Patents
Literature
45 results about "Thioanisole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
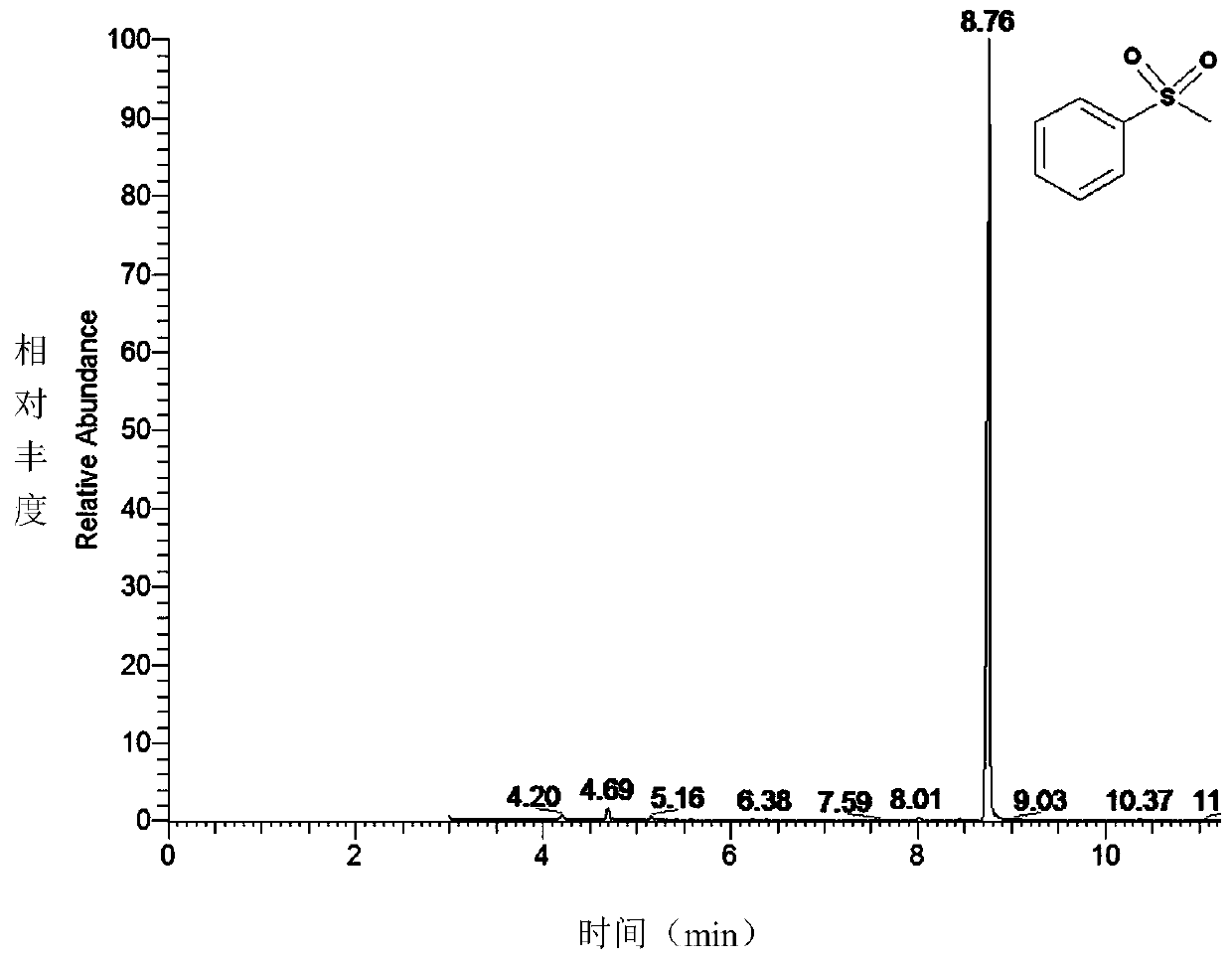
Thioanisole is an organic compound with the formula CH₃SC₆H₅. It is a colorless liquid that is soluble in organic solvents. It is the simplest alkyl–aryl thioether. The name indicates that this compound is the sulfur analogue—the thioether rather than the oxygen-centered ether—of anisole.
Method for synthesizing atosiban by solid phase
ActiveCN101696236AReduce poisonReduce pollutionOxytocins/vasopressinsPeptide preparation methodsRink amide resinTrifluoroacetic acid
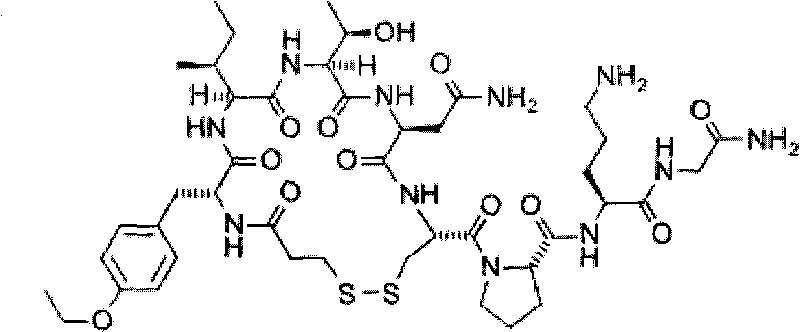
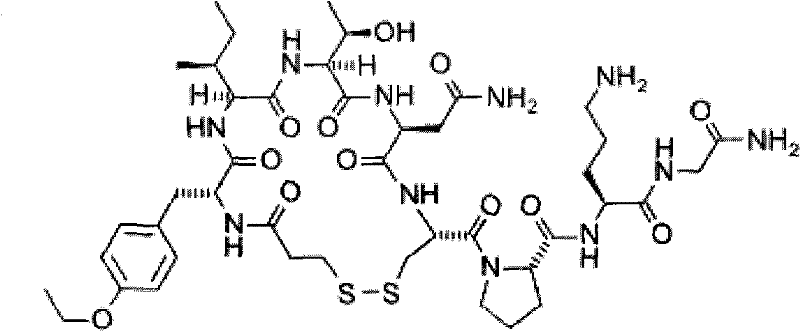
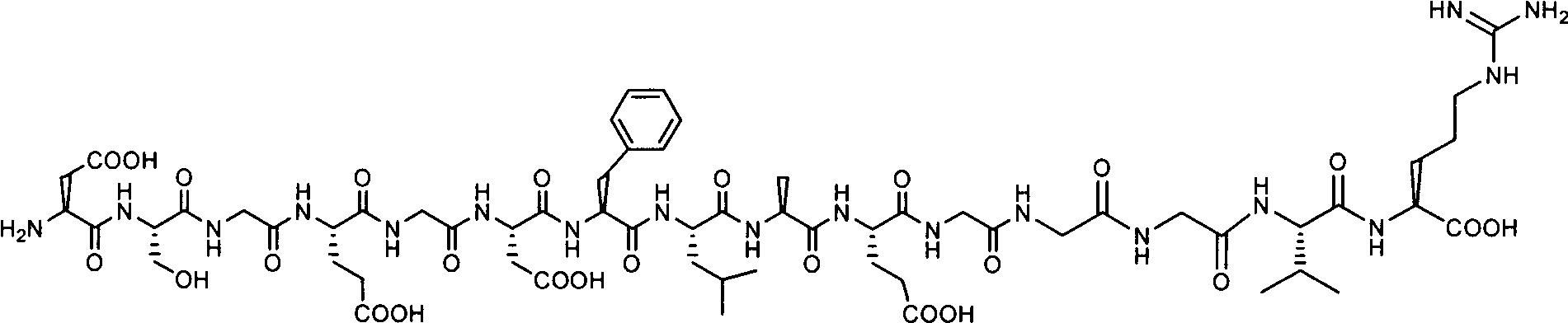
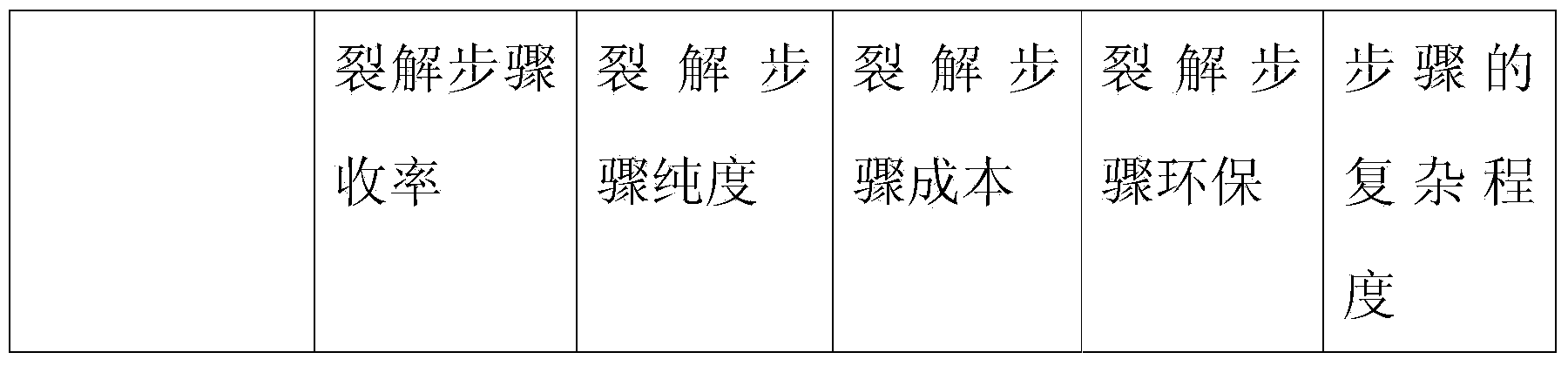
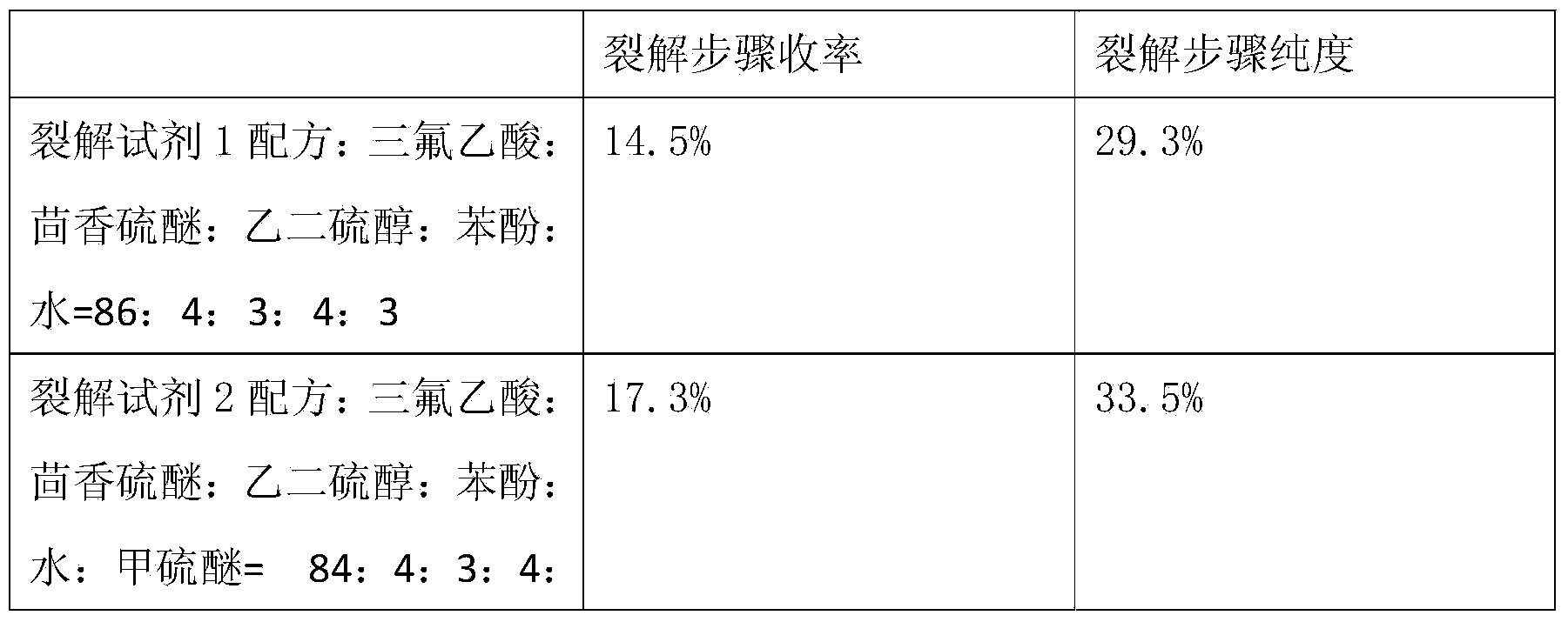
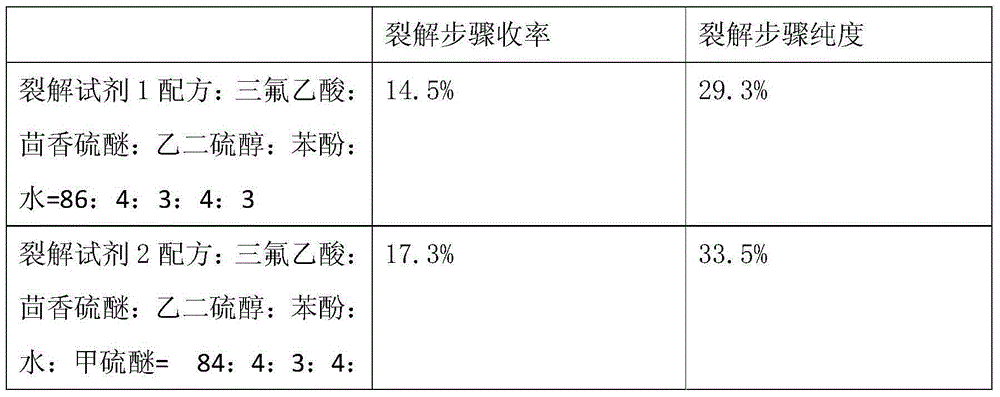
The invention relates to a method for synthesizing atosiban by a solid phase, which comprises the following steps: 1) de-Fmoc-protecting Rink Amide resin serving as a carrier to obtain H2N-Rink Amide resin; 2) connecting carboxyl of Fmoc-Gly-OH and amino of the resin by using HOBT and DIPCI as condensation reagents to obtain Fmoc-Gly (resin); 3) solid-phase synthesizing sequence residue amino acid in turn by adopting Fmoc strategy; 4) performing solid-phase cyclization by using iodine; 5) then cutting the sequence residue amino acid by using pyrolysis reagents (trifluoroacetic acid / thioanisole / 1, 2-dimercaptoethane / water), and settling the sequence residue amino acid by diethyl ether to obtain raw atosiban peptide; and 6) treating the raw product through HPLC preparation and separation to obtain a pure atosiban product.
