Quinazoline nitrogen-containing heterocyclic ring derivative as well as preparation method and application thereof
A quinazoline and derivative technology, applied in the field of medicinal chemistry synthesis, can solve the problems of inability to effectively prevent the onset of diabetes, unclear molecular mechanism, etc., and achieve the effects of high yield, easy repeatability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
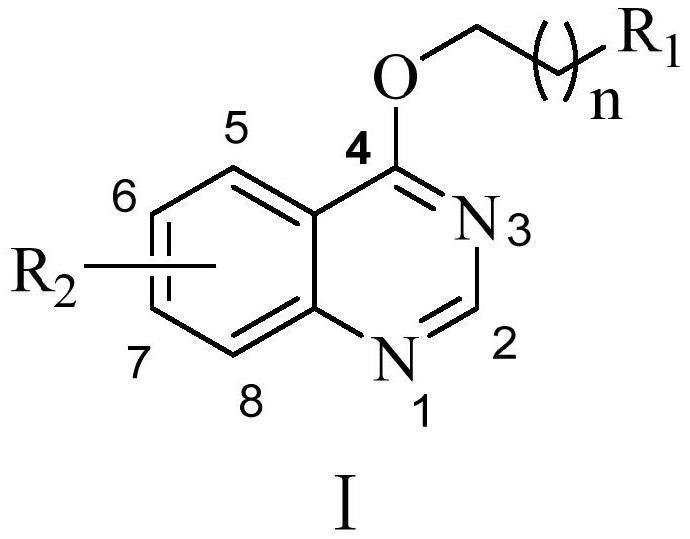
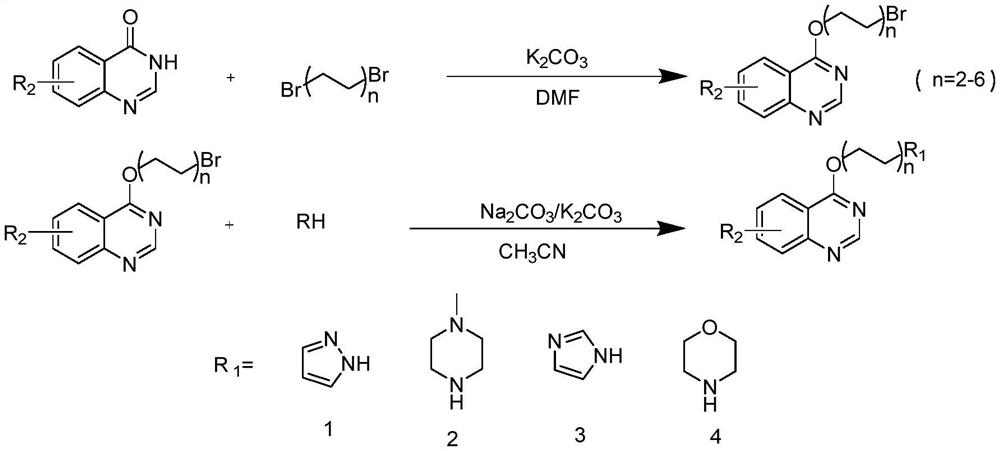
[0018] A kind of preparation method of quinazoline nitrogen-containing heterocyclic derivative of the present invention, specifically implement according to the following steps:
[0019] Step 1, brominated hydrocarbon, catalyst K 2 CO 3 In an eggplant-shaped bottle, add DMF, place it in a magnetic stirrer and stir to form a mixed solution; then dissolve 4-hydroxyquinazoline in DMF, slowly add it dropwise to the mixed solution at room temperature for reaction, and you can get Intermediate;
[0020] Brominated hydrocarbon is any one of 1,2-dibromoethane, 1,3-dibromopropane, 1,4-dibromobutane, 1,5-dibromopentane;
[0021] Step 2, the intermediate and nitrogen heterocyclic compound are dissolved in acetonitrile, and catalyst K is added 2 CO 3 , the reaction was carried out at 80 ° C, and the GF254 silica gel plate was used to monitor continuously during the reaction. After the reactant was completely reacted, water and ethyl acetate were added for extraction, and the organic l...
Embodiment 1
[0028] The preparation of embodiment 1 intermediate
[0029] a, 4-(2-bromoethoxy) quinazoline (A), the structural formula is as follows:
[0030]
[0031] First measure 1,2-dibromoethane (2.101mL, 24.63mmol) and catalyst K 2 CO 3 (4.17g, 30.17mmol) In an eggplant-shaped bottle, add about 30mL of DMF and stir in a magnetic stirrer. Then 4-hydroxyquinazoline (3.00 g, 20.12 mmol) was dissolved in 10 mL of DMF, and slowly added dropwise at room temperature. About 0.5 h after the DMF solution of 4-hydroxyquinazoline was added dropwise, the reaction was complete. After the solution was washed three times with 50 mL of water, it was washed with anhydrous Na 2 SO 4The organic layer was dried and concentrated under reduced pressure to obtain a reaction product. A small amount of the reaction product was dissolved in methanol, and detected by thin-layer chromatography (petroleum ether: acetone = 2:1) under ultraviolet light. The reaction product had two dark spots with large di...
Embodiment 2
[0050] Example 2 Preparation of 4-(2-(1H-pyrazol-1-yl)ethoxy)quinazoline (A-1), the structural formula of compound A-1 is as follows:
[0051]
[0052] Dissolve intermediate A (250mg, 0.99mmol) and pyrazole (80mg, 1.16mmol) in 10mL of acetonitrile, add catalyst K 2 CO 3 (410mg, 2.97mmol), reacted at 80°C. Continue to use GF during the reaction 254 The silica gel plate was used for monitoring, and after about 40 hours, the reactant reacted completely. Water (30mL) and ethyl acetate (3×30mL) were added for extraction, and the organic layer was washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. 200 mg of white crystal A-1 was obtained with a yield of 84.3%, which was identified as 4-(2-(1H-pyrazol-1-yl)ethoxy)quinazoline.
[0053] mp 117-119°C; 1 H-NMR (400MHz, CDCl 3 )δ8.15(1H,d,J=7.6Hz,H-8),8.08(1H,s,H-2),7.70(1H,t,J=7.6Hz,H-6),7.60(1H, s, H-5), 7.58 (1H, d, J=5.2Hz, pyrazole), 7.42 (1H, t, J=7.6Hz, H-7), 6.32 (1H, s, pyrazole), 4.14 (2H, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com