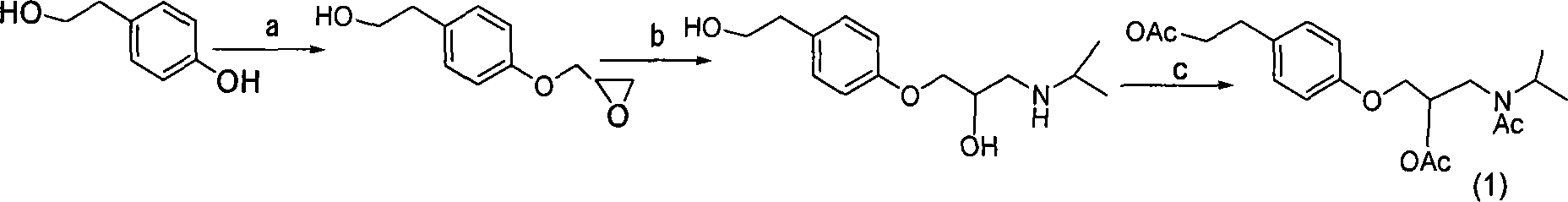
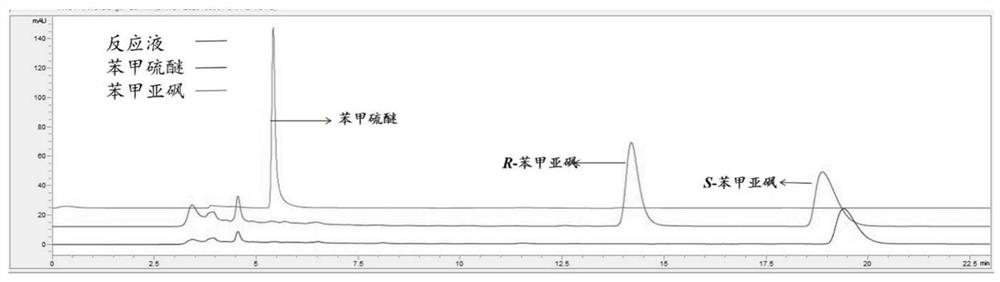
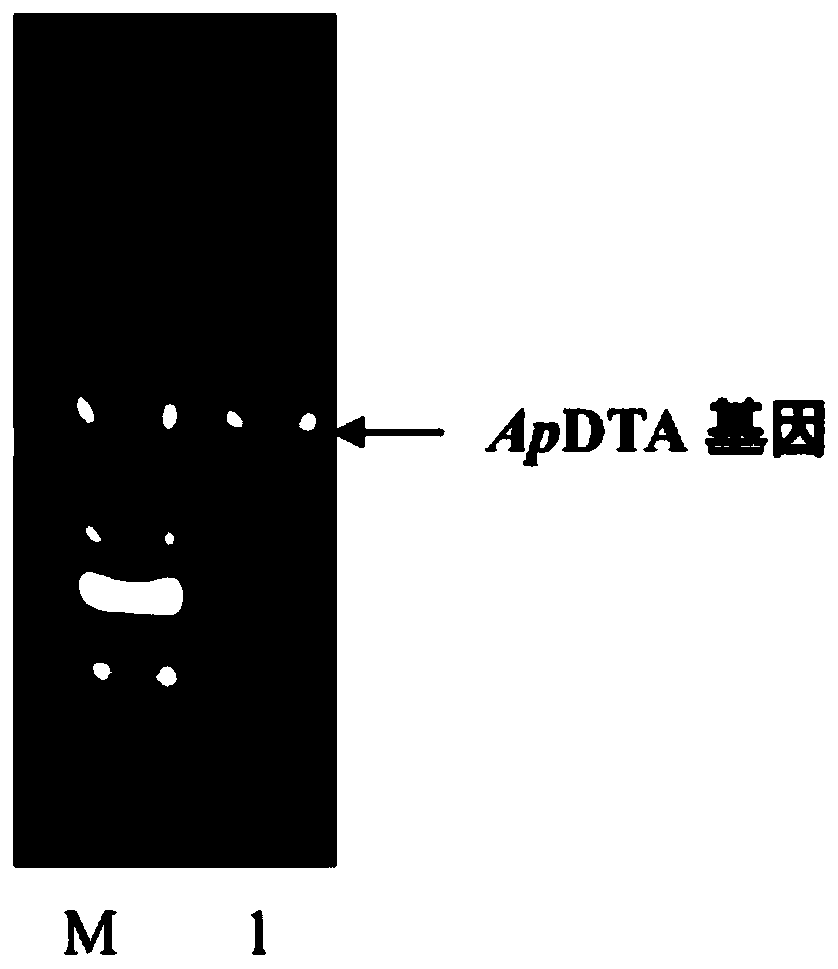
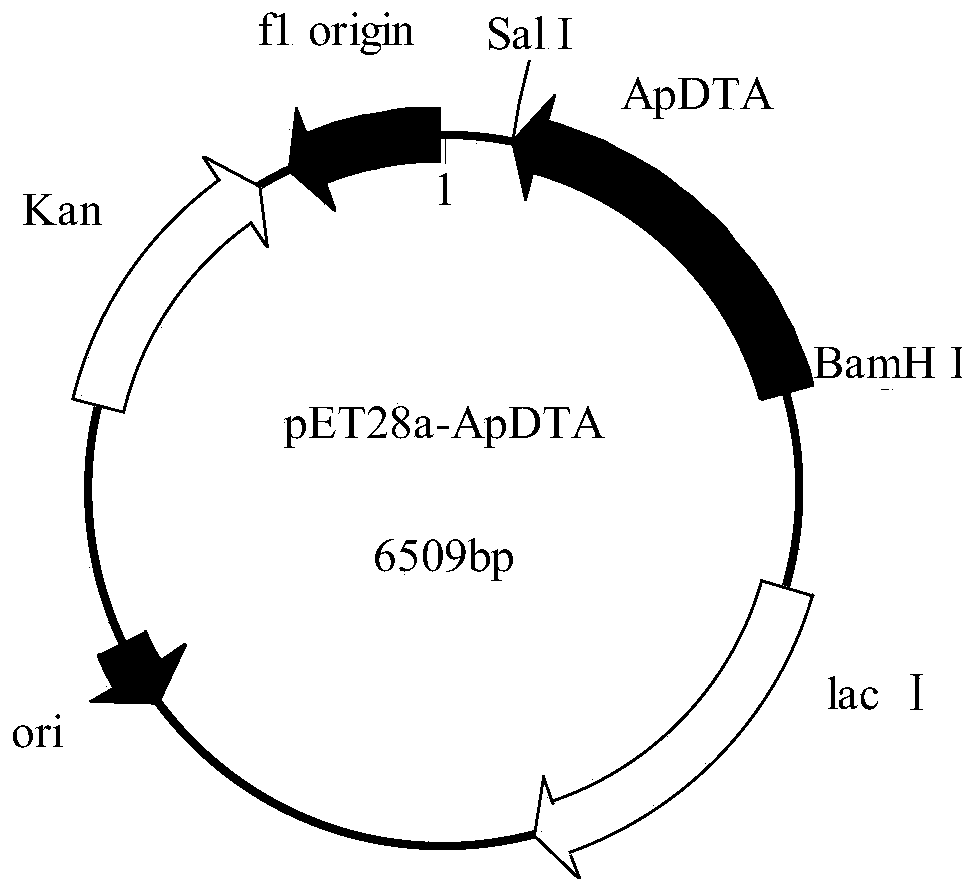
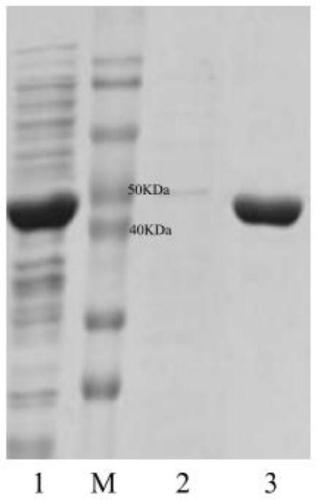
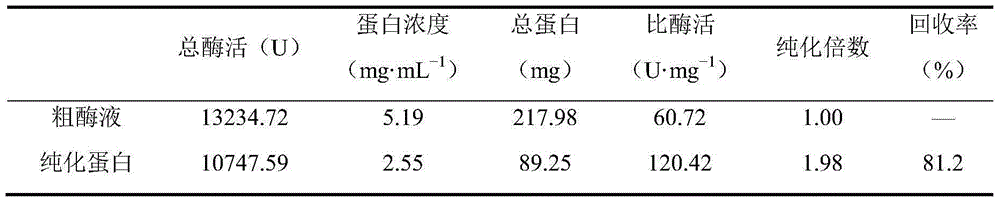
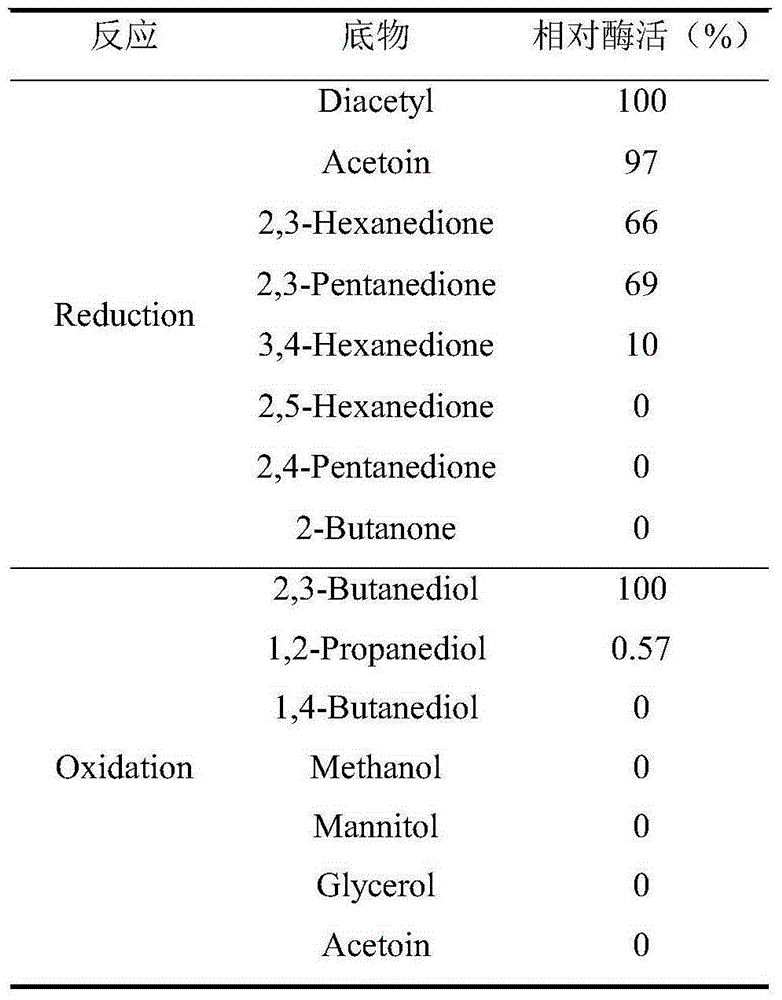
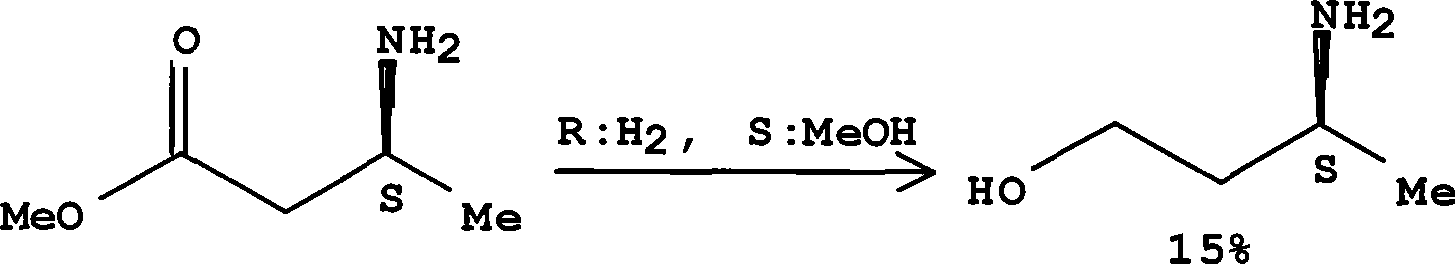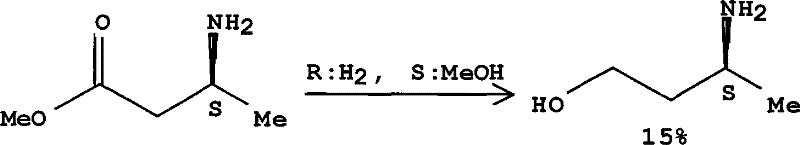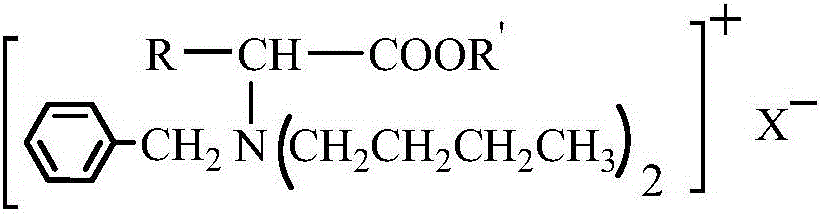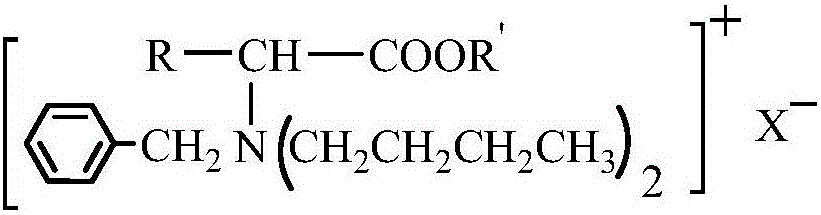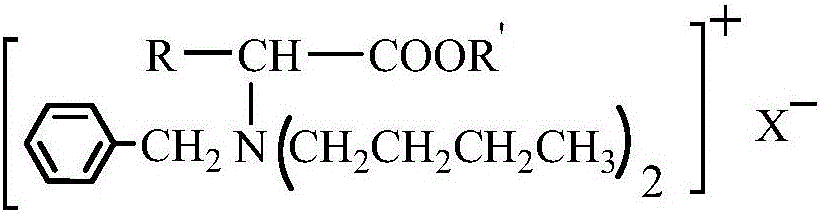Patents
Literature
117results about How to "Stereoselective" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
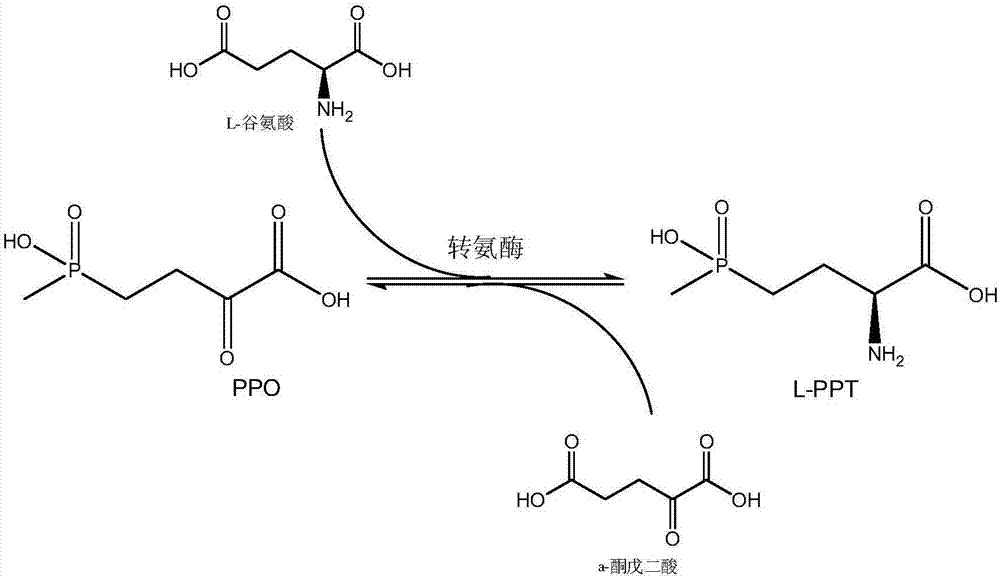
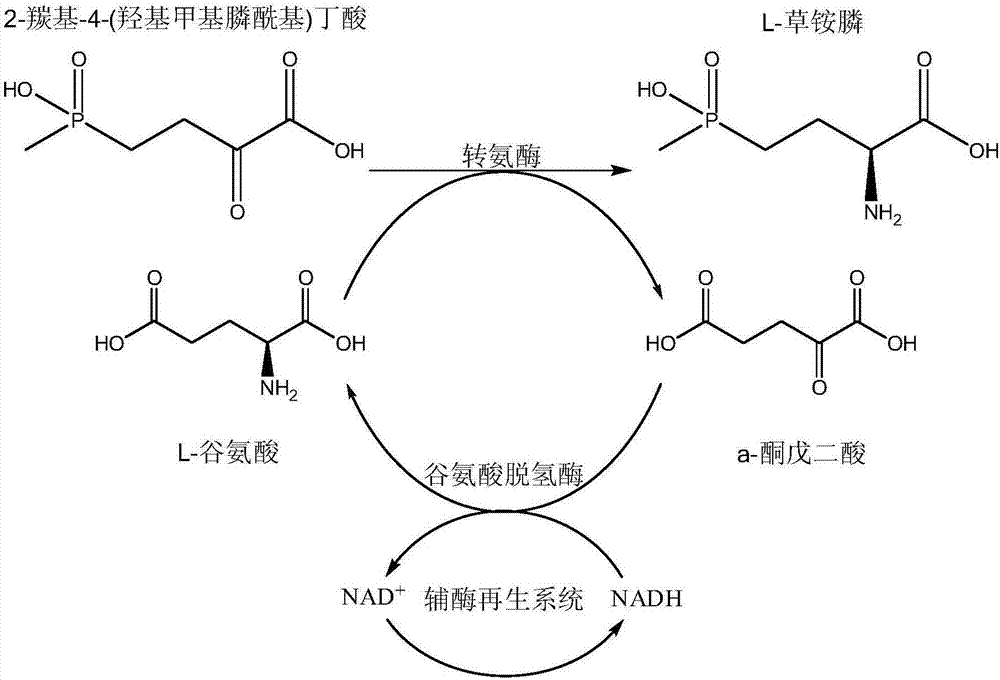
Production method of L-phosphinothricin
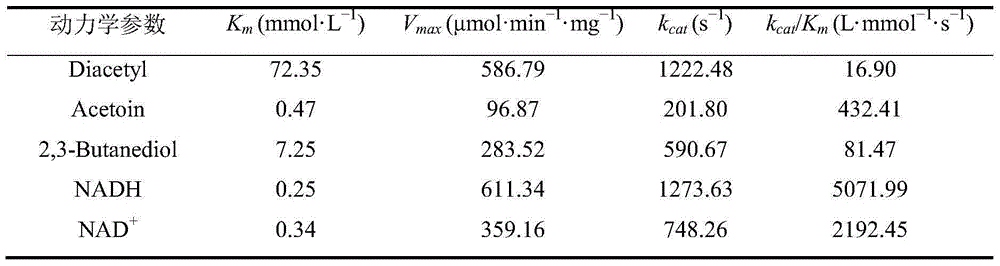
ActiveCN106916857AHigh catalytic activityStereoselectiveFermentationButyric acidGlutamate dehydrogenase
The invention discloses a production method of L-phosphinothricin. According to the method, 2-carbonyl-4-(hydroxymethylphosphonyl)butyric acid is used as a substrate and is catalyzed by an enzyme catalytic system to obtain L-phosphinothricin, and the enzyme catalytic system is composed of gamma-aminobutyric acid / alpha-ketoglutarate transaminase, glutamate dehydrogenase and a coenzyme regeneration system. The method utilizes the advantages of high catalytic activity, strong stereoselectivity and the like of transaminase, and also solves the problem of incomplete transaminase catalytic reaction, so that the catalytic reaction can completely convert the substrate 2-carbonyl-4-(hydroxymethylphosphonyl)butyric acid into L-phosphinothricin, and the conversion rate can reach 100%; no by-product alpha-ketoglutarate is accumulated in the final product of the method, and the residual amount of other substances such as the raw material glutamic acid in the product after the end of the reaction is extremely low, so the subsequent refining process of L-phosphinothricin is greatly simplified, and the total yield of the product is increased.
