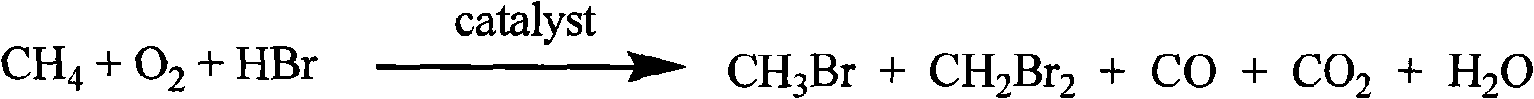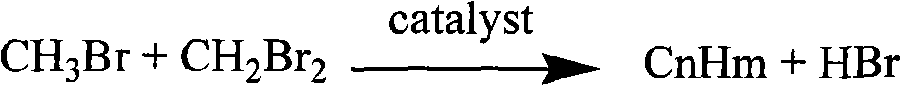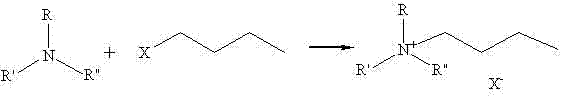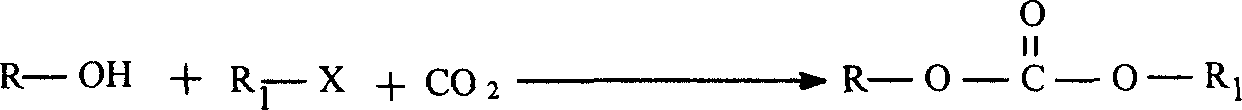Patents
Literature
30 results about "Brominated hydrocarbon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Brominated hydrocarbons — is hydrocarbons, which are substituted by bromine, one, two or more atoms of hydrogen. In most — liquid, very soluble in water. Some brominated hydrocarbons (methyl bromide, bromoform) have a strong odor, others (methyl bromide), even in high concentrations have a weak ethereal odor.
Bromomethane prepared by bromine oxidation of methane and catalyst for conversing the bromomethane into hydrocarbon
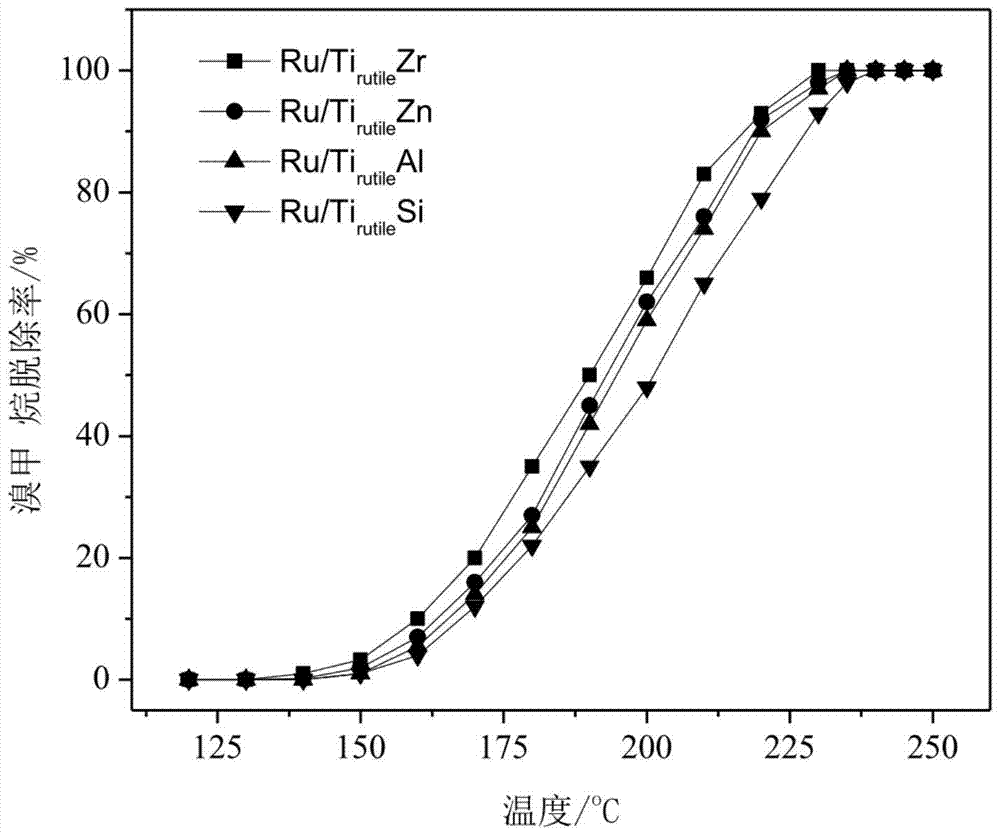
The invention discloses a catalyst which is used when methane is transformed into hydrobromic ether through the bromine oxidation reaction, and the catalyst which is used when preparing heavy hydrocarbons by using the hydrobromic ether further, and belongs to the technology field of the catalyst and the preparation method thereof. The first type of catalyst which is used when the methane is transformed into the hydrobromic ether through the bromine oxidation reaction comprises the preparation step that water-soluble metallic compound precursors such as Rh, Ru, Cu, Zn, Ag, Ce, V, W, Cd, Mo, Mn, Cr, La, etc. are mixed with a silica precursor, the mixture is processed through hydrolyzation, drying and roasting, and then the catalyst is obtained; the first type of catalyst can lead definite masses of reactant composed of the methane, HBr, H2O and oxygen sources (that is O2, air or oxygen-enriched air) to perform the catalytic bromine oxidation reaction, to generate target products such as CH3Br, CH2Br2, etc. The second type of catalyst, which is used when preparing the heavy hydrocarbons by using the hydrobromic ether further, includes the preparation step that molecular sieves such as HZSM-5, HY, H Beta, 3A, 4A, 5A, 13X, etc. load metallic compounds composed of Zn and Mg, so that the catalyst is obtained; the second type of catalyst can lead the methane bromination products composed of the CH3Br and the CH2Br2 to further react so as to generate the heavy hydrocarbons containing C3 to C13 and HBr, wherein, the HBr is used as a circular reaction medium.
Owner:MICROVAST POWER SYST CO LTD
Stainless steel degreasing agent
InactiveCN101979711AImprove solubilityImproves the properties of uniform corrosionActive agentBrominated hydrocarbon
The invention provides a stainless steel degreasing agent, which comprises the following components in percentage by weight: 5 to 25 percent of organic solvent, 2 to 5 percent of corrosion inhibitor, 10 to 25 percent of surfactant, 5 to 10 percent of inorganic salt, 1 to 3 percent of defoaming agent and the balance of deionized water, wherein the organic solvent is bromohydrocarbon; the corrosion inhibitor is sodium benzoate, ammonium benzoate, triethanolamine, ethanolamine or benzotriazole; the surfactant is one or more of alkylphenol polyoxyethylene ether and fatty alcohol-polyoxyethylene ether; the inorganic salt is alkaline salt; and the defoaming agent is tributyl phosphate, organic silicon, oleic acid or sodium oleate. The stainless steel degreasing agent can replace the currently used ODS cleaning agents, can achieve the cleaning effect of the ODS cleaning agents, does not damage the surface of the stainless steel, has obvious cleaning effect, can clean a large number of products, and does not harm a human body; and the preparation method is simple, safe, and environment-friendly.
Owner:JIANGSU TIANHENG NANO SCI & TECH
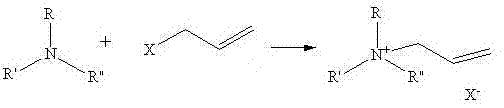
Method for synthesizing quaternary ammonium salt ionic liquid by microwave radiation heating
InactiveCN102344377AGood acid and alkali resistanceReduce volumeOrganic compound preparationAmino compound preparationHalohydrocarbonButyl chloride
The invention provides a method for synthesizing quaternary ammonium salt ionic liquid by microwave radiation heating, which comprises the following steps: mixing tertiary amine compounds (N,N-dimethylethanolamine, trimethylamine or triethanolamine) and halohydrocarbon compounds (allyl chloride, n-butyl chloride, n-butyl bromide, octadecyl chloride) with a molar ratio of 1:1.05-1:2 under a microwave radiation heating condition, putting the mixture into a microwave reactor to perform a microwave reaction with an adjusted microwave power of 100 W-400 W, a heating temperature of 30 DEG C-80 DEG C, and reaction time of 1 min-15 min so as to obtain a crude product, removing residual halohydrocarbon compounds in the crude product to obtain quaternary ammonium salt ionic liquid with halogens as anions; the halohydrocarbon compounds are chlorinated hydrocarbon compounds or brominated hydrocarbon compounds. The preparation method of the invention is simple, does not require the addition of any solvent except reaction raw materials, and can prepare high-purity quaternary ammonium salt ionic liquid simply, efficiently, rapidly, and economically.
