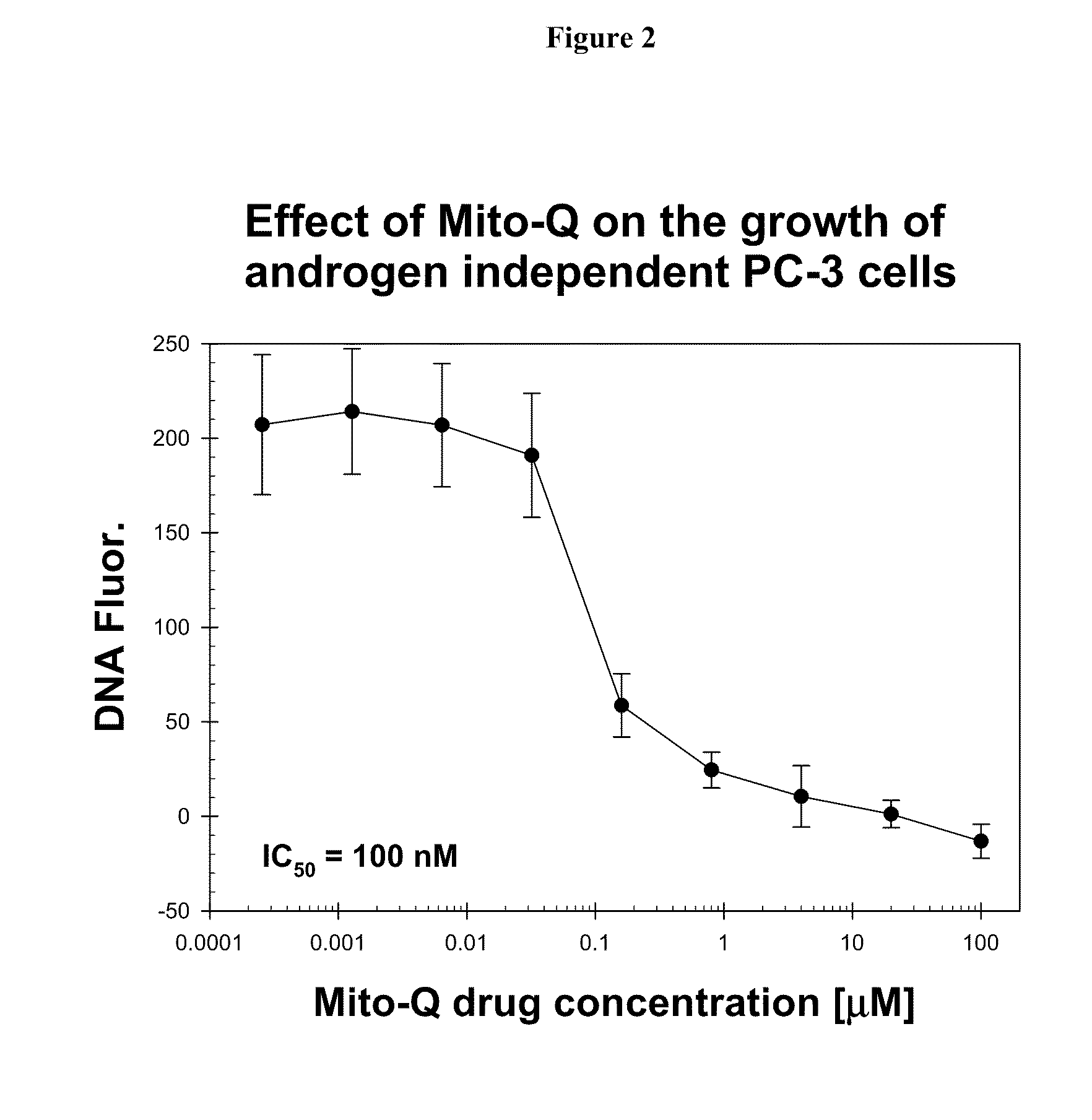
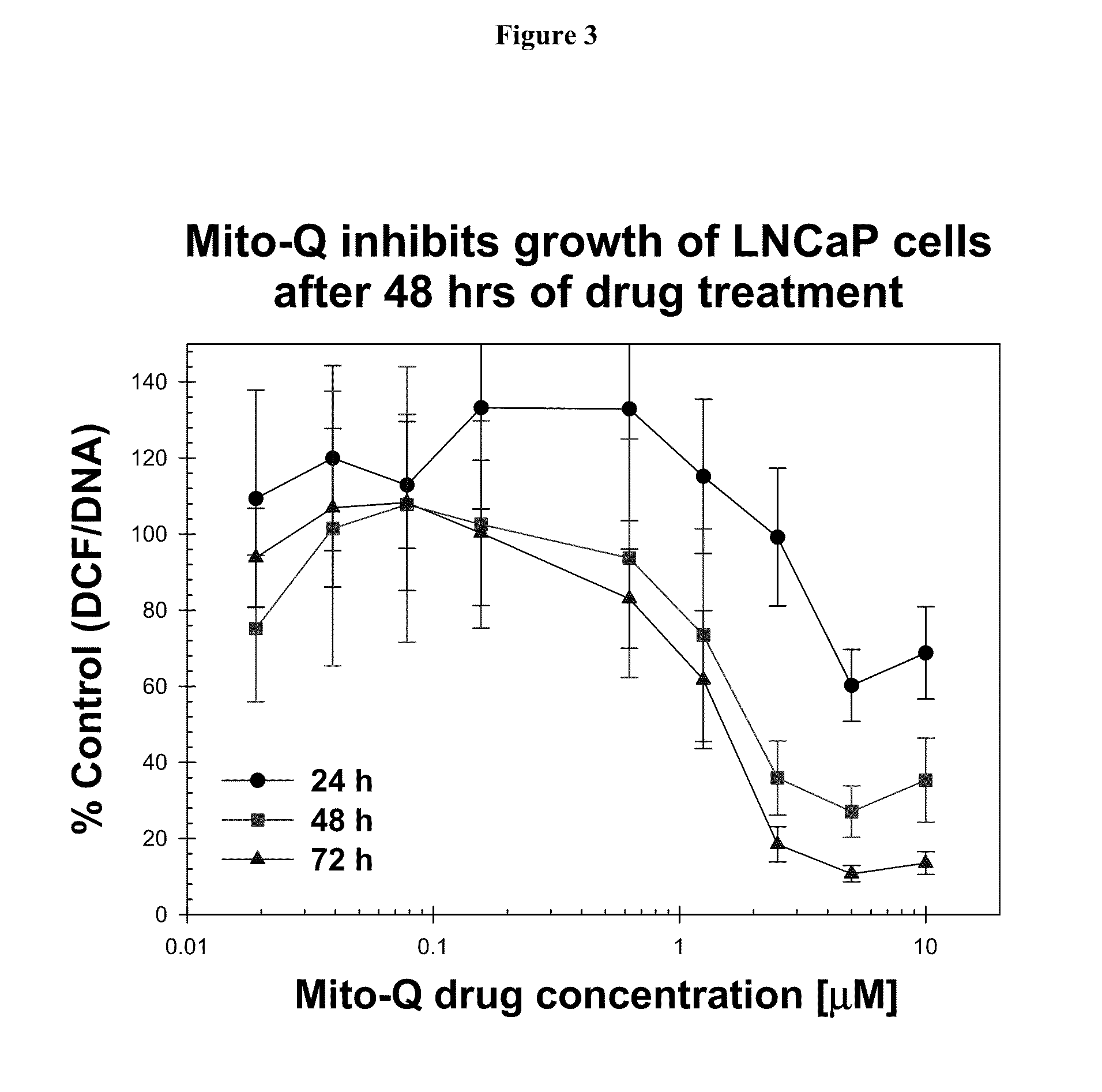
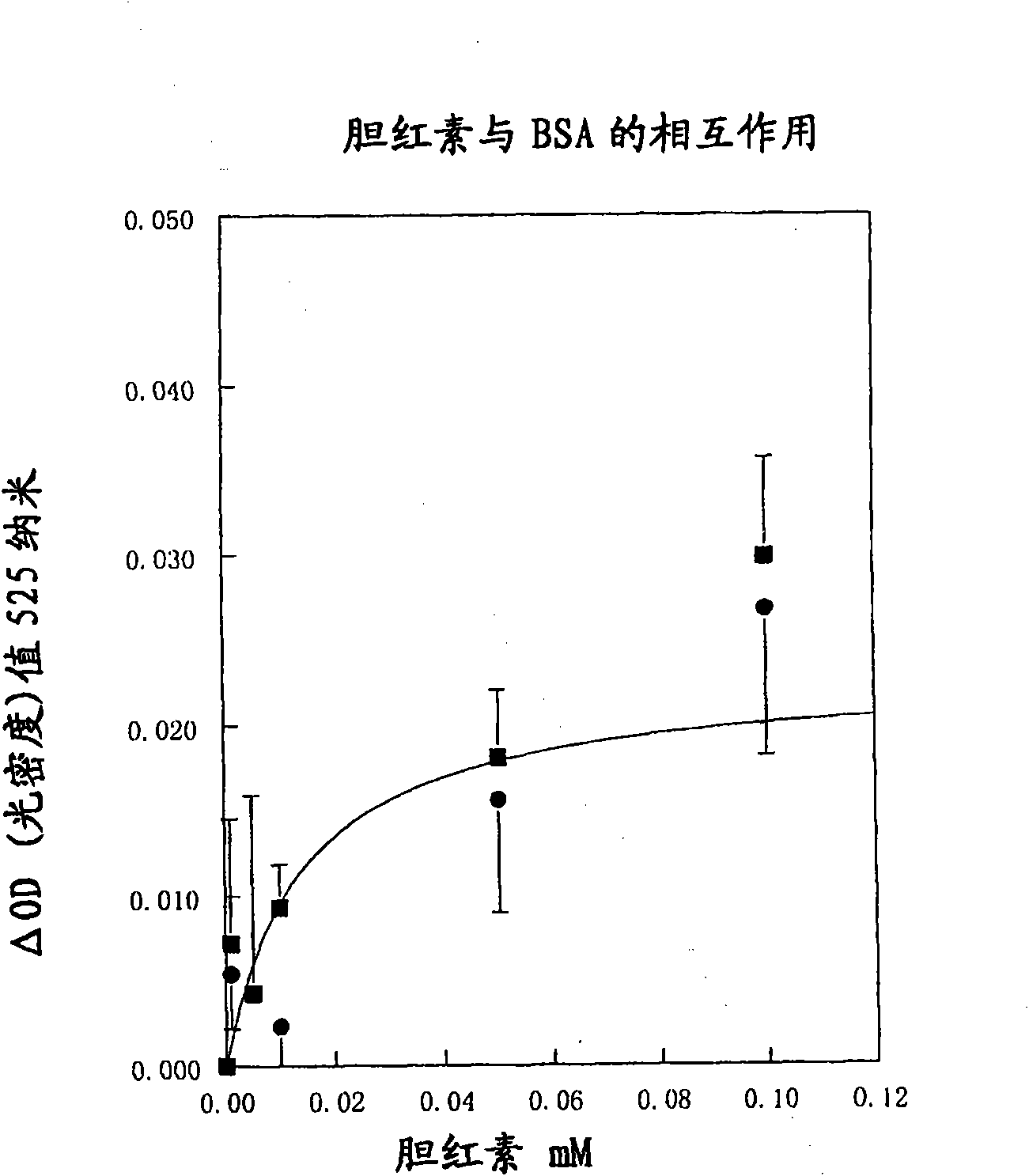
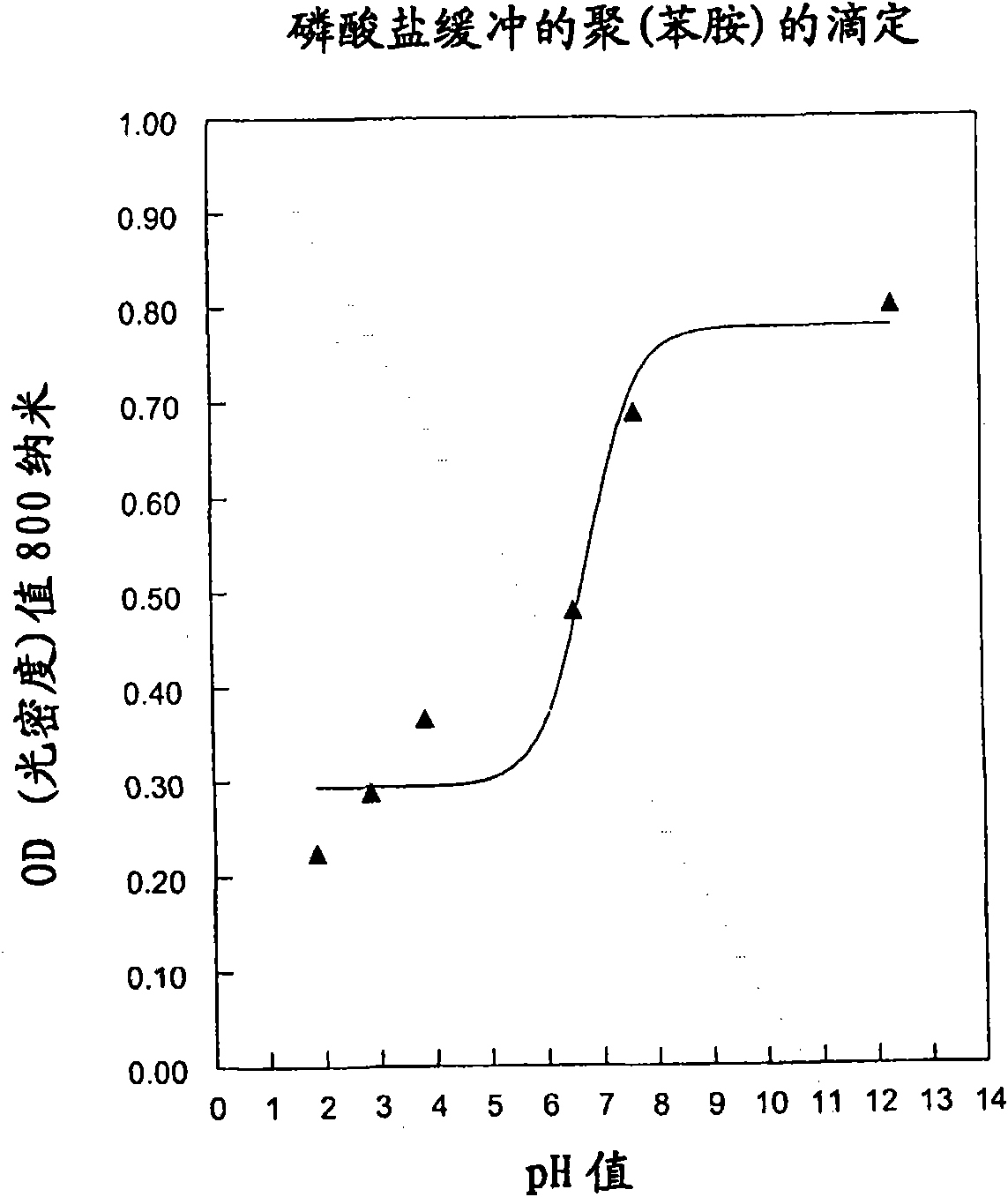
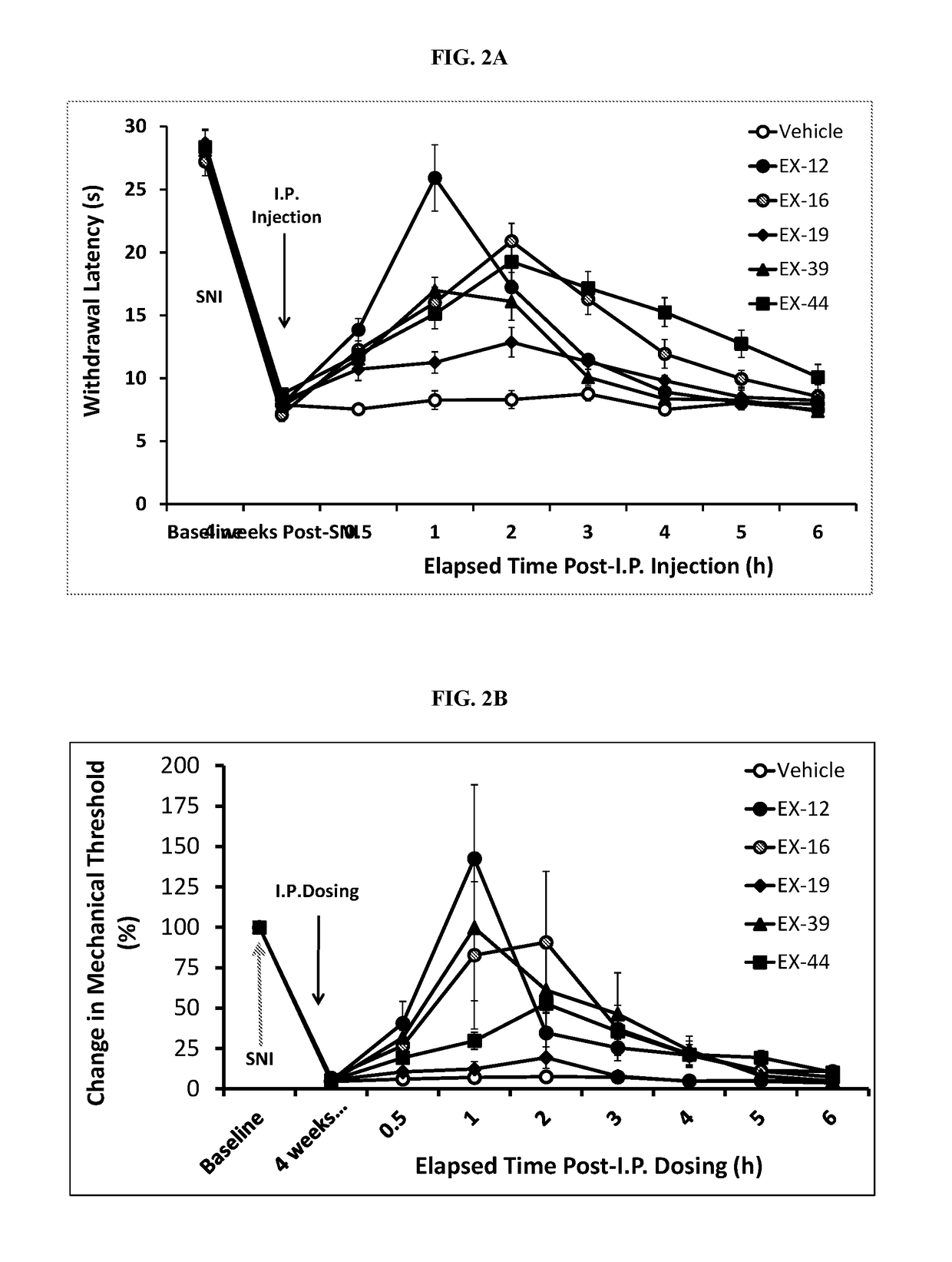