Owner:HAINAN ZHONGHE PHARM CO LTD
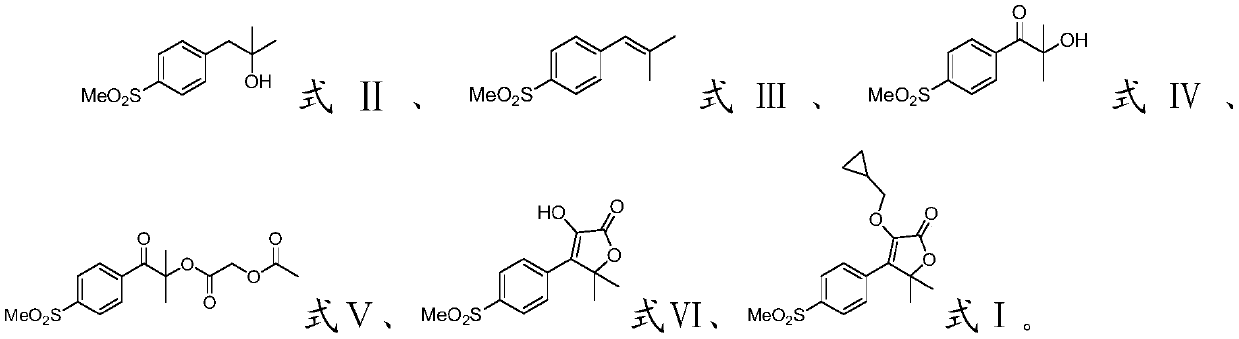
Firocoxib preparation method
InactiveCN105859664AEasy to operateHigh yieldOrganic chemistryChemical synthesisChromatographic separation
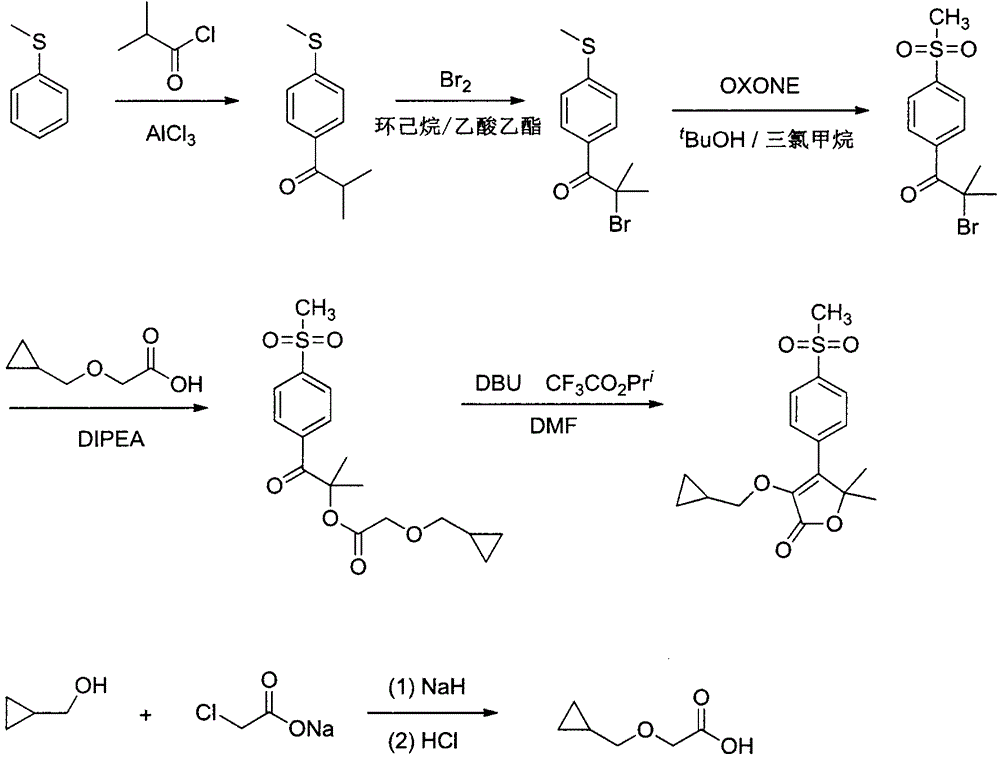
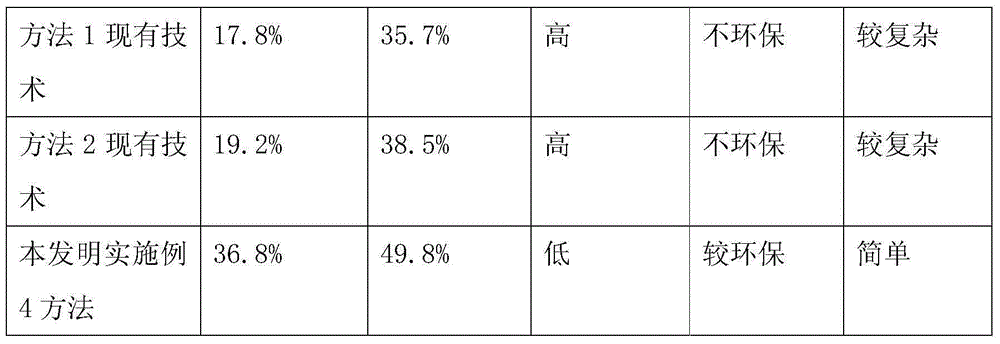
The invention discloses a firocoxib preparation method and relates to the technical field of chemical synthesis, in particular to a novel method for synthesizing firocoxib. The novel method for synthesizing the firocoxib includes subjecting thioanisole serving as a raw material to acylation reaction, bromination reaction, oxidizing reaction, esterification reaction and cyclization reaction sequentially so as to obtain the firocoxib. Compared with a traditional technology, the novel method for synthesizing the firocoxib has the advantages that an aftertreatment process is simple without column chromatography separation, and the method is high in yield, low in cost and suitable for industrial production.
Owner:CHINA PHARM UNIV
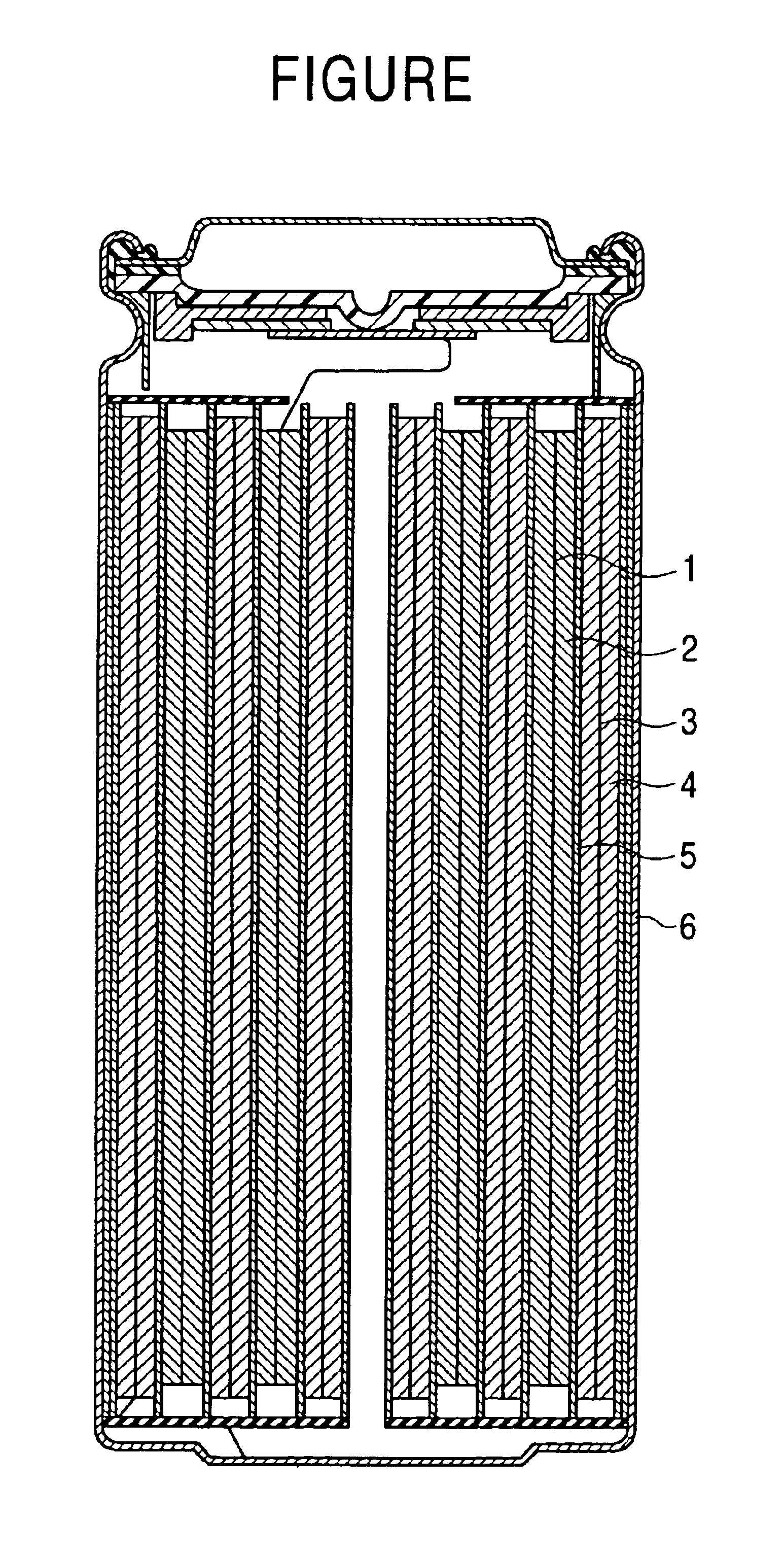
Nonaqueous electrolyte secondary battery
InactiveUS6913856B2Increase capacityImprove cycle lifeFinal product manufactureOrganic electrolyte cellsLithiumThiol
A nonaqueous electrolyte secondary battery includes a positive electrode having a positive electrode active material, a negative electrode containing a negative electrode active material capable of being doped / undoped with lithium, and a nonaqueous electrolyte. The nonaqueous electrolyte contains at least one of thiols, thiophenes, thioanisoles, thiazoles, thioacetates, aromatic sulfones, and the derivatives thereof. The capacity of the battery is not significantly degraded after cycling and its cycle life is significantly long.