Owner:SOUTH CHINA UNIV OF TECH
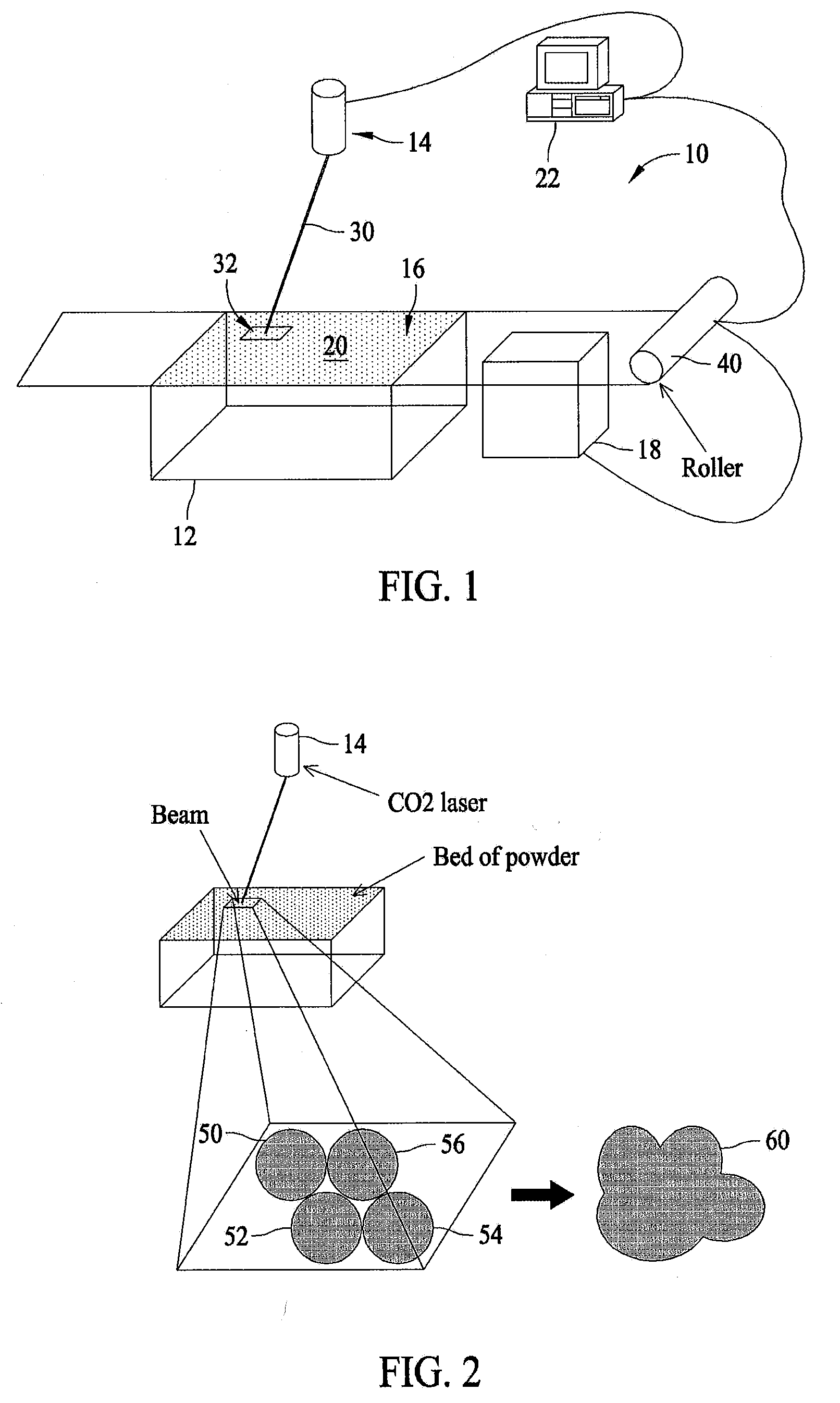
Methods and systems for fabricating fire retardant materials
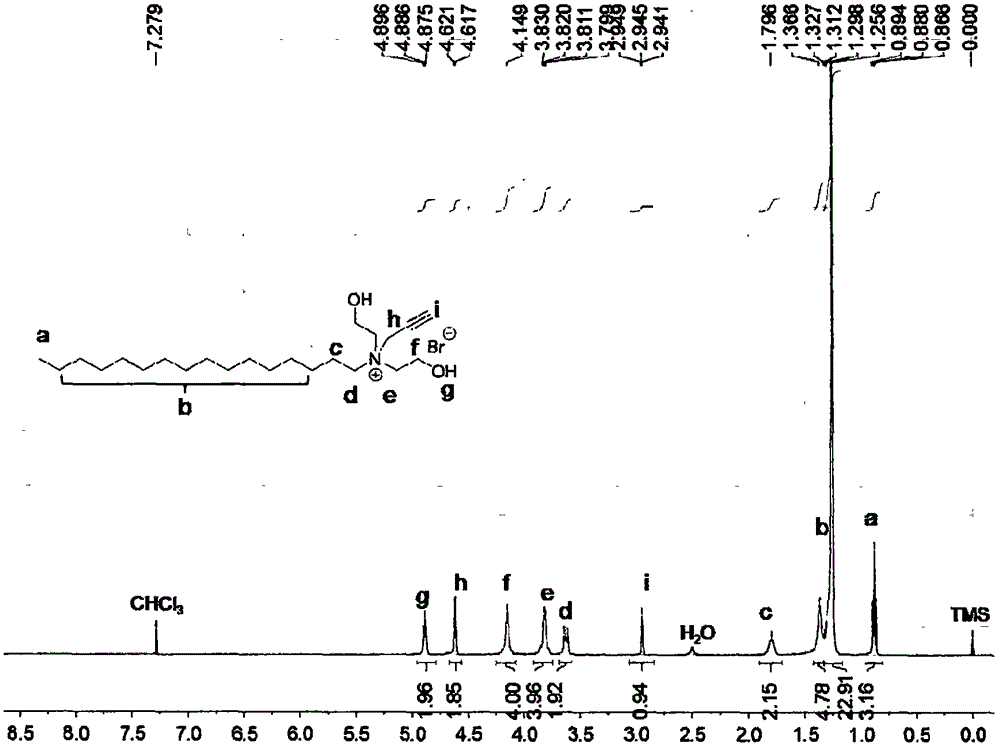
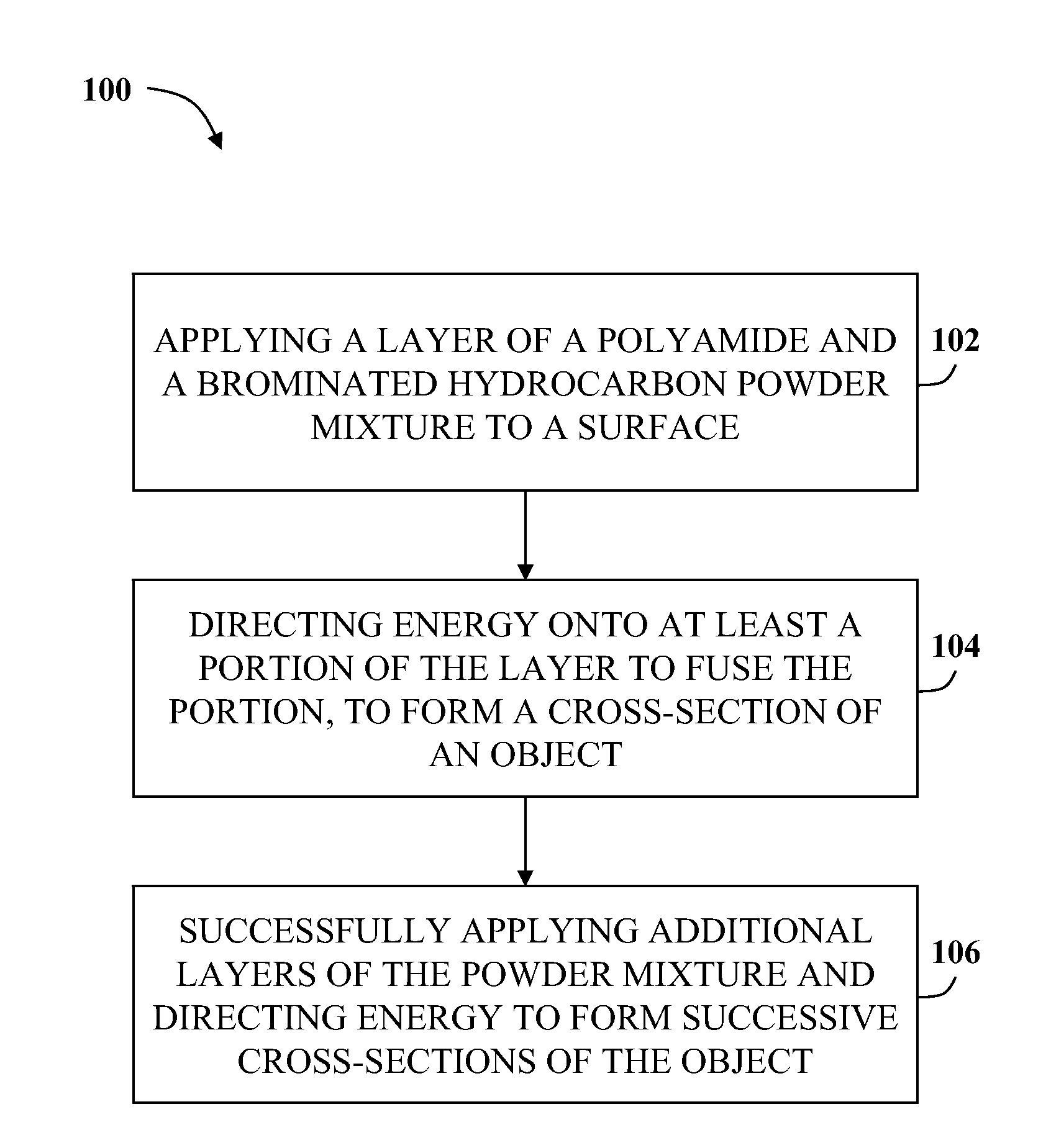
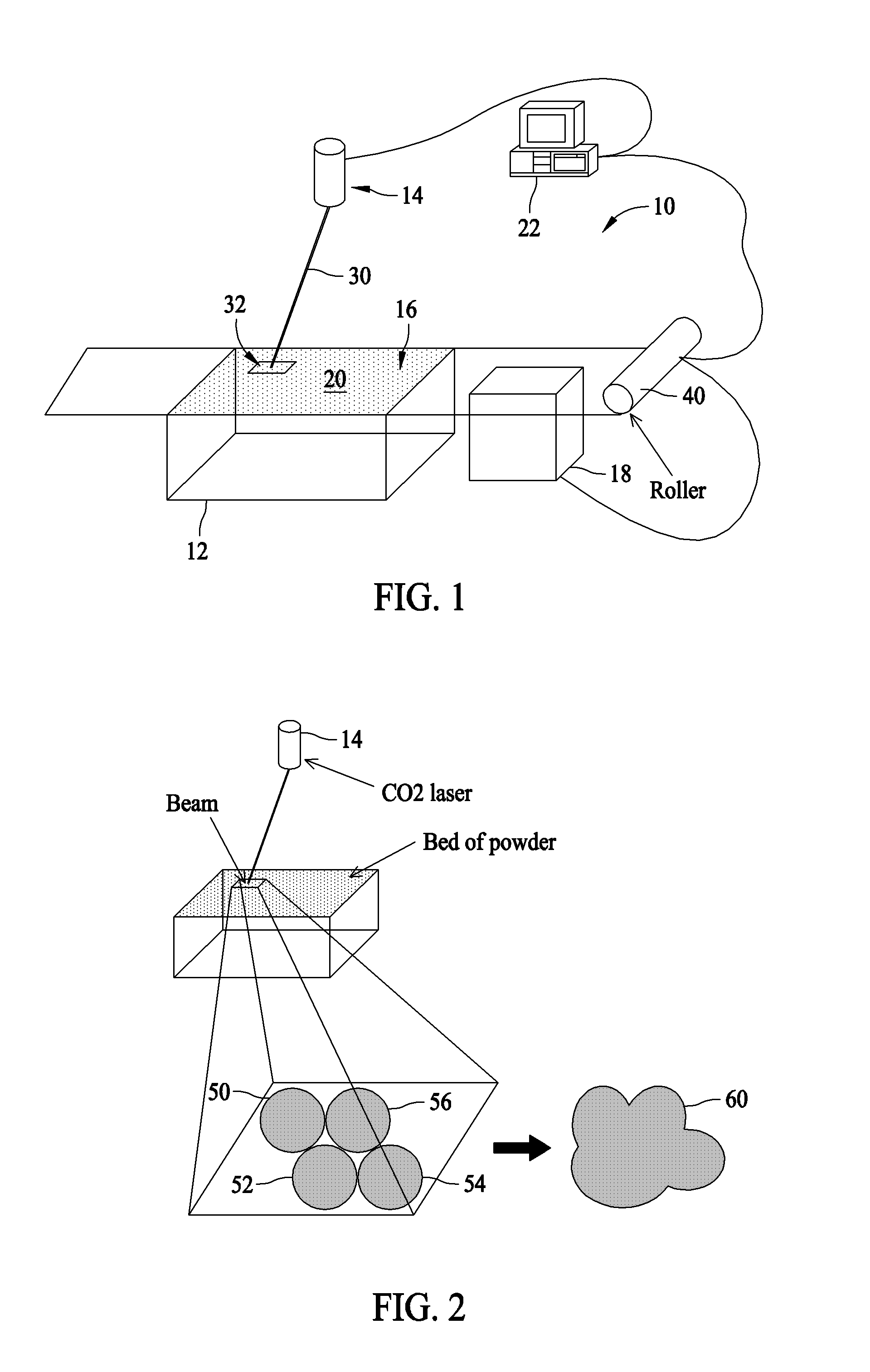
InactiveUS20080153947A1Additive manufacturing apparatusAdditive manufacturing with solidsPolyamideBrominated hydrocarbon
A fire retardant material is described that includes a polyamide and a brominated hydrocarbon. The brominated hydrocarbon makes up between about two percent and about 25 percent of the fire retardant material.
Owner:THE BOEING CO
Method for preparing methyl bromide, high-carbon hydrocarbon, methyl alcohol and dimethyl ether by methane
The invention discloses a method which is characterized in that methane is converted into bromohydrocarbon by bromine oxidation reaction, and bromohydrocarbon is further prepared into high-carbon hydrocarbon, methyl alcohol and dimethyl ether. Firstly, methane, HBr / H2O and oxygen react to generate bromo-alkane; the catalyst is a composite catalyst prepared by mixed hydrolysis, drying and roasting of silicon precursor and metallic compound precursor capable of dissolving in water; then, the bromo-alkane is performed with de-HBr to prepare high-carbon hydrocarbon, or bromo-alkane reacts with water to prepare methyl alcohol and dimethyl.
Owner:MICROVAST POWER SYST CO LTD
Alkynyl quaternary ammonium salt multifunctional surfactants and preparation method thereof
InactiveCN106187790AHas viscoelastic surfactant functionGood corrosion inhibitionOrganic compound preparationDrilling compositionViscous liquidQuaternary ammonium cation
The invention discloses a series of alkynyl quaternary ammonium salt multifunctional surfactants and a preparation method thereof. A structural formula of quaternary ammonium salt is shown in the description, wherein R represents a C4-C22 linear, branched or cycloalkyl, aryl or alkenyl group. The preparation method comprises the following steps: (1) carrying out alkylation on ethanol amine under alkaline and acetonitrile reflux conditions by virtue of bromo-hydrocarbon; and (2) carrying out further alkylation on tertiary amine under an ethanol reflux condition by virtue of propargyl bromide, so as to obtain alkynyl quaternary ammonium salt, namely reddish brown semitransparent viscous liquid or solid. The alkynyl quaternary ammonium salt multifunctional surfactants have very high surface activity, good corrosion resistance and excellent viscoelasticity, the preparation method is simple and feasible, the raw material cost is low, the operation is easy, the yield is high, and the surfactants are environmentally friendly.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Method for preparing solvent system capable of dissolving cellulose under room temperature condition
