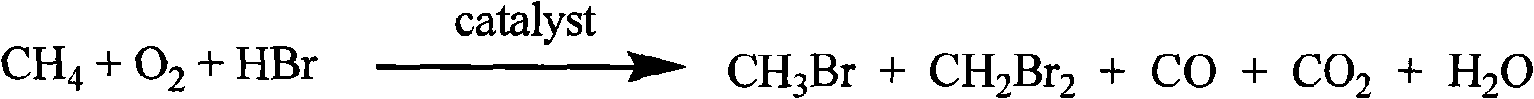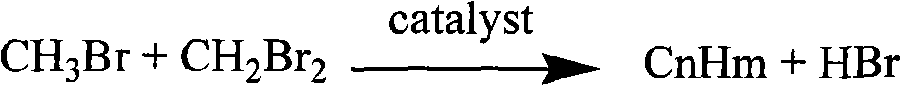Bromomethane prepared by bromine oxidation of methane and catalyst for conversing the bromomethane into hydrocarbon
A catalyst, bromine oxidation technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, molecular sieve catalyst, etc., can solve the problem of catalyst instability, high price, high single-pass conversion rate of methane 13.3% and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Preparation of catalyst:
[0025] Weigh 6.300g of oxalic acid solid and dissolve it in 100mL of deionized water to obtain solution A, and weigh the corresponding RhCl with 0.40% Rh metal content 3 ·NH 2 0.10224 g of O was dissolved in 50 mL of deionized water to obtain solution B, and 34.5831 g of tetraethoxysilane liquid was weighed and added to solution A to form two mutually immiscible liquids. After sealing and stirring for 1 hour, the solution becomes a uniform, colorless and transparent one-phase solution. Add solution B and continue to stir for 0.5 hours, then put the mixed solution in an oven at 120°C to dry to a colloidal solid, and then set the colloidal solid Put it into the muffle furnace and heat it to 900°C at a heating rate of 200°C / h. Maintain the temperature for 10 hours and then cool down. After the temperature drops to room temperature, take out the catalyst, sieving to 20-60 mesh, and finally obtain 0.40% Rh / SiO 2 -900-10 catalyst ("0.40%" means the mass...
example 2 to example 6
[0031] Preparation of catalyst:
[0032] According to the preparation method of the catalyst in Example 1, different concentrations of RhCl were used 3 ·NH 2 O solution 50.0mL deionized water to obtain solution B, of which RhCl 3 ·NH 2 The concentration of O solution is the converted amount of the required load of different mass fractions of Rh. Finally get 0.10% Rh / SiO 2 -900-10 (Example 2), 0.20% Rh / SiO 2 -900-10 (Example 3), 0.30% Rh / SiO 2 -900-10 (Example 4), 0.50% Rh / SiO 2 -900-10 (Example 5), 0.60% Rh / SiO 2 -900-10 (Example 6) catalyst.
[0033] Catalyst test:
[0034] According to the test conditions of the catalyst in Example 1, the reaction was carried out under the same reaction conditions. The experimental results are listed in Table 1.
[0035] Table 1 Experimental results of Example 1 to Example 6 (Effect of metal Rh loading)
[0036]
[0037]
example 7 to example 12
[0039] Preparation of catalyst:
[0040] According to the preparation method of the catalyst in Example 1, different calcination temperatures and times were used in the muffle furnace. Finally, catalysts with different specific surface areas are obtained. That is 0.40%Rh / SiO 2 -900-6 (Example 7), 0.40% Rh / SiO 2 -900-2 (Example 8), 0.40% Rh / SiO 2 -800-6 (Example 9), 0.40% Rh / SiO 2 -800-2 (Example 10), 0.40% Rh / SiO 2 -700-10 (Example 11), 0.40% Rh / SiO 2 -700-6 (Example 12) catalyst.
[0041] Catalyst test:
[0042] According to the test method of the catalyst in Example 1, the reaction was carried out under the same reaction conditions. The experimental results are listed in Table 2.
[0043] Table 2 Experimental results of Example 1, Example 7 to Example 12 (the influence of the calcination temperature during the preparation of the catalyst, the catalyst composition is 0.40% Rh / SiO 2 )
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com