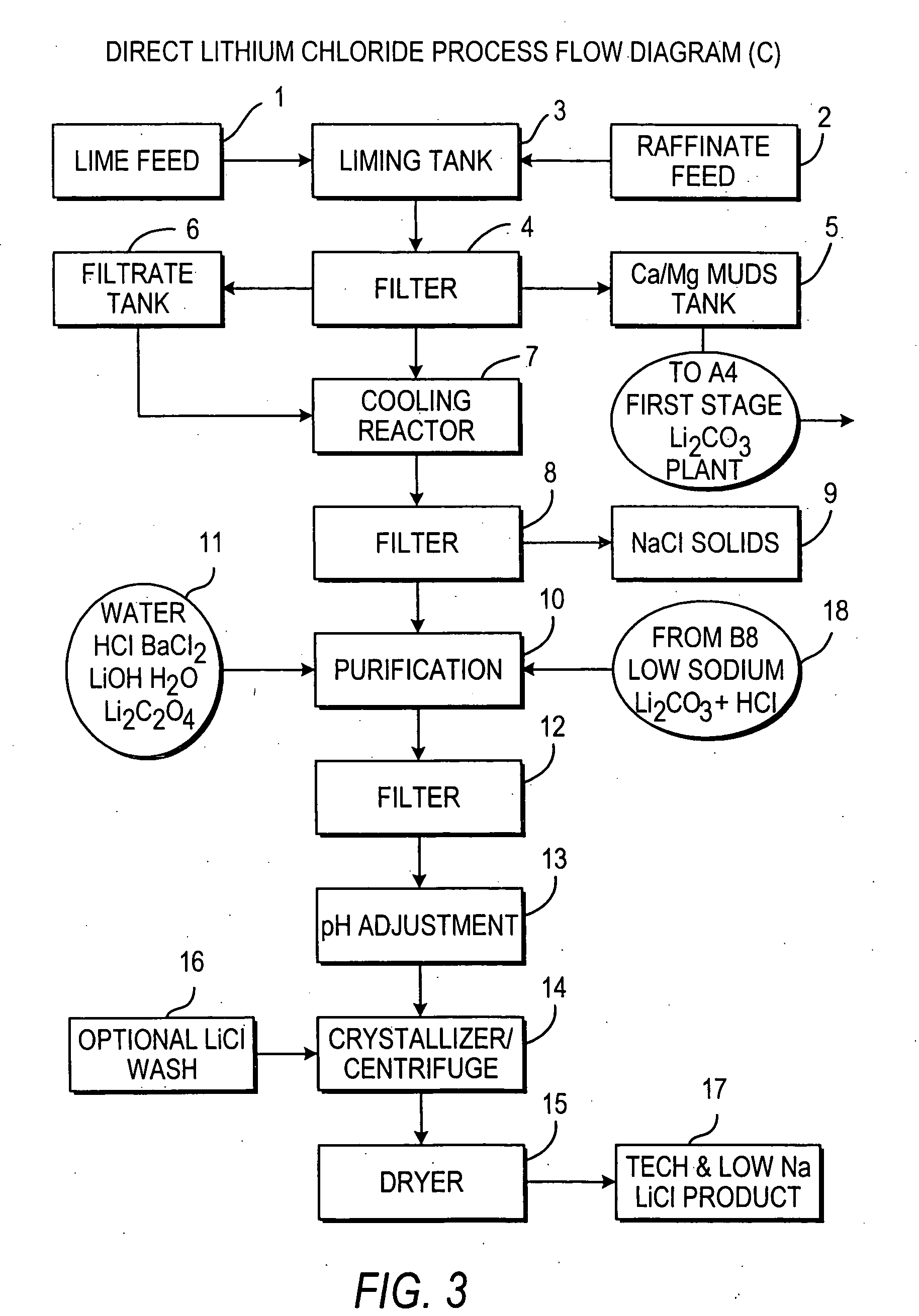
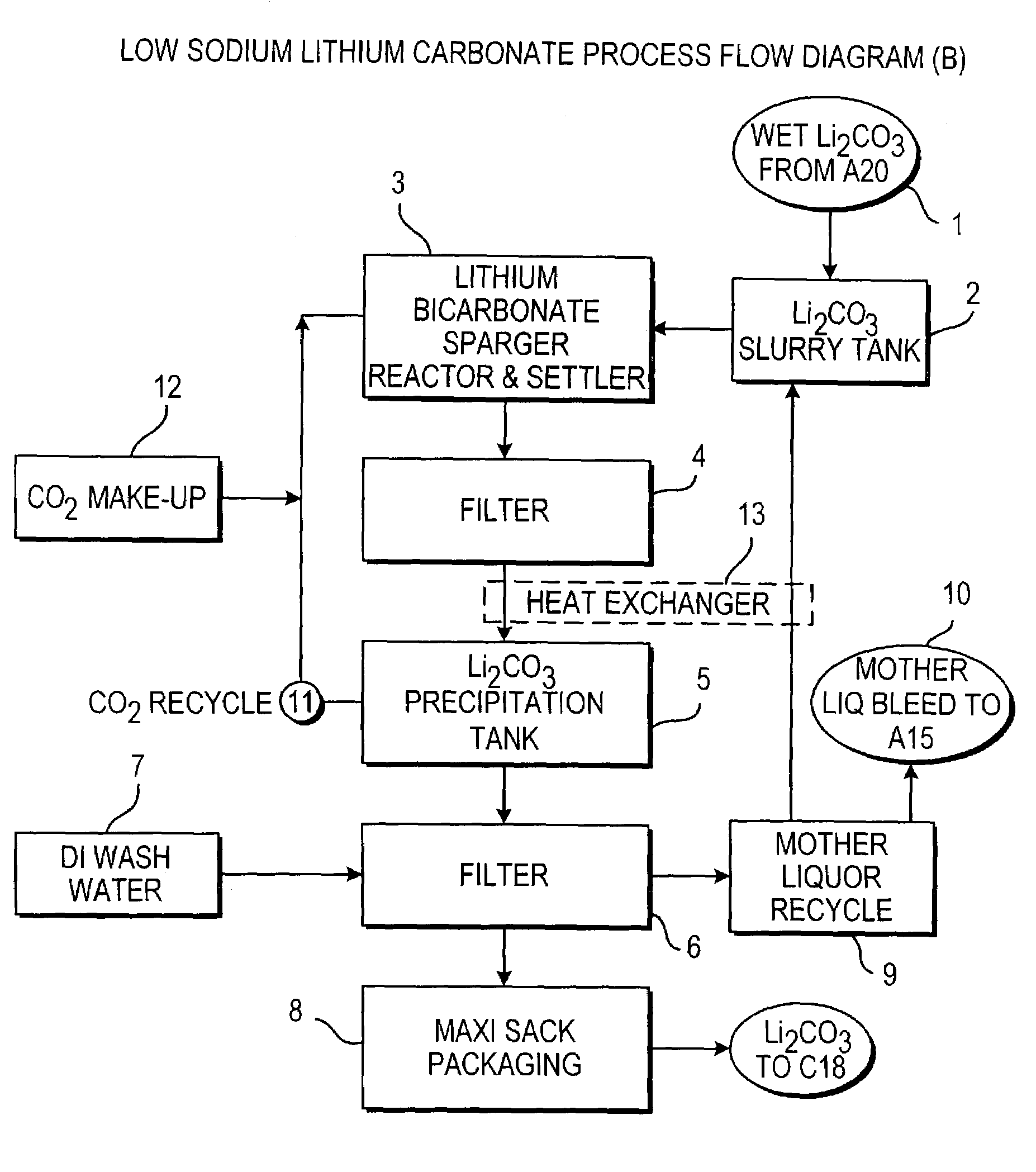
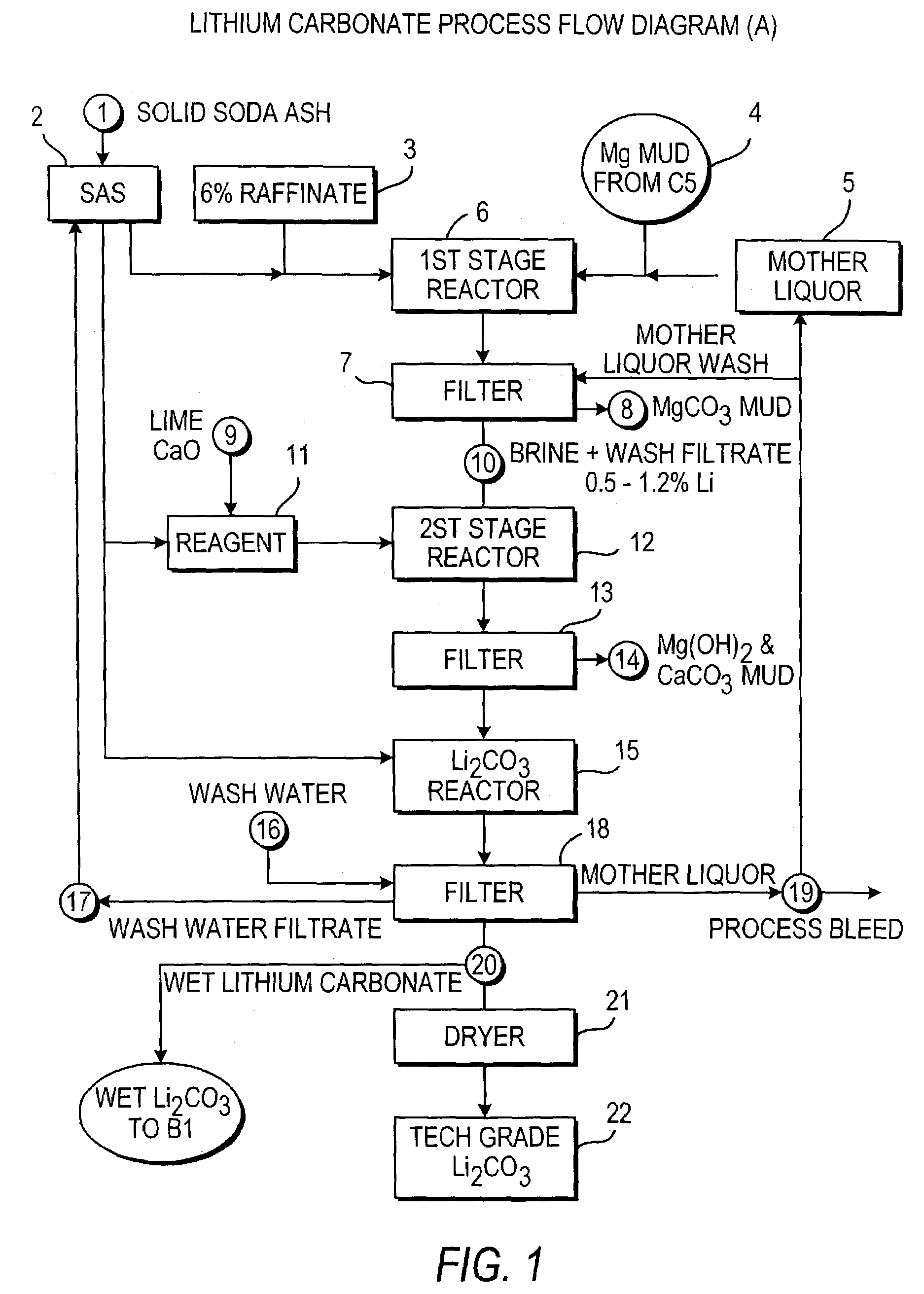
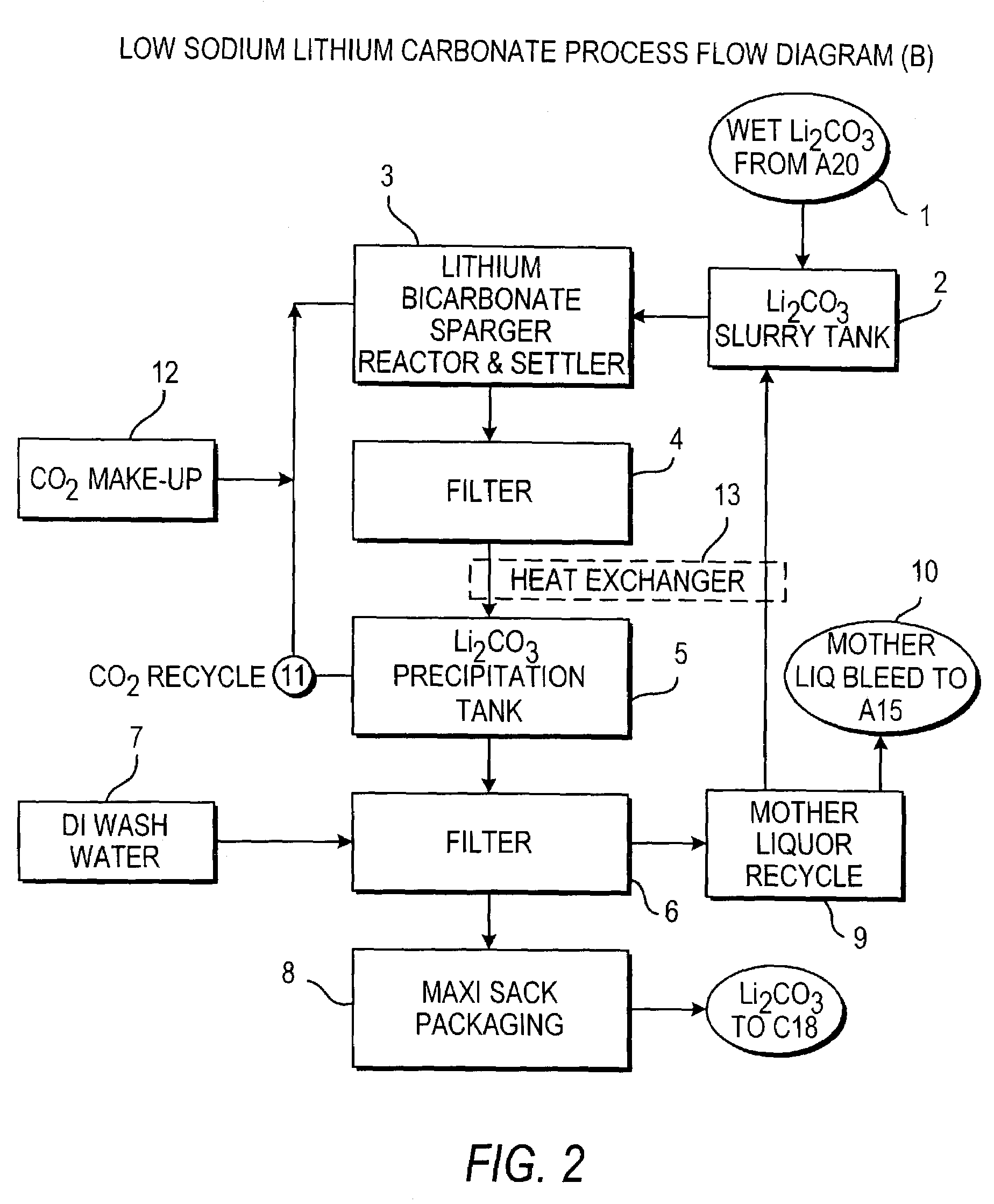
Production of lithium compounds directly from lithium containing brines
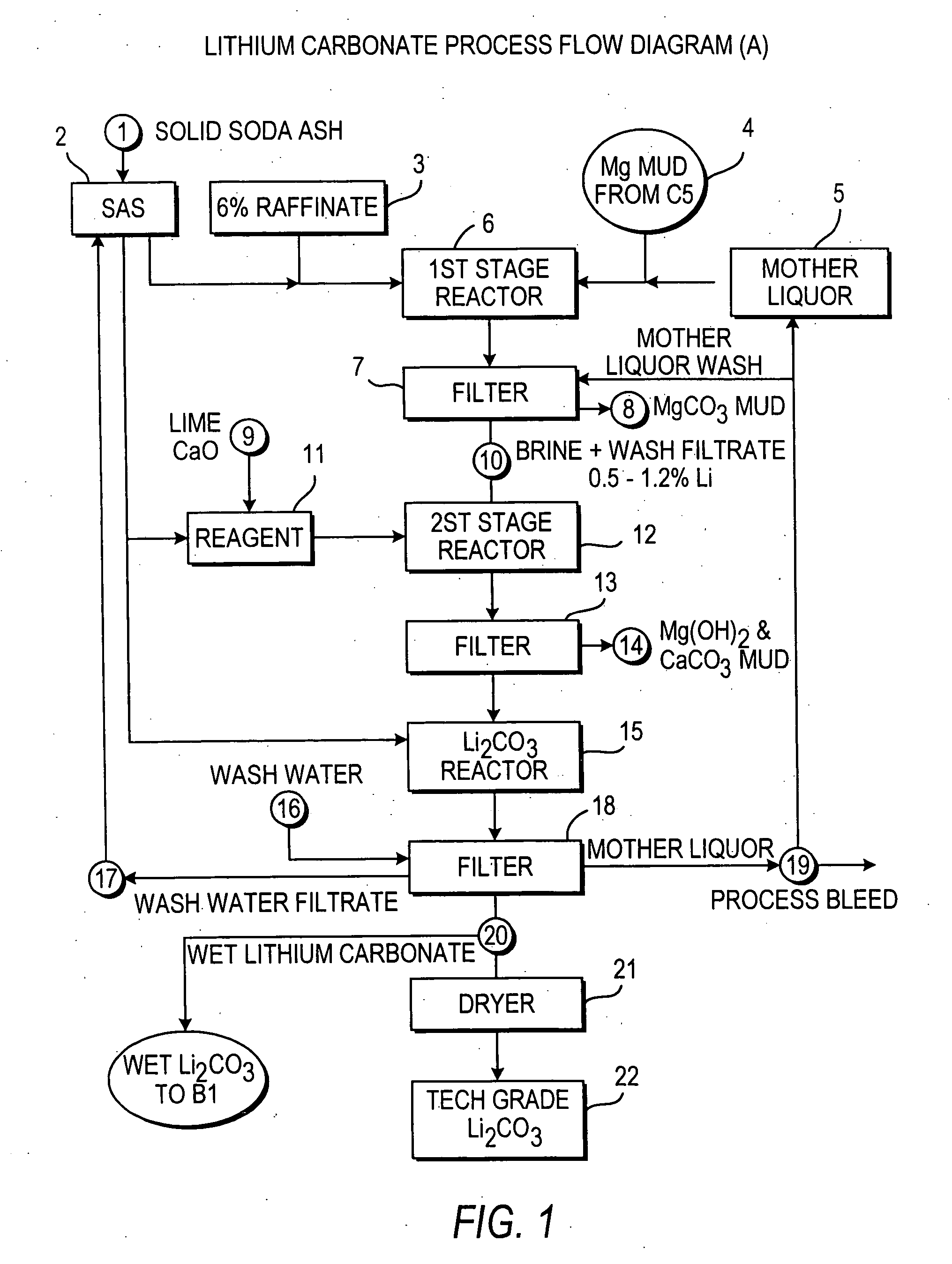
InactiveUS20060115396A1Promote absorptionConstant liquid volumeCalcium/strontium/barium carbonatesCalcium/strontium/barium sulfatesLithium chlorideLithium carbonate
Methods and apparatus for the production of low sodium lithium carbonate and lithium chloride from a brine concentrated to about 6.0 wt % lithium are disclosed. Methods and apparatus for direct recovery of technical grade lithium chloride from the concentrated brine are also disclosed.
Owner:BORYTA DANIEL ALFRED +2
Process for preparing nano-sized metal oxide particles
InactiveUS20050260122A1Efficiently provideNanosized metal oxide particles more efficientlyNanostructure manufactureGold compoundsHigh concentrationAlcohol