The invention discloses a method for preparing a solvent system capable of dissolving cellulose under a room temperature condition. The solvent system is composed of ionic liquid and dimethyl sulfoxide, wherein anions in the ionic liquids are acetate ions, cations are N-R1 and N-R2 imidazole cations; in the formula, R1 is methyl or ethyl, R2 is ethyl, allyl or butyl. The method comprises the following preparation steps: mixing and fully dissolving N-methylimidazole or N-ethylimidazole and the dimethyl sulfoxide; slowly dripping bromo-hydrocarbon R2Br under an ice bath condition to react; heating to carry out a reflux reaction at a temperature of 40-70 DEG C; and adding a saturated lead acetate or silver acetate solution, stirring to react, filtering precipitate, and removing water in filtrate through a rotary evaporation method to prepare the solvent system. The method disclosed by the invention has the advantages that the preparation method is short in reaction time, free of pollution and high in synthesis efficiency and conversion rate; and the solvent system can dissolve a large amount of non-activated or non-pretreated cellulose under the room temperature condition.
Owner:NANKAI UNIV +1
Methods and Systems for Fabricating Fire Retardant Materials
ActiveUS20100255327A1Additive manufacturing apparatusSynthetic resin layered productsPolyamideBrominated hydrocarbon
A fire retardant material is described that includes a polyamide and a brominated hydrocarbon. The brominated hydrocarbon makes up between about two percent and about 25 percent of the fire retardant material.
Owner:THE BOEING CO
Separation of components from methanol mixtures by extractive distillation
InactiveUS6383343B1Organic compound preparationOxygen compounds purification/separationExtractive distillationParaffin oils
A method of separating methanol and acetone, and methanol and methyl acetate involves distilling a mixture of the components by an extractive distillation process in the presence of an extractive distillation solvent. The extractive distillation solvent may be an amine, a chlorinated hydrocarbon, a brominated hydrocarbon, a paraffin, and an alkylated thiopene.
Owner:TRANS SCRIPTO
Amino-acid ester derivative cation type chiral ionic liquid and preparation method thereof
ActiveCN105152949AChiral substanceRich sourcesOrganic compound preparationAmino-carboxyl compound preparationMannich reactionBrominated hydrocarbon
The invention provides an amino-acid ester derivative cation type chiral ionic liquid and a preparation method thereof. A general formula of the amino-acid ester derivative cation type chiral ionic liquid is A<+>X<->, wherein A<+> represents an amino-acid ester cation derivative, and X<-> represents one of BF4<->, PF6<-> and HSO4<->. The preparation method comprises steps as follows: amino acid, methyl alcohol and thionyl chloride react at the low temperature, and amino acid ester hydrochloride is obtained; amino acid ester hydrochloride has a Mannich reaction with paraformaldehyde and acetone, and amino acid ester derivative Mannich base is obtained; amino acid ester derivative Mannich base reacts with bromo-hydrocarbon, and a bromate type chiral ionic liquid of the amino-acid ester derivative is obtained; bromide ions of the bromate type chiral ionic liquid of the amino-acid ester derivative are exchanged, and the amino-acid ester derivative cation type chiral ionic liquid is obtained. The amino-acid ester derivative cation type chiral ionic liquid has the multiple advantages of high selectivity of a chiral substance as well as non-volatility, non-toxicity and pollution prevention of an ionic liquid, and is suitable for large-scale production of the fine chemical industry.