Owner:ZHEJIANG UNIV
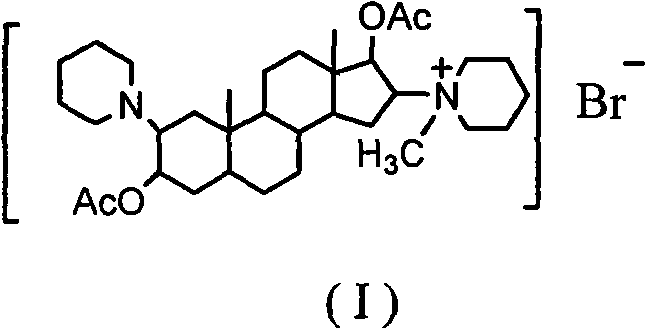
Synthesis process of vecuronium bromide
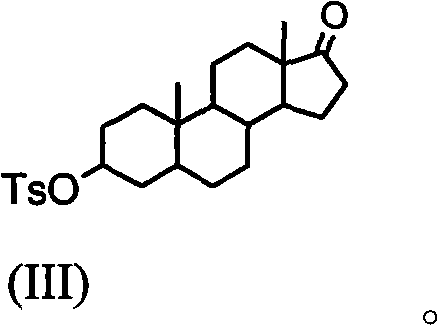
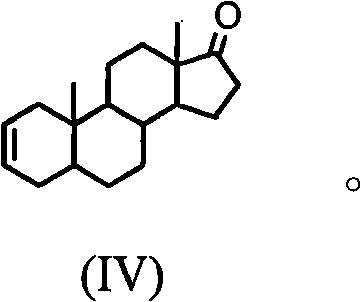
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
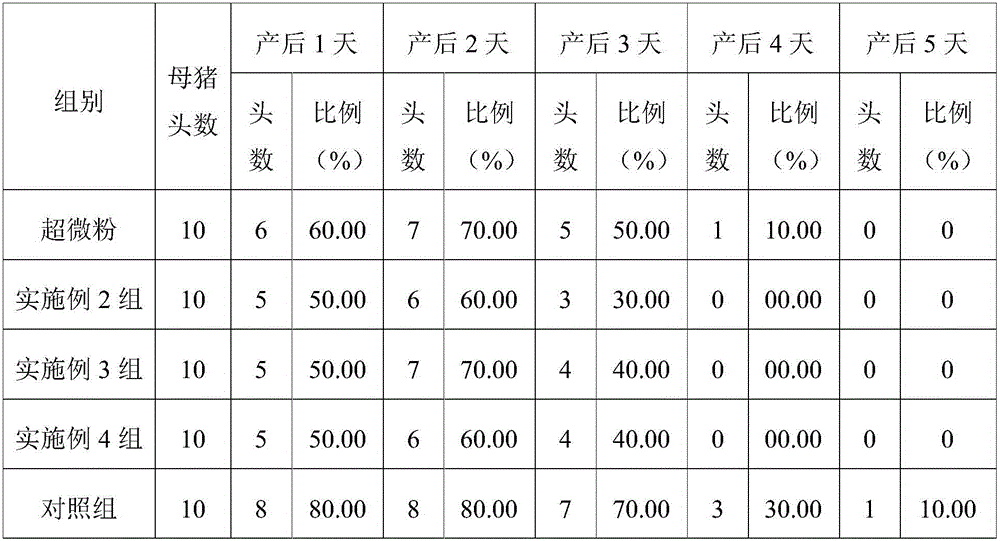
Traditional Chinese medicine fermented preparation for improving production performance of sows, preparation method and application thereof
InactiveCN106619941ASupplements or enhancementsHigh regional selectivityFood processingPharmaceutical delivery mechanismSide effectCodonopsis
The invention relates to a traditional Chinese medicine fermented preparation for improving production performance of sows, and belongs to the technical field of preparation for livestock. The preparation comprises the following traditional Chinese medicine components in parts by weight: 10-20 parts of astragali roots, 10-20 parts of codonopsis roots, 5-15 parts of large-headed atractylodes rhizomes, 5-15 parts of Chinese angelica, 10-15 parts of bupleurum roots, 5-10 parts of dried orange peels, 5-10 parts of cimicifuga roots, and 10-20 parts of licorice. The traditional Chinese medicine components selected by the method has the advantages of reasonable structure and proper proportion, selected Lactobacillus plantarum has the advantages of high activity, good stress resistance and the like; in the effects of the selected Lactobacillus plantarum, and efficacies of the original medicines are substantially improved; the fermented medicine can substantially improve production performance of sows, and is green and healthy without toxic and side effects.
Owner:哈尔滨中科生物工程有限公司
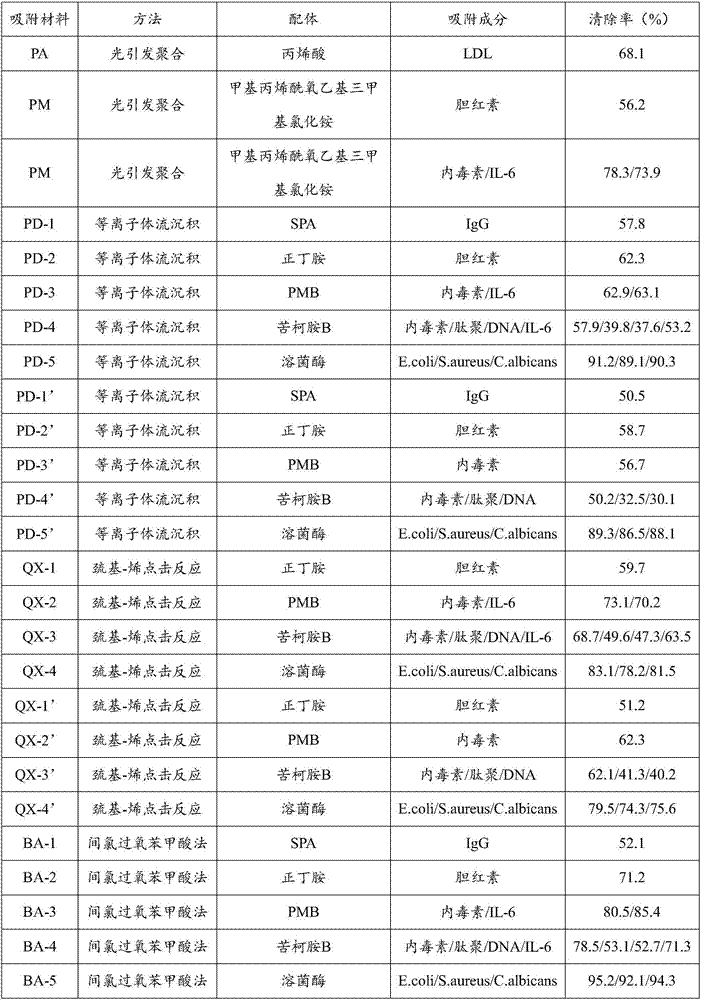
Adsorption material for blood purification and preparation method thereof
ActiveCN107486176ARealize functionRealize personalized functionOther blood circulation devicesOther chemical processesInflammatory mediatorSurface modified
