Baeyer-Villiger monooxygenase and application of Baeyer-Villiger monooxygenase
A monooxygenase and reaction system technology, applied in the field of bioengineering, can solve the problems of low conversion rate of cyclohexanone and product yield, low product ee value, low catalytic activity, etc., and achieve good application development prospects, three-dimensional Strong selectivity and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Construction of recombinant escherichia coli
[0037] The chemically synthesized nucleotide sequence is shown in SEQ ID NO.1 as the coding gene sequence of Baeyer-Villiger monooxygenase, which is named Rabvmo, and the amino acid sequence of the encoded protein is shown in SEQ ID NO.2.
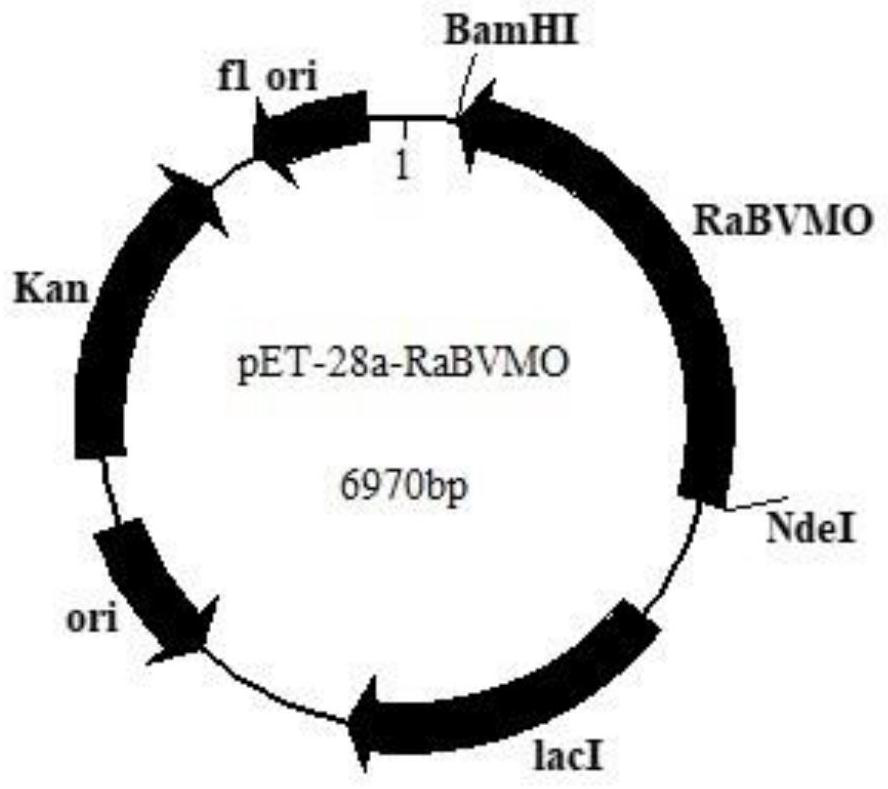
[0038] With the restriction endonuclease NdeI and BamHI after plasmid pET28a digested and the nucleotide fragment of Rabvmo, gene Rabvmo is connected with digested plasmid pET28 by T4 DNA ligase, obtains recombinant expression vector pET28a-Rabvmo( figure 1 ). Transform the constructed recombinant expression vector pET28a-Rabvmo into Escherichia coli BL21(DE3) competent, coat a solid plate containing kanamycin-resistant LB, and perform colony PCR verification after overnight culture. The positive clone is the recombinant large intestine Bacillus BL21(DE3) / pET28a-Rabvmo. Pick positive clones and culture them overnight in LB medium, transfer them into fresh LB culture at th...