The present invention is directed to novel sol-gel methods in which metal oxide precursor and an alcohol-based solution are mixed to form a reaction mixture that is then allowed to react to produce nanosized metal oxide particles. The methods of the present invention are more suitable for preparing nanosized metal oxide than are previously-described sol-gel methods. The present invention can provide for nanosized metal oxide particles more efficiently than the previously-described sol-gel methods by permitting higher concentrations of metal oxide precursor to be employed in the reaction mixture. The foregoing is provided by careful control of the pH conditions during synthesis and by ensuring that the pH is maintained at a value of about 7 or higher.
Owner:KANEKA CORP +1
Production of lithium compounds directly from lithium containing brines
InactiveUS7157065B2Reduce in quantityPromote absorptionCalcium/strontium/barium carbonatesChemical/physical/physico-chemical processesSlurryLithium compound
A continuous process for directly preparing high purity lithium carbonate from lithium containing brines by preparing a brine containing about 6.0 wt % lithium and further containing other ions naturally occurring in brines; adding mother liquor containing carbonate to precipitate magnesium; adding a solution of CaO and sodium carbonate to remove calcium and any residual magnesium; precipitating lithium carbonate from the purified brine by adding soda ash solution; filtering to obtain solid lithium carbonate; preparing an aqueous slurry of the lithium carbonate and introducing carbon dioxide gas at a temperature from at least minus 10 to +40° C.; passing the lithium bicarbonate solution through a filter to clarify the solution; introducing said filtered lithium bicarbonate solution into a reactor and adjusting the temperature of the solution to from 60–100° C. to precipitate ultra-pure lithium carbonate.
Owner:ROCKWOOD LITHIUM INC
Nanoplatelet copper hydroxides and methods of preparing same
Nanoplatelet forms of metal hydroxide and metal oxide are provided, as well as methods for preparing same. The nanoplatelets are suitable for use as fire retardants and as agents for chemical or biological decontamination.
Owner:AQUA RESOURCES CORP
Fine pore formation agent for porous resin film and composition containing the same for porous resin film
ActiveUS20090030100A1Uniform diameterDiameter is limitedCalcium/strontium/barium carbonatesSynthetic resin layered productsElectrical batteryInorganic particles
A fine pore formation agent for a porous resin film is provided which comprises inorganic particles satisfying (a) 0.1≦D50≦1.5 (μm) (D50: average particle diameter of particles in 50% cumulative total by weight from the larger particle side by micro-track FRA), (b) Da≦20 (μm) (Da: maximum particle diameter by micro-track FRA), (c) 3≦Sw≦60 (m2 / g) (Sw: BET specific surface area measured by nitrogen adsorption method), (d) Ir≦1.0×105 (Ω·cm) (Ir: volume resistivity (Ω·cm).The fine pore formation agent for a porous resin film is capable of providing a resin composition giving a porous resin film useful in uses for electric parts such as capacitors and battery separators.