Owner:SONY CORP
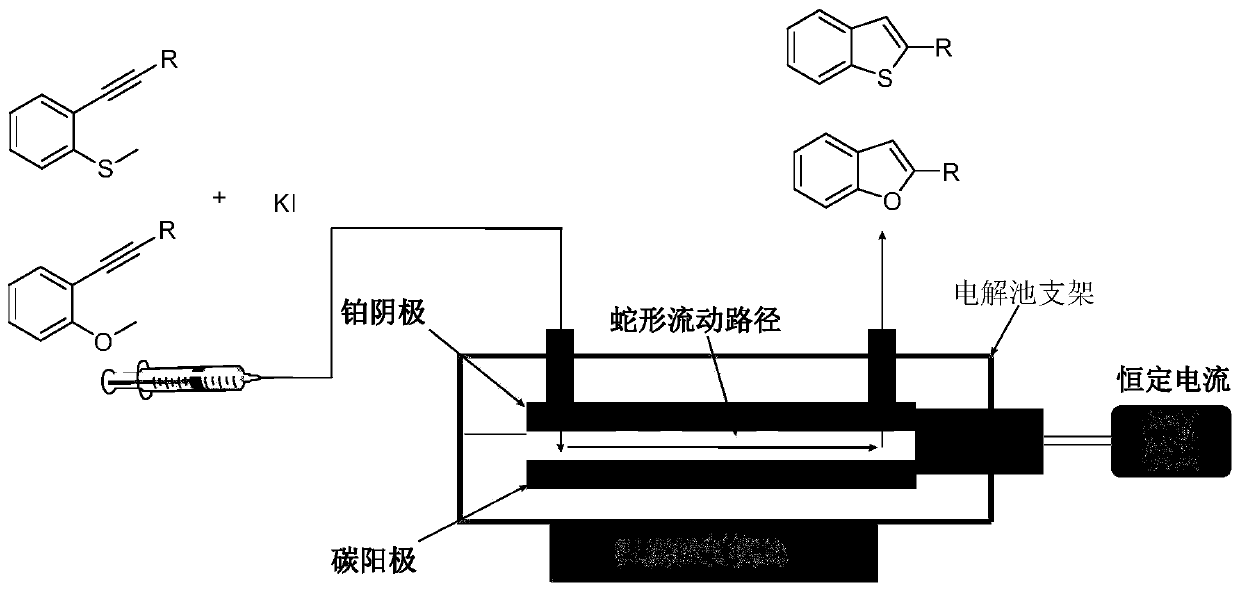
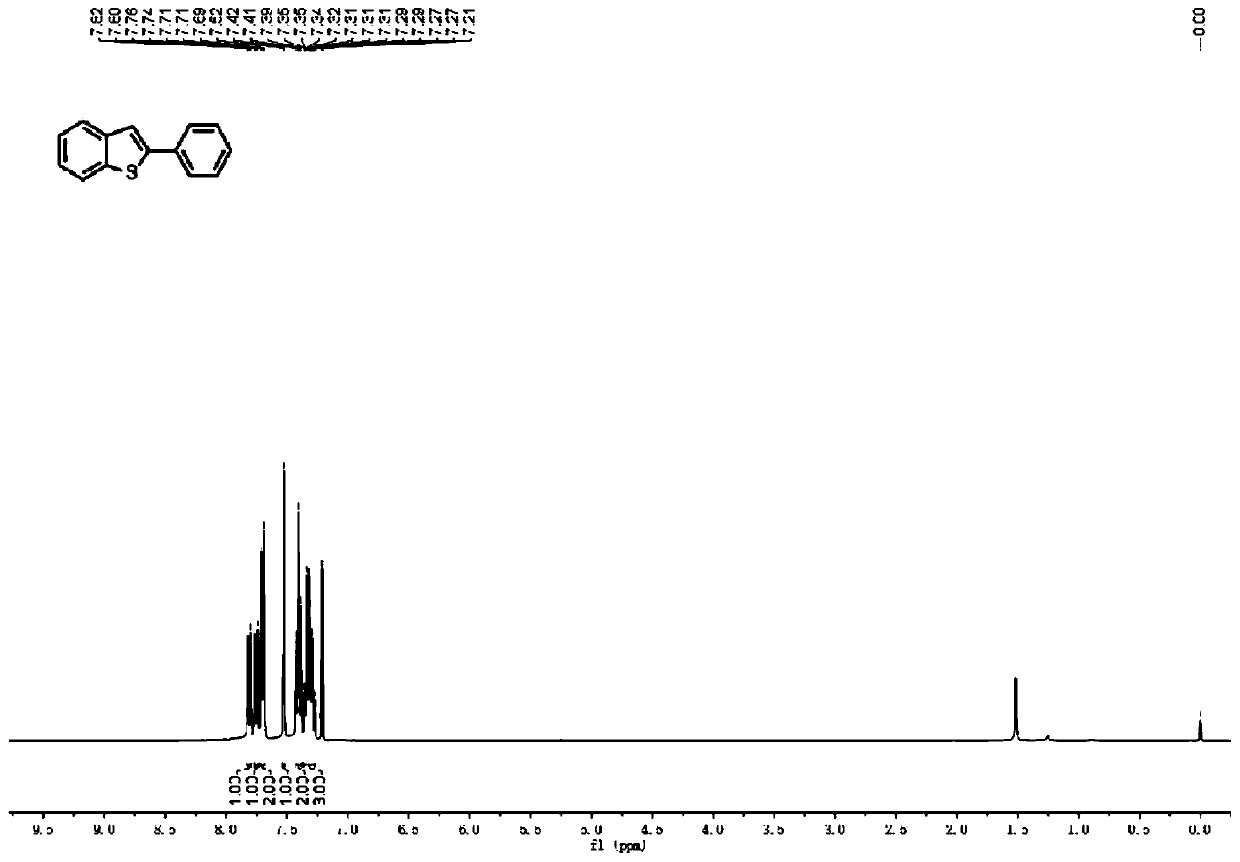
Method for continuously preparing 2-aryl-benzothiophene/furan compound by utilizing electrochemical micro-channel reaction device
ActiveCN110791775AAvoid disadvantagesHigh reaction yieldCellsElectrolytic organic productionFuranElectrochemistry
The invention discloses a method for continuously preparing a 2-aryl-benzothiophene / furan compound by utilizing an electrochemical micro-channel reaction device. The preparation method comprises the following steps: dissolving ethynyl thioanisole / phenylethynyl anisole and an iodine-containing electrolyte in water and acetonitrile to prepare a homogeneous solution, then introducing the prepared homogeneous solution into a feeding hole of an electrochemical micro-channel reaction device by utilizing an injection pump in single-strand sample introduction, and reacting under the action of a direct-current power supply to obtain the product 2-aryl-benzothiophene / furan compound; wherein the electrochemical micro-channel reaction device comprises an anode electrode, a cathode electrode, an electrolytic tank bracket, a reaction tank, the direct-current power supply and a temperature control module; wherein the reaction tank is positioned between the anode electrode and the cathode electrode, and a closed serpentine flow path is formed between the anode electrode and the cathode electrode; the anode electrode and the cathode electrode are arranged on the electrolytic tank bracket; one endsof the anode electrode and the cathode electrode are connected with each other and are connected with the direct-current power supply; and the temperature control module is embedded in the electrolytic tank bracket and is used for controlling the temperature of liquid in the reaction tank.
Owner:NANJING UNIV OF TECH
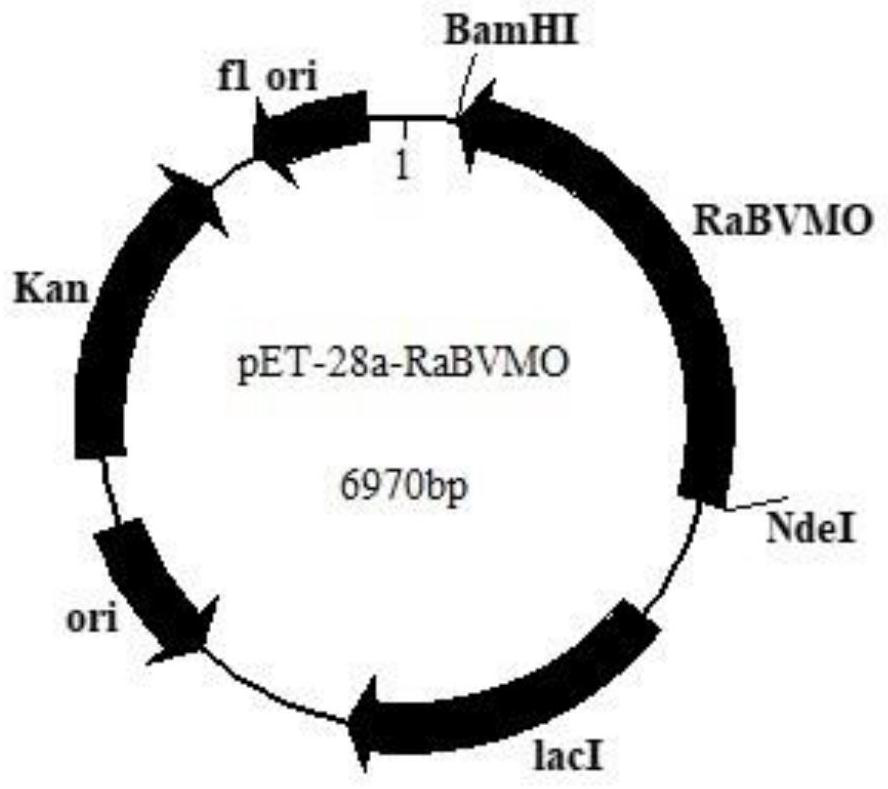
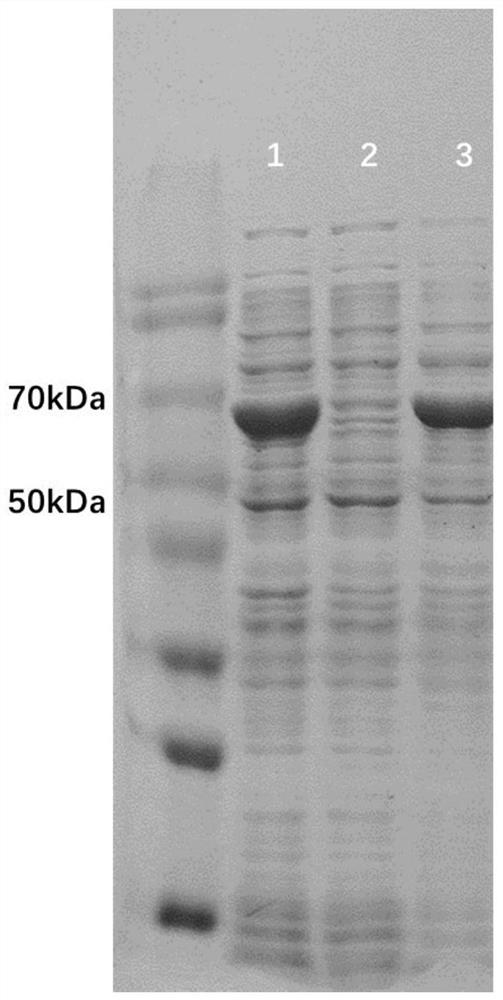
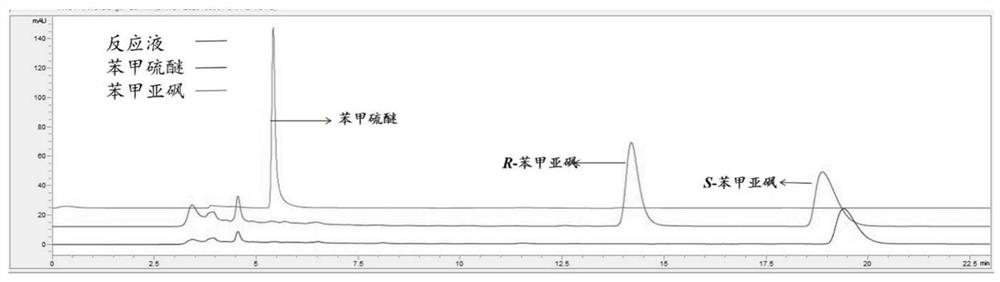
Baeyer-Villiger monooxygenase and application of Baeyer-Villiger monooxygenase
The invention discloses a Baeyer-Villiger monooxygenase and an application of the Baeyer Villiger monooxygenase, and belongs to the technical field of bioengineering. The invention provides another Baeyer-Villiger monooxygenase aiming at the problems of reported Baeyer-Villiger monooxygenase, such as low catalytic activity to substrate thioether, poor thermal stability, low ee value of a product and the like. The enzyme shows high stereoselectivity to linear ketone, cyclic ketone and thioether substrates, has high catalytic activity to cyclohexanone and thioanisole, and can be used for preparing high-purity products. Therefore, the problems of the existing monooxygenase can be directly and effectively solved. When diphenyl sulfide is used as a substrate to prepare S-benzosulfoxide, the conversion rate can reach 95% or above, and the yield can reach 99.5%. According to the present invention, a new thought and a new method are provided for industrial production of the optically active sulfoxide.
Owner:JIANGNAN UNIV
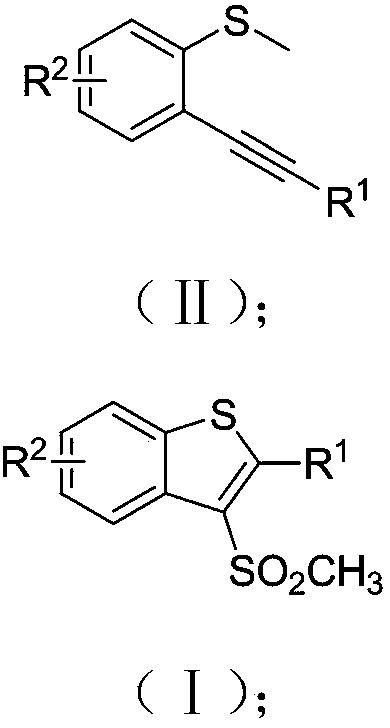
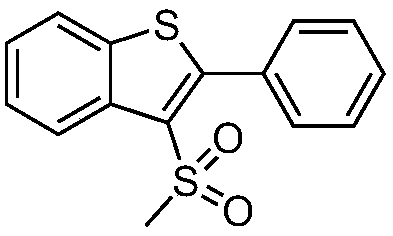
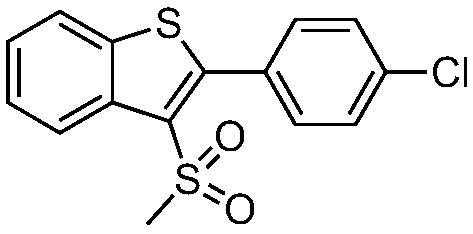
Preparation method of 3-methylsulfonyl-2-substituted benzothiophene compound
ActiveCN109336860ASolve the problem of using strong acid in the synthesisReduce usageOrganic chemistryChemical reactionFluorescent lamp
The invention discloses a preparation method of a 3-methylsulfonyl-2-substituted benzothiophene compound. The compound has a structure represented as the formula (I). The preparation method of the compound comprises following steps: sodium methanesulfonate is oxidized in an organic solvent by a photosensitizer in an excited state at the room temperature in an inert gas environment under the irradiation of a fluorescent lamp, methylsulfonyl free radicals are generated, the methylsulfonyl free radicals perform a addition ring closing reaction on o-alkynyl thioanisole, and the 3-methylsulfonyl-2-substituted benzothiophene compound with the structure represented as the formula (I) is obtained. According to the method, the conditions are mild, reaction raw materials are easily available, furthermore, reaction atom economy is good, organic raw materials in the reaction totally participate in the reaction, and high efficiency of the chemical reaction is shown.