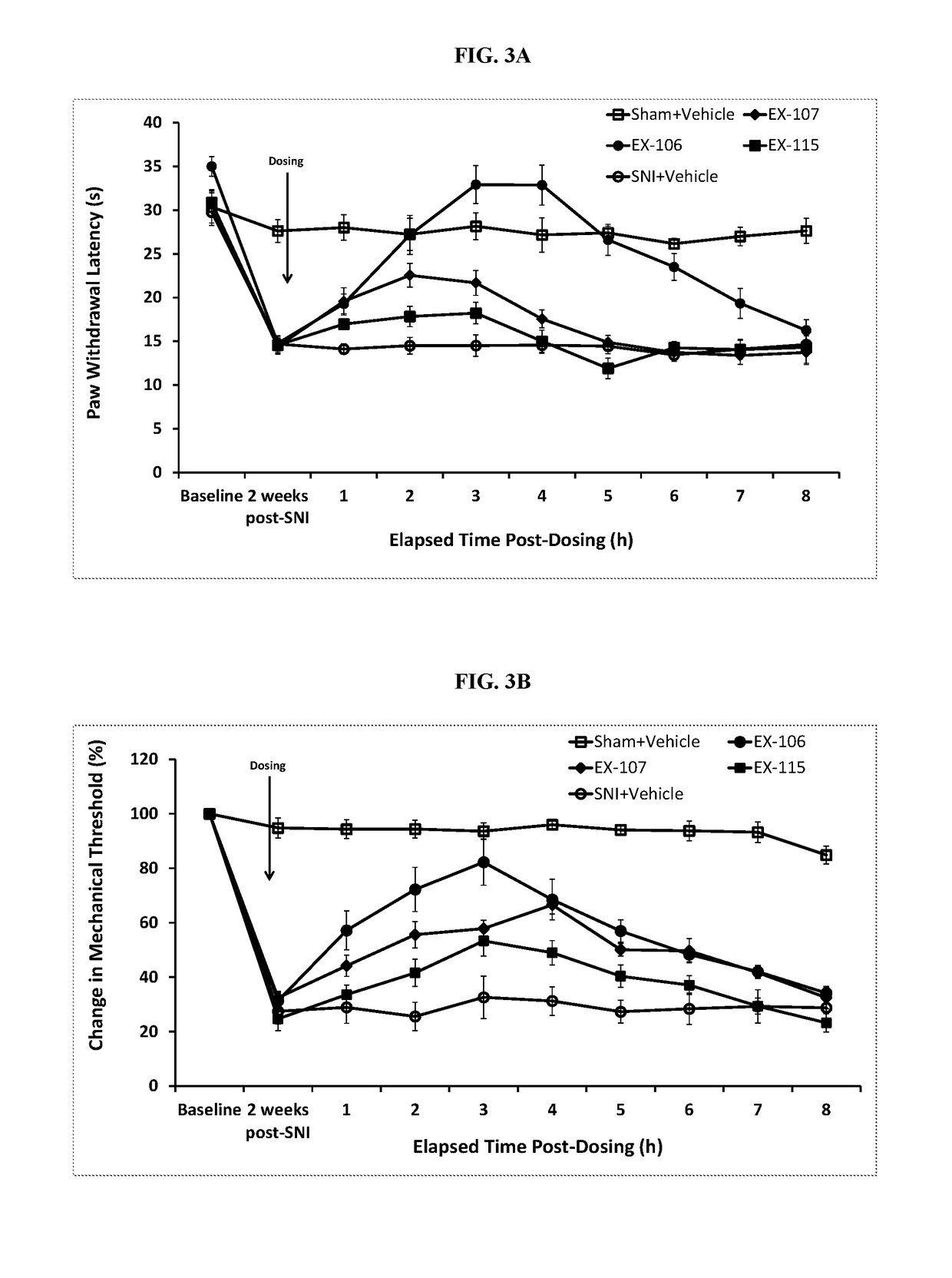
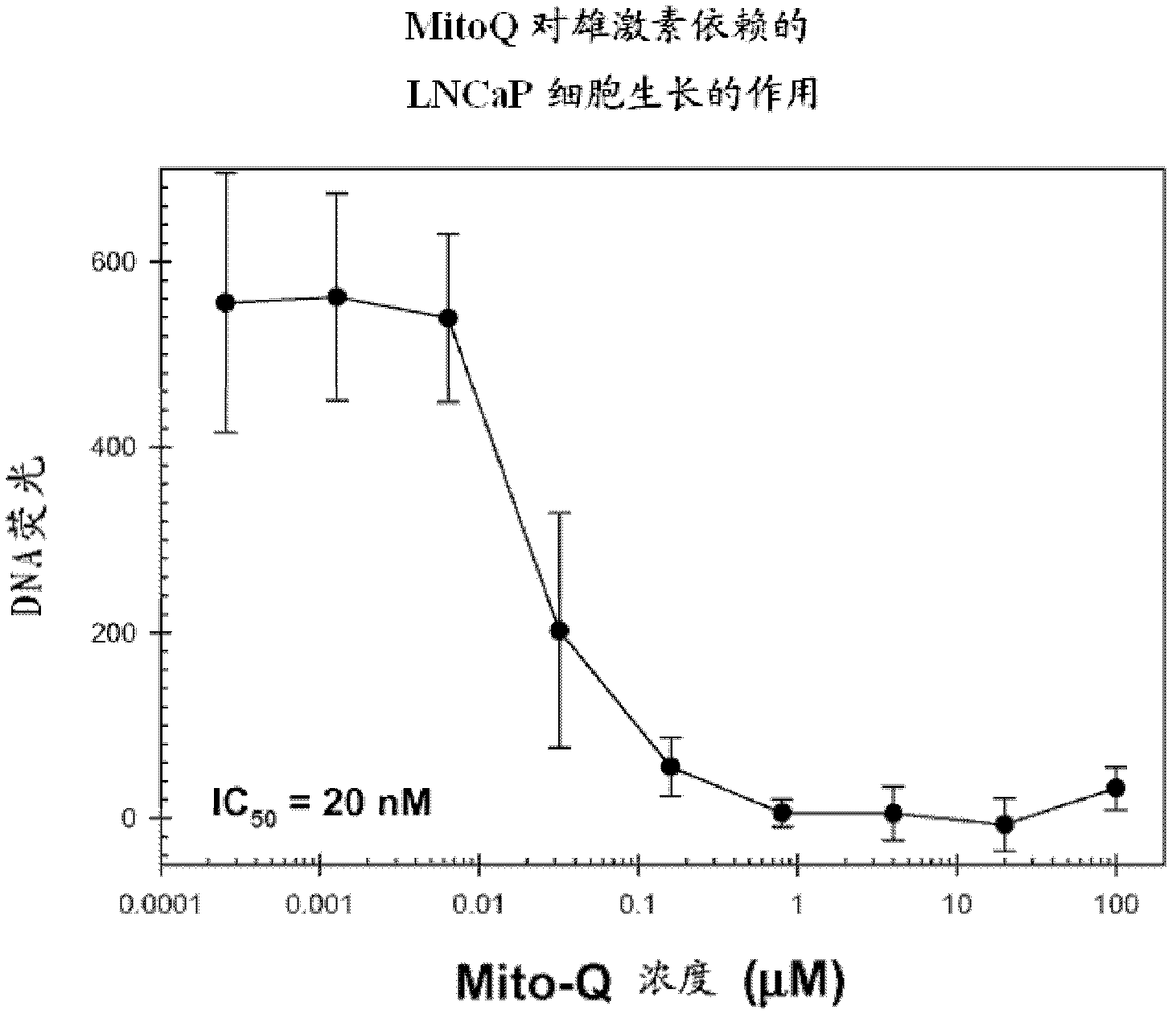
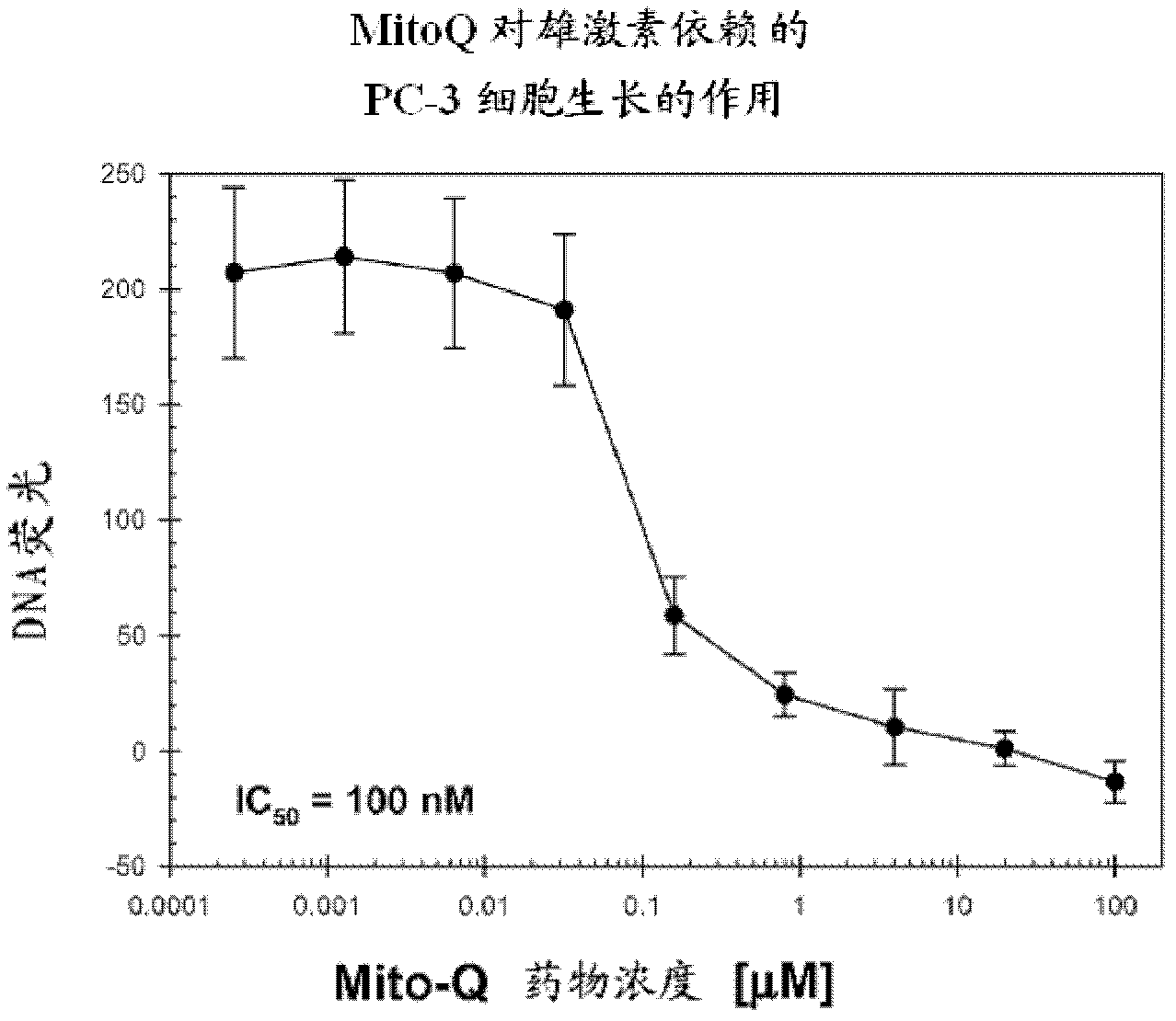
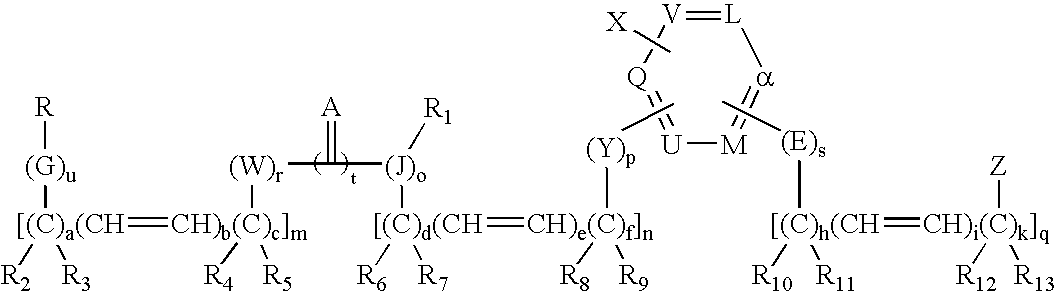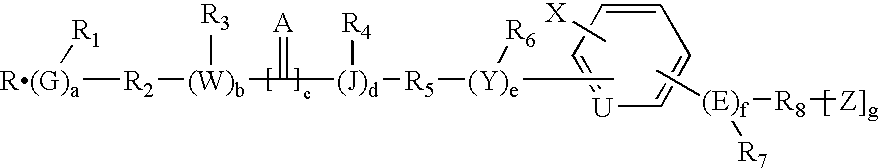Patents
Literature
64 results about "New chemical entity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A new chemical entity (NCE) is, according to the U.S. Food and Drug Administration, a drug that contains no active moiety that has been approved by the FDA in any other application submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act.