Owner:TANGSHAN NORMAL UNIV
Method for separating aromatic hydrocarbon and sulfur-containing aromatic hydrocarbon from heavy aromatic hydrocarbon
ActiveCN104119950AEfficient separationMeet the requirements of subsequent analysisTreatment with plural serial refining stagesStationary phaseBrominated hydrocarbon
A method for separating aromatic hydrocarbon and sulfur-containing aromatic hydrocarbon from heavy aromatic hydrocarbon. The method comprises the steps of: separating aromatic hydrocarbon components from heavy oil; adding an organic solution of PdCl2 into the separated aromatic hydrocarbon components and by fully mixing and reacting; then adding saturated hydrocarbon for liquid phase extraction; adding the saturated hydrocarbon solution obtained from liquid phase extraction into an extraction column filled with a stationary phase, so that the aromatic hydrocarbon components and aromatic sulfur-containing hydrocarbon components absorbed by the stationary phase; washing the stationary phase with saturated hydrocarbon to obtain a solution containing aromatics; then flushing the stationary phase with a polar solvent to obtain a solution containing sulfur-containing aromatic solution; respectively evaporating solvents in the solution containing aromatic hydrocarbon and the sulfur-containing aromatic solution, so as to obtain the aromatic hydrocarbon and sulfur-containing aromatic hydrocarbon. The solvent in the organic solution of PdCl2 is methanol and / or cetonitrile, and the stationary phase is aminopropyl silica gel and / or mercapto propyl silica gel. The method can effectively separate aromatic and aromatic hydrocarbon and sulfur-containing aromatic hydrocarbon in the aromatic hydrocarbon components, and has simple convenient and quick operation.
Owner:CHINA PETROLEUM & CHEM CORP +1
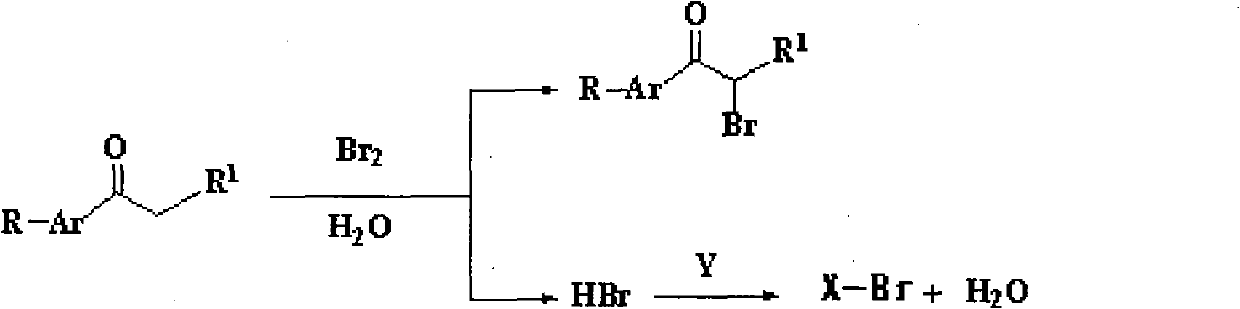
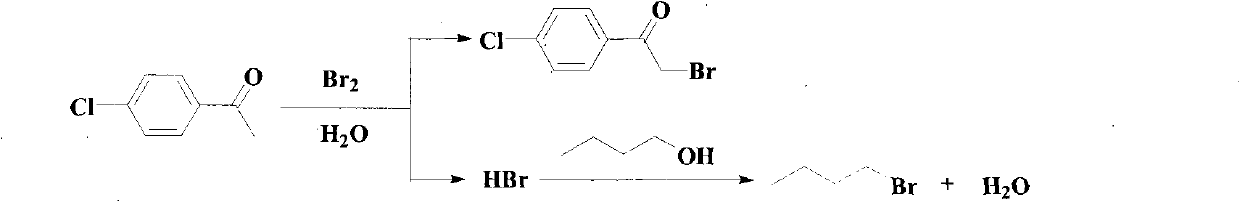
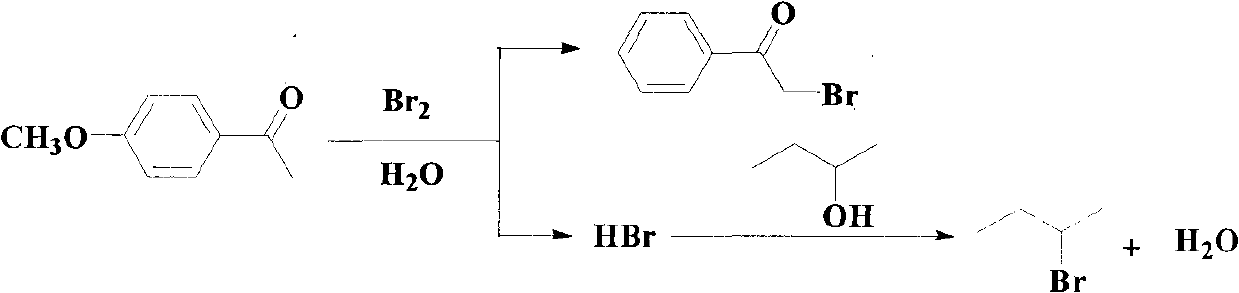
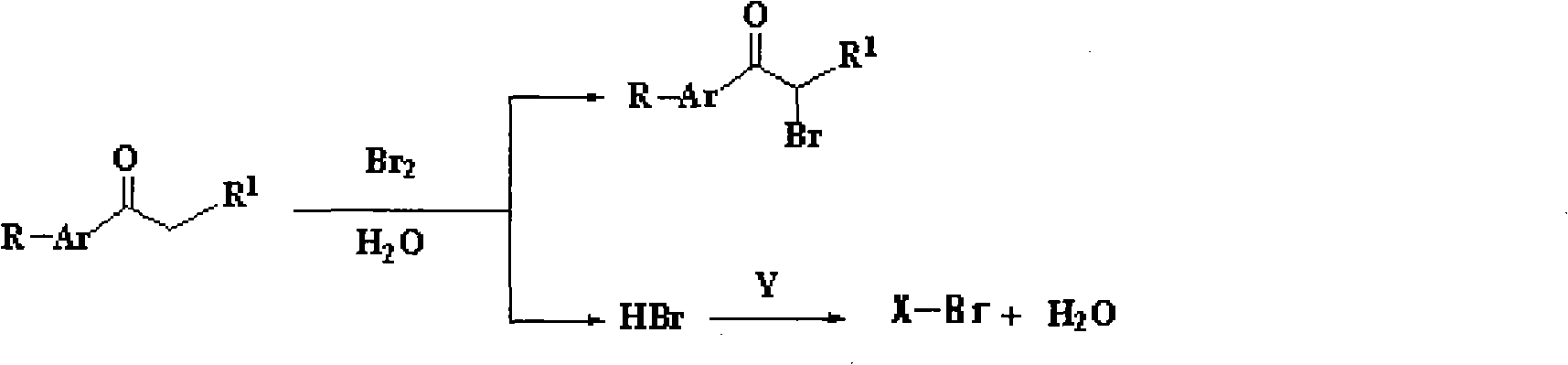
Method for synthesizing alpha-bromoketone and coproducing bromohydrocarbon
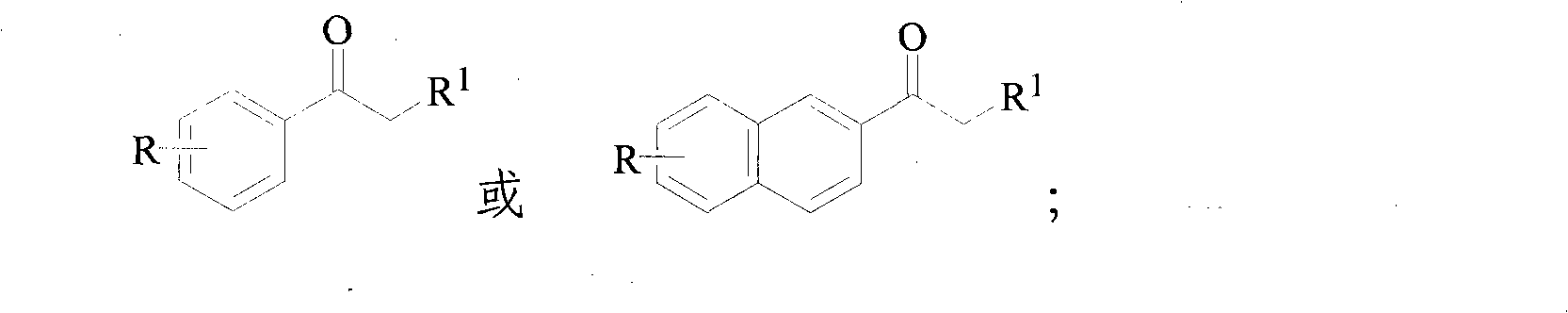
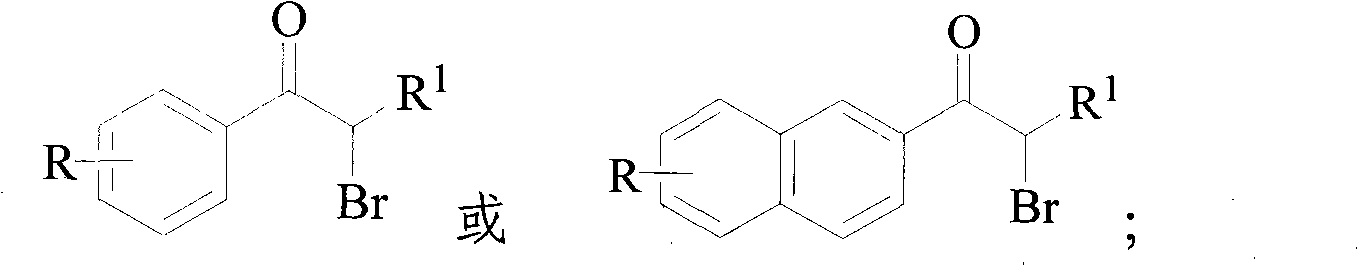
InactiveCN101948374AIncrease profitAvoid emissionsOrganic compound preparationPreparation by OH and halogen introductionWater vaporOrganic layer
The invention discloses a method for synthesizing alpha-bromoketone and coproducing bromohydrocarbon. The technical scheme comprises the following steps of: adding arylalkyl ketone compounds, alcohol or epoxy compounds and water into a flask first; dripping bromine into the flask slowly under the conditions of stirring and heating; performing a bromo-reaction on the bromine and the ketone compounds to obtain alpha-bromoketone and bromine hydride; dissolving the bromine hydride in the water to form hydrobromic acid; performing a reaction on the hydrobromic acid and the alcohol or epoxy compounds to obtain bromohydrocarbon ketone compounds; after the reaction is completed, performing steam distillation to obtain a raffinate serving as an alpha-bromoketone crude product after distillation; cooling for separating crystals out; washing and drying the crystals to obtain an alpha-bromoarylalkyl ketone product; separating an aqueous layer from a distillate serving as a bromohydrocarbon crude product; washing an organic layer until the organic layer is neutral; drying by using anhydrous calcium chloride; and distilling to obtain a bromohydrocarbon product. In the method, the water is takenas a solvent, and the bromohydrocarbon is coproduced while the alpha-bromoketone compounds are synthesized; and the gross products of the reaction are the alpha-bromoketone, the bromohydrocarbon and the water, so the method has the characteristics that: the high atom utilization rate of the reaction is high, the generation or the discharging of noxious substances such as bromine hydride gas, strong acid waste water and the like is avoided, and the effects of environmental protection, energy conservation and reduction emission are achieved.