Embodiment 2
[0040] Example 2: Properties of Baeyer-Villiger monooxygenases
[0041] (1) Separation and purification of Baeyer-Villiger monooxygenase
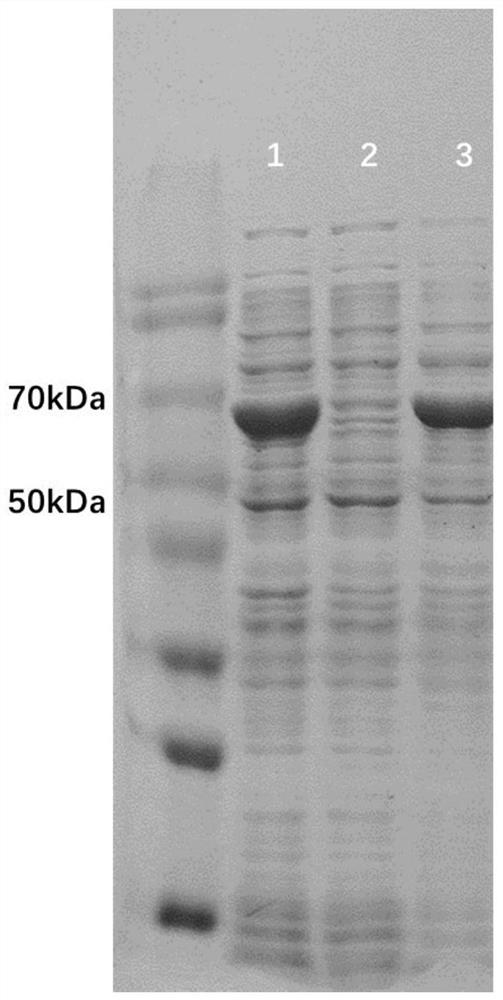
[0042] Suspend the recombinant cells in solution A (20mmol·L -1 Sodium phosphate, 500mmol·L -1 NaCl, 20mmol·L -1 imidazole, pH 7.4), the crude enzyme solution was obtained after sonication and centrifugation. The column used for purification is an affinity column HisTrap FF crude (nickel column), which is accomplished by using the histidine tag on the recombinant protein for affinity binding. First, use solution A to equilibrate the nickel column, load the crude enzyme solution, continue to use solution A to elute the breakthrough peak, and after equilibrium, use solution B (20mmol L -1 Sodium phosphate, 500mmol·L - 1 NaCl, 1000mmol·L -1 imidazole, pH 7.4) for gradient elution to elute the recombinant protein bound to the nickel column to obtain recombinant Baeyer-Villiger monooxygenase. The purified protein was assayed for enzyme a...
Embodiment 3
[0072] Embodiment 3: Baeyer-Villiger monooxygenase is applied to the preparation of S-phenylmethyl sulfoxide
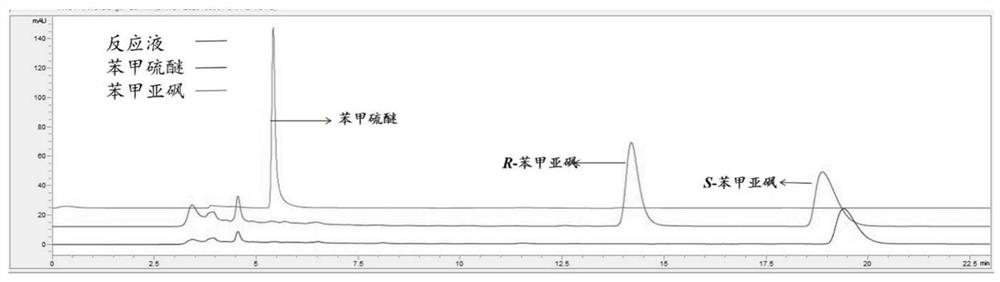
[0073] Take 2-500 U of the obtained recombinant Baeyer-Villiger monooxygenase and 2-500 U of glucose dehydrogenase (GDH, purchased from Novizyme, 5 U / g) in Tris-HCl buffer (pH 9, 100 mmol L -1), add 5% methanol, the concentration in the reaction system is 30~100mmol·L -1 The sulfide anisole (Table 5), the total volume of the reaction solution is 10mL. The reaction is placed at 30°C, and the conversion process is detected by sampling. The conditions are as follows: chiral OD-H chromatographic column (250mm×4.6mm×5μm), the detection wavelength is 254nm, and the mobile phase is n-hexane:isopropanol (90:10 ), the flow rate is 0.2-1mL / min.
[0074] The reaction process is sampled and detected in real time, and when it is detected that the product no longer increases, the reaction ends.
[0075] The results are shown in Table 5, Baeyer-Villiger monooxygenase can maintain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com