Lipid mediated screening of drug candidates for identification of active compounds
InactiveUS20030198664A1Reduce solubilityMicroencapsulation basedMicrobiological testing/measurementLipid formationSolubility
The subject invention provides liposome formulations that are capable of specifically targeting cell types. The subject invention also provides for the encapsulation of new chemical entities (NCE) or other drug candidate molecules (DCM) within liposomes that specifically target a particular cell type. The subject invention, advantageously, solubilizes compounds, with low solubility in aqueous environments, and permits screening of these compounds against intact cells for biological activity in the absence of detergents that can damage cell membranes. Also provided are methods of preparing liposome formulations that target a specific cell type and methods of delivering therapeutic agents to target cells.
Owner:BRISTOL MYERS SQUIBB CO +1
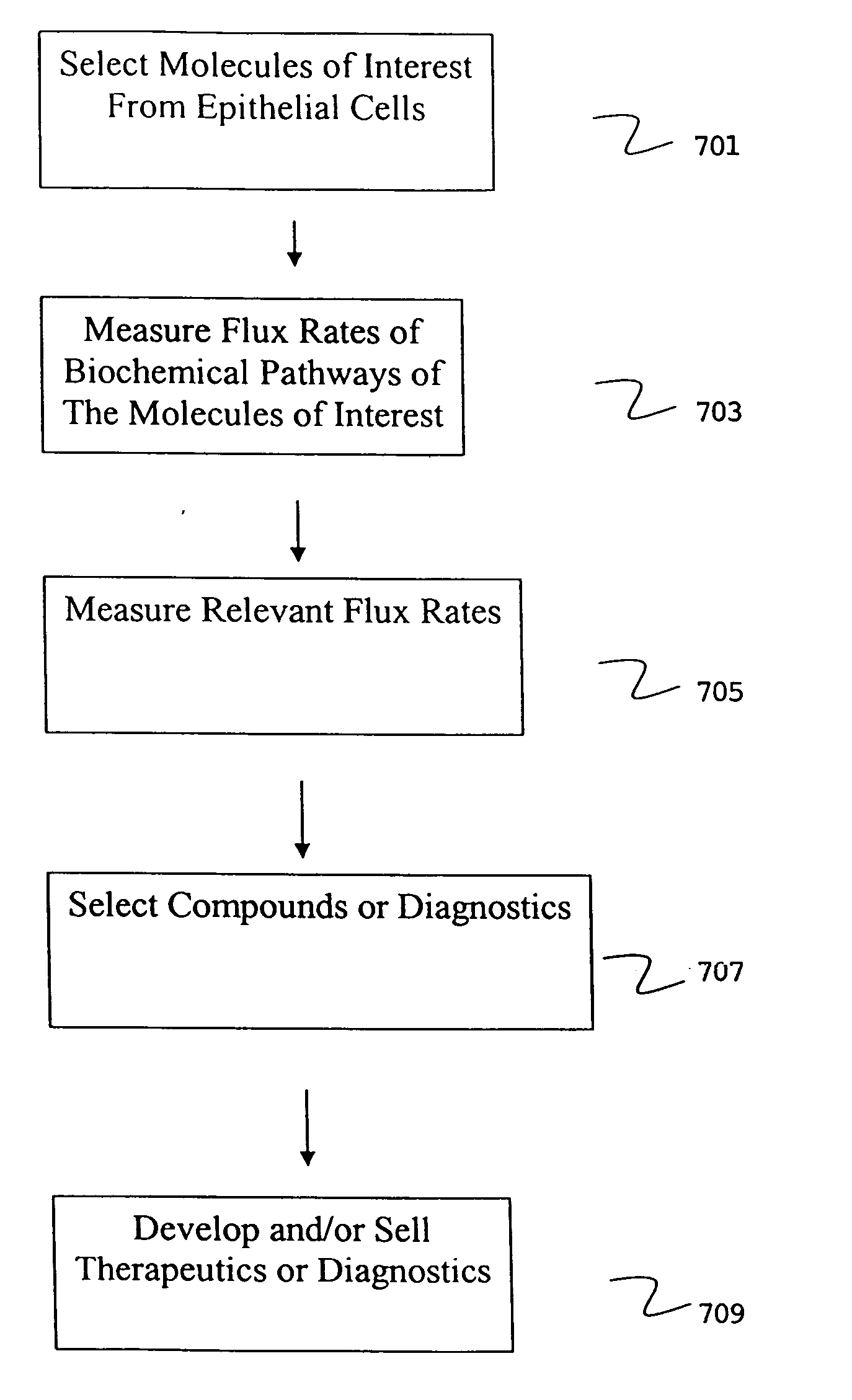
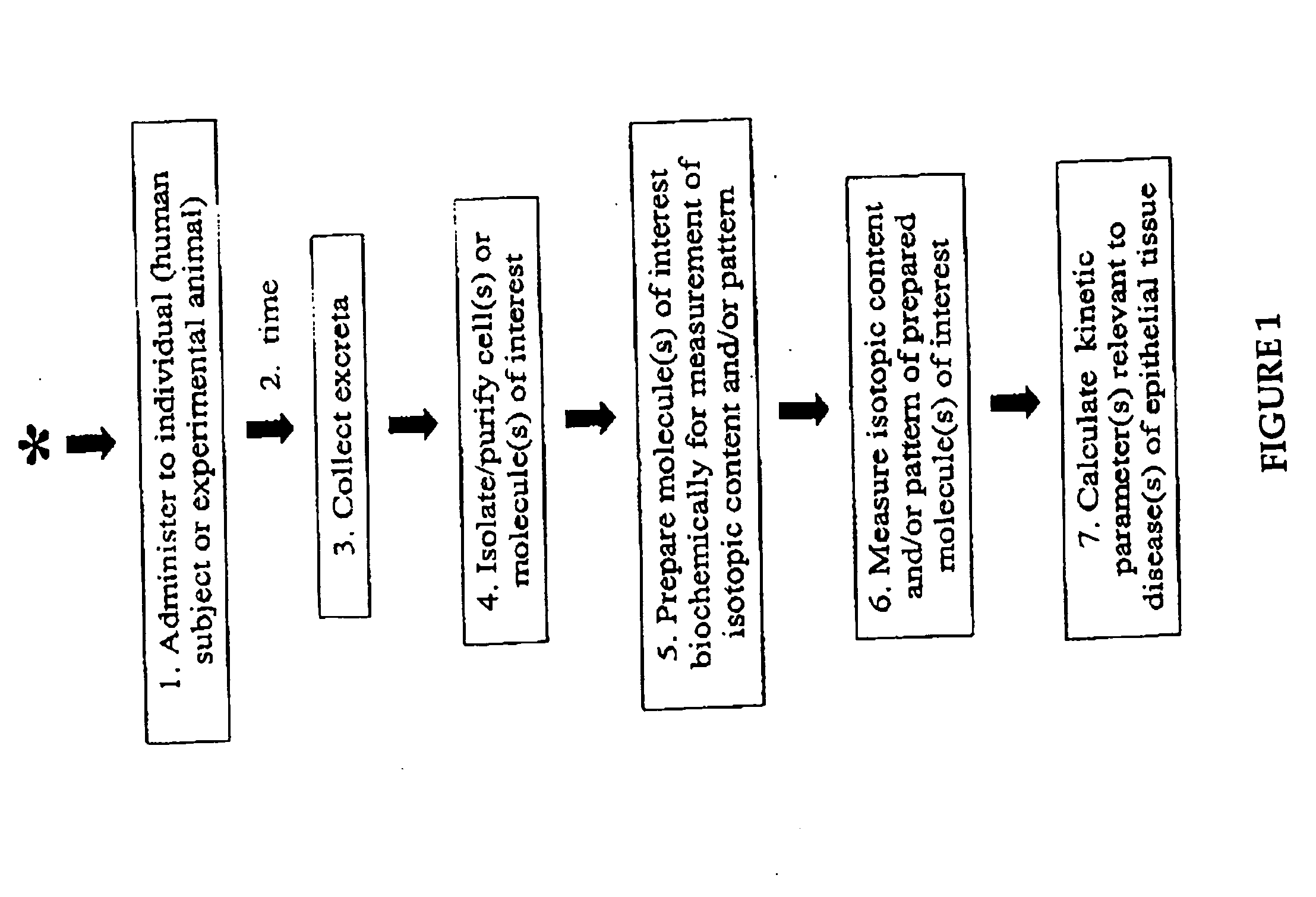
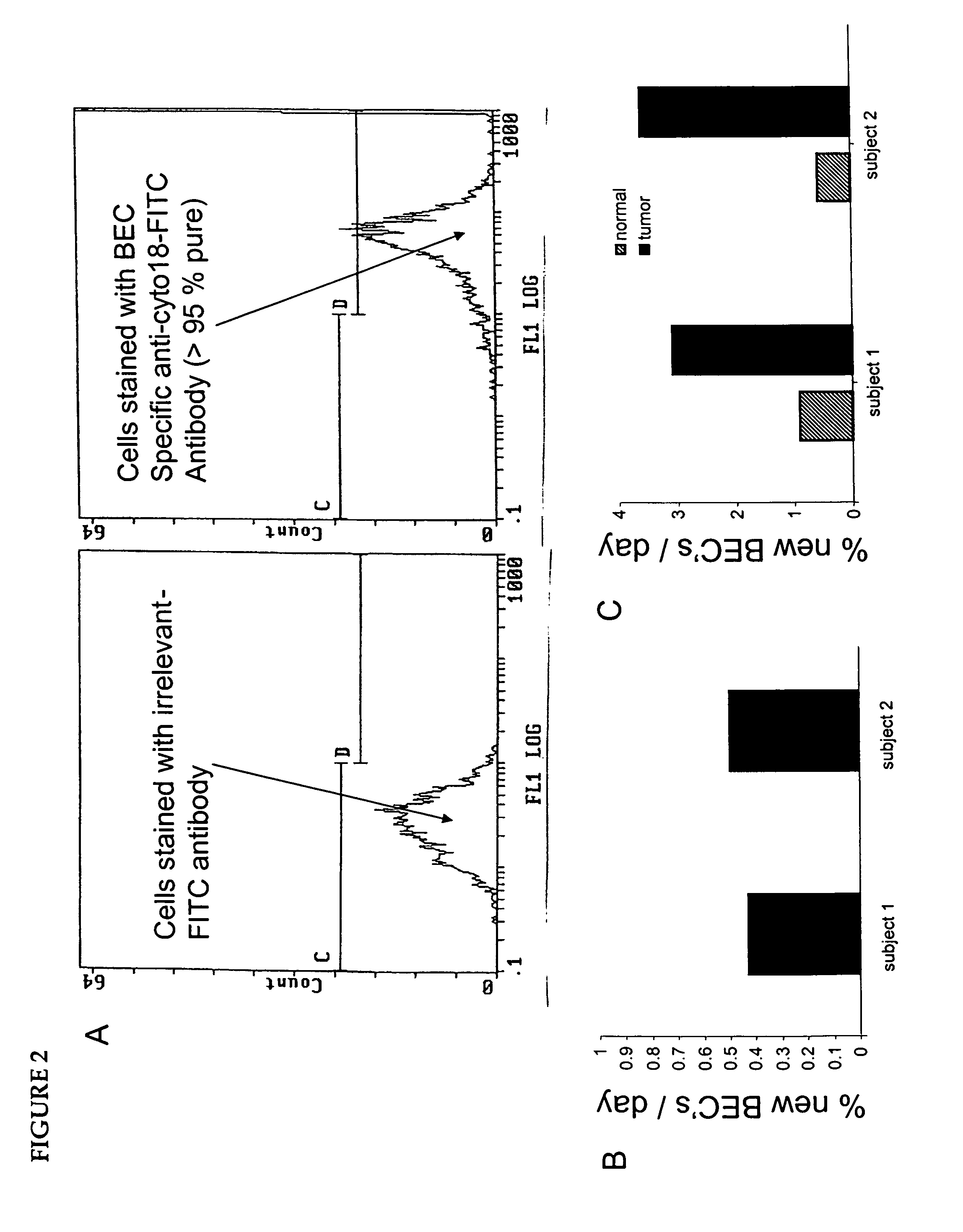
Isolation of epithelial cells or their biochemical contents from excreta after in vivo isotopic labeling
The methods of the present invention allow for the non-invasive or minimally invasive isolation of epithelial cells or their biochemical contents from excreta after in vivo isotopic labeling. Once isolated, the labeled epithelial cells or their labeled biochemical contents find use as kinetic biomarkers of diseases of