The invention discloses an adsorption material for blood purification and a preparation method thereof. The adsorption material is prepared by coupling a functionalization area with at least one ligand, wherein the functionalization area is formed by a carrier with 0.05-2mm particle size alone or by performing surface modifying on the carrier with 0.05-2mm particle size; and the ligand is capable of binding to at least one pathogenic component in blood or plasma, so that at least one pathogenic composition in whole blood or plasma can be selectively adsorbed. For the adsorption material disclosed by the invention, functional polymers and different ligands are introduced onto the surface of the carrier by using different methods according to different blood purification requirements, so as to achieve an effect of reducing circulating pathogenic substances and harmful pro-inflammatory mediators in a human body, and achieve personalized and functional aims of the adsorption material for blood purification. The adsorption material has the good market value and application prospect.
Owner:GUANGZHOU KONCEN BIOSCI
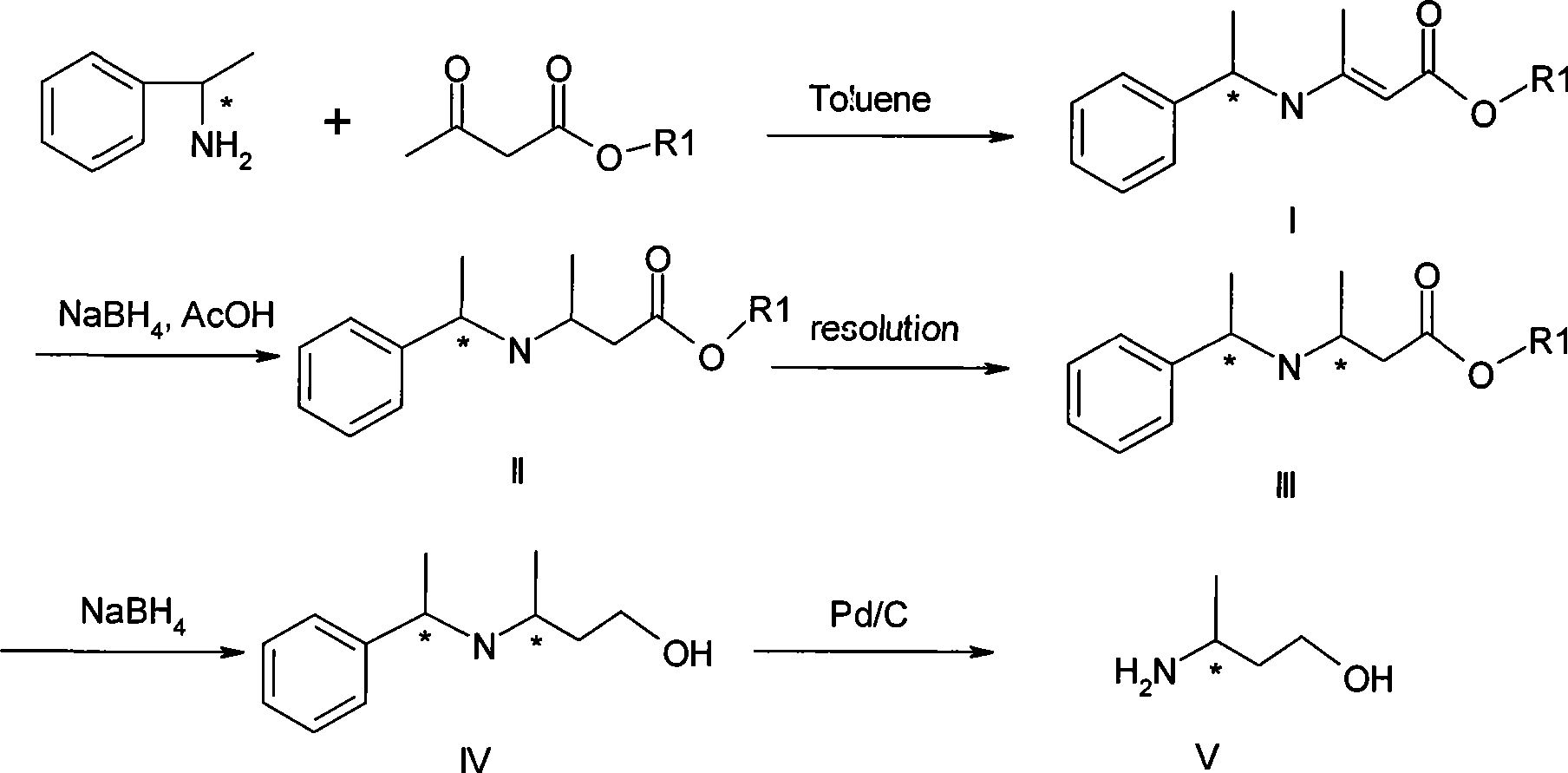
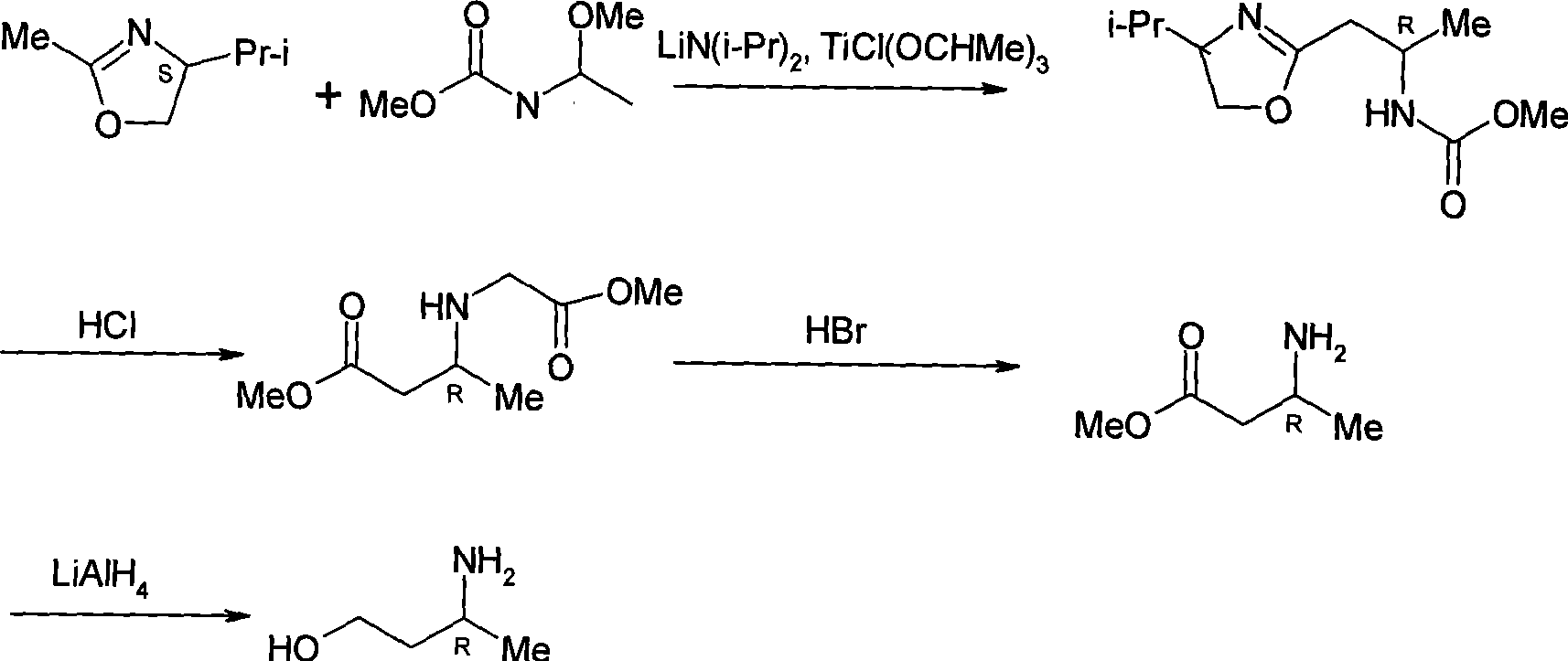
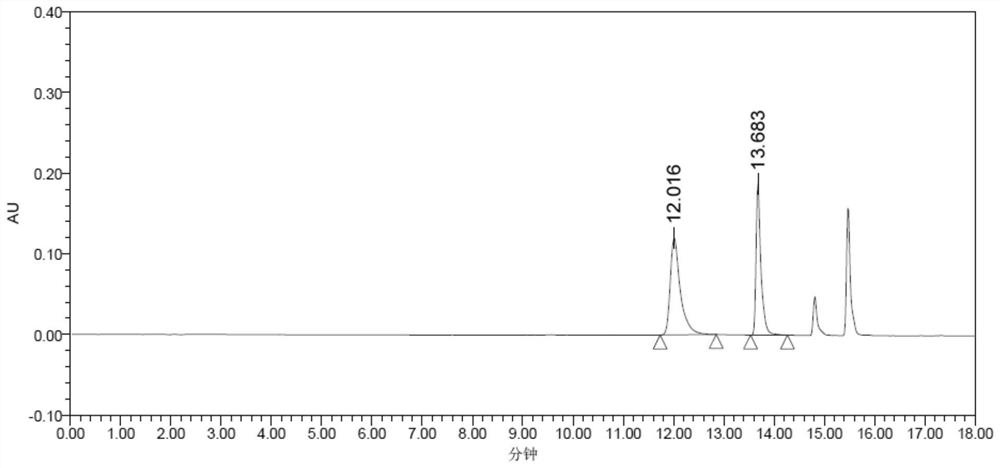
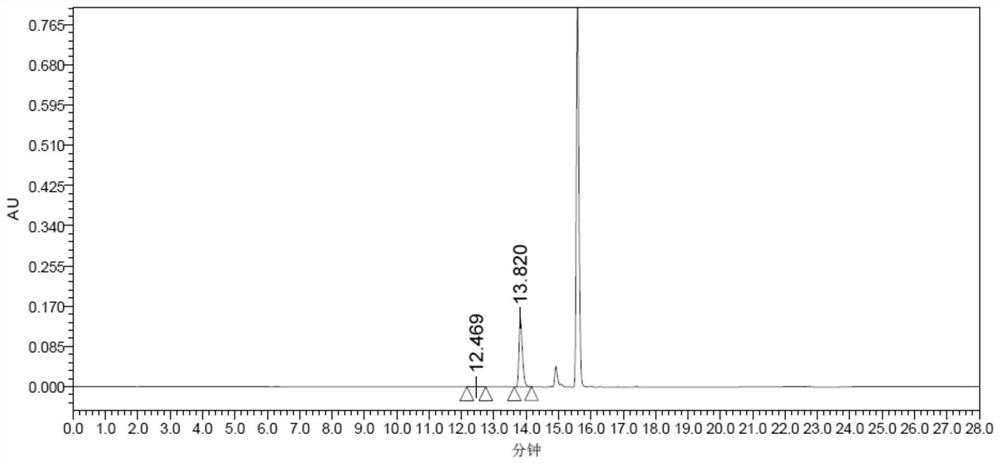
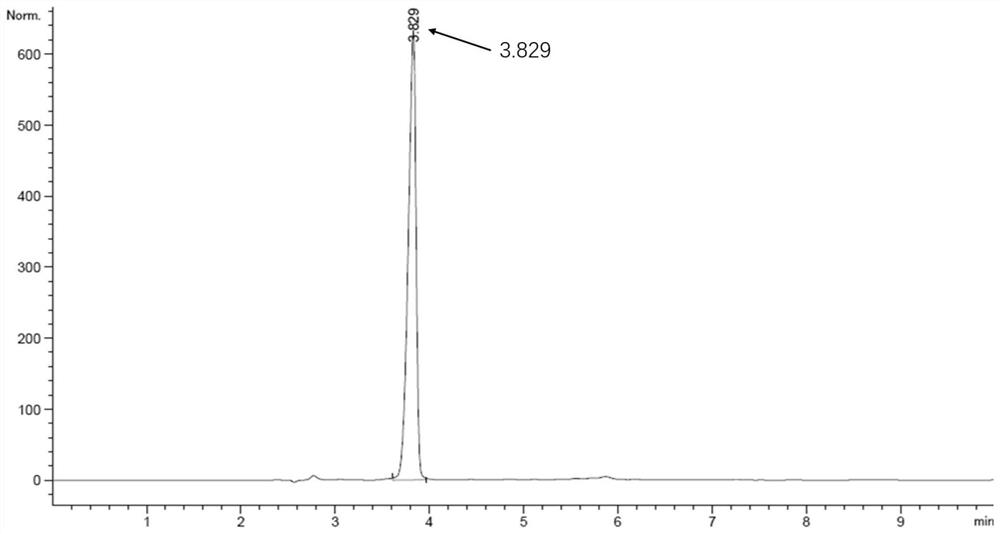
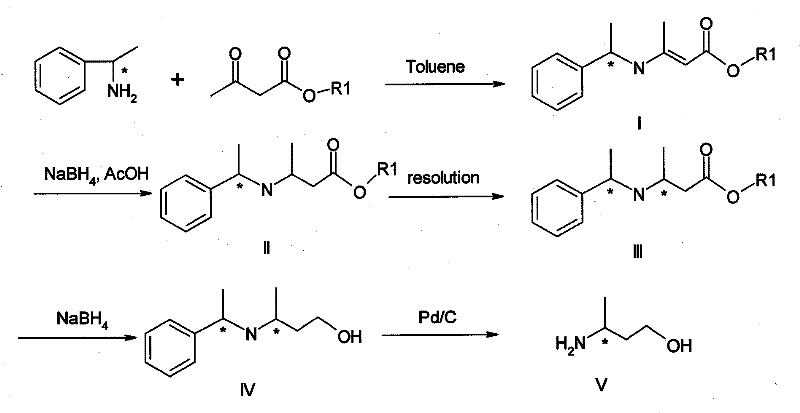
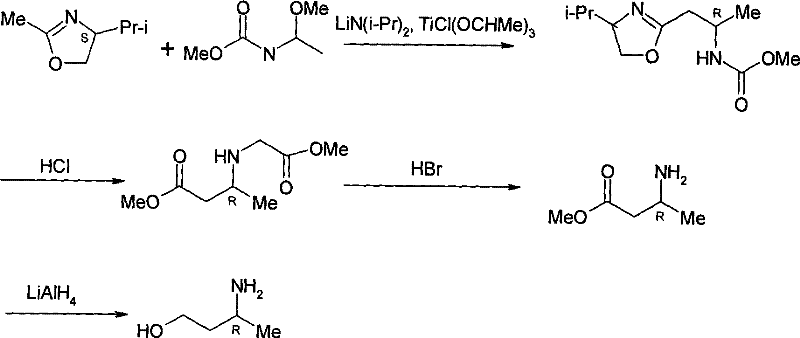
Method for preparing optically pure 3-amino butyl alcohol
ActiveCN101417954ASuitable for industrial productionStereoselectiveOrganic compound preparationAsymmetric synthesesAcetoacetatesPotassium borohydride
The invention discloses a method for preparing optical pure 3-aminobutanol, which comprises the following steps: acetylacetic ester and chiral phenethylamine react to generate 3-(1'-benzylmethylamine)-2-crotonate enantiomer, namely 3-(1'-benzylmethylamine)-2-crotonate; potassium borohydride triacetate or sodium borohydride triacetate is used to reduce the 3-(1'-benzylmethylamine)-2-crotonate enantiomer into a 3-(1'-benzylmethylamine)-2-butyric ester enantiomer; then chiral pure 3-(1'-benzylmethylamine)-2-butyric ester is obtained through salification and resolution; and the 3-(1'-benzylmethylamine)-2-butyric ester is subject to reducing debenzylation by palladium-carbon to obtain the optical pure 3-aminobutanol. The method has low cost and high product purity, and is suitable for industrialized production.