Owner:MARUO CALCIUM COMPANY +1
Powdered lime composition, method of preparing same and use thereof
ActiveUS7744678B2Improve performanceImprove efficiencyGas treatmentOxide/hydroxide preparationDesorptionFlue gas
Powdered lime composition having a BET specific surface area that is equal to or greater than 25 m2 / g and a BJH total pore volume obtained from nitrogen desorption that is equal to or greater than 0.1 cm3 / g, and furthermore comprising an alkali metal. The alkali metal content is equal to or greater than 0.2% and equal to or less than 3.5% based on the total weight of the composition. There is also described a method of preparing the composition and use thereof to reduce flue gases.
Owner:LHOIST RECH & DEV SA
Additive for plastic and plastic
InactiveUS20060188428A1Small amountCalcium/strontium/barium carbonatesMagnesium carbonatesCALCIUM CARBONATE/MAGNESIUM CARBONATEMagnesium carbonate / Magnesium Oxide
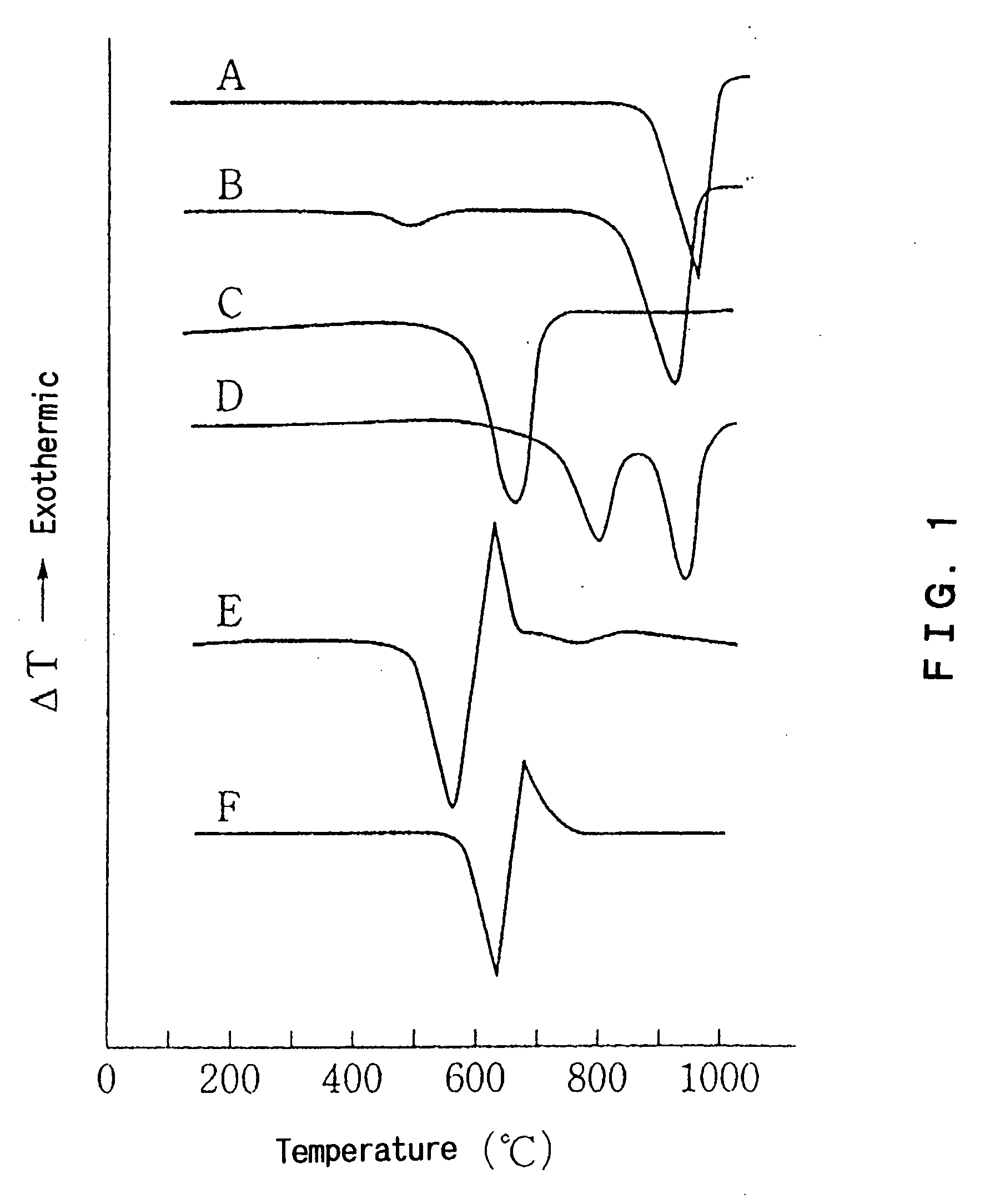
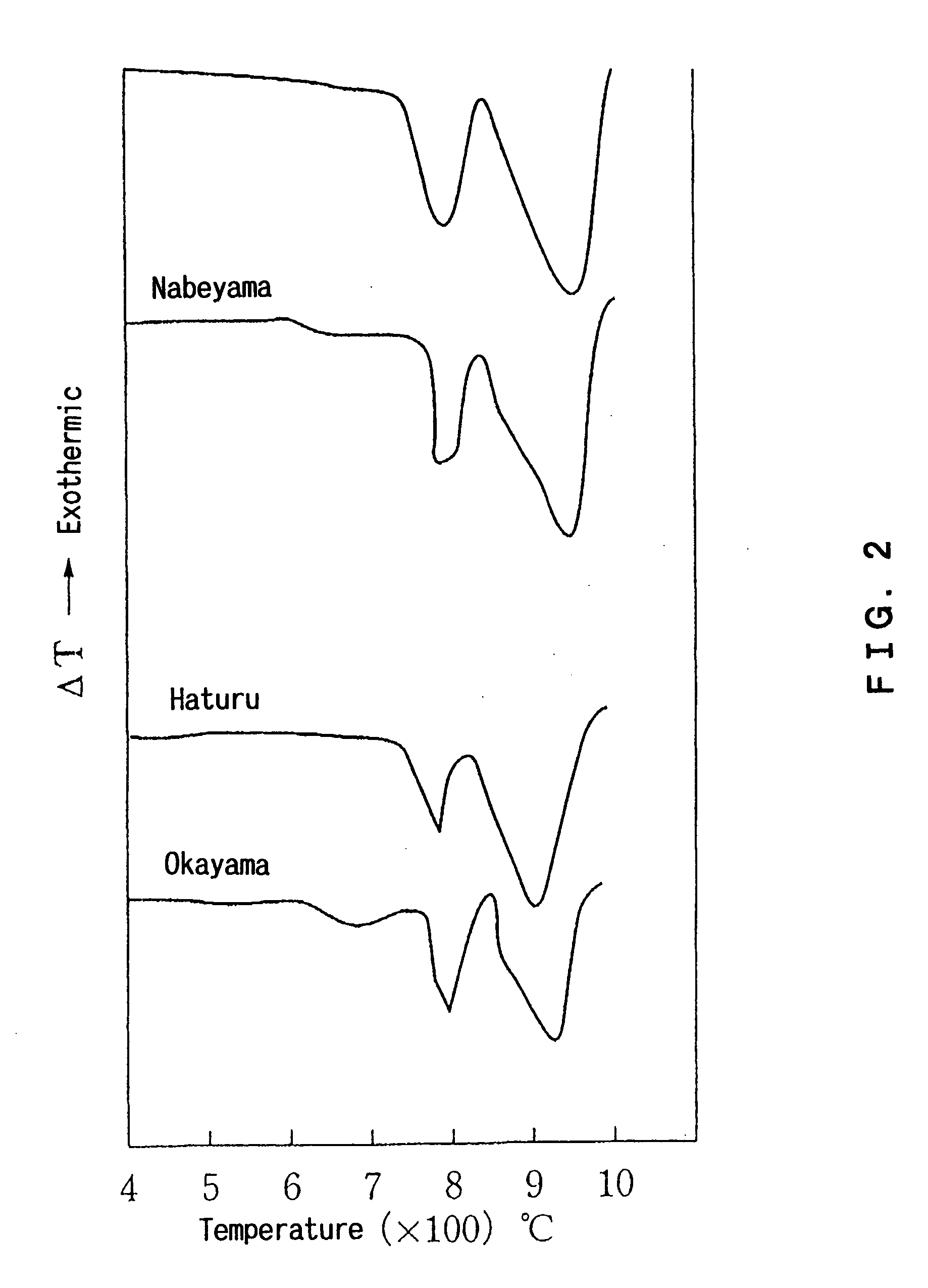
Disclosed is an additive for a plastic, comprising fine particles obtained by calcination and slaking of a dolomite which exhibits two endothermic peaks in the differential thermal analysis, said fine particles containing calcium carbonate, magnesium carbonate, magnesium oxide, calcium hydroxide and magnesium hydroxide as main chemical components and also containing an ignition loss component in an amount of 10 to 40% by weight based on the weight of said fine particles. A plastic hating hydrogen chloride scavenging properties and antimicrobial properties imparted by incorporating the additive for a plastic is also disclosed.
Owner:OSAKA MUNICIPAL TECHN RES INST +2
Method for separating magnesium from lithium and extracting lithium from brine
InactiveCN101538057ASolve the difficult technical problems of filtrationSimple technical processMultistage water/sewage treatmentSolution crystallizationFiltrationHigh energy