epithelial tissues including epithelial cancers and epithelial proliferative disorders such as psoriasis and benign prostate hyperplasia. The biomarkers are useful in diagnosing a disease of epithelial tissue origin, monitoring therapeutic efficacy of compounds or combinations of compounds used to treat diseases of epithelial tissue origin, screen new chemical entities or biological factors or combinations of chemical entities or biological factors, or mixtures thereof, for therapeutic activity in animal models of diseases of epithelial tissue origin or in human clinical trials, or test for toxicity on epithelial tissues from exposure to therapeutic compounds or new chemical entities or environmental chemicals such as industrial or occupational chemicals, environmental pollutants, food additives, and cosmetics.
Owner:RGT UNIV OF CALIFORNIA
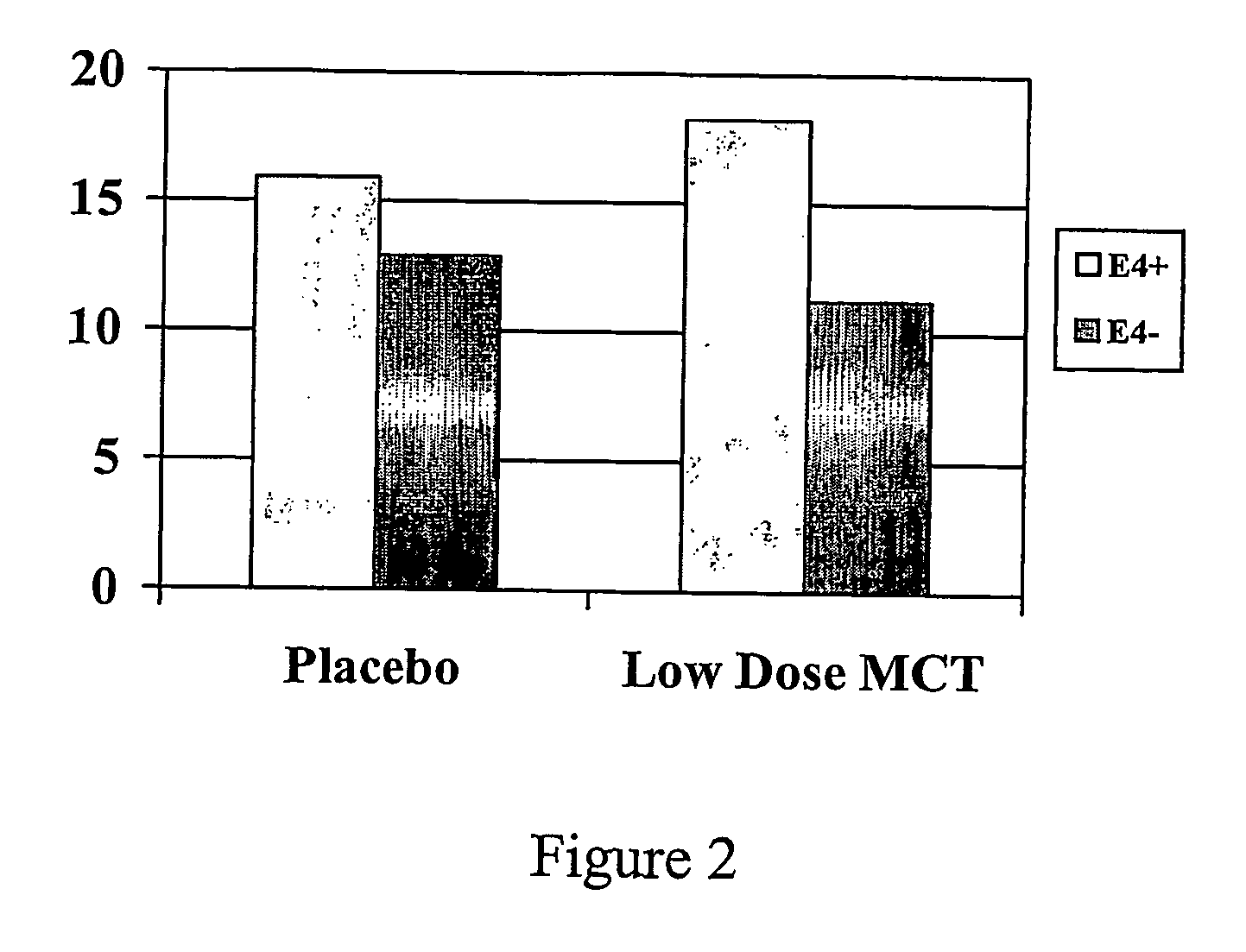
Novel chemical entities and methods for their use in treatment of metabolic disorders
InactiveUS20060189545A1Improve cognitive abilityIncreased ketone body levelBiocideHeavy metal active ingredientsPhysiologyCompound (substance)
Methods and composition for treating or preventing, the occurrence of senile dementia of the Alzheimer's type, or other conditions arising from reduced neuronal metabolism and leading to lessened cognitive function are described. In a preferred embodiment the administration of novel esterified saccharide compounds to said patient at a level to produce an improvement in cognitive ability.