Owner:HEBEI UNIVERSITY
Amino-acid ester bromide type chiral ionic liquid and preparation method thereof
InactiveCN105061236AAdd new varietiesLess freedomOrganic compound preparationAmino-carboxyl compound preparationNon toxicityMannich reaction
The invention discloses amino-acid ester bromide type chiral ionic liquid and a preparation method thereof. The general formula of the amino-acid ester bromide type chiral ionic liquid is A<+>Br<->, wherein A<+> is an amino-acid ester positive ion. The preparation method comprises the following steps: performing reaction on amino acid, methyl alcohol and thionyl chloride under low temperature to obtain amino-acid ester hydrochloride; performing mannich reaction on amino-acid ester hydrochloride, paraformaldehyde and acetone to obtain amino-acid ester mannich base; performing reaction on amino-acid ester mannich base and bromo-hydrocarbon to obtain the amino-acid ester chiral ionic liquid. The chiral amino-acid ester ionic liquid prepared through the preparation method disclosed by the invention has the advantages of high selectivity and chiral induction effect of a chiral substance, and non-volatilization, non-toxicity and no pollution of ionic liquid and is suitable for scale production of the fine chemical industry.
Owner:海客迈斯生物科技(上海)有限公司
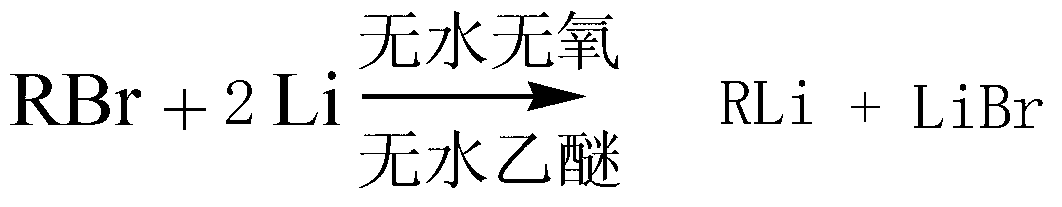
Alkyl-substituted disilane and preparation method thereof
InactiveCN103288860AMeet performance requirementsImprove heat resistanceSilicon organic compoundsWater bathsLiquid waste
The invention discloses alkyl-substituted disilane expressed as Rn Si-Si(CH3)6-n (I) in a formula (I) and a preparation method thereof. The preparation method of the compound comprises: adding magnesium powder, diethyl ether and iodine in a reactor, dropwise adding a diethyl ether solution of brominated hydrocarbon into the reactor for initiation, dropwise adding the residual diethyl ether solution of brominated hydrocarbon into the reactor, and reacting for 2-3hours after the dropwise adding operation to prepare a Grignard reagent; putting the reaction system in ice water bath, dropwise adding a diethyl ether solution of chlorosilane liquid waste with high boiling point into the Grignard reagent, heating up to room temperature to react for 1.5-2hours after the dropwise adding operation, and heating up to 50-60 DEG C to react for 5-6hours; adding water to separate an organic phase from an aqueous phase, extracting the aqueous phase through petroleum ether, mixing the organic phase, drying, concentrating, performing silicagel column chromatography, and concentrating the leacheate to obtain the remaining material which is the product (alkyl-substituted disilane). Industrial waste is used as a raw material in the invention, and the product has good thermal stability and strong heat resistance, thereby being applied to hydraulic oil, diffusion pump oil and heat transfer oil.
Owner:SOUTHWEAT UNIV OF SCI & TECH
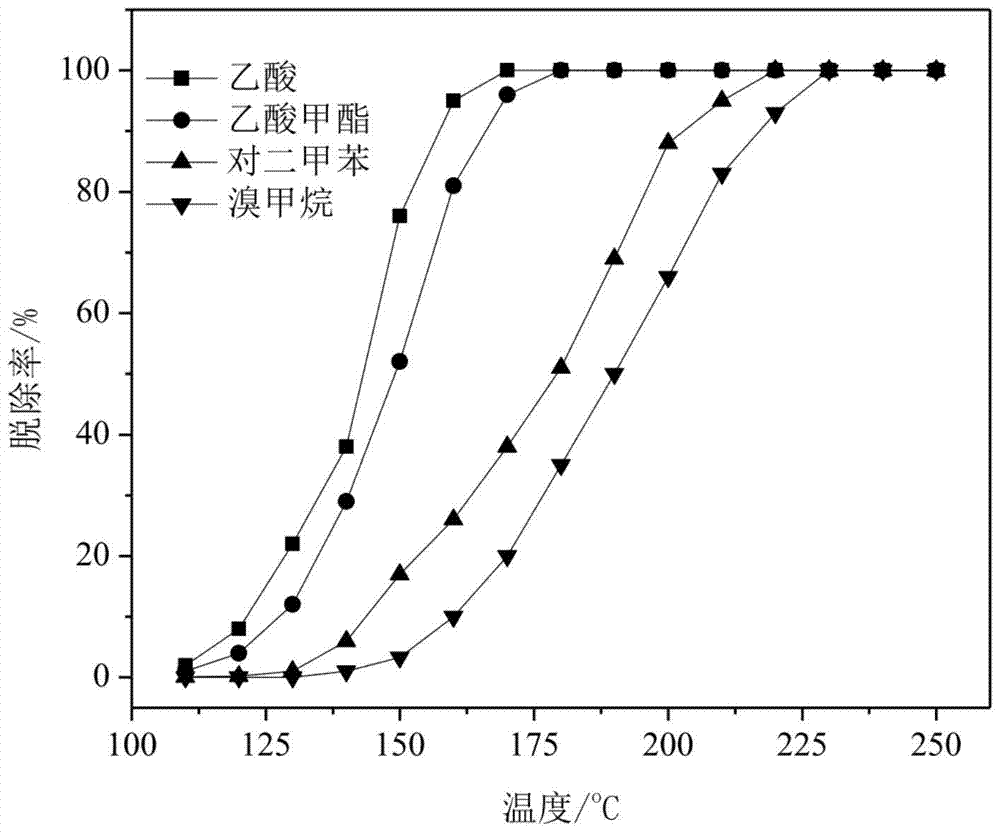
A kind of monolithic ruthenium catalyst for PTA oxidation tail gas purification, preparation method and use thereof
ActiveCN105126834BSimple preparation processRaw materials are easy to getDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCatalytic oxidationBrominated hydrocarbon
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of self-cleaning coating on surface of light-induced solar cell
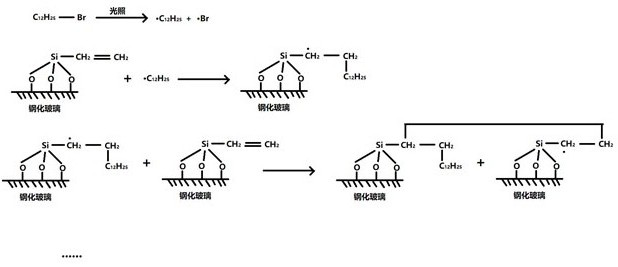
ActiveCN112038421ASave raw materialsLow costFinal product manufacturePhotovoltaic energy generationBrominated hydrocarbonSolar battery
The invention discloses a preparation method of a light-induced solar self-cleaning coating. The method comprises the following steps of adding a vinyl silane coupling agent and ammonia water into water, and sufficiently stirring to form uniform liquid; coating the liquid on the surface of a tempered glass plate of the solar cell, and airing at room temperature in a dark place; uniformly coating brominated hydrocarbon on the surface of the toughened glass plate of the solar cell; irradiating for a certain time under a light source, and combining brominated hydrocarbon with the vinyl silane coupling agent through a free radical addition reaction to form a hydrophobic self-cleaning coating; and washing off unreacted micromolecular organic matters on the surface with ethyl acetate to finish the construction of the self-cleaning coating. According to the present invention, the advantages of the in-situ polymerization reaction are fully utilized, the raw materials are saved, and the cost isreduced; the properties of the hydrophobic coating on the surface of the solar cell can be conveniently regulated and controlled by using different alkyl halides.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Synthesis method of dimethyl octenone
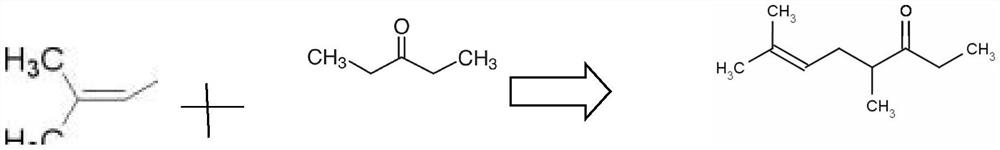
PendingCN112321403ASynthetic Method AdvantagesSimple process routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHalohydrocarbonPtru catalyst
The invention provides a synthesis method of dimethyl octenone. The method comprises the following steps: (1) adding 3-pentanone, 50 wt% of basic catalyst, and a phase transfer catalyst into a four-neck flask with a stirring function, and heating to 20-50 DEG C while stirring; (2) dropwise adding 3, 3-dimethyl allyl bromide, continuously stirring for 1 hour after dropwise adding, sampling and detecting, stopping stirring when the 3, 3-dimethyl allyl bromide is completely reacted, and standing for layering to obtain an organic phase and a water phase; and (3) extracting the water phase obtainedin the step (2) by using 3-pentanone to obtain an organic phase, combining the organic phase with the organic phase obtained in the step (2), and distilling to obtain a dimethyl octenone finished product. According to the synthesis method of the dimethyl octenone, a bimolecular nucleophilic substitution reaction (SN2) of halogenated hydrocarbon is carried out, and a-site hydrogen of carbonyl is substituted with long-chain branched chain brominated hydrocarbon; the method is simple in process route, capable of being completed in one step and mild in condition.