Owner:ABA CHEM SHANGHAI +1
Immobilized recombinant penicillin G acylase and application thereof
ActiveCN104120120AGood operational stabilityWide variety of sourcesChemical industryOn/in organic carrierGlycerolImmobilized enzyme
The invention discloses an immobilized recombinant penicillin G acylase and an application thereof to preparation of (S)-2-aryl-amino acid and cephalosporin antibiotics. The immobilized recombinant penicillin G acylase disclosed by the invention is capable of converting an S-type substrate into an S-type product within shorter time, high in substrate tolerance and strict in S selectivity for the substrate and structural analogues thereof; due to the addition of cobalt ions as well as a protective agent with phenylacetic acid and glycerin as enzyme active centers, the immobilized recombinant penicillin G acylase disclosed by the invention is prevented from being successfully subjected to multipoint covalent immobilization on the surface of an epoxy resin carrier under the condition that enzyme molecules are seriously inactivated in an immobilization process, and the immobilization method is simple and feasible, cheap in raw materials and suitable for large-scale operation; the immobilized recombinant penicillin G acylase can be used for synthesizing cephalosporin antibiotics and splitting the prepared (S)-2-aryl-amino acid, and can be continuously used for more than 50 batches before the activity of the immobilized enzyme is not remarkably reduced.
Owner:ZHEJIANG UNIV OF TECH
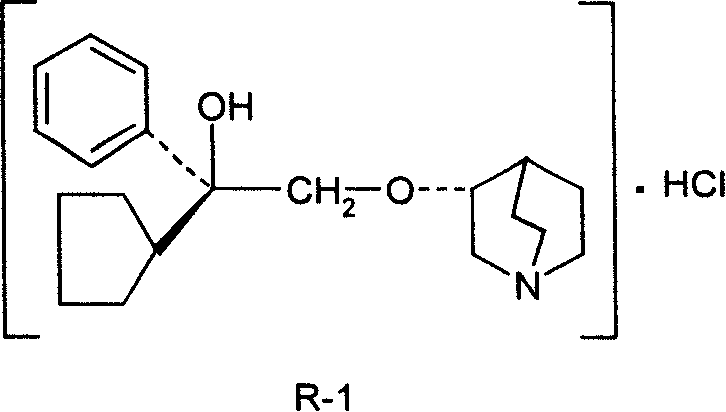
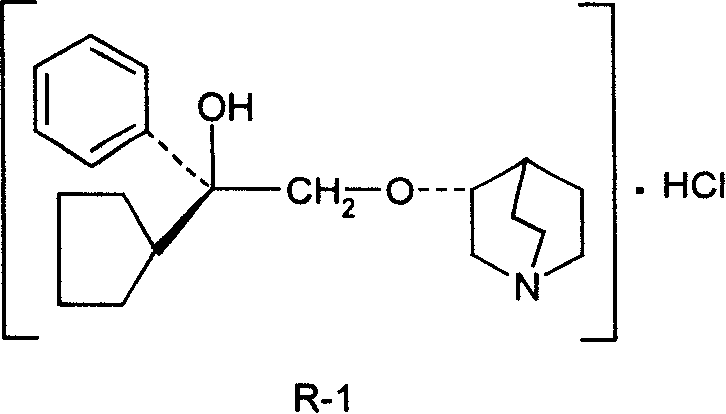
Pharmaceutical composition containing (3S, 2'R)-3-(2'-hydroxy-2'-cyclopentyl-2'-phenylethoxy) quinuclidine hydrochloride and uses thereof
InactiveCN101234108AHigh optical purityStereoselectiveOrganic active ingredientsUrinary disorderDiseaseSide effect
The invention discloses medical composition containing active component of (3S, 2'R)-3-(2'-hydroxyl-2'-cyclopentyl-2'-phenylethoxy) quinine quinuclidine chloride and application of the medical composition in preparing drugs for treating chronic obstructive lung disease, incontinentia urinae, diversified shock diseases and preparing adjuvant drugs used before anaesthesia. The active component of the medical composition of the invention has high optical purity, strong stereoselectivity in vivo process, definite curative effects and high pertinency to the disease, thus effectively avoiding generation of toxic and side effects.
Owner:CHENGDU LIST PHARMA
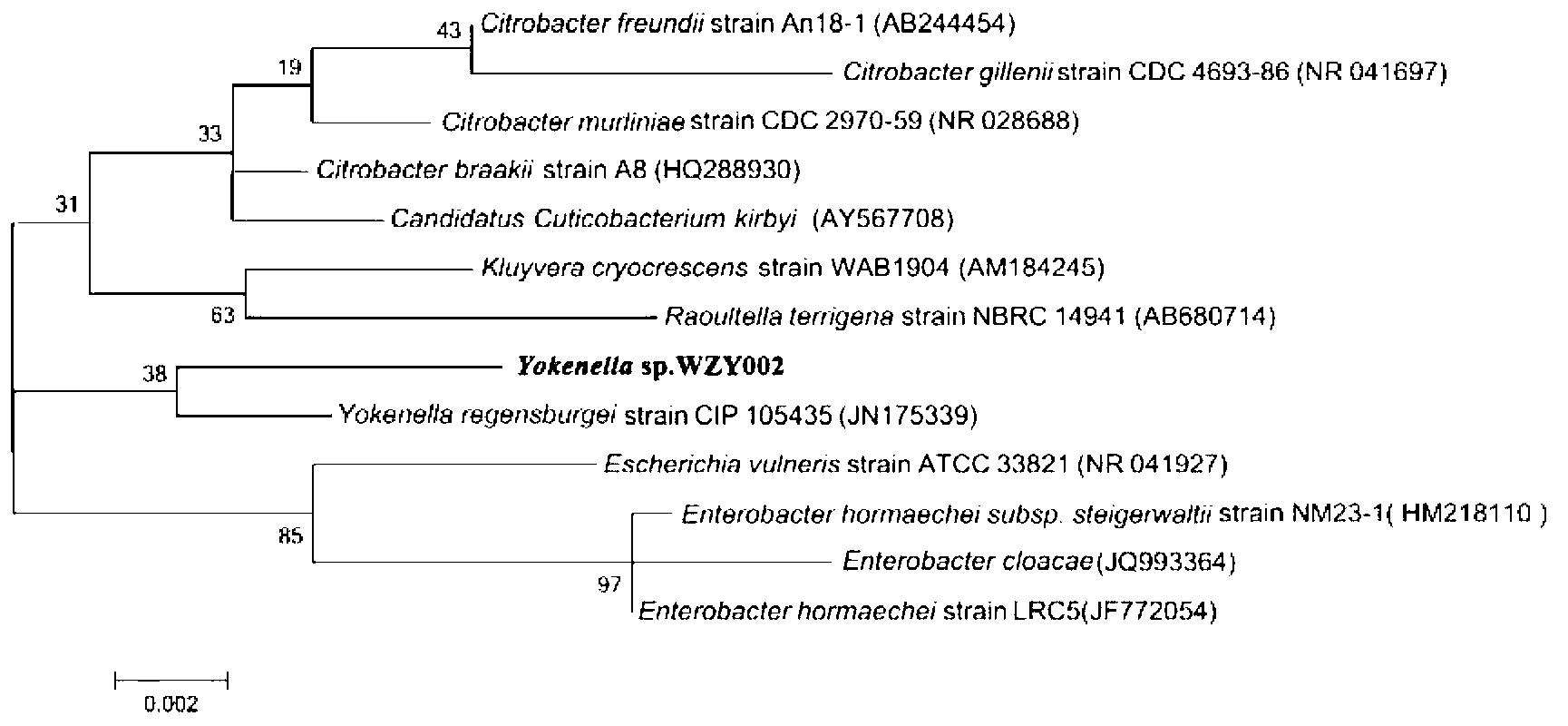
Yokenella sp. and application thereof in preparing alpha, beta-unsaturated enol and aromatic alcohol
ActiveCN103289922AHigh regional selectivityHigh stereoselectivityBacteriaMicroorganism based processesReaction temperatureKetone
The invention provides a novel bacterial strain-Yokenella sp. WZY002 and an application thereof in preparing alpha, beta-unsaturated olefine aldehyde (ketone) through regioselective reduction and aromatic alcohol through aromatic aldehyde (ketone) reduction. The bacterial strain is preserved in the China general microbiological culture collection center (CCTCC); the address is 430072, Wuhan University, Wuhan, China; the preservation number is CCTCC No: M2013099; the preservation date is March 22, 2013. The novel bacterial strain has the main beneficial effects of high regioselectivity, high stereoselectivity and high enzymatic activity, regioselective reduction of the alpha, beta-unsaturated enol is catalyzed so as to obtain various alpha, beta-unsaturated olefine aldehydes, and reduction of the aromatic aldehyde (ketone) can also be catalyze so as to obtain the aromatic alcohol. The bacterial strain is served as a biocatalyst and has high regioselectivity, high stereoselectivity and strong catalytic activity, the catalytic reaction does not need to add coenzyme, the reaction temperature is moderate, and the novel bacterial strain has a relatively high application value for industrial production.
Owner:ZHEJIANG UNIV OF TECH
Saccharomycete with stereoselectivity lipase liveness and application in producing S- type betaxolol hydrochloride with biological split method thereof
InactiveCN101220336ALow priceMild reaction conditions for production conversionFungiHydrolasesBacterial strainSalbutamol sulfate
The invention discloses a yeast which has the stereoselective lipase activity and the application of the yeast in the preparation of an S-type betaxolol hydrochloride by using a biological separation method. The method selects one yeast with the stereoselective lipase activity by screening from the soil, utilizes an immobilized cell which is obtained by immobilizing the wet bacteria or sodium alginate-activated carbon-polyethylenimine as an enzyme preparation, carries out an enantiomer separation to a substrate which contains acyl group and prepares the S-type betaxolol hydrochloride. The conversion method is simple, the cost is low and the stereoselectivity is better. The usage of the bacterial strain can carry out the chiral separation of Beta-receptor blocker of betaxolol etc., ephedrine hydrochloride, epinephrine, levodropropizine, salbutamol sulfate, captopril, zofenopril and other compounds which contain hydroxy group or acyl group at the chiral center, thereby having important application value for promoting the development process of the chiral drugs of China.