Owner:ACCERA INC
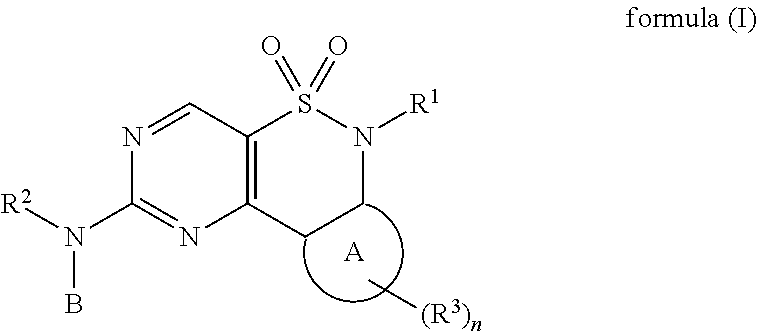
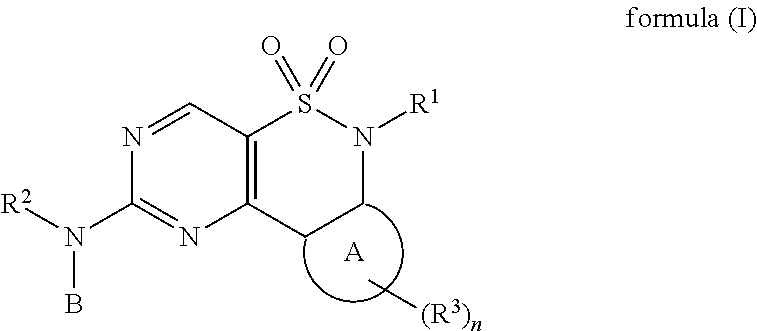
New Chemical Entities To Be Used For Wee1 Inhibition For The Treatment Of Cancer
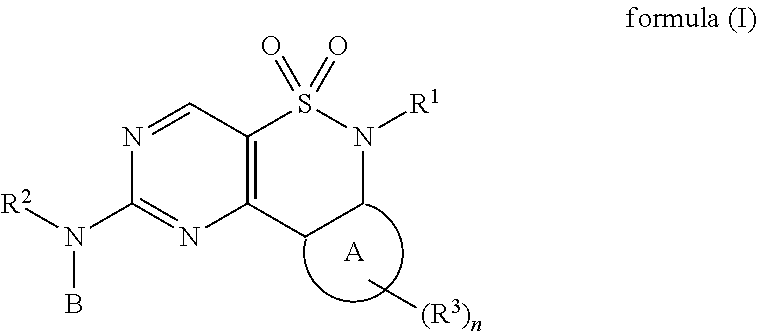
The present invention relates to compounds of formula (I) or pharmaceutical acceptable salts,wherein R1, R2, R3, A, B, and n are defined in the description. The present invention relates also to compositions containing said compounds which are useful for inhibiting kinases such as wee-1 and methods of treating diseases such as cancer.
Owner:ABBVIE INC
In vitro cell-based methods for biological validation and pharmacological screening of chemical entities and biologicals
InactiveUS7198895B2Increase in motilityOrganic active ingredientsBiocidePharmacometricsClinical trial
This patent describes a novel in vitro cell-based method for biological validation and pharmacological screening of drugs, new chemical entities (NCEs) and biologics, which is predictive of in vivo testing for efficacy and adverse events in patients, as occurs in clinical trials. The same method can be used to create an in vitro cell-based assay to identify the ‘right marketed medication for the right patient’ (personalized medicine), and to identify responders / non-responders in ongoing clinical trials with NCEs. In addition this approach can be used to identify new indications for existing medicines and new indications for NCEs that were unsuccessful in their intended uses.
Owner:MOHANLAL RAMON W
Novel chemical entities affecting neuroblastoma tumor-initiating cells
InactiveUS20070123448A1Low cytotoxicityReducing high rate of mortalityBiocidePeptide/protein ingredientsAbnormal tissue growthRegimen
Disclosed are neuroblastoma tumor-initiating cell inhibiting compositions comprising chemical entities capable of affecting neuroblastoma tumor-initiating cells. Pharmaceutical preparations that include these chemical entities are also provided for the treatment of neuroblastoma. These pharmaceutical preparations are suitable for the treatment of humans, and are particularly suited for the treatment of children of 12 years of age or younger having neuroblastoma. The compositions and pharmaceutical preparations posses reduced normal cell cytotoxicity. The compositions and pharmaceutical preparations may be used alone or together with other conventional neuroblastoma preparations as part of a clinical regimen in the treatment and management of neuroblastoma.
Owner:HOSPITAL FOR SICK CHILDREN
Process for preparing antrodia camphorata polysaccharide and antrodia camphorata triterpene with micro-prorous adsorptive resin and its product
InactiveCN1683418AOrganic active ingredientsAdsorption purification/separationChromatographic separationAlcohol
The present invention provides preparation process of antrodia camphorata polysaccharide and antrodia camphorata triterpene with adsorptive microporous resin and its product, and relates to chromatographic separation technology with adsorptive microporous resin and extraction of effective Chinese medicine component. Antrodia camphorata sporophore or mycelium is extracted with hot water and sectional eluted in microporous resin with water and alcohol to obtain antrodia camphorata polysaccharide and antrodia camphorata triterpene separately. The products of the present invention are used in preparing medicine and antrodia camphorata triterpene has excellent liver cancer cell resisting activity.
Owner:敖宗华 +2
Polyisoprenylated Benzophenones and Their Isomers as Inhibitors of Histone Acetyltransferases and Uses thereof
In this patent we describe the purification of prenylated benzophenones from the fruit rinds of Garcinia species and its evaluation as an inhibitor for Histone acetyltransferases p300 and PCAF. We have found that prenylated benzophenones are potent HAT inhibitors of p300 (IC50-1 μM) and pCAF (IC50-15 μM). The inhibitors significantly repress the p300 HAT dependent transcriptional activation from in vitro assembled chromatin template but had no effect on transcription from DNA. These results suggest that the compounds could be specific to HATs. Thus these compounds should be useful as biological switching molecule for evaluating the role of p300 and PCAF in cellular functions and may be useful as new chemical entities for the development of anticancer drugs.
