PD-L1IgV-targeting affinity peptide D1 with anti-tumor activity
An anti-tumor activity and targeted technology, applied in anti-tumor drugs, medical preparations containing active ingredients, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
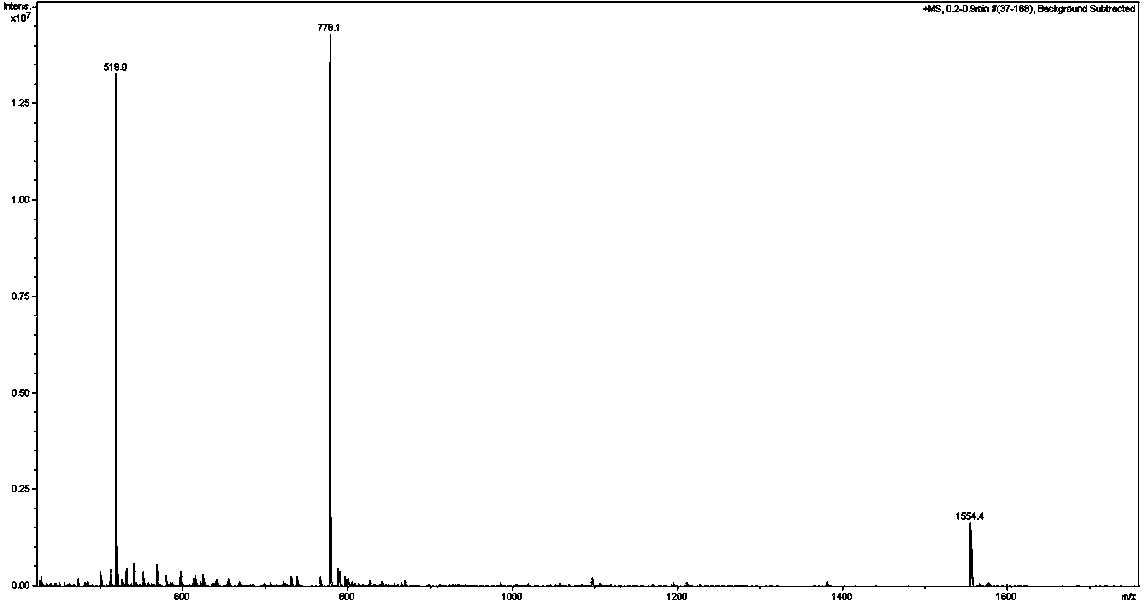
[0024] The D-configuration affinity peptide D1 targeting PD-L1 IgV with anti-tumor activity provided by the present invention specifically binds to the PD-L1 IgV region and is screened by mirror-image phage display peptide library technology. Its amino acid sequence It is: Asn-Tyr-Ser-Lys-Pro-Thr-Asp-Arg-Gln-Tyr-His-Phe, namely N-Y-S-K-P-T-D-R-Q-Y-H-F, with a molecular weight of 1554.7.
[0025] Since the peptides obtained by conventional phage display peptide library screening are composed of natural amino acid residues, they are easily degraded by enzymes and have a short half-life in vivo. Therefore, we selected the PD-L1 IgV region protein artificially synthesized from D-configuration amino acids as the target, and screened D-configuration antagonistic peptides through mirror phage display technology, thereby greatly improving its ability to resist enzymatic degradation. Prolong the half-life of action in vivo. For the convenience of those skilled in the art to implement ...
Embodiment 2
[0059] The D-configuration affinity peptide D1 of the PD-L1 IgV with anti-tumor activity is synthesized by Fomc solid-phase peptide synthesis method.
[0060] The main reagents used in the synthesis process are:
[0061] Heading liquid: acetic anhydride / pyridine solution (1:1 v / v);
[0062] Indene detection reagent: A. Ninhydrin / ethanol solution (5% w / v), B. Phenol / ethanol (4:1 w / v), C. Potassium cyanide / pyridine (2% v / v);
[0063] Deprotection solution: piperidine / DMF solution (20% v / v);
[0064] Cleavage reagent: by volume, TFA (82.5%), H 2 O (5%), phenol (5%), thioanisole (5%), ethanedithiol (2.5%).
[0065] The synthetic steps are briefly described as follows:
[0066] (1) Swell the resin, add the first amino acid
[0067] A. Swelling resin: Take 0.3~0.5 g Rink resin (the C-terminal amino acid of the peptide connected to the resin is an amide) and place it in a cleaned and dried peptide synthesizer, add an appropriate amount of DMF, soak for about 30 minutes to ful...
Embodiment 3
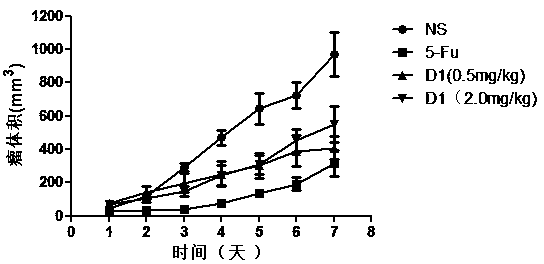
[0109] Taking the D-configuration affinity peptide D1 targeting PD-L1 IgV with anti-tumor activity prepared in Example 2 as an example, the inventors conducted further in vivo experiments on tumor-bearing mice. The specific experimental process is as follows:
[0110] (1) Affinity peptide D1 inhibits the growth of transplanted tumors in mice bearing CT26 colon cancer
[0111] Select 24 experimental Balb / c mice, adjust the cell concentration to 5×10 mouse colon cancer (CT26) cells with normal saline (NS) 6 cells / mL, 0.1 mL cell suspension (containing 5×10 5 cells) were inoculated subcutaneously in the armpit of the right forelimb of each Balb / c mouse, and the growth of the subcutaneous tumor was continuously observed.
[0112] The affinity peptide D1 prepared in Example 2 was dissolved in physiological saline to make a polypeptide drug, which was subpackaged and stored at -20°C for future use. Nine days after inoculation with mouse-derived colon cancer (CT26) cells, the mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com