The invention provides a method for separating magnesium from lithium and extracting the lithium from high magnesium-lithium ratio brine (brine from a saline lake, from underground and from an oil-gas field). The method comprises: sodium salt and potassium and magnesium mixed salt are separated from the brine by evaporation of a saltpan; after boron extraction, sodium hydroxide is used for precipitating Mg<2+> from obtained old brine, and crystallized Mg(OH)2 is obtained by modification and precipitation condition control; filtration and separation are carried out to remove the Mg(OH)2 to realize separation of magnesium and lithium; after filtered mother solution is vaporized and concentrated for 2-4 times, Na2SO4 and NaCl are separated by crystallization, and pure caustic soda can be added to form lithium carbonate from lithium; or the operation of further evaporation is carried out until Na2SO4 and NaCl are separated by multiple times of natural evaporation or forced evaporation concentration and multiple times of cooling crystallization; the operations of evaporation and concentration are carried out until LiCl saturation, and LiCl products can be prepared after the operation of cooling crystallization is carried out. Compared with the prior art for separating the magnesium from the lithium and extracting the lithium from the brine, the method obtains the crystallized Mg(OH)2 by modification and precipitation condition control, solves the existing technical problem of hard filtration of Mg(OH)2, solves the defects of high energy consumption, complex process and high cost of the existing calcination method, and solves the fundamental defects of low Li<2+> recovery ratio and complex technical process of the traditional precipitation method. The Li<2+> recovery ratio ranges from 85-93%, Mg<2+> removal ratio is more than 99.5%, and the method solves the problem of extracting Li<+> and Mg<2+> from high-magnesium and low-lithium brine with Mg<2+> / Li<+>>=20 mass ratio.