Owner:TIANJIN ANKAITE CATALYST
Preparation method of carbonate
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
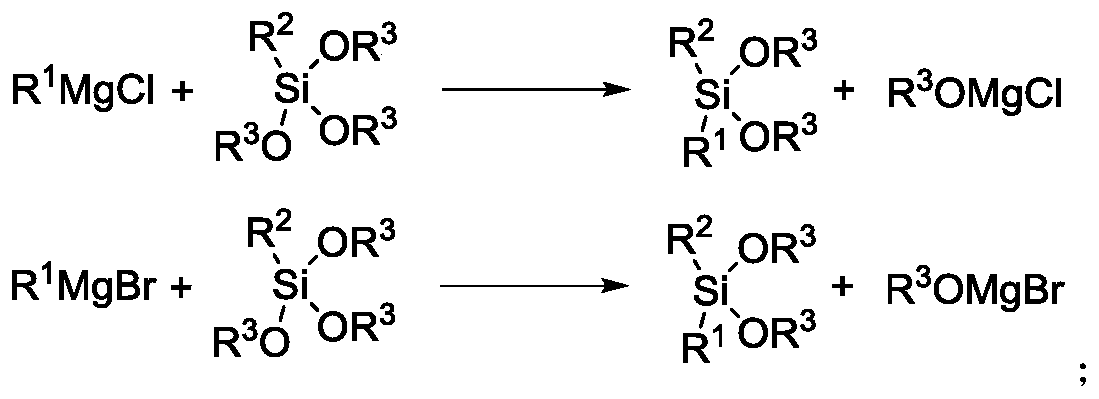
Method for industrially producing dialkyl dialkoxy silane
ActiveCN110964051AImprove conversion rateHigh yieldGroup 4/14 element organic compoundsChemical recyclingPtru catalystAlkoxy group
The invention discloses a method for industrially producing dialkyl dialkoxy silane, which comprises the following steps: in the presence of a catalyst and a solvent A, heating brominated hydrocarbonA and metal magnesium to reflux, and initiating reaction to obtain a first reaction solution; synchronously and dropwise adding chlorinated hydrocarbon and brominated hydrocarbon B which are diluted by a solvent B into the first reaction solution, and continuing reaction to obtain a second reaction solution; dropwise adding the second reaction solution into alkyl trialkoxy silane, and carrying outreflux reaction to obtain a third reaction solution; and cooling the third reaction solution, carrying out solid-liquid separation, and finally, carrying out washing and rectifying to obtain the dialkyl dialkoxy silane. The Grignard reaction of the chlorinated hydrocarbon is promoted by utilizing the high activity of the brominated hydrocarbon, so that on one hand, the overall conversion rate ofthe Grignard reaction can be remarkably improved, byproducts are reduced, the yield of a final product is improved, and on the other hand, the production cost can be reduced.
Owner:HUBEI HUABANG CHEM
Quinazoline nitrogen-containing heterocyclic ring derivative as well as preparation method and application thereof
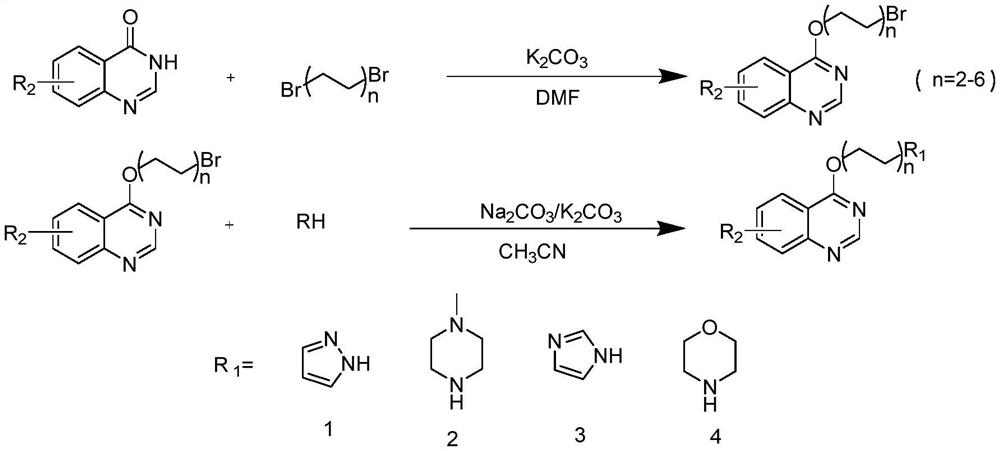
ActiveCN114315802AMild reaction conditionsEasy to repeatOrganic active ingredientsOrganic chemistryIslet cellsBrominated hydrocarbon
The invention discloses a quinazoline nitrogen-containing heterocyclic derivative as well as a preparation method and application thereof, and the preparation method specifically comprises the following steps: adding brominated hydrocarbon and K2CO3 into DMF (Dimethyl Formamide), and stirring to form a mixed solution; dissolving 4-hydroxyquinazoline in DMF (Dimethyl Formamide), and dropwise adding into the mixed solution for reaction to obtain an intermediate; the intermediate and a nitrogen heterocyclic compound are dissolved in acetonitrile, K2CO3 is added for a reaction, after reactants completely react, water and ethyl acetate are added for extraction, drying and vacuum concentration, and the quinazoline nitrogen heterocyclic derivative can be obtained. The quinazoline nitrogen-containing heterocyclic ring derivative is synthesized through two-step reaction, the reaction condition is mild, the method is easy to repeat, and the yield is high. The islet cell protection activity of the quinazoline nitrogen-containing heterocyclic ring derivative is researched, and the quinazoline nitrogen-containing heterocyclic ring derivative is a potential new chemical entity for treating diabetes. The important significance is realized on the research and development of anti-diabetic medicines.
Owner:XIAN MEDICAL UNIV
Alkyl substituted disilane and its preparation method
InactiveCN103288860BMeet performance requirementsImprove heat resistanceSilicon organic compoundsLiquid wasteIce water
Owner:SOUTHWEAT UNIV OF SCI & TECH
Amino acid ester derivative cationic chiral ionic liquid and preparation method thereof
ActiveCN105152949BChiral substanceRich sourcesOrganic compound preparationAmino-carboxyl compound preparationNon toxicityMannich reaction
A novel amino acid ester derivative cationic chiral ionic liquid and a preparation method thereof. The general formula of the amino acid ester derivative cationic chiral ionic liquid is A+X-, wherein A+ is an amino acid ester cationic derivative, and X-is BF4-, PF6-, HSO4-. The preparation method is: react amino acid with methanol and thionyl chloride at low temperature to obtain amino acid ester hydrochloride; react amino acid ester hydrochloride with paraformaldehyde and acetone through Mannich reaction to obtain amino acid ester derivative Mannich base Amino acid ester derivative Mannich base reacts with brominated hydrocarbon to obtain bromide salt chiral ionic liquid of amino acid ester derivative; amino acid ester derivative is obtained by exchanging the bromide ion of the bromide salt chiral ionic liquid of amino acid ester derivative Cationic chiral ionic liquid. The invention has many advantages such as high selectivity of chiral substances, non-volatile, non-toxic, non-polluting ionic liquid, and is suitable for large-scale production in the fine chemical industry.
Owner:TANGSHAN NORMAL UNIV
A kind of preparation method of light-induced solar cell surface self-cleaning coating
ActiveCN112038421BSave raw materialsLow costFinal product manufacturePhotovoltaic energy generationAlkaneElectrical battery
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Method for synthesizing alpha-bromoketone and coproducing bromohydrocarbon
InactiveCN101948374BIncrease profitAvoid emissionsOrganic compound preparationPreparation by OH and halogen introductionWater vaporStrong acids
The invention discloses a method for synthesizing alpha-bromoketone and coproducing bromohydrocarbon. The technical scheme comprises the following steps of: adding arylalkyl ketone compounds, alcohol or epoxy compounds and water into a flask first; dripping bromine into the flask slowly under the conditions of stirring and heating; performing a bromo-reaction on the bromine and the ketone compounds to obtain alpha-bromoketone and bromine hydride; dissolving the bromine hydride in the water to form hydrobromic acid; performing a reaction on the hydrobromic acid and the alcohol or epoxy compounds to obtain bromohydrocarbon ketone compounds; after the reaction is completed, performing steam distillation to obtain a raffinate serving as an alpha-bromoketone crude product after distillation; cooling for separating crystals out; washing and drying the crystals to obtain an alpha-bromoarylalkyl ketone product; separating an aqueous layer from a distillate serving as a bromohydrocarbon crude product; washing an organic layer until the organic layer is neutral; drying by using anhydrous calcium chloride; and distilling to obtain a bromohydrocarbon product. In the method, the water is takenas a solvent, and the bromohydrocarbon is coproduced while the alpha-bromoketone compounds are synthesized; and the gross products of the reaction are the alpha-bromoketone, the bromohydrocarbon and the water, so the method has the characteristics that: the high atom utilization rate of the reaction is high, the generation or the discharging of noxious substances such as bromine hydride gas, strong acid waste water and the like is avoided, and the effects of environmental protection, energy conservation and reduction emission are achieved.
