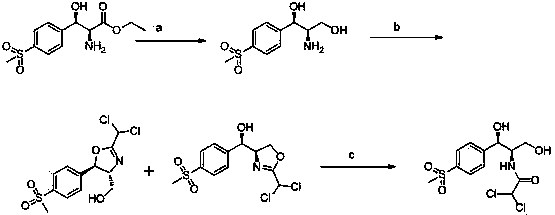
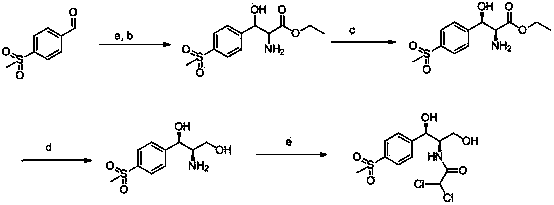
Chiral catalytic synthesis method of thiamphenicol
A technology of thiamphenicol and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high cost, time-consuming and long route of wastewater treatment, and achieve cost reduction and environmental impact. pollution, avoid splitting process, avoid the effect of waste water pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
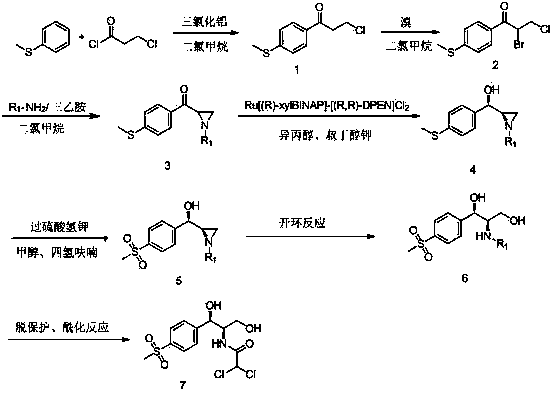
[0030] Synthesis of 3-chloro-1-(4-(methylthio)phenyl)-1-acetone: Add aluminum trichloride (35.7 g, 268 mmol) and 200 ml of dichloromethane to a 500 ml three-necked flask at room temperature. Anhydrous calcium chloride pre-drying), connect the system to the drying tube, stir, pour 3-chloropropionyl chloride (37.2 g, 293 mmol) into the reaction flask, cool to 0 ℃ in an ice bath, add anisole (27.7 g, 223 mmol), drip in 2 hours (control the liquid temperature between -5-10 ℃), after dripping, move the reaction flask into an oil bath and heat up to 25 ℃ for 1-3 hours. Pour the reaction solution into a 1000 ml beaker, add 100 ml of water while stirring under ice bath, separate the layers, extract with dichloromethane, combine the organic phases, wash with saturated sodium bicarbonate once, wash twice with water, dry with anhydrous sodium sulfate, and filter The sodium sulfate was removed, the filter cake was washed with dichloromethane, the filtrate was recrystallized with dichlorome...
Embodiment 2
[0032] Synthesis of compound 2-bromo-3-chloro-1-(4-(methylthio)phenyl)-1-acetone: Add 3-chloro-1-(4-(methylthio) to a 500 ml three-necked flask at room temperature ) Phenyl)-1-acetone (16.0 g, 74.5 mmol), 100 ml of dichloromethane (pre-dried with anhydrous calcium chloride), stir. Add 4.0 ml of bromine (12.5 g, 78 mmol) and 100 ml of dichloromethane (pre-dried with anhydrous calcium chloride) into a 250 ml beaker, stir and mix well, pour it into a constant pressure dropping funnel, and place it on the reaction flask . The reaction flask was cooled in an ice-water bath, and when the liquid temperature dropped to 0 ℃, the dichloromethane solution of bromine was added dropwise. Keep the liquid temperature at about 0 ℃. After dripping within 1-2 h, the reaction was kept at 0 ℃, followed by TLC. After the addition, react for 1 hour. Add saturated sodium bicarbonate solution under ice bath until the water phase is alkaline, separate liquids, add saturated sodium thiosulfonate solut...
Embodiment 3
[0034] Synthetic compound 1-benzyl-2-(4-(methylthio)phenyl) formyl aziridine: add 2-bromo-3-chloro-1-(4-methylthiophenyl) to a 250 ml three-necked flask )-1-acetone (5.0 g, 17 mmol), 100 ml of dichloromethane, stir to dissolve, add benzylamine (1.8 g, 17 mmol), triethylamine (3.6 g, 36 mmol) and dichloromethane dropwise at room temperature The 50 ml mixed solution was reacted at room temperature for 3 hours, quenched by adding water, the organic phase was washed three times with water, dried over anhydrous sodium sulfate, and the organic solvent was removed to obtain 4.7 g of light yellow solid with 95.5% detected by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com