Owner:钟辉
Magnesium hydroxide particles, process for producing the same, and resin composition containing the particles
InactiveUS6676920B1Maintain good propertiesOxide/hydroxide preparationSilicaShell moldingSingle crystal
Owner:KYOWA CHEM IND
Method for making single or mixed metal oxides or silicon oxide
This invention relates to a method of manufacturing a product based on a simple or mixed metal oxide, or silicon oxide, from a charge of one or more precursors comprising one or more organic precursors. These oxides can be, for example, oxides of Ti, Al, Mg, Th, Si, Ba, Bc or Zr etc. The method comprises bringing the charge of organo-metallic precursors into contact with a reaction medium that comprises supercritical C02, at a temperature of from 31 to 100° C. and a supercritical pressure of from 3x107 to 5x107 Pa, in order to form from the precursor, a product based on a simple or mixed metal oxide, or silicon oxide, from the reaction medium by reducing the pressure of the supercritical C02 to a pressure lower than the supercritical pressure.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Cathode active material for lithium rechargeable battery, manufacturing method thereof and lithium rechargeable battery
ActiveUS20080118428A1Inhibition of attachmentExcellent cycle characteristicsCell electrodesLithium compoundsHydrogen phosphatePhosphate
A method for manufacturing a cathode active material for a lithium rechargeable battery, including: selecting a first metal compound from a group consisting of a halide, a phosphate, a hydrogen phosphate and a sulfate of Mg or Al; selecting a second metal compound from a group consisting of an oxide, a hydroxide and a carbonate of Mg or Al; combining the first metal compound and the second metal compound to obtain a metal compound, the metal compound containing either Mg or Al atoms; mixing a lithium compound, a transition metal compound and the metal compound to obtain a mixture; and sintering the mixture.
Owner:NIPPON CHECMICAL IND CO LTD
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
Porous material process of producing the porous material, catalyst for purifying exhaust gas comprising the porous material, method of purifying exhaust gas
InactiveUS6926875B2Excellent characteristicsImprove heat resistanceNitrogen compoundsInternal combustion piston enginesHigh resistanceMeson
Disclosed are a porous material comprising particles without substantial fibrous structure and having pores, the pores having a mean pore diameter in a meson-pore region, sharp pore size distribution, and at least a part of the pores being connected three-dimensionally to form a three-dimensional network structure with random passages, the porous material preferably being of alumina and having a spongy structure or the porous material preferably being an aggregate of particles having an aspect ratio of 3 or less; a process of producing the porous material which includes a step of aging a system capable of becoming an oxide on thermal decomposition; a catalyst for exhaust gas purification having excellent NOx removal performance, high resistance against sulfur poisoning, and satisfactory high-temperature durability which comprises the porous material as a carrier having supported thereon a noble metal and an NOx storage component; and a method of exhaust gas purification using the catalyst.
Owner:TOYOTA CENT RES & DEV LAB INC
Process for producing magnesium hydroxide and calcium carbonate by dolomite conversion method
InactiveCN102225775AReduce consumptionGood environmental benefitsCalcium/strontium/barium carbonatesMagnesium hydroxideEvaporationCarbonization
