Compound peniroquesine A with anti-inflammatory activity as well as preparation method and application of compound peniroquesine A
A compound and active technology, applied in the field of anti-inflammatory active compound peniroquesine A and its preparation, can solve the problems of difficult to fully utilize the anti-inflammatory activity of sesquiterpene compounds, high cost of sesquiterpene compounds, abuse of antibiotics, etc., to achieve It is easy to realize industrialization, the production method cycle is short, and the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of the anti-inflammatory compound peniroquesine A according to the above technical scheme, which comprises the following steps:
[0043] (1) Inoculating Penicillium Louvum into the fermentation medium and fermenting to obtain the fermentation product of Penicillium Louvum;
[0044] The preservation number of the said Penicillium Louvum is CGMCC NO.14140;
[0045] (2) Mixing the fermented product of Penicillium Louvum from the step (1) with an alcohol solution and then ultrasonically extracting to obtain a crude extract of peniroquesine A;
[0046] (3) Purify the crude extract of peniroquesine A by chromatography to obtain the anti-inflammatory active compound peniroquesine A.
[0047] In the present invention, the Penicillium Louvum with the preservation number of CGMCC NO. 14140 is inoculated into the fermentation medium and fermented to obtain the fermentation product of Penicillium Louvum. The fermentation temperature i...
Embodiment 1
[0065] Example 1 Preparation of anti-inflammatory compound peniroquesine A
[0066] 1. Strain activation: Inoculate Penicillium Loudiflorum (preservation number CGMCC NO.14140) on the PDA slant medium, culture at 28°C for 3-7 days, and store in a refrigerator at 4°C for later use.
[0067] 2. Preparation of fermentation medium: Take the washed potatoes, divide them into potato pieces with a diameter of 1 cm, and place them in a tissue culture flask, 50g / bottle. After the tissue culture flask is capped, sterilize it at 120°C for 30 minutes at high temperature, and then cool it. Fermentation medium.
[0068] 3. Inoculate the activated Penicillium louboutin in step 1 into the fermentation medium prepared in step 2 at an inoculum of 1%, cover it and incubate it at 28° C. for 30 days to obtain a fermentation product of Penicillium louboutin.
[0069] 4. Take 50g of the fermented product of Peniroquesine obtained in step 3 and mix with 100mL of methanol according to the mass-volume ratio. T...
Embodiment 2
[0071] Example 2 Identification of structure
[0072] Take the anti-inflammatory compound peniroquesine A prepared in Example 1, and identify the compound peniroquesine by 1D / 2D NMR (one-dimensional nuclear magnetic resonance spectroscopy and two-dimensional nuclear magnetic resonance spectroscopy) and HR-ESI-MS (high resolution electrospray ionization mass spectrometry) A and its structure.
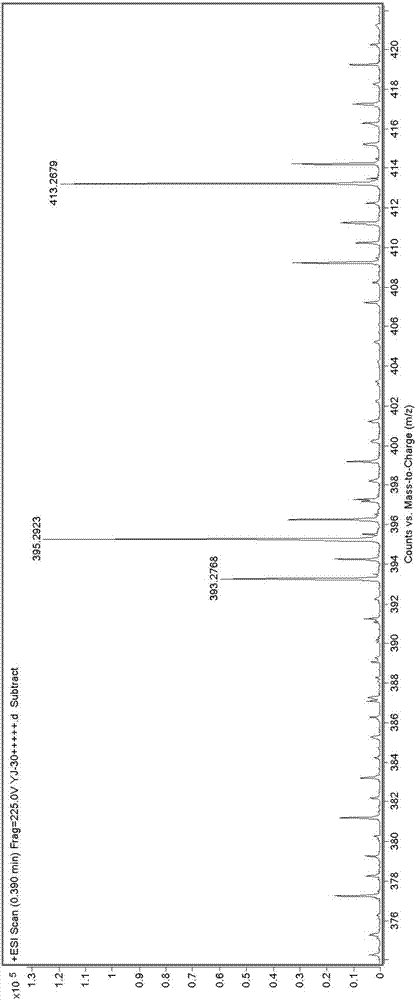
[0073] (1) The HR-ESI-MS spectrum of peniroquesine A is attached figure 1 , See the results figure 2 :
[0074] According to the attached figure 1 And figure 2 It can be seen that the molecular formula of the compound is C 25 H 40 O 2 (m / z:395.2923[M+Na] + , Calculated value: 395.2926), the degree of unsaturation is 6.
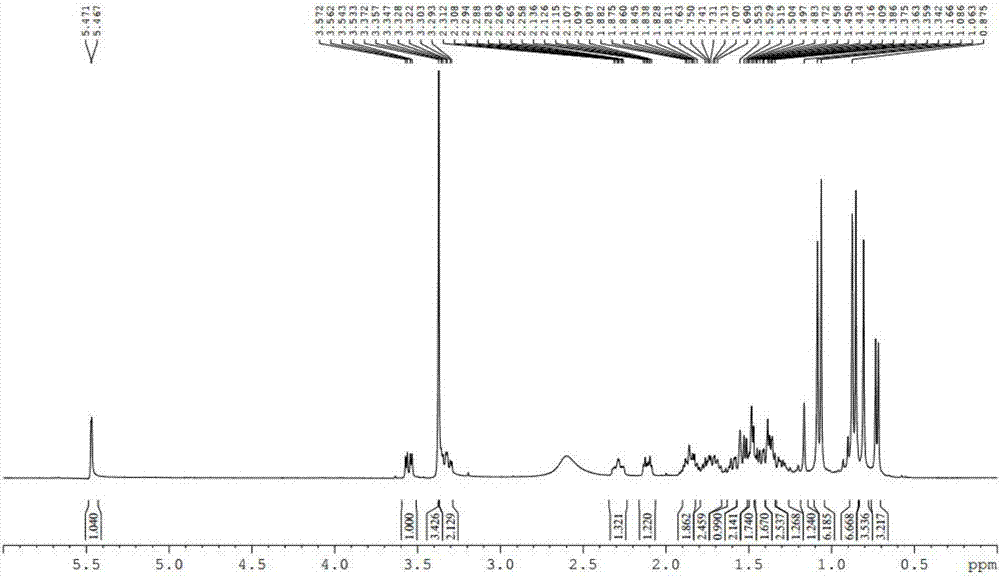
[0075] (2) The results of 1D / 2DNMR detection are as attached Figure 3~7 As shown,
[0076] By the attachment image 3 , 4 It can be seen that the compound 1 H and 13 C NMR data show that it contains a double bond (δ H : 5.57s, 1H; δ C : 135.5d), suggesting that the compound co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com