Owner:JIAXING UNIV
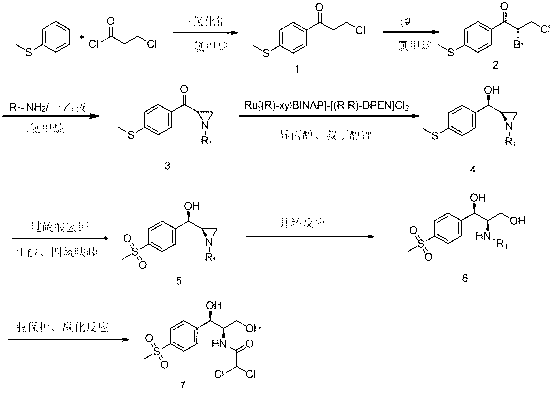
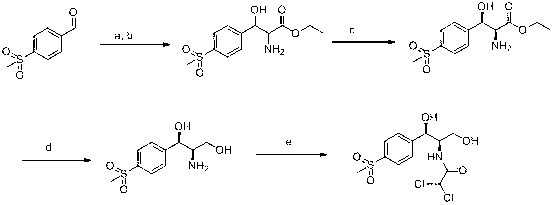
Chiral catalytic synthesis method of thiamphenicol
ActiveCN102863361AAvoid pollutionReduce pollutionOrganic chemistryOrganic compound preparationPtru catalystKetone
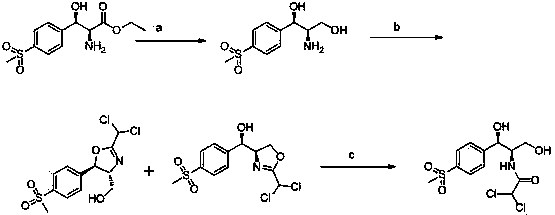
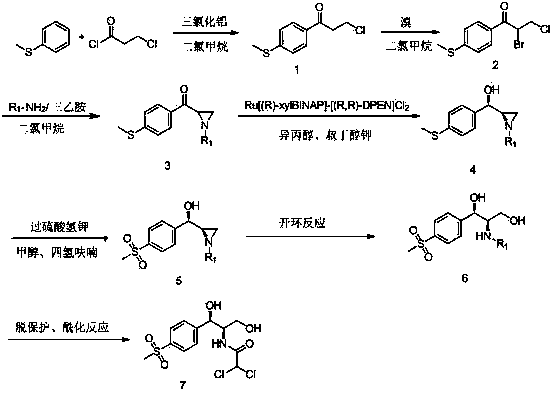
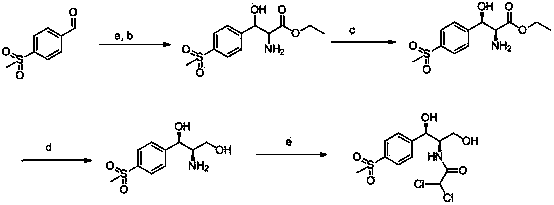
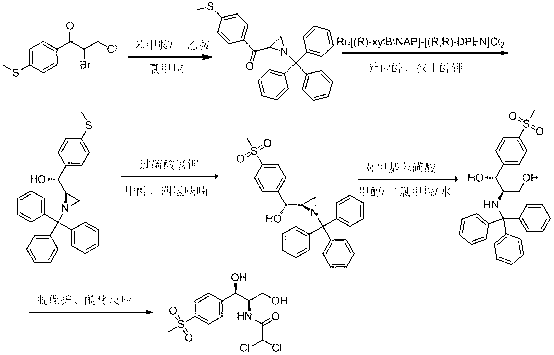
The invention relates to a chiral catalytic synthesis method of thiamphenicol which is a chloramphenicol broad-spectrum antibiotic. Thioanisole serves as an initating raw material, acylation and bromo are achieved, nitrogen iridine containing substituent groups is synthetized, and a qualified product which meets the requirement of drug administration is synthetized through chiral catalytic reduction, oxidizing reaction, acidification loop opening, deprotection and acylation reaction. The chiral catalytic reduction includes that under the action of a catalyst trans-RuC12[(R)-xylbinap][(S)-DPEN], [1-substituent group nitrogen iridine-2-group] [4-(methylthio group) phenyl group] ketone is subjected to hydrogenation reduction to obtain [1-substituent group nitrogen iridine-2-group] [4-(methylthio group) phenyl group] ketone with a high ee value and a high de value. D-methylsulfonylphenyl serine ethyl ester used in industrial production serves as a raw material to synthetize the thiamphenicol, the D-methylsulfonylphenyl serine ethyl ester is obtained through chemical chiral resolution by using a racemic compound, and the other half of the raw material is wasted. According to the unsymmetrical chiral catalytic dynamic reduction method, waste of the other half of the raw material is avoided, the utilization rate of a material is improved, and the production cost is reduced.
Owner:MASTEAM BIO TECH
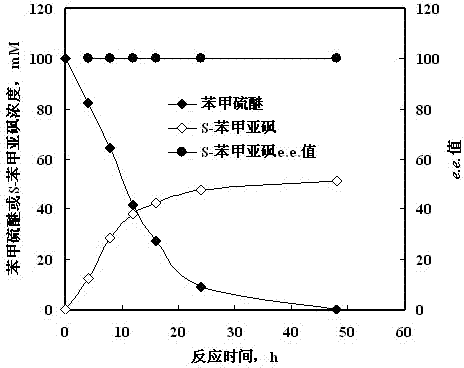
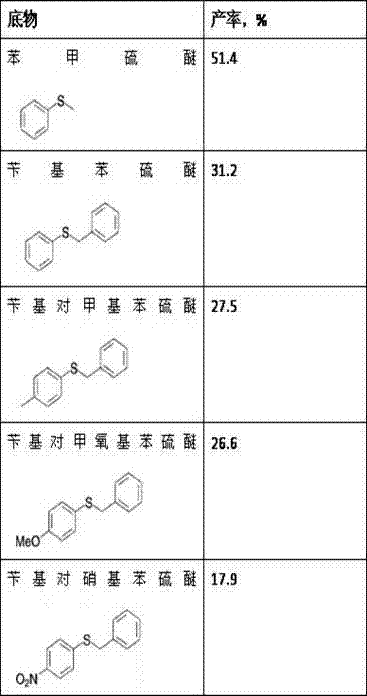
Method for synthesizing chiral sulfoxide from thioether under catalytic action of Rhodococcus
InactiveCN103114109ASimple process routeMild reaction conditionsMicroorganism based processesFermentationPtru catalystCatalytic effect
The invention discloses a method for synthesizing chiral sulfoxide from thioether under the catalytic action of Rhodococcus, belonging to the technical field of microorganisms. By using a resting cell of Rhodococcus sp. CCZU10-1 of which the collection number is CGMCC NO.4911 as a biocatalyst, asymmetric catalytic oxidation is performed on prochiral thioanisole and derivatives thereof, thus synthesizing chiral S-methyl phenyl sulfoxide (e.e.>99.9%) and derivatives thereof. The strain disclosed by the invention and the stereoselective biological oxidation method using the same have the characteristics of favorable catalytic effect, simple operating process route, mild reaction conditions, no pollution and the like.
Owner:临泉县嘉鸿装饰工程有限公司
A kind of solid phase synthesis atosiban method
ActiveCN101696236BReduce poisonReduce pollutionOxytocins/vasopressinsPeptide preparation methodsRink amide resinAtosiban
Owner:HAINAN ZHONGHE PHARM CO LTD
Preparation method of antitumor small peptides FpAT
InactiveCN103172704AInhibition formationSimple operation processPeptide preparation methodsSmall peptideEther
The invention relates to a preparation method of antitumor small peptides FpAT. A technical scheme is characterized in that the preparation method comprises the following steps: adopting Fmoc / tBu solid-phase peptide synthesis technique and adopting 2-chlorotrityl chloride resin (CTC Resin) as a carrier to prepare Fmoc-Arg(Pbf)-CTC Resin having a substitutivity of 0.4-0.7mmol / g; sequentially condensing Fmoc protected amino acids having a sequence from the C-terminal to the N-terminal of FpAT through the solid-phase peptide synthesis technique until the condensation of the N-terminated aspartic acid is completed to obtain an FpAT fully-protected peptide resin; obtaining crude FpAT peptides through using a TFA / thioanisole / EDT / anisole as a cracking reagent and settling with anhydrous ether; and adding water to the crude FpAT peptides for dissolving, adjusting the pH value to 3-4, purifying through using preparative reverse phase HPLC, collecting a target product having a qualified purify, and lyophilizing to obtain the finished antitumor small peptides FpAT. The preparation method has the advantages of simple operation, high production efficiency, low equipment requirement, total yield reaching above 35%, realization of the large-scale production, and good economic and social values and application prospect.
Owner:苏州中科天马肽工程中心有限公司
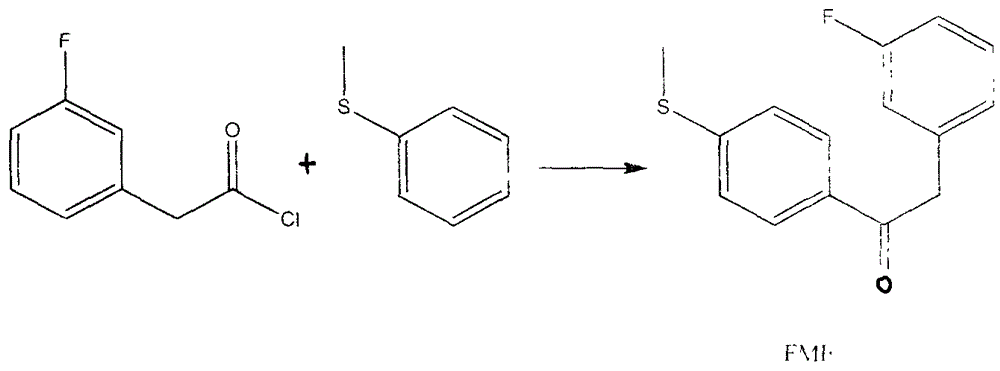
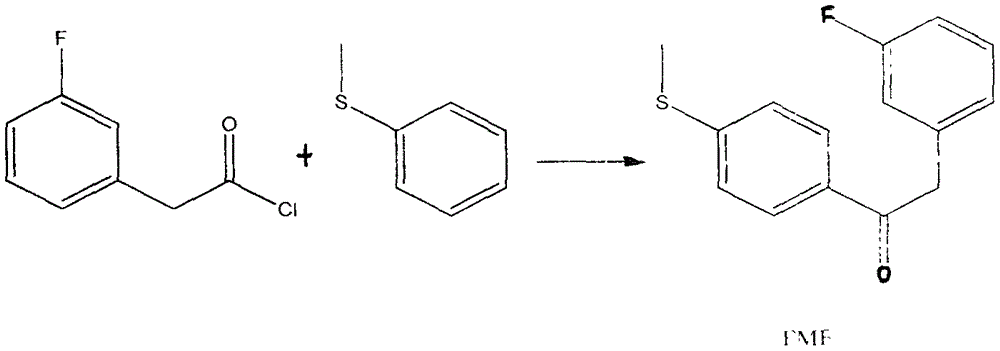
Preparation method for 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl) furan-3(2H)-ketone
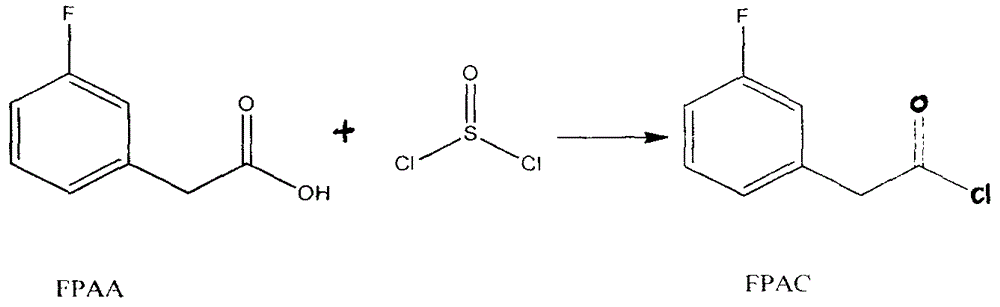
The invention discloses a preparation method for 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl)furan-3(2H)-ketone. The preparation method comprises the following steps: m-fluorobenzene acetylchloride is generated under the existence of fluorobenzene acetic acid and thionyl chloride; 2-bromine-2-methyl propionyl-bromine is reacted with trimethyl cyanogen silane to generate 3-bromine-3-methyl-2-oxo butyl cyanide; m-fluorobenzene acetylchloride is reacted with thioanisole to generate 2-(3-fluorine phenyl)-1-(4-(methylmercapto-)phenyl) acetone under the existence of aluminium trichloride and dichloromethane; the 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl)furan-3(2H)-ketone is generated by the 2-(3-fluorine phenyl)-1-(4-( methylmercapto-)phenyl)acetone and the 3-bromine-3-methyl-2-oxo butyl cyanide; a best product is obtained through separation, crystallization, filtering and drying.
Owner:NANTONG HUAFENG CHEM
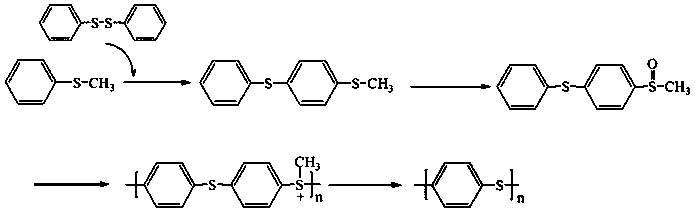
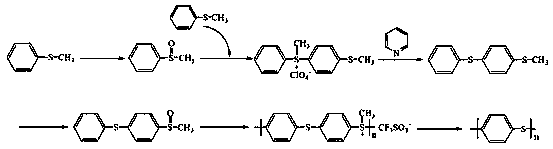
Synthetic method of high-molecular-weight linear polyphenylene sulfide
The invention discloses a synthetic method of high-molecular-weight linear polyphenylene sulfide. The synthesis method comprises the following steps: synthesizing 4-(thiophenyl)thioanisole by taking polyphenylene sulfide and diphenyl disulfide as raw materials, further synthesizing 4-(sulfophenyl)methyl phenyl sulfoxide through oxidation by using hydrogen peroxide, then carrying out a cationic polymerization reaction on the 4-(sulfophenyl)methyl phenyl sulfoxide under the action of trifluoromethanesulfonic acid to synthesize a polymethyl[4-(thiophenyl)-phenyl]sulfonium trifluoromethanesulfonate; and finally, removing methyl by a nucleophilic reagent pyridine to obtain the polyphenylene sulfide. The polyphenylene sulfide synthesized by the method is high in purity and high in molecular weight and is in linear, the reaction system is mild in condition, operation of the whole process is relatively stable, and the method has an industrial application prospect.