Owner:HEBEI UNIVERSITY
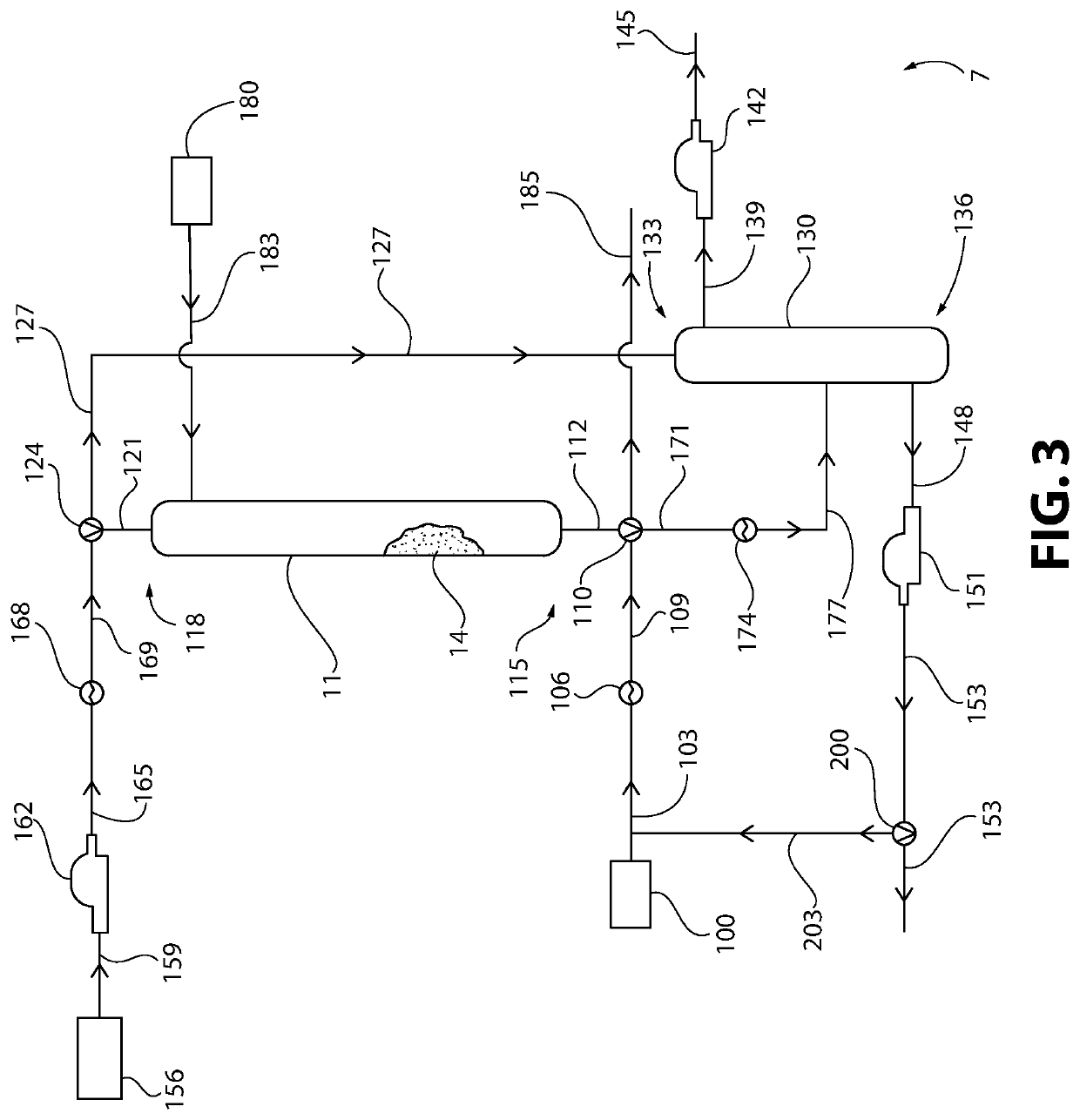
Method of Converting a Brominated Hydrocarbon to a Chlorinated Hydrocarbon
ActiveUS20210047250A1Ion-exchange column/bed processesOrganic anion exchangersIon exchangeWater chlorination
The present invention provides a method of converting a brominated hydrocarbon to a chlorinated hydrocarbon that involves contacting together the brominated hydrocarbon and a chlorinated ion exchange resin that has a water content of less than or equal to 30 percent by weight, based on the total weight of the chlorinated ion exchange resin and the water. The brominated hydrocarbon includes at least one replaceable bromo group, where each replaceable bromo group is independently covalently bonded to an sp3 hybridized carbon. Contact between the brominated hydrocarbon and the chlorinated ion exchange resin results in replacement of at least one replaceable bromo group of the brominated hydrocarbon with a chloro group, and correspondingly conversion of at least a portion of the brominated hydrocarbon to the chlorinated hydrocarbon.
Owner:EAGLE US 2 LLC
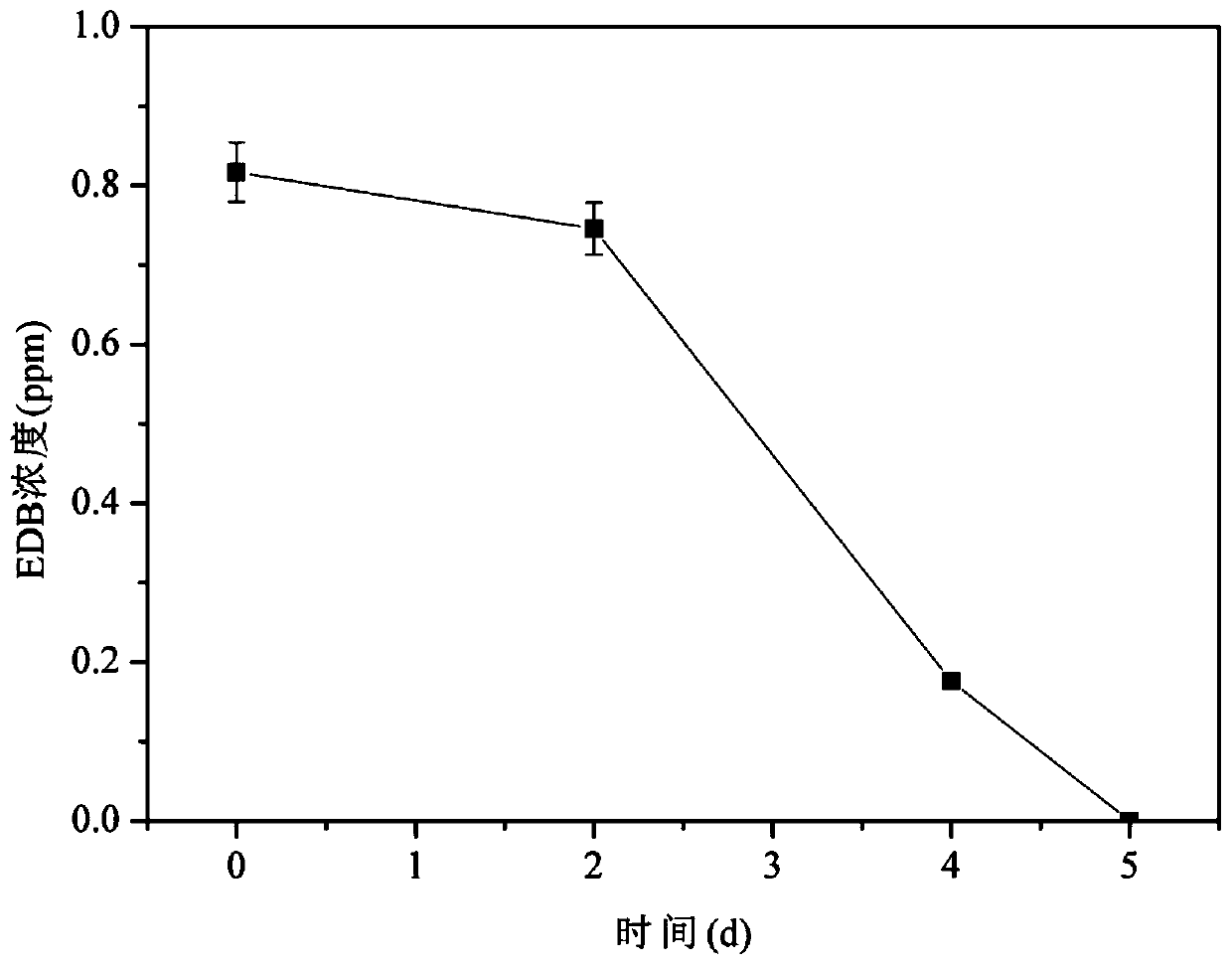
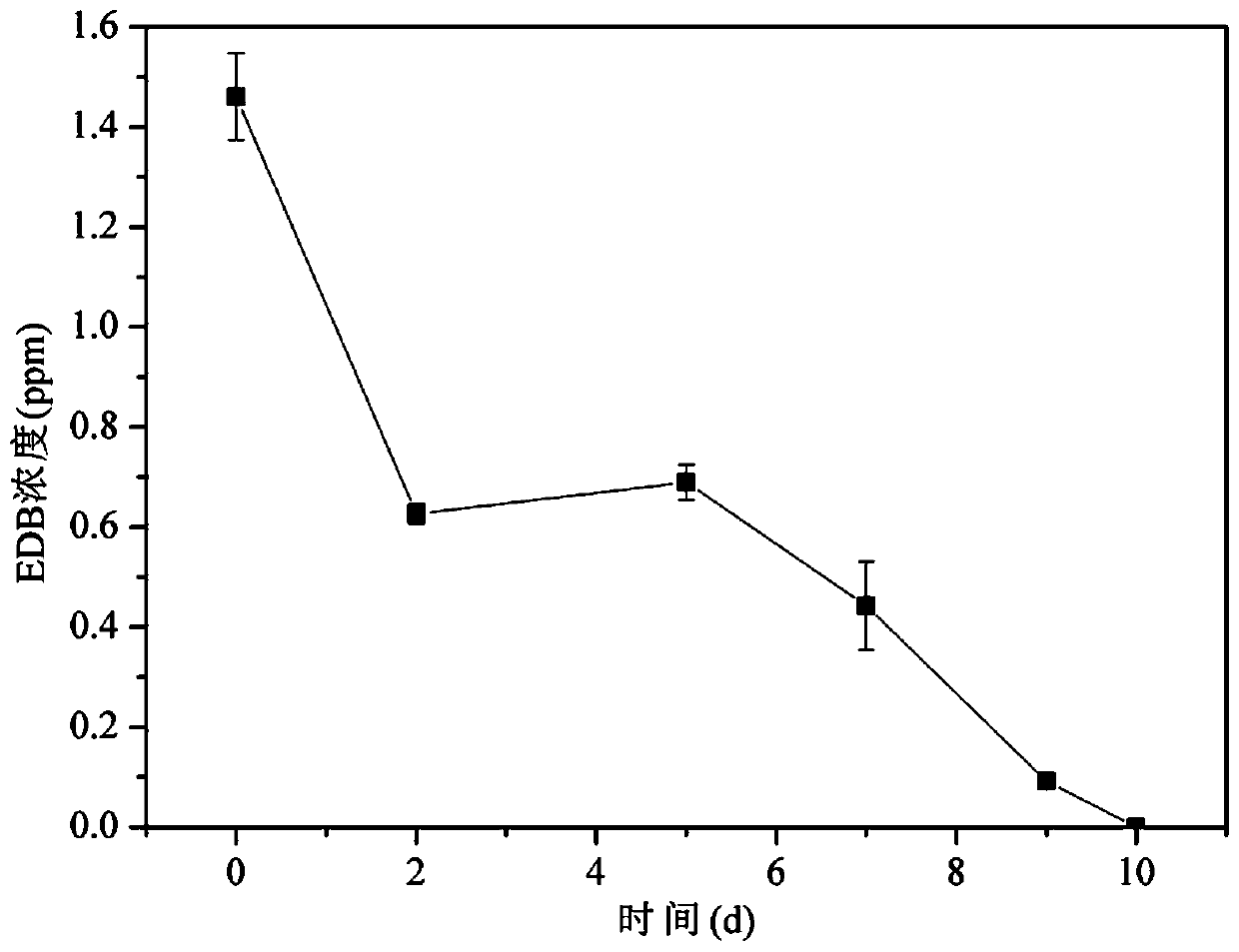
Preparation method and application of a kind of brominated hydrocarbon anaerobic degradation bacterial agent
ActiveCN107267435BPractical and operableNo secondary pollutionBacteriaWater contaminantsBrominated hydrocarbonBioremediation
The invention discloses a preparation method and application of a bromo-hydrocarbon anaerobic degrading bacterial agent. The preparation method comprises the following steps: adding an inorganic salt medium in an anaerobic bottle, removing dissolved oxygen in the medium by utilizing a nitrogen evaporator, after high-temperature and high-pressure sterilization of the medium, adding anaerobic sludge from urban sewage treatment plants in an anaerobic cultural incubator, sealing the anaerobic bottle with a cover presser, injecting an EDB standard liquid in the anaerobic bottle, placing the anaerobic bottle in a constant-temperature shaking incubator for anaerobic culture under the condition with the temperature being 30 DEG C, carrying out transferring after microorganisms reproduce to a logarithmic growth phase, basically removing solid particulates in the anaerobic bottle after transferring for 4-5 times, and effectively enabling anaerobic degrading bacteria to be enriched. The anaerobic degrading bacterial agent provided by the invention can efficiently degrade EDB and is suitable for bioremediation of groundwater contaminated by bromo-hydrocarbon.
Owner:INST OF SOIL SCI CHINESE ACAD OF SCI +1
Method for preparing methyl bromide, acetyl bromide, acetic acid and acetic ester by methane
InactiveCN101723794AImprove stabilityImprove catalytic performancePreparation from carboxylic acid halideOrganic compound preparationAlkaneAcetic acid
