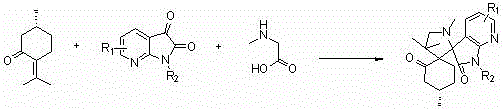
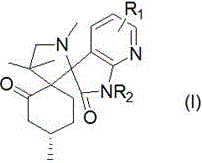
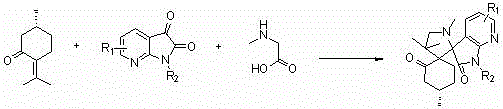
A kind of 7-azaisatin double spiro compound with antitumor activity and its synthesis method
A technology of compound and heteropolyacid, which is applied in the field of medicine, can solve problems such as treatment failure, and achieve the effect of less steps, high yield and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The influence of different solvent systems on the yield of the synthesized compound (1-2) was investigated, and the results are shown in Table 1:
[0050]
[0051] The reaction conditions are: the molar ratio of pulegone, 7-azaisatin derivative and sarcosine is 1:1:1, the reaction temperature is 80°C, the reaction time is 10 hours, and the solvents are toluene and ethyl acetate respectively. , ethanol and water, the results show that the yield of ethanol is the best under the condition of no catalyst.
Embodiment 2
[0053] Taking the synthetic compound (1-2) as an example, the influence of different catalyst ratios on the yield of the target product was investigated, and the results are shown in Table 2:
[0054]
[0055] The reaction conditions are as follows: the molar ratio of pulegone, 7-azaisatin derivatives and sarcosine is 1:1:1, ethanol is used as solvent, the reaction temperature is 80°C, and the reaction time is 10 hours. Polyacid - phosphotungstic acid (H 3 PW 14 o 4 ), organic acid—acetic acid, Lewis acid—zinc chloride (ZnCl 2 ), the organic base - triethylamine, the molar ratio of the amount of catalyst to the amount of pulegone is 0.1 (that is, 0.1 times the equivalent), the results show that the reaction can be better under the catalysis of heteropoly acid - phosphotungstic acid yield.
Embodiment 3
[0057] Taking the synthetic compound (1-2) as an example, the influence of different reaction temperatures on the yield of the target product was investigated, and the results are shown in Table 3:
[0058]
[0059] The reaction conditions are: the molar ratio of pulegone, 7-azaisatin derivative and sarcosine is 1:1:1, ethanol is used as solvent, phosphotungstic acid is used as catalyst, the reaction time is 8 hours, and the reaction temperature is respectively 25°C, 60°C, 70°C, and 80°C. The results showed that the yield was the highest when the reaction temperature was 80°C (reflux temperature).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com