Owner:TAIYUAN UNIV OF TECH
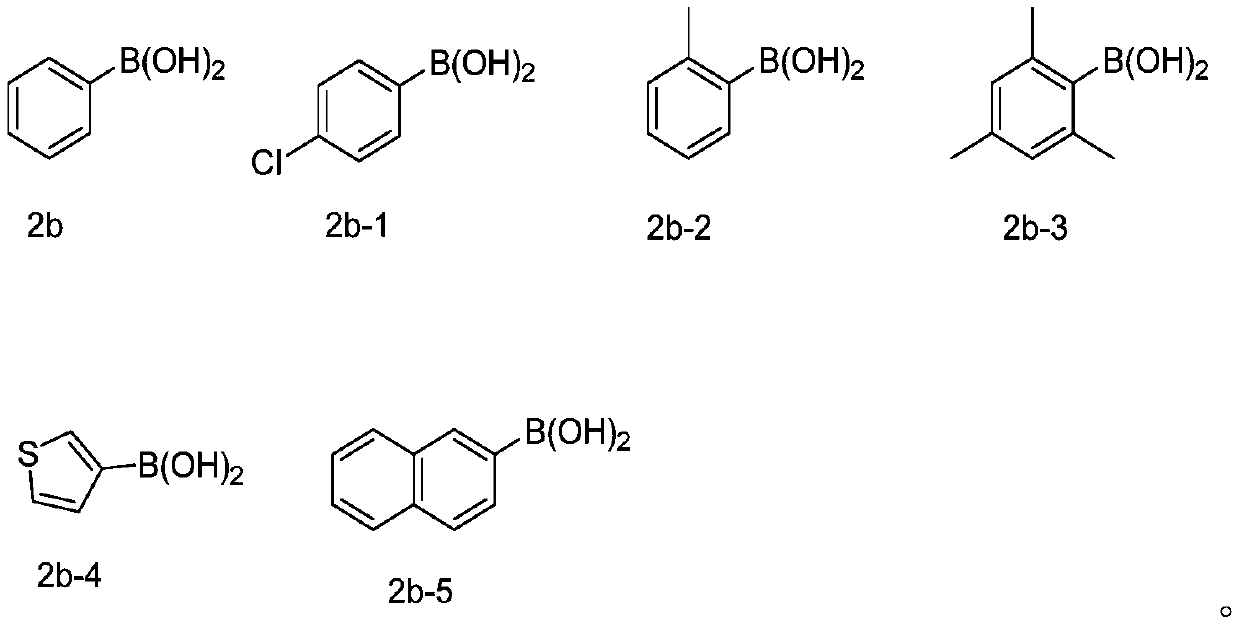
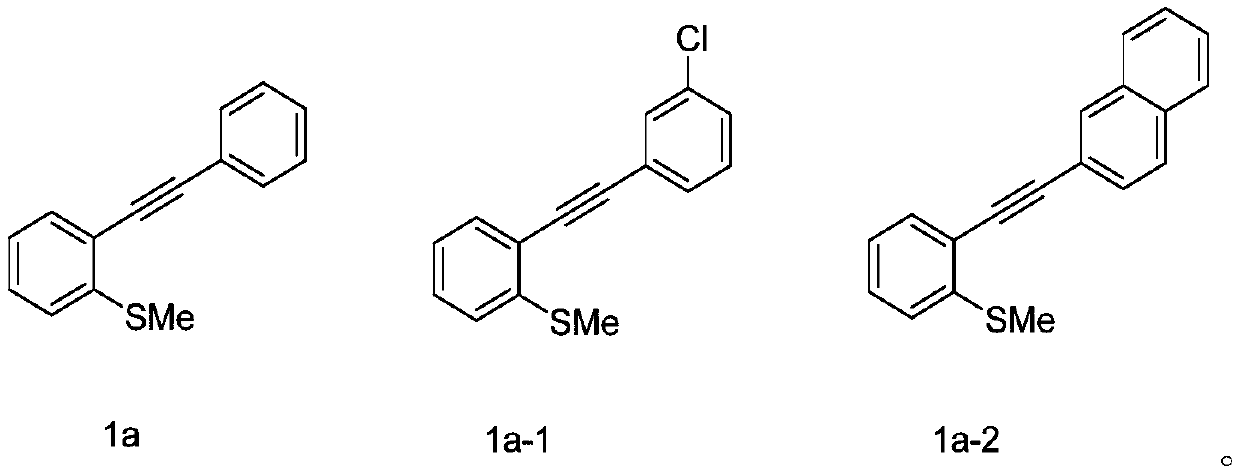
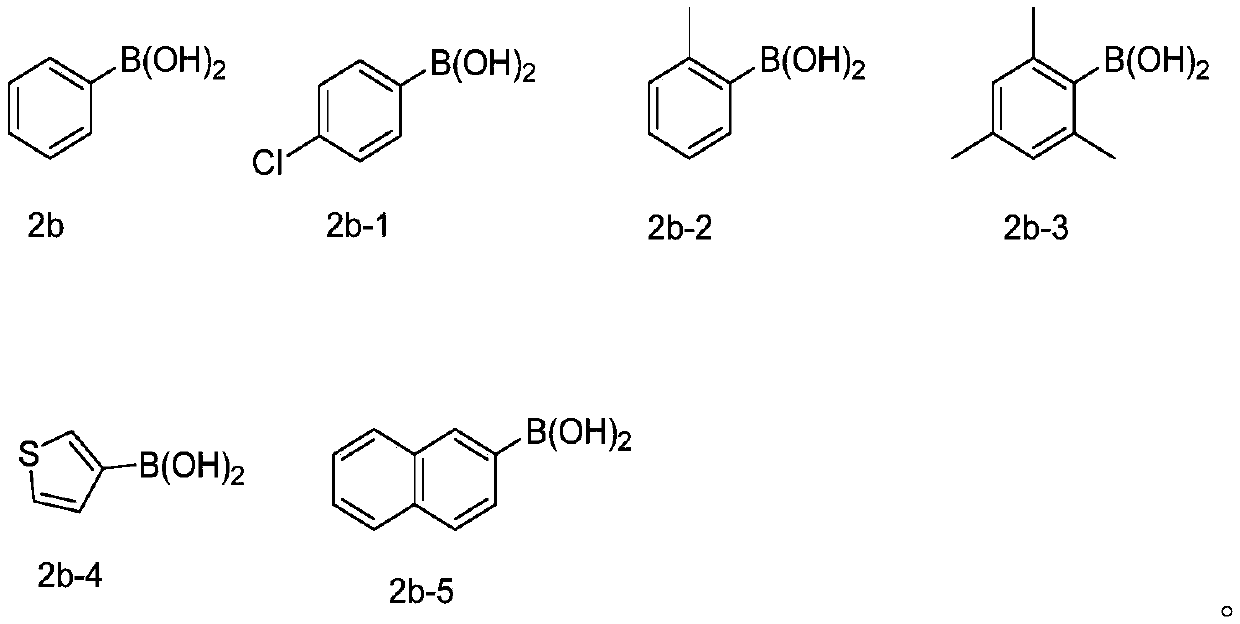
Method for synthesizing selenized benzothiophene compound
The invention relates to a method for synthesizing a selenized benzothiophene compound. According to the method, in an organic solvent, under the condition of oxygen, arylboronic acid and selenium powder generate a benzene selenium-based free radical in situ under the action of a silver catalyst, and the benzene selenium-based free radical and alkynyl thioanisole are further subjected to a free radical tandem cyclization reaction to obtain the selenized benzothiophene compound. The present invention further discloses a selenized benzothiophene compound prepared by the method. According to the present invention, the method has characteristics of easily available alkynyl thioanisole as the raw material, inexpensive and easily available elemental selenium, wide reaction substrate range, good functional group tolerance, simple reaction condition and high yield and high purity of product, develops the new synthesis rote and method for selenized benzothiophene compounds, and further has good application potential and research value.
Owner:WENZHOU UNIVERSITY
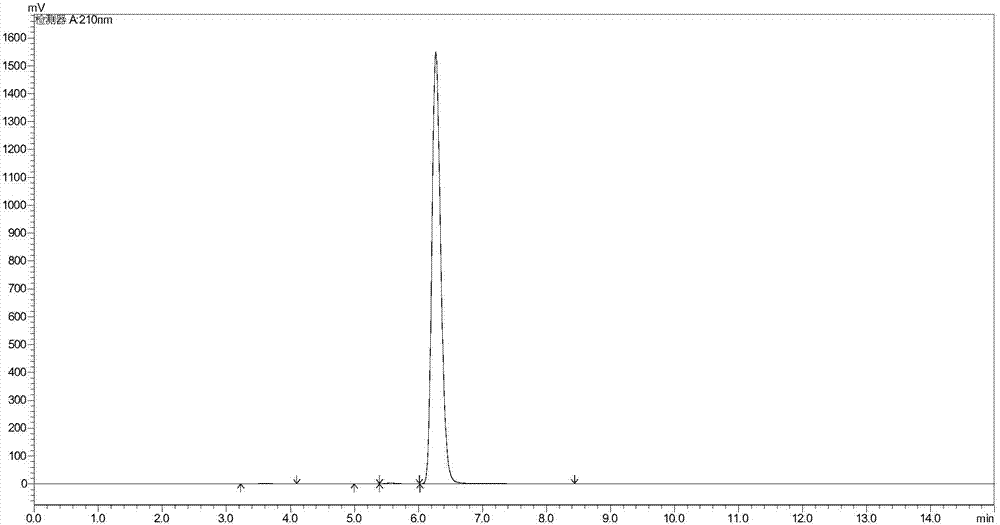
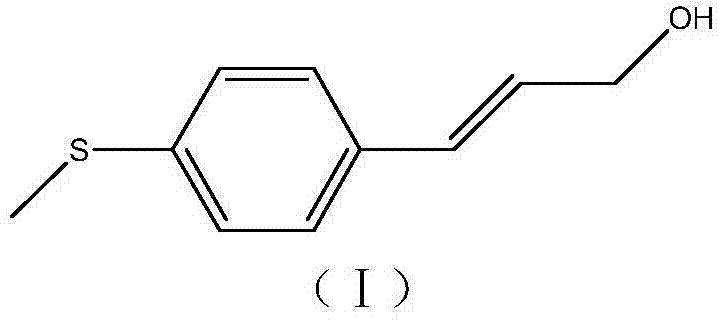
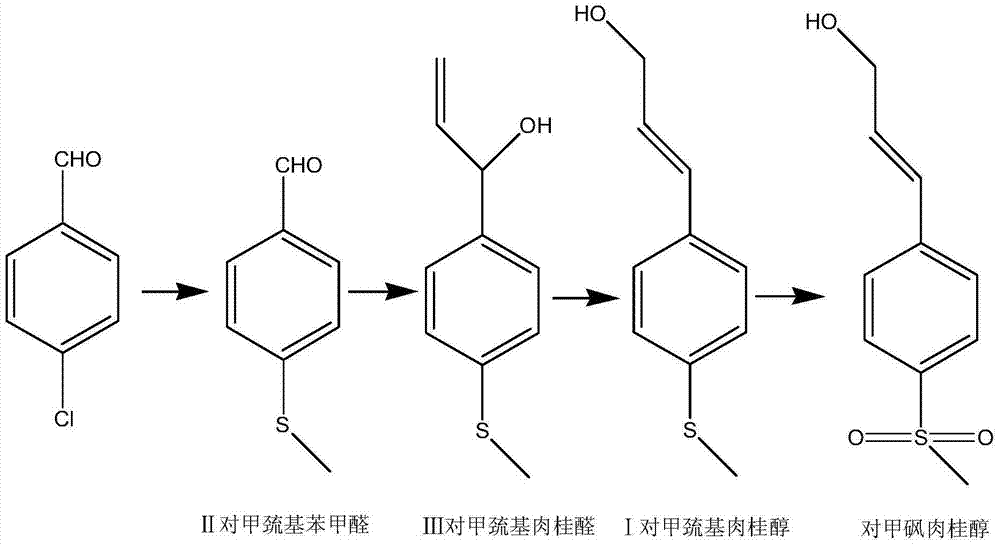
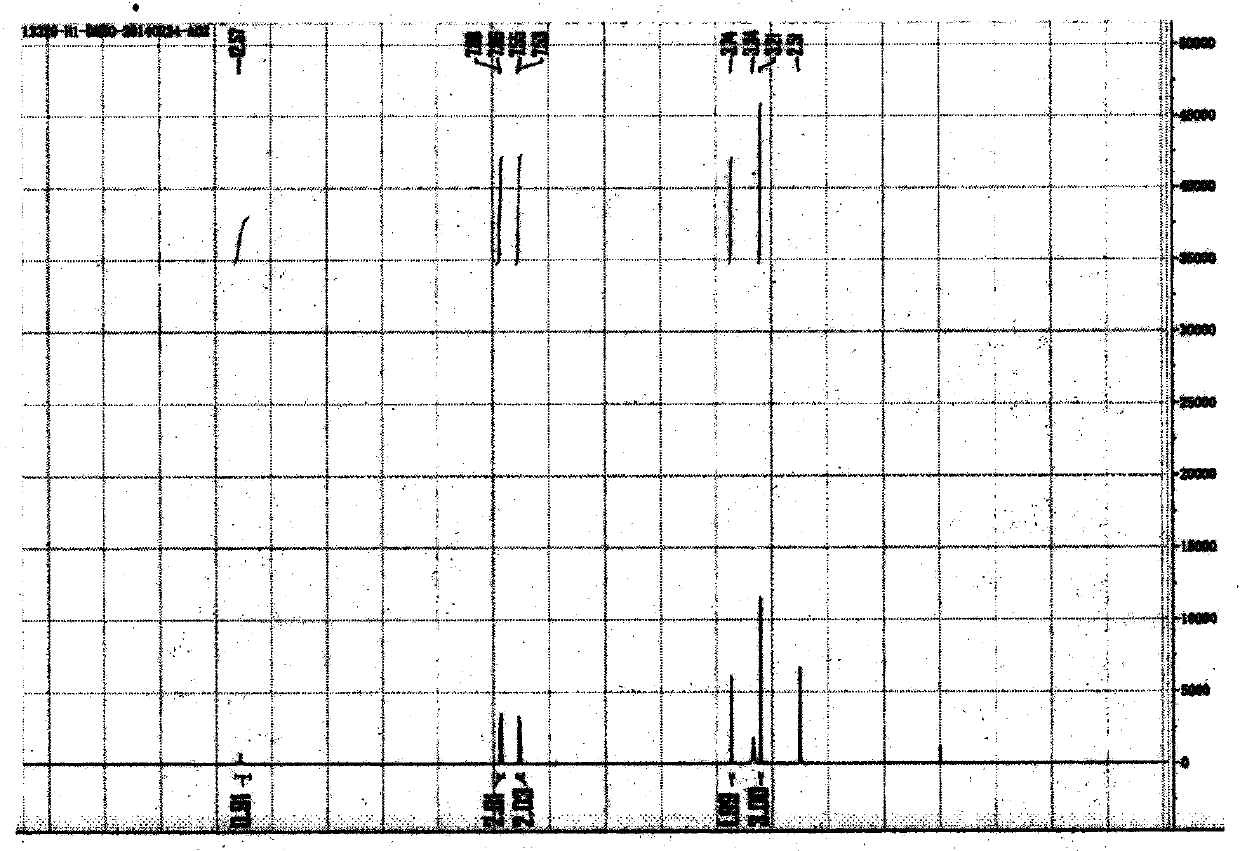
Simple synthetic method of trans-p-methylthiocinnamyl alcohol used for industrial production
ActiveCN103787936AReduce usageEasy to operateOrganic compound preparationSulfide preparationAlcoholFormylation reaction
The invention relates to a simple synthetic method of trans-p-methylthiocinnamyl alcohol used for industrial production. The method comprises the following steps: performing a formylation reaction on thioanisole and a formylation reagent to prepare p-methylthiobenzaldehyde; condensing the p-methylthiobenzaldehyde and acetaldehyde in the presence of a catalyst to prepare trans-p-methylthiocinnamyl aldehyde; reducing the trans-p-methylthiocinnamyl aldehyde to obtain the trans-p-methylthiocinnamyl alcohol. The method has the advantages of readily-available raw materials, easiness in operation of reaction conditions, high reaction selectivity, low product cost, safety, environmental friendliness and suitability for industrial production.