Owner:ZHENGZHOU UNIV
Preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction
InactiveCN102206686AHigh catalytic efficiencyIncrease productivityBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The present invention discloses a preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction and used recombinant vectors and genetically engineered bacteria. The preparation method comprises the step of carrying out biotransformation reaction on methyl o-chlorobenzoylformate used as the substrate with genetically engineered bacteria wet cells or freeze-dried cells capable of coexpressing recombinant reductase and recombinant glucose dehydrogenase as the catalyst at pH 6-8 in the presence of glucose, wherein the recombinant reductase is a recombinant aldo-keto reductase. The genetically engineered bacteria whole cells can simultaneously express the aldo-keto reductase and glucose dehydrogenase and can achieve high-efficiency regeneration of intracellular coenzyme NADP<+>. By using the preparation method, high-concentrations methyl o-chlorobenzoylformate can be catalyzed and completely transformed into methyl (R)-o-chloromandelate with a single conformation, without adding a coenzyme. Because of expensive coenzyme, the preparation method provided by the invention greatly lowers the production cost, has mild reaction conditions, is environmentally-friendly and simple to operate, and has good industrial application prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
A kind of lipase gene and its recombinant enzyme and its application in preparing optically active mandelic acid
InactiveCN102260657AHigh activityGood prospects for industrial application developmentFungiBacteriaAspergillus fumigatusMandelic acid
The invention discloses a lipase and a gene thereof, a recombinant expression vector, recombinant expression transformant and recombinase containing the gene, and a preparation method of the recombinase. In addition, the invention also discloses application of thallus containing the recombinant lipase as a catalyst in preparing an optically active substance from alpha-acetoxyphenylacetic acid and2-Cl-alpha-acetoxyphenylacetic acid in an asymmetric deacylation mode. The recombinant lipase gene is derived from aspergillus fumigatus Af293. The recombinase can be used as a catalyst for asymmetric deacylation to prepare optically active mandelic acid. The recombinase has the advantages of high catalytic efficiency, strong stereoselectivity, mild applicable reaction conditions and environmental friendliness. The recombinase disclosed by the invention has high catalytic activity, and has wide prospects in industrial application and development.
Owner:EAST CHINA UNIV OF SCI & TECH
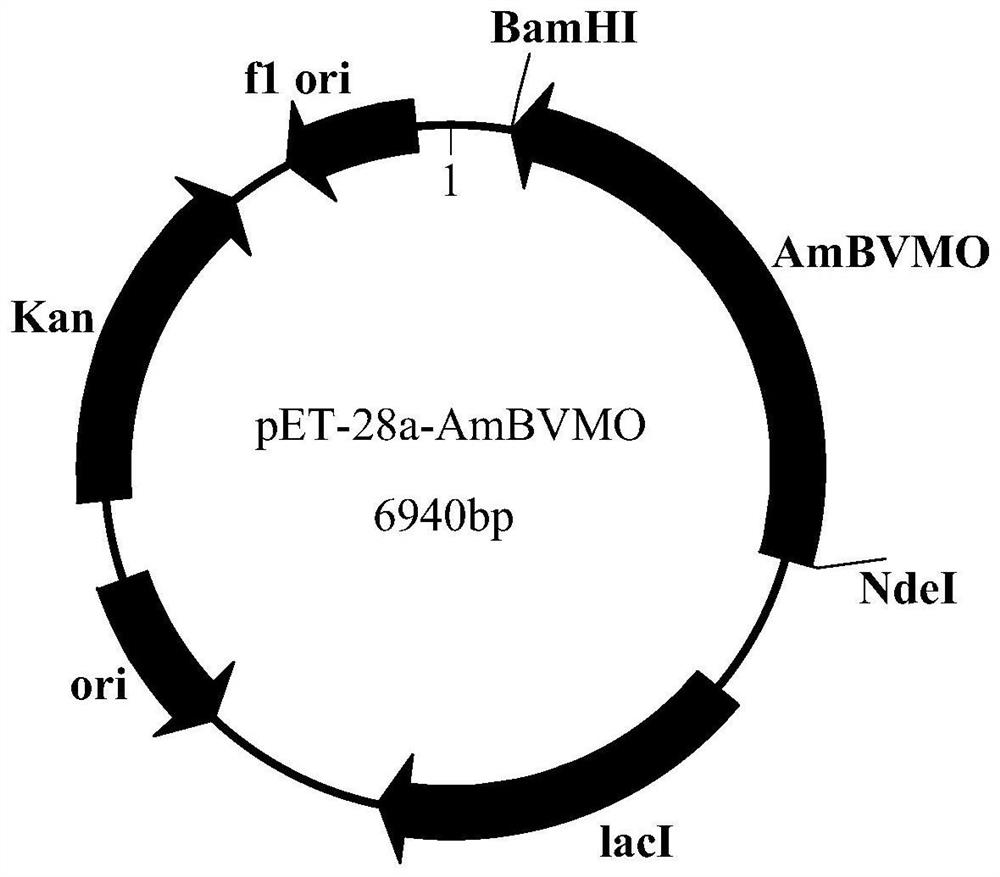
Baeyer-Villiger monooxygenase and application of Baeyer-Villiger monooxygenase
The invention discloses a Baeyer-Villiger monooxygenase and an application of the Baeyer Villiger monooxygenase, and belongs to the technical field of bioengineering. The invention provides another Baeyer-Villiger monooxygenase aiming at the problems of reported Baeyer-Villiger monooxygenase, such as low catalytic activity to substrate thioether, poor thermal stability, low ee value of a product and the like. The enzyme shows high stereoselectivity to linear ketone, cyclic ketone and thioether substrates, has high catalytic activity to cyclohexanone and thioanisole, and can be used for preparing high-purity products. Therefore, the problems of the existing monooxygenase can be directly and effectively solved. When diphenyl sulfide is used as a substrate to prepare S-benzosulfoxide, the conversion rate can reach 95% or above, and the yield can reach 99.5%. According to the present invention, a new thought and a new method are provided for industrial production of the optically active sulfoxide.
Owner:JIANGNAN UNIV
Recombinant bacterium expressing D-threonine aldolase and construction method and application of recombinant bacterium
ActiveCN110272856AGood catalyticWide substrate applicabilityBacteriaMicroorganism based processesCatalytic effectThreonine aldolase
The invention discloses a recombinant bacterium expressing a D-threonine aldolase and a construction method and application of the recombinant bacterium, and belongs to the technical field of enzyme engineering. The recombinant bacterium of the present invention expresses the D-threonine aldolase with an amino acid sequence as shown in SEQ ID NO. 2. The invention provides the D-threonine aldolase which can be used as a catalyst for the synthesis of chiral beta-hydroxy-alpha-amino acid, the catalytic efficiency (conversion rate) is high to 65%, the stereoselectivity is high with (e.e.) of 99% and (d.e.) of 95%, and the suitable reaction condition is mild and environment-friendly. According to the present invention, the D-threonine aldolase has good catalytic effect, wide substrate applicability, and good application and development prospect.
Owner:JIANGNAN UNIV
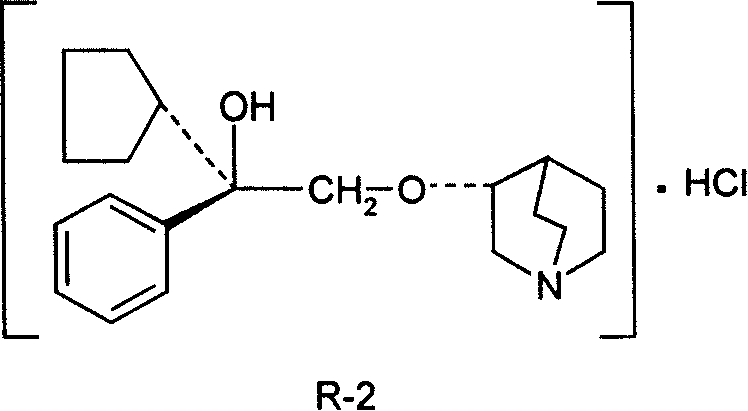
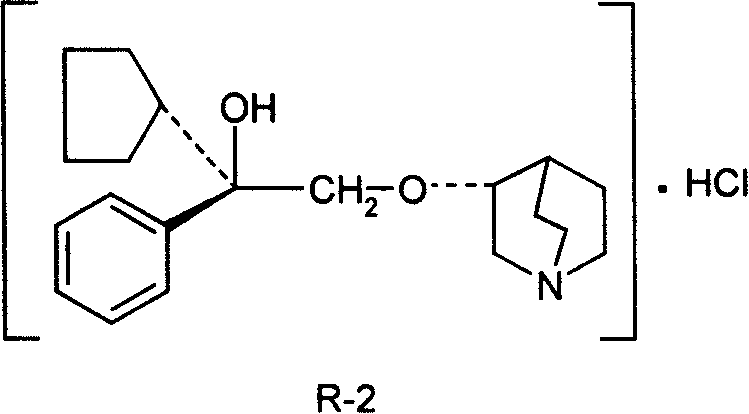
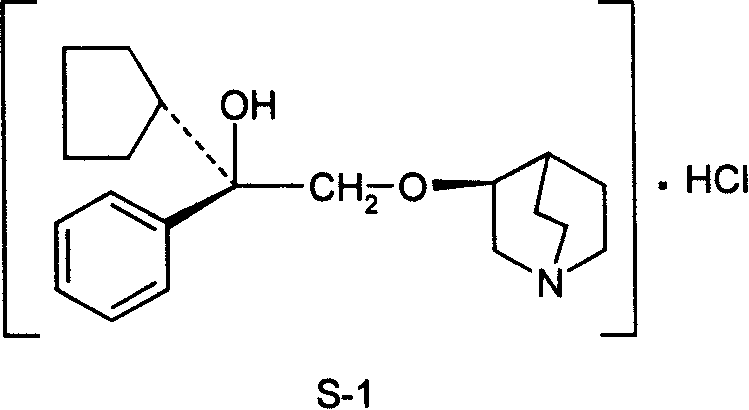
Pharmaceutical composition containing (3S, 2í»S)-3-(2í»-hydroxy-2í»-cyclopentyl-2í»-phenylethoxy) quinuclidine hydrochloride and uses thereof
InactiveCN101234107AHigh optical purityStereoselectiveOrganic active ingredientsUrinary disorderSide effectDisease
The invention discloses medical composition containing active component of (3S, 2' S)-3-(2'-hydroxyl-2'-cyclopentyl-2'-phenylethoxy) quinine quinuclidine chloride and application of the medical composition in preparing drugs for treating chronic obstructive lung disease, incontinentia urinae, diversified shock diseases and preparing adjuvant drugs used before anaesthesia. The active component of the medical composition of the invention has high optical purity, strong stereoselectivity in vivo process, definite curative effects and high pertinency to the disease, thus effectively avoiding generation of toxic and side effects.
