Ziziphora clinopodioides Lam fingerprint and establishment method thereof
A technique for fingerprinting and establishing methods, which is applied in the field of drug analysis, can solve problems such as difficult to achieve effective quality control of lip vanilla, insufficient quality stability, etc., and achieve high sensitivity and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
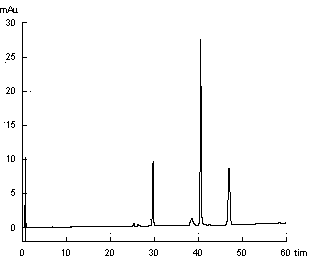
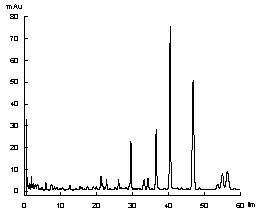
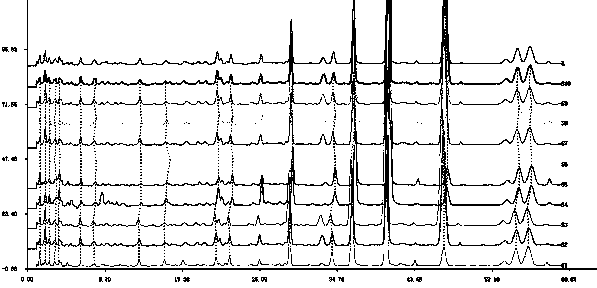
[0032] 1. Source of medicinal materials:
[0033] Sources and similarity of 10 samples of lip vanilla from different origins
[0034]
[0035] Lip vanilla was identified as Ziziphora clinopodioides Lam. by Li Yonghe, chief pharmacist of the Department of Traditional Chinese Medicine, College of Traditional Chinese Medicine, Xinjiang Medical University.
[0036] 1. Preparation of standard solution
[0037] The standard reference substances used in this method are diosmin, mongoside, pulegone
[0038] Weighed an appropriate amount of the standard substance and dissolved them in methanol. It was found that pulegone could be well dissolved in methanol, while diosmin and mongoside had very little solubility in methanol and were almost insoluble. So add co-solvent DMSO to help it dissolve. Standards were prepared in 25% v / v DMSO in methanol. The solutions with concentrations of 0.122 mg / ml, 0.276 mg / ml, and 0.063 mg / ml of diosmin, mongoside and pulegone, respectively, were us...
Embodiment 2
[0058] (1) Preparation of standard solution:
[0059] Weigh the standard products of diosmin, montanoside and pulegone respectively, dissolve them in a methanol solution containing 20% v / v of dimethyl sulfoxide and settle to volume, and make diosmin, montanoside and pulegone Menthone concentrations of 0.122mg / ml, 0.276mg / ml, and 0.063mg / ml were used as standard solutions.
[0060] (2) Preparation of the test solution:
[0061] Accurately weigh 1.000g of lip vanilla coarse powder from different origins, put it into a 30mL conical flask with a stopper, use 8mL of 20% DMSO methanol as solvent, soak for 0.5h, ultrasonically extract for 30min, filter, wash with 20% DMSO methanol and dilute to 10mL Put it in a volumetric flask, shake well, pass through a 0.22μm microporous membrane, and obtain.
[0062] All the other operations are the same as in Example 1.
Embodiment 3
[0064] (1) Preparation of standard solution:
[0065] Weigh the standard products of diosmin, montanoside and pulegone respectively, dissolve them with 30% v / v methanol solution containing dimethyl sulfoxide and make the diosmin, montanoside and pulegone Menthone concentrations of 0.122mg / ml, 0.276mg / ml, and 0.063mg / ml were used as standard solutions.
[0066] (2) Preparation of the test solution:
[0067] Accurately weigh 1.000g of lip vanilla coarse powder from different origins, put it into a 30mL conical flask with a stopper, use 15mL of 30% DMSO methanol as solvent, soak for 1.5h, ultrasonically extract for 1h, filter, wash with 30% DMSO methanol and dilute to 10mL Put it in a volumetric flask, shake well, pass through a 0.22μm microporous membrane, and obtain.
[0068] All the other operations are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com