Owner:XINFA PHARMA
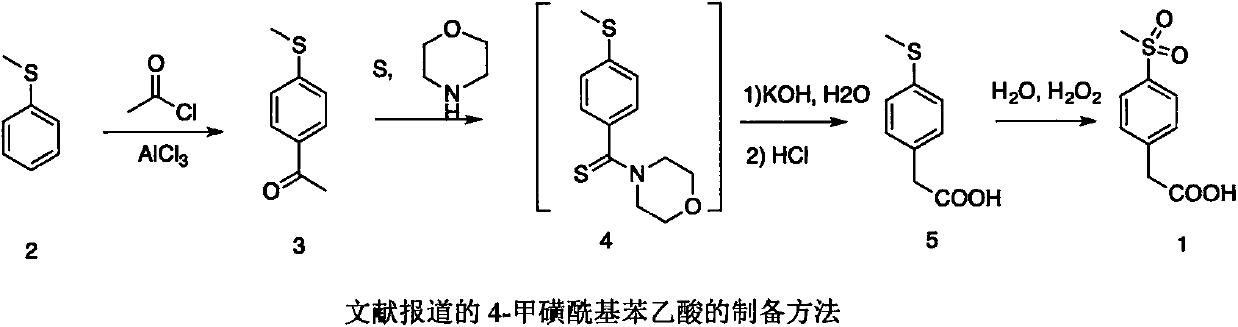
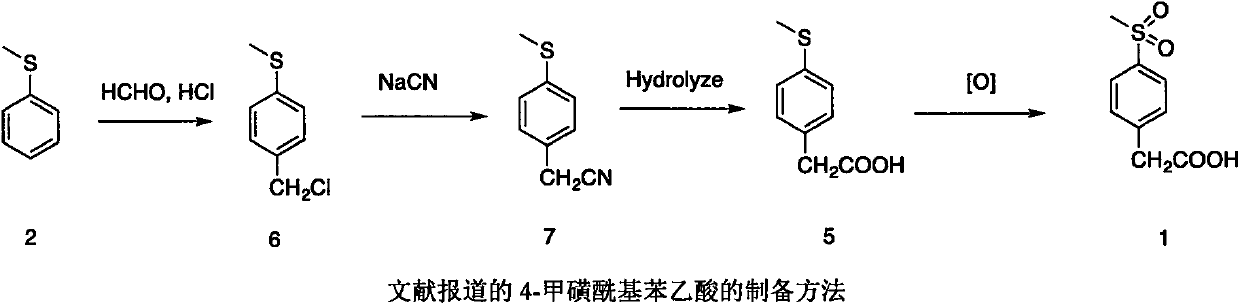
Preparation method of 4-methylsulphonylphenylacetic acid
InactiveCN107556223AReduce harmAvoid damageOrganic compound preparationSulfide preparationBenzeneBenzaldehyde
The invention belongs to the field of organic synthesis and provides a preparation method of 4-methylsulphonylphenylacetic acid. According to the preparation method, 4-methylthio benzaldehyde is prepared from thioanisole as a starting material through a Vilsmeier reaction and subjected to a Darzen reaction; a product is subjected to hydrolysis and decarboxylation, and 4-methylthio phenylacetaldehyde is obtained; 4-methylsulphonylphenylacetic acid is prepared from 4-methylthio phenylacetaldehyde through oxidization.
Owner:孙家强
Cytochrome P450 119 and mutant and purpose thereof
ActiveCN110241095AHigh catalytic efficiencyImprove catalytic performanceOxidoreductasesFermentationCytochrome P450Wild type
The invention provides a cytochrome P450 119 and a mutant and purpose thereof. The mutant of the cytochrome P450 119 provided by the invention is T213G+F153G. The cytochrome P450 119 and the mutant thereof provided by the invention can efficiently catalyze the oxidization of thioether type substrates into sulfoxide and sulphone at normal temperature; and besides, n-caprylic acid is added to a reaction system, the catalysis capacity of the mutant for performing enzyme catalysis on an oxidation reaction of thioanisole is improved by 2.38 times than that of a wild type mutant. The invention also finds that the mutation enzyme can increase the chemical selectivity of a reaction, namely increasing the generation of sulfoxide and reducing the generation of the sulphone. The invention provides the new purpose of the cytochrome P450 119, and also provides the new purpose of the mutant of the cytochrome P450 119 to improvement of the catalysis capacity of the thioether type oxidation reaction and improvement of the chemical selectivity of the reaction, excellent economic benefits can be obtained, and the cytochrome P450 119 has great application prospects.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Method for pyrolysis of thymosin beta-4
ActiveCN103724422AEfficient removalPrevent oxidationThymosin peptidesPeptide preparation methodsSolventPhenol
The invention discloses a method for pyrolysis of thymosin beta-4. The method comprises the step of carrying out pyrolysis on thymosin beta-4 peptide resin to obtain crude peptide, wherein a mixed solvent of trifluoroacetic acid, thioanisole, dithioglycol, phenol, water, dimethyl sulfide and ammonium iodide is used as a pyrolysis reagent in the pyrolysis process. According to the method for pyrolysis of thymosin beta-4, the purity of crude peptide is greatly improved, meanwhile the total yield is greatly increased, the production cost is reduced, and the method is simple in process and convenient for industrial production.
Owner:哈尔滨吉象隆生物技术有限公司
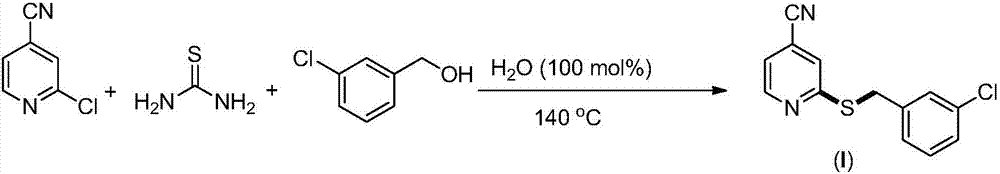
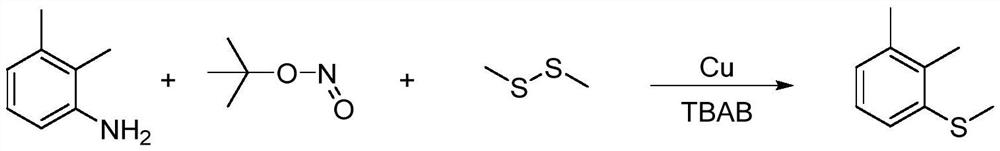
Synthesis method of 2-(4-cyano) pyridyl (3-cyano) thioanisole
The invention provides a synthesis method of 2-(4-cyano) pyridyl (3-cyano) thioanisole. The invention also provides a synthesis method of 4-(2-formamido) pyridyl (3-bromo) thioanisole. According to the technical scheme, 2-chloro-4-cyanopyridine / 4-chloropyridine-2-formamide, micromolecular sulfur compounds and 3-chloro / bromobenzyl alcohol are used for directly synthesizing 2-(4-cyano) pyridyl (3-cyano) thioanisole (I) and 4-(2-formamido) pyridyl (3-bromo) thioanisole (II) under the condition of no additional catalysts. The method has the advantages that the reaction conditions are simple; inert gas protection is not needed; solvents are not needed; the operation is easy. By the method, the 20-time amplification production can be conveniently realized; the product gram grade preparation is performed, so that certain study and industrial application prospects are realized.
Owner:WENZHOU UNIVERSITY
Method for preparing firocoxib
The invention relates to the technical field of medicines and particularly relates to a method for preparing firocoxib. Raw materials used in the preparation method are conventional reagents in the market, thioanisole which is easily volatized and has strong odor, isobutyryl chloride with high corrosion and liquid bromine with high toxicity, easy volatilization and strong corrosion are abandoned.The method is suitable for industrial production.
Owner:SHANDONG LUKANG SHELILE PHARMA
Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole
The invention discloses a novel synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole and belongs to the technical field of organic chemical synthesis. The method comprises the following steps: carrying out reaction on thioanisole and acetyl chloride to generate 4-methylthioacetophenone, carrying out reaction on 4-methylthioacetophenone and methyl benzoate to generate 1-(4-methylthiophenyl)-3-phenyl-1,3-dione, carrying out reaction on 1-(4-methylthiophenyl)-3-phenyl-1,3-dione and tert-butyl nitrite to generate 1-(4-methylthiophenyl)-2-phenyl-1,2-dione, carrying out reaction on 1-(4-methylthiophenyl)-2-phenyl-1,2-dione and trifluoroacetaldehyde methyl hemiacetal to generate 4-[4-(methylthio)phenyl]-5-phenyl-2-(thrifluoromethyl)-1H-imidazole, and finally oxidizing to generate 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole. According to the invention, the synthesis method is adopted, the utilization of cyanide and the damage of KMnO4 to carbon-carbon double bond are avoided, so that the method has industrial application values.
Owner:ZHEJIANG UNIV
Brevibacillus borstelensis strain having capability of degrading thioanisole and application thereof
ActiveCN103013877BHas the ability to degrade sulfide anisoleEfficient degradationBacteriaWater contaminantsMicroorganismBrevibacillus borstelensis
The invention belongs to the technical field of microorganisms, and discloses a Brevibacillus borstelensis strain having a capability of degrading thioanisole and application thereof. The Brevibacillus borstelensis strain having a capability of degrading thioanisole is named as Brevibacillus borstelensis GIGAN1 and was collected at China Center for Type Culture Collection in Wuhan University in Wuhan, Hubei province, China on November 8, 2012; and the collection number is CCTCC NO:M 2012451. The capability of the strain, of degrading thioanisole in the environment, can be up to 96.2%; and the strain can be used for degrading thioanisole in the environment, thereby achieving the purpose of environment renovation.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
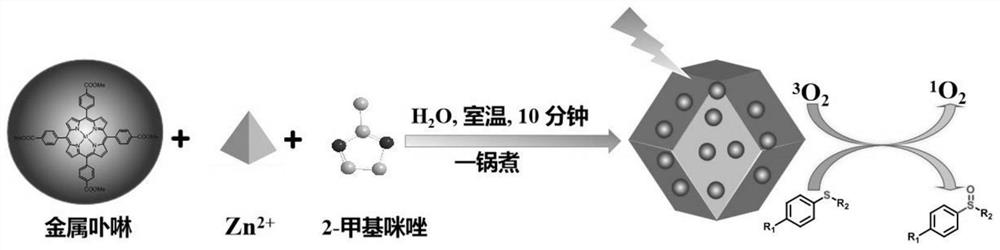
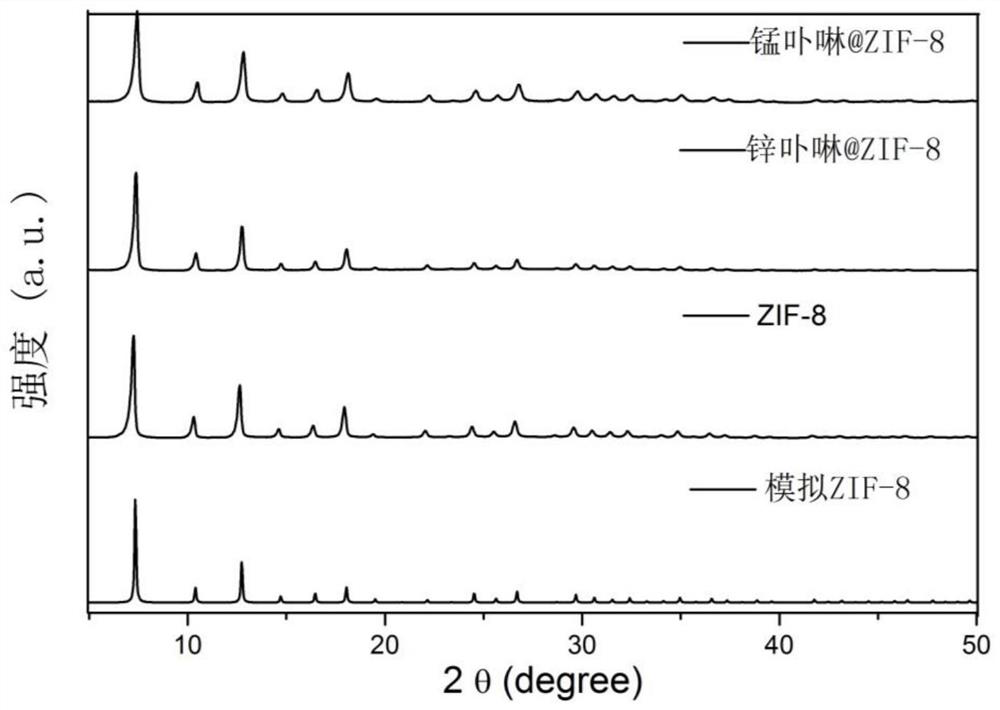
Metalloporphyrin@metal organic framework material heterogeneous photosensitizer as well as preparation method and application thereof
InactiveCN112844480AImprove stabilityLarge specific surface areaOrganic compound preparationQuinone preparation by oxidationPtru catalystCatalytic oxidation
The invention discloses a heterogeneous photosensitizer based on a metal organic framework material as a carrier as well as a preparation method and application of the heterogeneous photosensitizer, and belongs to the technical field of preparation of environment-friendly functional composite materials. The heterogeneous photosensitizer disclosed by the invention is a metalloporphyrin@ZIF-8 composite material, wherein a metalloporphyrin compound which has strong absorption in a visible light region and can efficiently generate singlet oxygen (1O2) is loaded into a ZIF-8 metal organic framework material pore channel through a simple "one-pot" self-assembly method. Finally, the prepared heterogeneous photosensitizer is used for catalytic oxidation of thioanisole, then the catalyst can be recycled through simple centrifugation, washing and drying, and the heterogeneous photosensitizer is circularly used for catalytic oxidation of thioanisole and degradation of bisphenol A. The preparation method is simple, easy to operate, environmentally friendly, low in cost, good in repeatability and short in consumed time; the heterogeneous photosensitizer has strong absorption in a visible region, and 1O2 can be efficiently generated, so that thioether compounds can be selectively oxidized into sulfoxide under the condition of stirring at room temperature, the conversion rate is high, and the reusability is good.