Owner:CHENGDU LIST PHARMA
Reductase, reductase gene, recombinant enzyme, preparation method of recombinant enzyme and application
InactiveCN101979527AGood catalyticWide substrate applicabilityBacteriaOxidoreductasesEntire cellWild type
The invention discloses reductase, a reductase gene, a recombinant expression vector containing the gene, a recombinant expression transformant containing the gene, a recombinant enzyme of the reductase, a preparation method for the recombinant enzyme and application of the reductase or the recombinant enzyme of the reductase which serves as a catalyst to preparing optical active chiral alcohol from an asynchronous reductive prochiral carbonyl compound. The reductase or the recombinant enzyme of the reductase can be derived from Bacillus sp. China General Microbiological Culture Collectio Center (CGMCC) No. 2549 and applied to preparing the optical active chiral alcohol from the asynchronous reductive prochiral carbonyl compound when used as the catalyst, and has high catalysis efficiency and stereoselectivity, and the reaction conditions are mild and environment-friendly. Compared with the entire cell of the wild Bacillus sp. CGMCC No. 2549, the reductase of the invention has better catalysis effect, wider substrate applicability and excellent industrial application development prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Amino-acid ester derivative cation type chiral ionic liquid and preparation method thereof
ActiveCN105152949AChiral substanceRich sourcesOrganic compound preparationAmino-carboxyl compound preparationMannich reactionBrominated hydrocarbon
The invention provides an amino-acid ester derivative cation type chiral ionic liquid and a preparation method thereof. A general formula of the amino-acid ester derivative cation type chiral ionic liquid is A<+>X<->, wherein A<+> represents an amino-acid ester cation derivative, and X<-> represents one of BF4<->, PF6<-> and HSO4<->. The preparation method comprises steps as follows: amino acid, methyl alcohol and thionyl chloride react at the low temperature, and amino acid ester hydrochloride is obtained; amino acid ester hydrochloride has a Mannich reaction with paraformaldehyde and acetone, and amino acid ester derivative Mannich base is obtained; amino acid ester derivative Mannich base reacts with bromo-hydrocarbon, and a bromate type chiral ionic liquid of the amino-acid ester derivative is obtained; bromide ions of the bromate type chiral ionic liquid of the amino-acid ester derivative are exchanged, and the amino-acid ester derivative cation type chiral ionic liquid is obtained. The amino-acid ester derivative cation type chiral ionic liquid has the multiple advantages of high selectivity of a chiral substance as well as non-volatility, non-toxicity and pollution prevention of an ionic liquid, and is suitable for large-scale production of the fine chemical industry.
Owner:TANGSHAN NORMAL UNIV
Coding gene of meso-2,3-butanediol dehydrogenase, recombinase and preparation method and application of recombinase
InactiveCN105200068AGood catalyticWide substrate applicabilityBacteriaMicroorganism based processesBacillus licheniformisDiol
The invention discloses a coding gene of meso-2,3-butanediol dehydrogenase, a recombinant expression vector and recombinant expression transformant with the gene, a preparation method of recombinase, and application of the recombinase as a catalyst in an asymmetrically reducing prochiral dicarbonyl compound for preparing optical activity chiral diols. The meso-2,3-butanediol dehydrogenase comes from Bacillus licheniformis CICIMB0109, can be used as a catalyst for efficiently synthesizing chiral diols, is high in catalytic activity and stereoselectivity, mild in suitable reaction condition and friendly to the environment, and has quite good application and development prospects.
Owner:JIANGNAN UNIV
Synthetic method for 2-hydroxyl-1-indanone compounds
ActiveCN108129306AMild reaction conditionsLow costOrganic compound preparationCarboxylic acid esters preparationQuinidineSolvent
The invention belongs to the technical field of organic chemistry, and specifically relates to a synthetic method for 2-hydroxyl-1-indanone compounds. The method uses an indanone compound as a raw material, a peroxide as an oxidant and a quinidine or a quinine derivative as a catalyst, in the presence of a solvent, the indanone compound and the peroxide are subjected to an asymmetric hydroxylation, and therefore the 2-hydroxyl-1-indanone compounds with stereoselectivity are obtained. Compared with a previous method, the method provided by the invention has the advantages that the catalyst canbe quantitatively recovered, the reaction conditions are mild, the costs are low, the yield is high, and the stereoselectivity is strong, and is suitable for industrialized production.
Owner:金华奥布朗医药科技有限公司
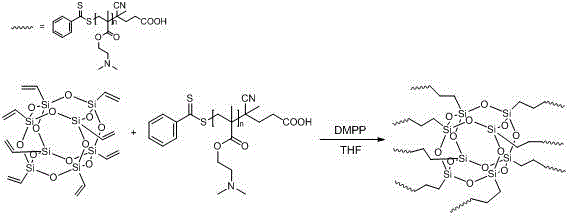
Preparation method of temperature and pH sensitive organic/inorganic hybrid material POSS-PDMAEMA
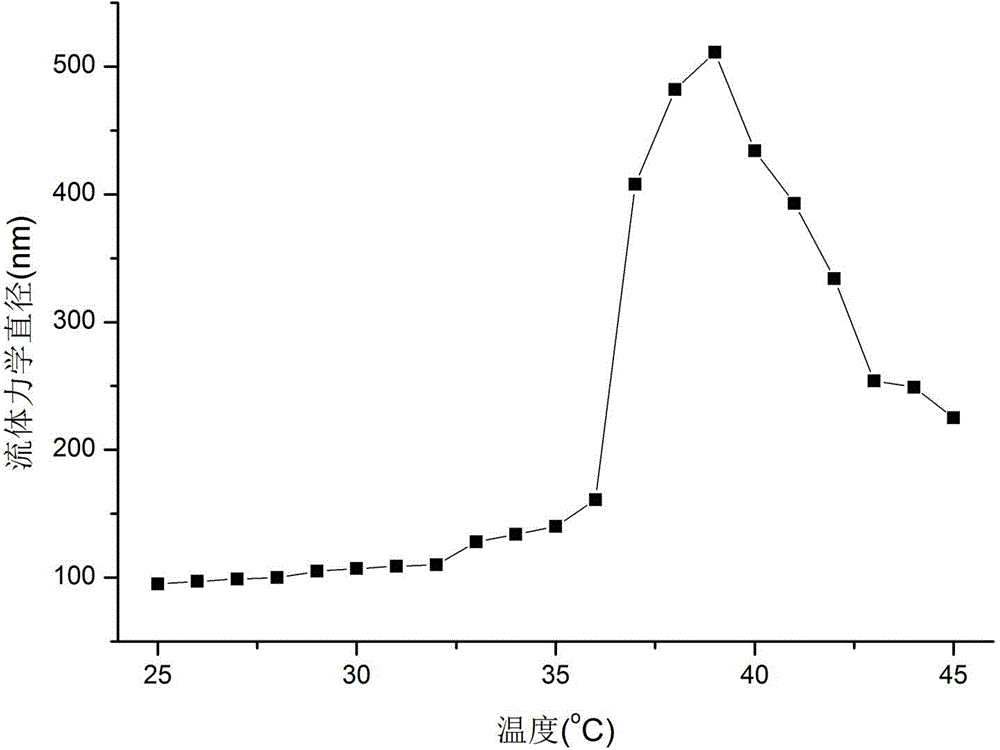
The invention belongs to the technical filed chemical engineering and new materials, and concretely relates to a preparation method of a temperature and pH sensitive organic / inorganic hybrid material POSS-PDMAEMA. The method comprises the following steps: synthesizing polydimethylaminoethyl methacrylate through adopting a reversible addition-fragmentation chain transfer polymerization technology, and reacting polydimethylaminoethyl methacrylate with octavinyl POSS through a thiol-ene click chemical reaction to generate the organic / inorganic hybrid material POSS-PDMAEMA. In an aqueous solution, the POSS-PDMAEMA self-assembles with the change of the temperature and the pH value and forms micelle. The method has the characteristics of simplicity, convenience, high preparation yield and no pollution to environment, and the prepared organic / inorganic hybrid material POSS-PDMAEMA has temperature and pH sensitivity, is a new-generation high-performance intelligent material product, and can be applied to medicinal fields of organic dye adsorption, heavy metal adsorption and in vivo delivery of insoluble anticancer medicines.
Owner:TONGJI UNIV
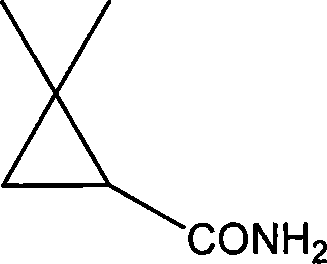
Epidermis brevibacterium ZJB-07021 and use for preparation of (S)-2,2-dimethyl cyclopropane formamide thereof
The invention provides a novel strain which is Brevibacterium epidermidis ZJB-07021 and the application in the microbiological preparation of (S)-2, 2-dimethylcycloproprane carboxamide. The beneficial effects of the invention mainly lie in that the invention provides a novel strain which has stereo selectivity in the production of optically pure (S)-2, 2-dimethylcycloproprane carboxamide; and the strain can be used for the production of optically pure (S)-2, 2-dimethylcycloproprane carboxamide. The invention obtains a novel strain which is different from the previous research through screening; the invention provides the basis for the investigation on the variety of microbial strain in the chiral resolution aspect and the further comparison of the difference of transformation pathway and reaction mechanism among various strains.
Owner:ZHEJIANG UNIV OF TECH +1
Cyclohexanone monooxygenase and application thereof
PendingCN112410312AImprove solubilityHigh catalytic efficiencyOxidoreductasesFermentationCyclohexanonePtru catalyst
The invention discloses a cyclohexanone monooxygenase and application thereof, and belongs to the technical field of bioengineering. The cyclohexanone monooxygenase disclosed by the invention is derived from Amycolatopsis methanolica, and can be used as a catalyst for efficient synthesis of thioether. According to the invention, thioether such as cyclic ketone and linear ketone can be taken as a substrate, and the prepared cyclohexanone monooxygenase can tolerate high concentration of substrates, and has high catalytic activity and strong stereoselectivity, which is environmentally friendly and has a good application and development prospect.