The invention discloses a method which is characterized in that methane is converted into bromohydrocarbon by bromine oxidation reaction, and bromohydrocarbon is further prepared into acetyl bromide, acetic acid and acetic ester. Firstly, methane, HBr / H2O and oxygen react to generate bromo-alkane and CO; the catalyst is a composite catalyst prepared by mixed hydrolysis, drying and roasting of silicon precursor and metallic compound precursor capable of dissolving in water; then, bromo-alkane and CO react to prepare acetyl bromide; then, acetyl bromide and water react to generate acetic acid, or the acetyl bromide reacts with alcohol to generate acetic ester.
Owner:MICROVAST POWER SYST CO LTD
Amino acid ester bromide salt type chiral ionic liquid and preparation method thereof
InactiveCN105061236BAdd new varietiesRich sourcesOrganic compound preparationAmino-carboxyl compound preparationNon toxicityMannich reaction
The invention discloses amino-acid ester bromide type chiral ionic liquid and a preparation method thereof. The general formula of the amino-acid ester bromide type chiral ionic liquid is A<+>Br<->, wherein A<+> is an amino-acid ester positive ion. The preparation method comprises the following steps: performing reaction on amino acid, methyl alcohol and thionyl chloride under low temperature to obtain amino-acid ester hydrochloride; performing mannich reaction on amino-acid ester hydrochloride, paraformaldehyde and acetone to obtain amino-acid ester mannich base; performing reaction on amino-acid ester mannich base and bromo-hydrocarbon to obtain the amino-acid ester chiral ionic liquid. The chiral amino-acid ester ionic liquid prepared through the preparation method disclosed by the invention has the advantages of high selectivity and chiral induction effect of a chiral substance, and non-volatilization, non-toxicity and no pollution of ionic liquid and is suitable for scale production of the fine chemical industry.
Owner:海客迈斯生物科技(上海)有限公司
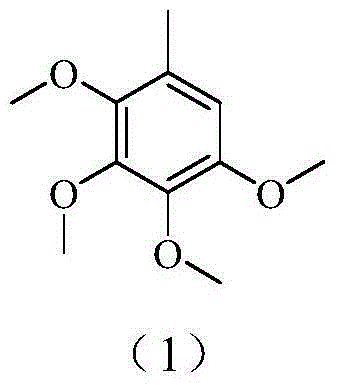
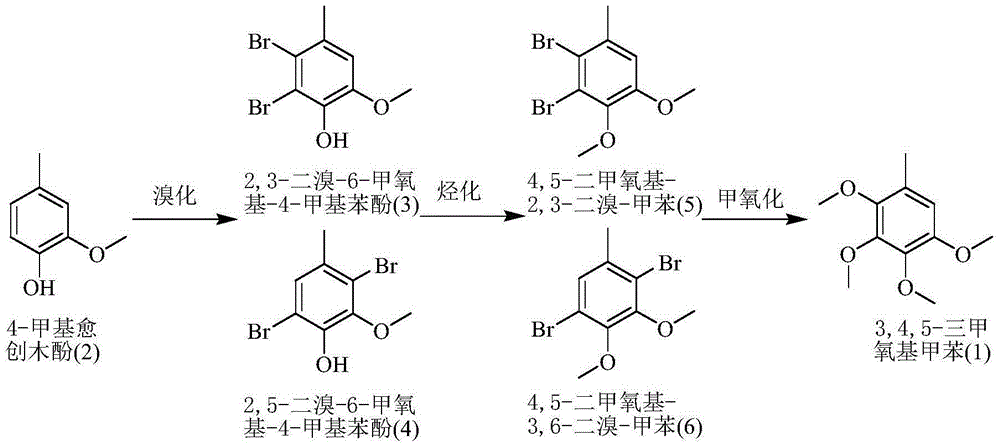
A kind of method for preparing 2,3,4,5-tetramethoxytoluene
InactiveCN103833531BWide variety of sourcesHigh reaction yieldOrganic compound preparationEther preparation by ester reactionsBenzeneProcess engineering
The invention provides a method for preparing 2, 3, 4, 5-tetramethoxytoluene by taking 4-hydroxy-3-methoxy-1-methyl-benzene as a raw material. The method comprises the process flows of brominating raw materials twice; then, carrying out hydrocarbonylation; and next, carrying out methoxymethylation to obtain 2, 3, 4, 5-tetramethoxytoluene. According to the invention, natural 4-hydroxy-3-methoxy-1-methyl-benzene extracted from biological oil is used as the raw material, so that the source of the raw material is relatively wide. The method is novel in ideal, simple and convenient in synthesis process, not high in requirements for reaction equipment and reaction conditions, relatively low in production cost and relatively high in industrial application value.
Owner:UNIV OF SCI & TECH OF CHINA
Method of converting a brominated hydrocarbon to a chlorinated hydrocarbon
ActiveUS10968153B2Ion-exchange column/bed processesOrganic anion exchangersPhysical chemistryIon exchange
Owner:EAGLE US 2 LLC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com