Owner:PINGDINGSHAN UNIVERSITY
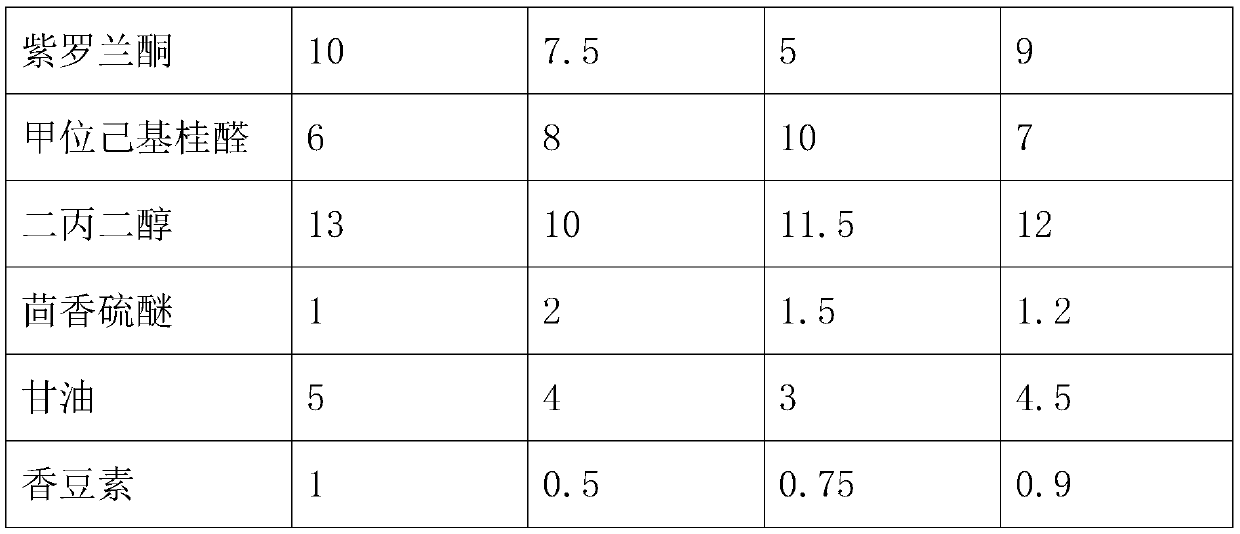
Lasting fragrance type essence and preparation method thereof
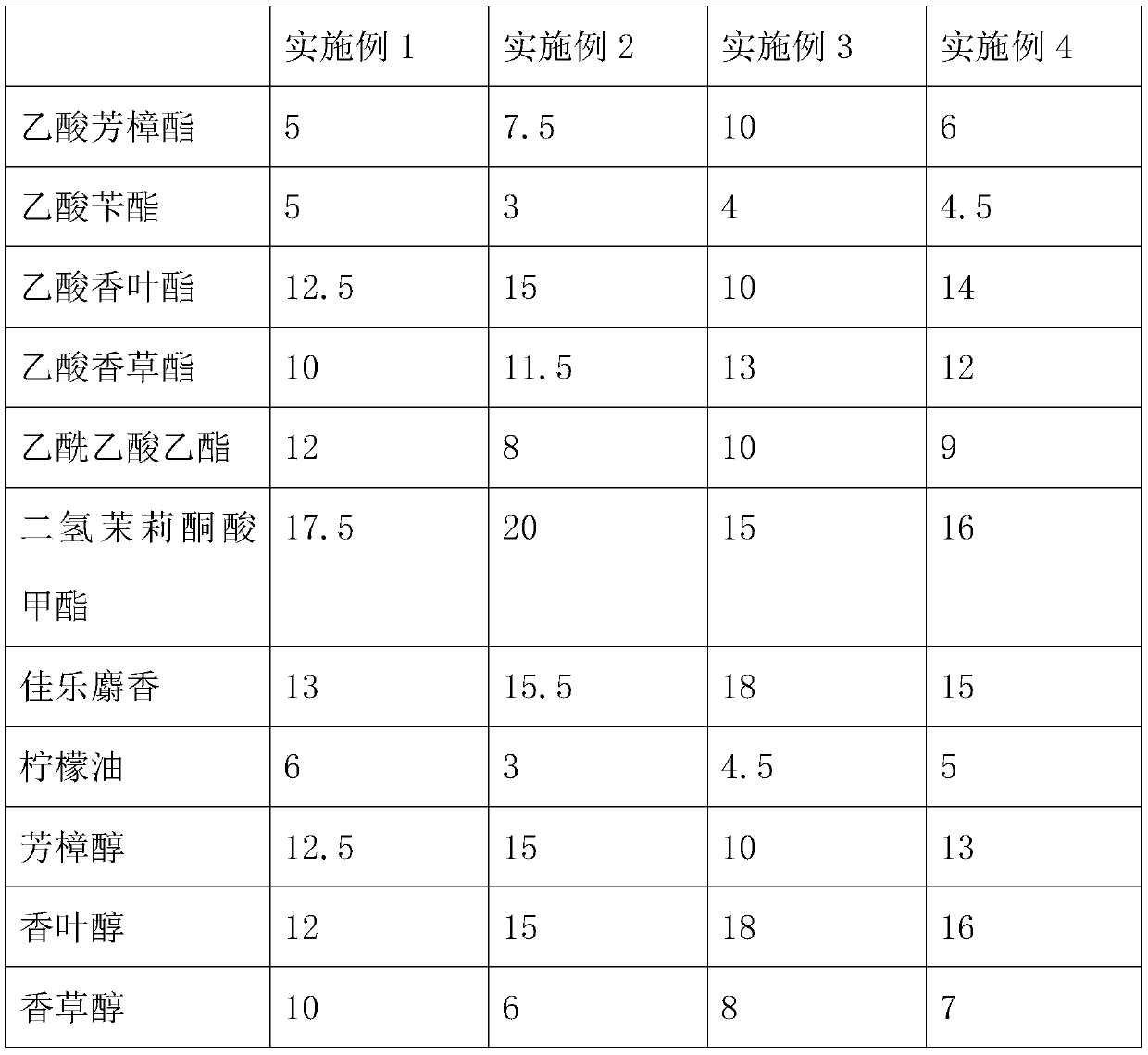
PendingCN111575114AImprove fragrance retentionImprove stabilityEssential-oils/perfumesBiotechnologyGalaxolide
The invention relates to the technical field of essences, and relates to a lasting fragrance type essence, which comprises the following components by mass: 5-10 parts of linalyl acetate; 3-5 parts ofbenzyl acetate; 10 to 15 parts of geranyl acetate; 10 to 13 parts of vanillate acetate; 8 to 12 parts of ethyl acetoacetate; 15 to 20 parts of methyl dihydrojasmonate; 13 to 18 parts of galaxolide; 3-6 parts of lemon oil; 10 to 15 parts of linalool; 12 to 18 parts of geraniol; 6 to 10 parts of vanillyl alcohol; 5 to 10 parts of ionone; 6 to 10 parts of alpha-hexylcinnamaldehyde; 10 to 13 parts ofdipropylene glycol; 1 to 2 parts of thioanisole; 3-5 parts of glycerol; and 0.5-1 part of coumarin. The invention has the effect of prolonging the fragrance retention time of the essence.
Owner:广东香龙香料有限公司
Method for preparing 2, 3-dimethyl thioanisole
ActiveCN112694427AHigh purityMild reaction conditionsSulfide preparationDimethylaniline N-oxidePtru catalyst
Owner:JIANGXI TIANYU CHEM CO LTD
A method for splitting thymosin β4
ActiveCN103724422BEfficient removalPrevent oxidationThymosin peptidesPeptide preparation methodsSolventPhenol
Owner:哈尔滨吉象隆生物技术有限公司
Synthesis method of etoricoxib intermediate 4-methylsulfonyl acetophenone
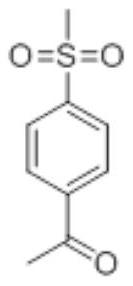
InactiveCN113292461ASolve instabilityLow reaction temperatureOrganic compound preparationSulfide preparationSodium bicarbonateAcetophenone
The invention discloses a synthetic method of an etoricoxib intermediate 4-methylsulfonyl acetophenone, wherein the comprises the following steps: step 1, adding anhydrous aluminum trichloride and acetyl chloride in a dichloromethane system to obtain a reaction solution; step 2, dropwise adding the reaction solution into thioanisole into the reaction solution; step 3, standing for liquid separation, extracting a water phase by using dichloromethane, combining organic phases, and washing the combined organic phases by using a 10% sodium bicarbonate aqueous solution; step 4, evaporating the washed organic phase to dryness, adding n-heptane, crystallizing, washing, and drying to obtain acetylphenylmethyl sulfide; and step 5, adding the acetylphenylmethyl sulfide obtained in the step 4 into an acetone system, and further reacting to obtain 4-methylsulfonyl acetophenone; or adding the acetylphenylmethyl sulfide obtained in the step 4 into an acetic acid system, and stirring for dissolving to obtain a reaction mixture; and further reacting to obtain the 4-methylsulfonyl acetophenone. The energy consumption is reduced, the reaction is more stable, and the benefit is high.