Owner:JIANGNAN UNIV
New (R)-transaminase from Fusarium oxysporum and application thereof
InactiveCN104630171ABroad amino donor substrate spectrumIncrease vitalityBacteriaTransferasesFusarium oxysporumNucleotide
The invention discloses a new (R)-transaminase and application thereof. The (R)-transaminase derives from Fusarium oxysporum Fo5176 (named HFO), the gene nucleotide sequence is shown as SEQ ID No.1 and the amino acid sequence is shown as SEQ ID No.2. The new (R)-transaminase HFO is a strict (R) stereoselective Omega-transaminase, has a broad substrate spectrum, and has great application potential in bio-production of chiral amines and unnatural amino acids.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
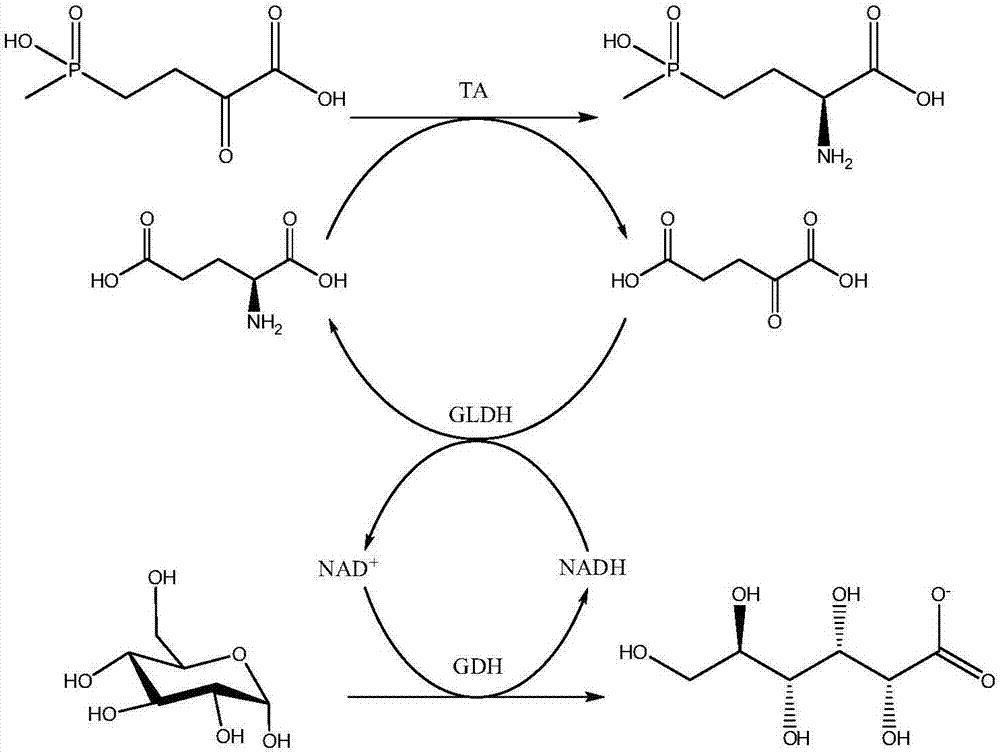
L-glutamate dehydrogenase mutant and application thereof
ActiveCN111979208AImprove efficiencyImprove conversion rateMicroorganism based processesOxidoreductasesAcyl CoA dehydrogenaseGlutamate Dehydrogenase (NADP+)
The invention discloses an L-glutamate dehydrogenase mutant. The sequence of the L-glutamate dehydrogenase mutant is obtained by mutating a 175 amino acid residue A of the sequence shown in SEQ IDNO. 1 into G and mutating a 386 amino acid residue V into an amino acid residue with smaller steric hindrance. The invention also discloses application of the L-glutamate dehydrogenase mutant inpreparation of L glufosinate-ammonium or salts thereof. When the L-glutamate dehydrogenase mutant is used for preparing the L-glufosinate-ammonium or salts thereof, compared with the L-glutamate dehydrogenase mutant which is only mutated at the 175 site or 386 site, the L-glutamate dehydrogenase mutant has higher enzyme activity, thereby improving the action efficiency of the enzyme, decreasing the reaction cost and being beneficial to industrial production.
Owner:SHANGHAI QIZHOU ZIYUE BIOTECHNOLOGY CO LTD
Method for preparing optically pure 3-amino butyl alcohol
ActiveCN101417954BStereoselectiveHigh optical purityOrganic compound preparationAsymmetric synthesesAcetoacetatesPotassium borohydride
The invention discloses a method for preparing optical pure 3-aminobutanol, which comprises the following steps: acetylacetic ester and chiral phenethylamine react to generate 3-(1'-benzylmethylamine)-2-crotonate enantiomer, namely 3-(1'-benzylmethylamine)-2-crotonate; potassium borohydride triacetate or sodium borohydride triacetate is used to reduce the 3-(1'-benzylmethylamine)-2-crotonate enantiomer into a 3-(1'-benzylmethylamine)-2-butyric ester enantiomer; then chiral pure 3-(1'-benzylmethylamine)-2-butyric ester is obtained through salification and resolution; and the 3-(1'-benzylmethylamine)-2-butyric ester is subject to reducing debenzylation by palladium-carbon to obtain the optical pure 3-aminobutanol. The method has low cost and high product purity, and is suitable for industrialized production.
Owner:雅本(绍兴)药业有限公司
Amino acid ester cationic chiral ionic liquid and preparation method thereof
ActiveCN106397239AStereoselectiveWide liquid rangeThiol preparationOrganic compound preparationStereoselectivityStructural diversity
The invention provides an amino acid ester cationic chiral ionic liquid and a preparation method thereof. The amino acid ester cationic chiral ionic liquid has a structural formula shown in the description. In the structural formula, R is a variable group of alpha-amino acid, R' is a C1-3 alkyl group, and X is one of BF4, PF6 and HSO4. The chiral position is in an amino acid cation, the amino group is connected with a large-volume group, so the amino acid ester cationic chiral ionic liquid has the advantages of high stereoselectivity, structure diversity and good designability.
Owner:TANGSHAN NORMAL UNIV
Compound peniroquesine A with anti-inflammatory activity as well as preparation method and application of compound peniroquesine A
ActiveCN107954839ARich diversityGood anti-inflammatory activityOrganic chemistryAntipyreticSesterterpenesStereochemistry
The invention relates to the technical field of terpenes and provides a compound peniroquesine A with anti-inflammatory activity. The structure of the compound is shown as formula I in the description. The compound belongs to sesterterpenes and has remarkable anti-inflammatory activity. The compound peniroquesine A with the anti-inflammatory activity is sesterterpenes with novel 5 / 6 / 5 / 6 / 5 five-ring skeleton structure, and variety of the sesterterpenes is enriched. The invention further provides a preparation method of the compound peniroquesine A with anti-inflammatory activity. The compound peniroquesine A is prepared from Penicillium roqueforti by fermenting. The method is simple, fast, low in cost and suitable for large-scale industrial production.
Owner:YUNNAN UNIV
Biosynthesis method of L-carnitine
The invention discloses a biosynthesis method of L-carnitine. The biosynthesis method comprises: 1) dissolving a substrate ethyl 4-chloroacetoacetate in a MOPS buffer solution, adding ketoreductase-containing engineering bacteria whole cells, coenzyme, butylene glycol, a surfactant and an additive, and carrying out a reaction to generate (R)-4-chloro-3-hydroxy-butyric acid ethyl ester, wherein thepH value is controlled at 6.5-7.5; and 2) dissolving sodium hydroxide in a trimethylamine aqueous solution with a mass ratio of 25% to form a mixed solution, slowly adding the mixed solution to the (R)-4-chloro-3-hydroxy-butyric acid ethyl ester generated in the step 1) at a temperature of -5-5 DEG C in a dropwise manner, carrying out a reaction for 20-30 h at a temperature of -5-5 DEG C, stopping the reaction, adjusting the pH value to 5-7, and purifying to obtain L-carnitine. According to the present invention, the biosynthesis method has characteristics of strong stereoselectivity, mild reaction conditions, low cost, low pollution, high efficiency, high yield and high optical purity of the product.
Owner:武汉佰施达生物技术有限公司
Soluble graphdiyne derivative and preparation method and application thereof
ActiveCN111574465AOvercoming dispersionOvercome solubilityOrganic chemistryChlorobenzeneOrganic solvent
The invention belongs to the technical field of functional materials, and particularly relates to a soluble graphdiyne derivative, and a preparation method and application thereof. Compared with graphdiyne, the dissolvability of the graphdiyne derivative disclosed by the invention in an organic solvent is greatly improved. For example, the graphdiyne derivative provided by the invention has good solubility in polar organic solvents such as dichloromethane, chlorobenzene, N-methylpyrrolidone (NMP) and tetrahydrofuran, and the concentration of the graphdiyne derivative is up to 5mg.ml<-1> in chlorobenzene; and therefore, the problem that graphdiyne is difficult to disperse or dissolve in a solution is solved, and the graphdiyne derivative has great application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for preparing R-o-chloromandelic acid methyl ester through biocatalysis dynamic kinetic resolution
ActiveCN103820521AGood technical effectHigh yieldBacteriaMicroorganism based processesEnzyme systemMethyl o-chloromandelate
The invention relates to the field of biological engineering and discloses a method for preparing R-o-chloromandelic acid methyl ester through biocatalysis dynamic kinetic resolution. Under a condition that pH is 7-8, recombined racemase and recombined esterase are linked or gene engineering bacteria capable of respectively expressing the recombined racemase and the recombined esterase are linked to implement conversion by taking racemized o-chloromandelic acid methyl ester as a substrate; the recombined racemase is recombined mandelic acid racemase, and the recombined esterase is recombined BioH esterase. The method disclosed by the invention has the advantages that the recombined racemase and the recombined esterase are linked or the gene engineering bacteria containing the recombined racemase and the recombined esterase are linked, so that the aim of preparation is fulfilled; the technology is simple; the conversion efficiency is high; the problem of instability caused by a double-enzyme system is solved; the method has higher application value.
Owner:ZHEJIANG UNIV +1
Preparation method for polyhydroxy functional POSS hybrid material
InactiveCN109180943ANo pollution in the processOvercome the disadvantages of poor biocompatibilityUltraviolet lightsPolyethylene glycol
The invention discloses a preparation method for a polyhydroxy functional POSS hybrid material. The preparation method is characterized by the following steps: adding reactants, namely, polyethylene glycol derivative PEG (C=CH2) containing unsaturated carbon-carbon double bond at one end, olefinic organism (RHC=CH2), octa-sulfydryl POSS and dissolvent into a reactor; adding radical initiator afterthe added reactants dissolve and uniformly disperse; reacting for 40min-4h under the nitrogen shielding condition at 30-80 DEG C or under an ultraviolet light condition, thereby acquiring the POSS hybrid material; removing the remained radical initiator and unreacted reactants, and drying, thereby acquiring the polyhydroxy functional POSS hybrid material. The preparation method disclosed by the invention has the advantages of simplicity in operation, easily acquired reaction materials, fast reaction and mild reaction condition. The polyhydroxy functional POSS hybrid material prepared according to the invention has the advantages of high biocompatibility, controllable hydrophily and hydrophobicity, controllable form under condensed state, multifunctional reaction group and the like.
Owner:DONGHUA UNIV
Popular searches
Simple refining process Raw materials are easy to get Simple production process Suitable for large-scale industrial production applications Residue reduction The process route is green and environmentally friendly Reasonable design Easy post-processing Mild conditions No need for high temperature and high pressure
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com