The invention provides a process for producing magnesium hydroxide and calcium carbonate by the dolomite conversion method. The process comprises the following steps: calcining dolomite, carrying out a digestion reaction on dolomite and water and refining so as to obtain a refined slurry of calcium hydrate and magnesium hydroxide; reacting the slurry with an ammonium chloride solution to enable calcium hydrate to react with ammonium chloride; carrying out ammonia evaporation so as to obtain a fluid suspension of a calcium chloride solution, ammonia gas and magnesium hydroxide; carrying out filtering and washing so as to obtain a magnesium hydroxide product; carrying out a carbonization reaction on the calcium chloride aqueous solution which has absorbed ammonia gas with kiln gas generated by the calcination of dolomite so as to obtain calcium carbonate; carrying out filtering and washing so as to obtain a calcium carbonate product. In the invention, washing water is used for the digestion of dolomite ashes so as to totally separate magnesium from calcium in dolomite and produce magnesium hydroxide and calcium carbonate products, thereby fully utilizing dolomite. The process provided in the invention enables cyclic utilization of the intermediate product ammonium chloride and no discharge of the three wastes, being in accordance with the developmental requirements for green chemicals in modern society.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
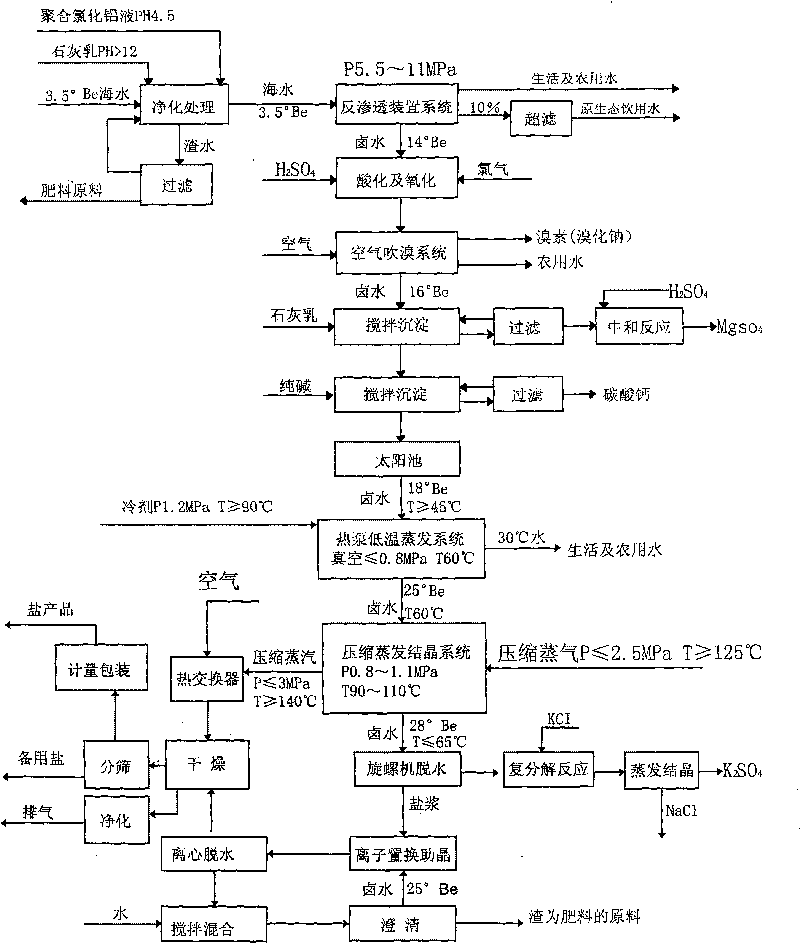
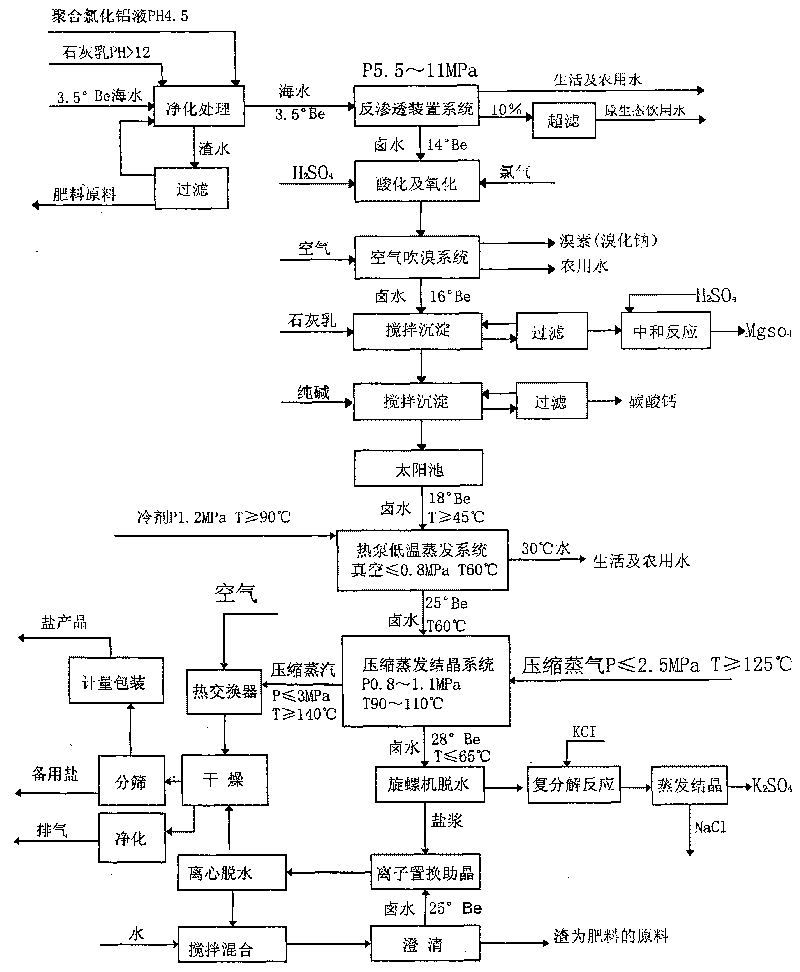
Technique for desalting sea water, making salt and comprehensively using by-products thereof by utilizing wind energy, solar energy and heat pump technologies
ActiveCN101704560AIncrease volumeAchieve balanceGeneral water supply conservationEnergy inputWater desalinationSolar pond
The invention relates to a technique for desalting sea water, making salt and comprehensively using by-products thereof by utilizing wind energy, solar energy and heat pump technologies, the main procedures comprise desalting sea water, air-blowing bromine, removing impurities for clarification, heating solar pond and storing halogen, evaporating for concentration, compressing and crystallizing for salt making, brine chemical engineering, etc. the invention desalts sea water, makes salt and comprehensively uses by-products thereof by utilizing wind energy, solar energy and heat pump technologies, organically combines sea water desalting, salt making and comprehensive use of dregs and waste liquor, takes wind energy, solar energy and other renewable energy resources as main energy sources, changes sea salt production from drying on beach in the open air manually to mechanized and automated operation, puts an end to the backward state of sea salt enterprises, can save energy consumption and reduce pollution, can save investment by about 50% and occupied area by 95%, not only lowers production cost, but also recovers 35m<2> of fresh water by producing one ton of salt, thus protecting fresh water resource.
Owner:ZHANJIANG NONGHAI TECHNOLOGY CO LTD
Popular searches
Hydrocarbon by hydrocarbon condensation Bulk chemical production Hydrocarbon oils treatment products Aluminium oxides/hydroxides Tungsten oxides/hydroxides Organic-compounds/hydrides/coordination-complexes catalysts Heterogenous catalyst chemical elements Hydrocarbon preparation catalysts Catalyst activation/preparation Hydrocarbons
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com