Owner:SCINOPHARM CHANGSHU PHARMA
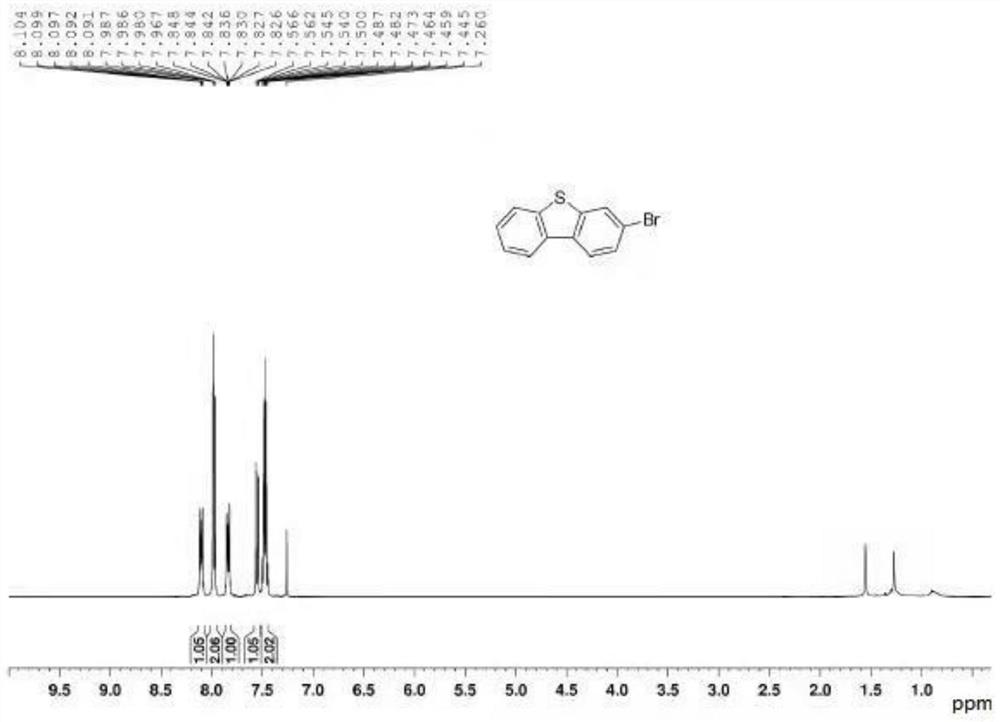
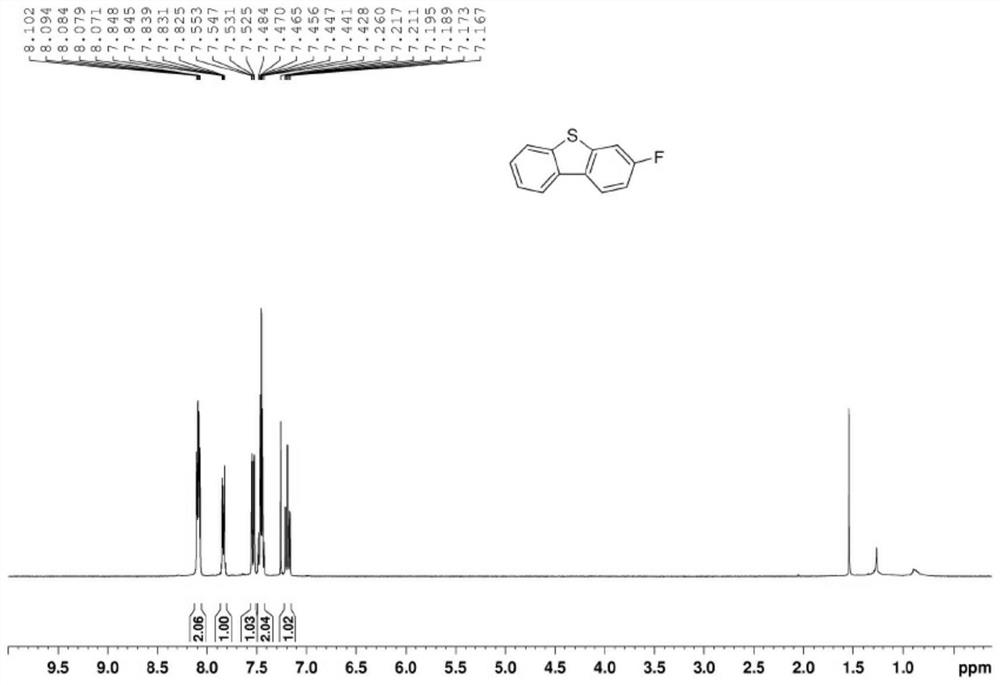
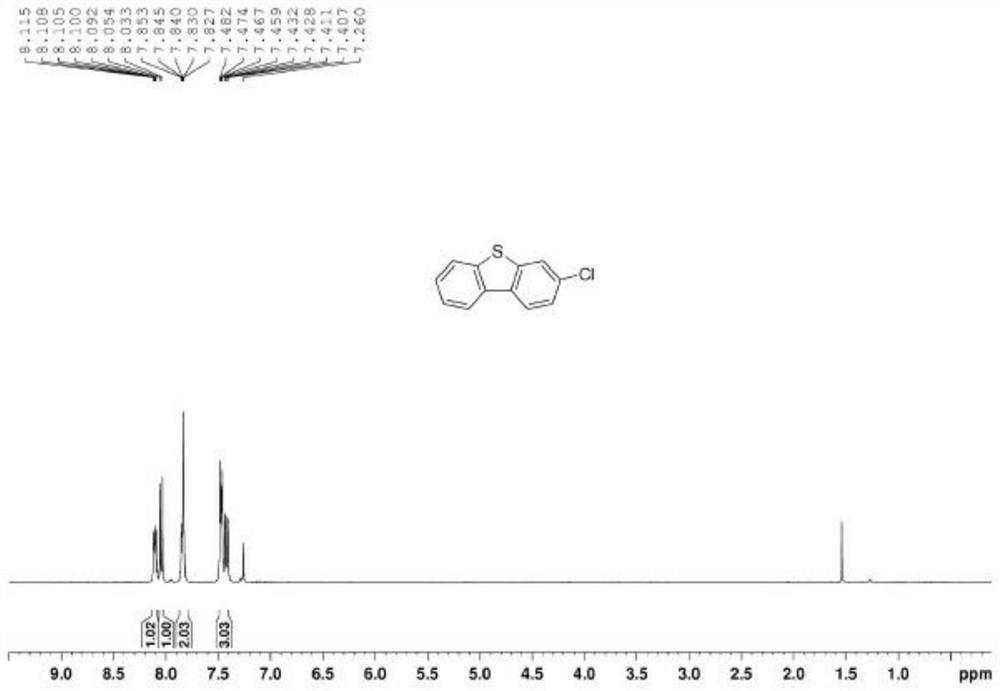
3-substituted dibenzothiophene and synthesis method thereof
The invention discloses 3-substituted dibenzothiophene and a synthesis method thereof. The synthesis method comprises the steps of 1, taking o-iodoanethiol sulfide and 4-substituted phenylboronic acid as starting raw materials, and performing a coupling reaction to obtain an intermediate a, wherein the molar ratio of the o-iodoanethiol sulfide to the 4-substituted phenylboronic acid is 1: (1-1.4); 2, enabling the intermediate a, p-toluenesulfonic acid and hydrogen peroxide to be subjected to an oxidation reaction, and obtaining an intermediate b, wherein the molar ratio of the intermediate a to the p-toluenesulfonic acid to the hydrogen peroxide is 1: 0.5: (1-1.2); and 3, enabling the intermediate b and an Eaton reagent to be subjected to a ring closing reaction, and obtaining the 3-substituted dibenzothiophene, wherein the molar ratio of the intermediate b to the Eaton reagent is 1: (1-1.5).
Owner:西安欧得光电材料有限公司
Solid Phase Fragment Synthesis of Thymosin α1
ActiveCN104558149BLow purityExtend consumption timeHormone peptidesPeptide preparation methodsFreeze-dryingSynthesis methods
The invention discloses a synthesis technology of thymosin alpha1, which comprises the following steps: synthesizing multiple segments according to the difficult-synthesis sequence of thymosin alpha1, wherein a solid-phase segment condensation method is adopted for the area of difficult sequences so as to improve the synthesis quality and shorten the synthesis time, and a conventional step-by-step condensation method is adopted for the area of easy-synthesis sequences; obtaining all-protected thymosin alpha1 peptide resin on Wang Resin; cracking the peptide resin to obtain crude peptide by taking TFA / m-cresol or TFA / thioanisole / EDT / anisole as a cracking reagent; dissolving the crude peptide and filtering; and performing preparative reversed phase HPLC purification, desalting, rotary evaporation and freeze drying to obtain a finished product of thymosin alpha1 of which the purity can exceed 99%. According to the technology disclosed by the invention, the purity of the synthesized crude peptide can reach about 80%, the purification is easy, and the total yield can reach 30%. The total yield and product quality of thymosin alpha1 produced by the method disclosed by the invention are both much remarkably improved than those of other methods reported in documents; and moreover, the production cycle can be shortened by 40-50%, the production cost is reduced, and the method is suitable for large-scale preparation of high-purity thymosin alpha1.
Owner:苏州天马医药集团天吉生物制药有限公司
Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole
The invention discloses a method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole. The method includes: thioanisole is reacted with acetyl chloride to generate 4-methylthio acetophenone which is reacted with 4-methyl fluorobenzoate to generate 1-(4-fluorophenyl)-3-(4-methylthio benzene)-1,3 diketone which is reacted with tert-butyl nitrite to generate 1-(4-fluorophenyl)-2-(4-methylthio benzene)-1,2 diketone which is reacted with 2-chlorobenzaldehyde to generate 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(methylthio) phenyl]thiazole which is oxidized to generate 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole. The method avoids usage of nitrine reagent and has important application value.
Owner:ZHEJIANG UNIV
Chiral catalytic synthesis method of thiamphenicol
ActiveCN102863361BAvoid pollutionReduce pollutionOrganic chemistryOrganic compound preparationPtru catalystKetone
The invention relates to a chiral catalytic synthesis method of thiamphenicol which is a chloramphenicol broad-spectrum antibiotic. Thioanisole serves as an initating raw material, acylation and bromo are achieved, nitrogen iridine containing substituent groups is synthetized, and a qualified product which meets the requirement of drug administration is synthetized through chiral catalytic reduction, oxidizing reaction, acidification loop opening, deprotection and acylation reaction. The chiral catalytic reduction includes that under the action of a catalyst trans-RuC12[(R)-xylbinap][(S)-DPEN], [1-substituent group nitrogen iridine-2-group] [4-(methylthio group) phenyl group] ketone is subjected to hydrogenation reduction to obtain [1-substituent group nitrogen iridine-2-group] [4-(methylthio group) phenyl group] ketone with a high ee value and a high de value. D-methylsulfonylphenyl serine ethyl ester used in industrial production serves as a raw material to synthetize the thiamphenicol, the D-methylsulfonylphenyl serine ethyl ester is obtained through chemical chiral resolution by using a racemic compound, and the other half of the raw material is wasted. According to the unsymmetrical chiral catalytic dynamic reduction method, waste of the other half of the raw material is avoided, the utilization rate of a material is improved, and the production cost is reduced.
Owner:MASTEAM BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

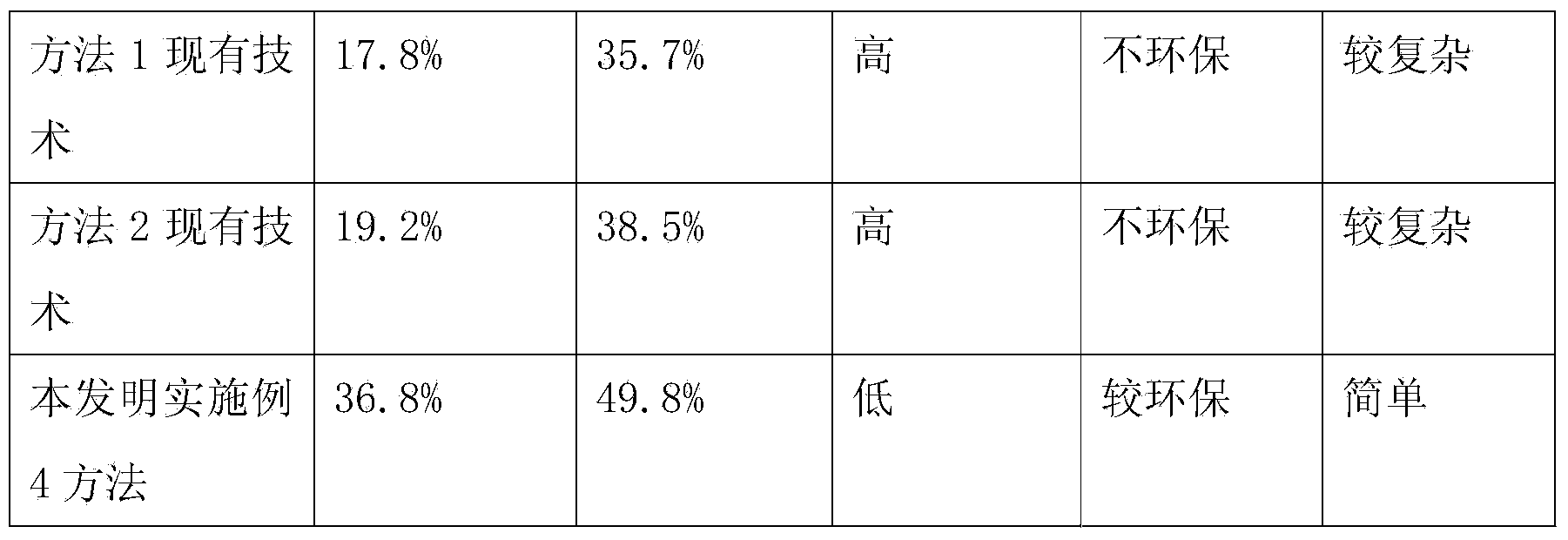
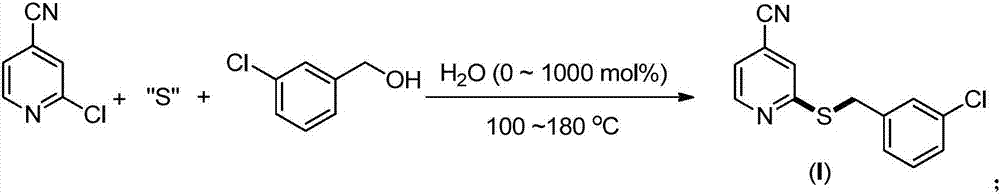
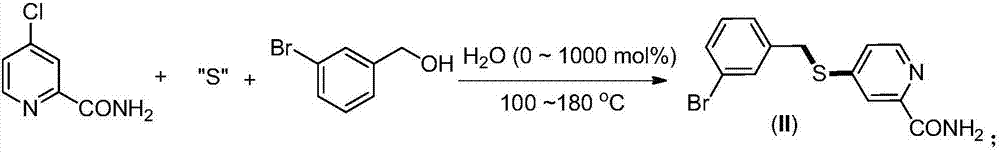
![Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole](https://images-eureka.patsnap.com/patent_img/8674a998-19e1-4a9d-9033-5aae23376041/BDA0000145780070000011.png)
![Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole](https://images-eureka.patsnap.com/patent_img/8674a998-19e1-4a9d-9033-5aae23376041/BDA0000145780070000021.png)
![Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole Synthesis method of 4-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)-1H-imidazole](https://images-eureka.patsnap.com/patent_img/8674a998-19e1-4a9d-9033-5aae23376041/BDA0000145780070000022.png)


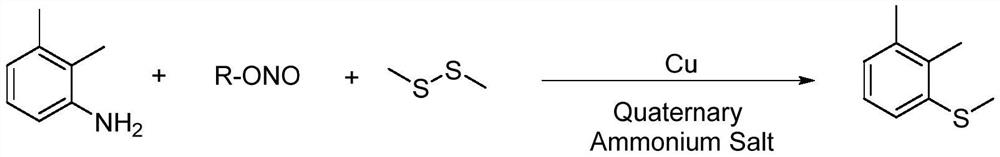
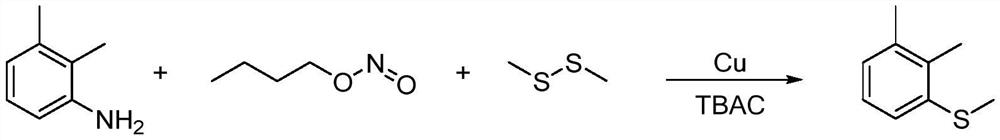

![Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole](https://images-eureka.patsnap.com/patent_img/7722080c-e73e-4cb2-b2d6-814e9c104347/GDA0000394526180000011.PNG)
![Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole](https://images-eureka.patsnap.com/patent_img/7722080c-e73e-4cb2-b2d6-814e9c104347/GDA0000394526180000021.PNG)
![Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole Method for synthesizing 2-(2-chlorophenyl)-4-(4-fluorophenyl)-5-[4-(mesyl) phenyl] thiazole](https://images-eureka.patsnap.com/patent_img/7722080c-e73e-4cb2-b2d6-814e9c104347/GDA0000394526180000051.PNG)