Owner:JAWAHARLAL NEHRU CENT FOR ADVANCED SCI RES
Method and apparatus for evaluating new chemical entities
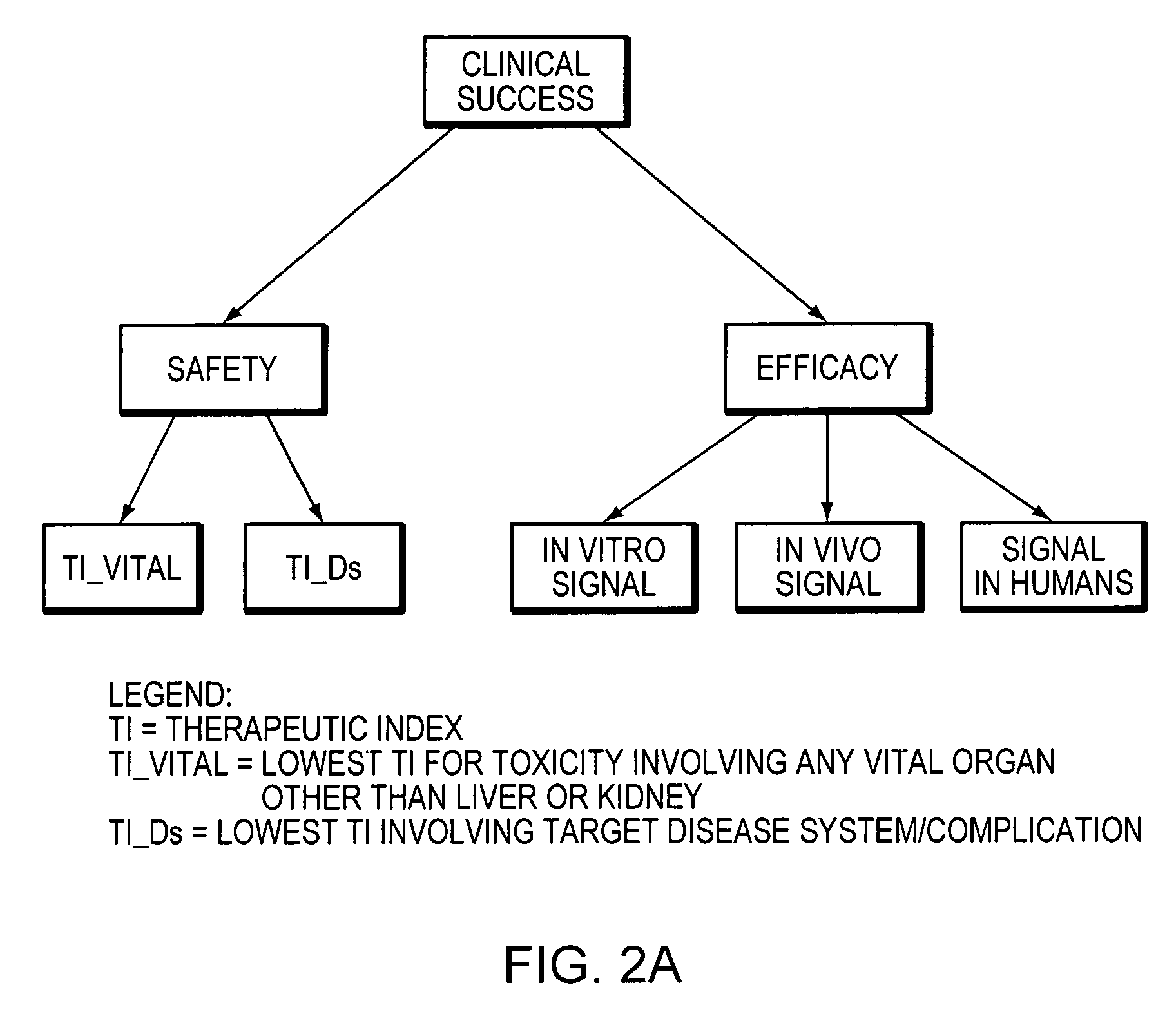
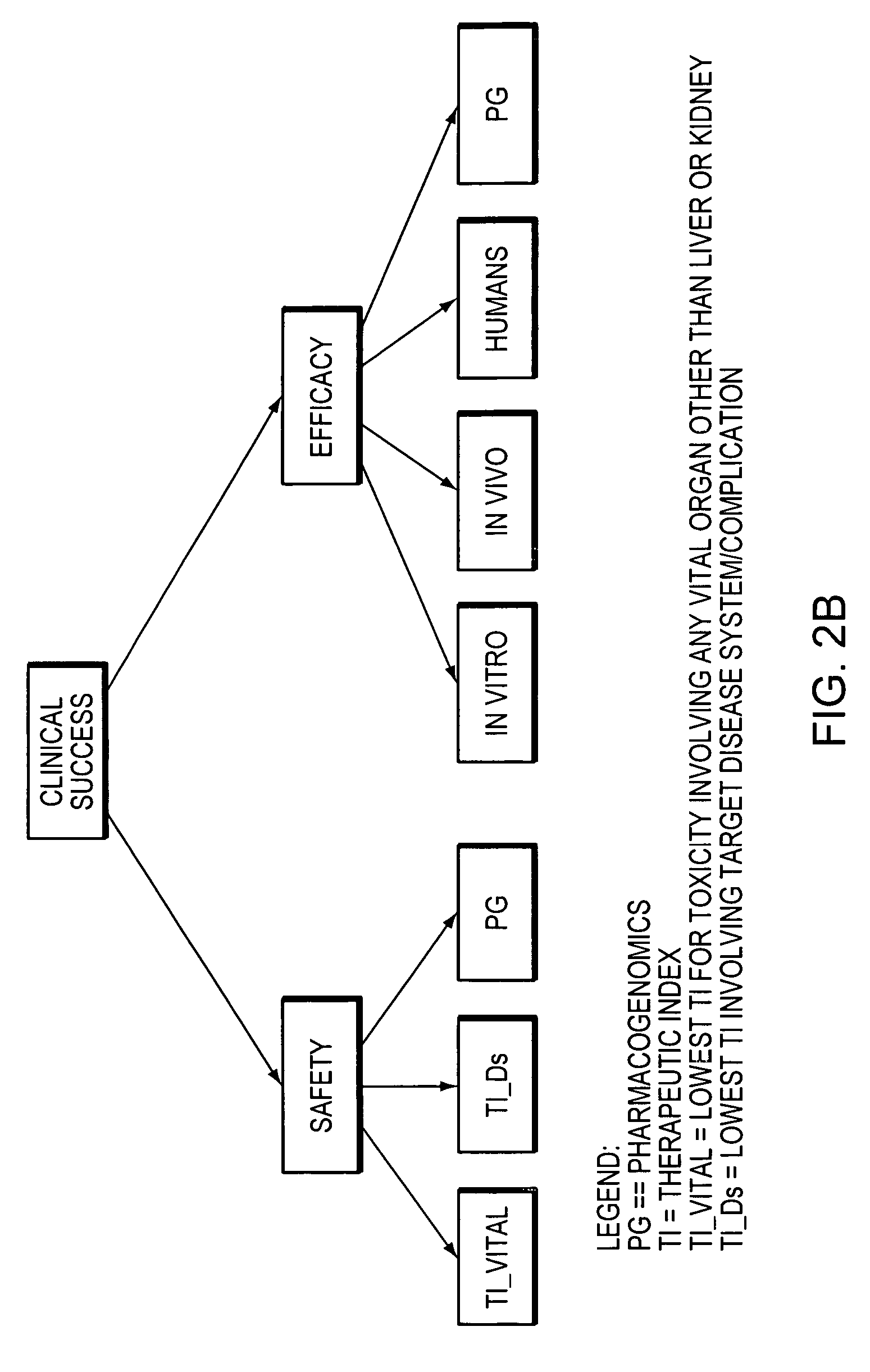
InactiveUS20050021237A1Chemical property predictionBiological testingCompound (substance)Computer science
A method for predicting the success of a new chemical entity, including the steps of providing a signal related to the new chemical entity, providing a therapeutic index, providing a conditional probability table, providing a prior probability distribution, providing a prior N, and calculating a posterior probability distribution for the new chemical entity. An apparatus for predicting the success of a new chemical entity including an input for a signal related to the new chemical entity, a therapeutic index, a conditional probability table, a prior probability distribution, a prior N, and a processor calculating a posterior probability distribution for the new chemical entity.
Owner:SCHACHTER ASHER DANIEL +1
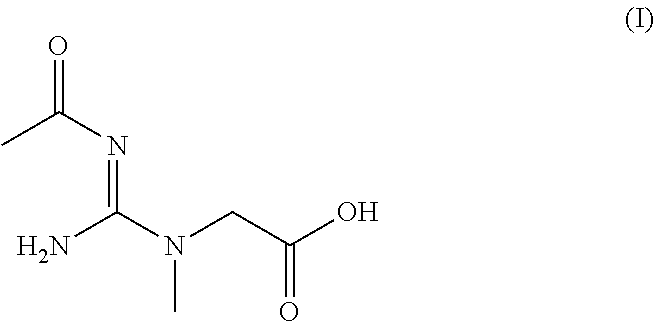
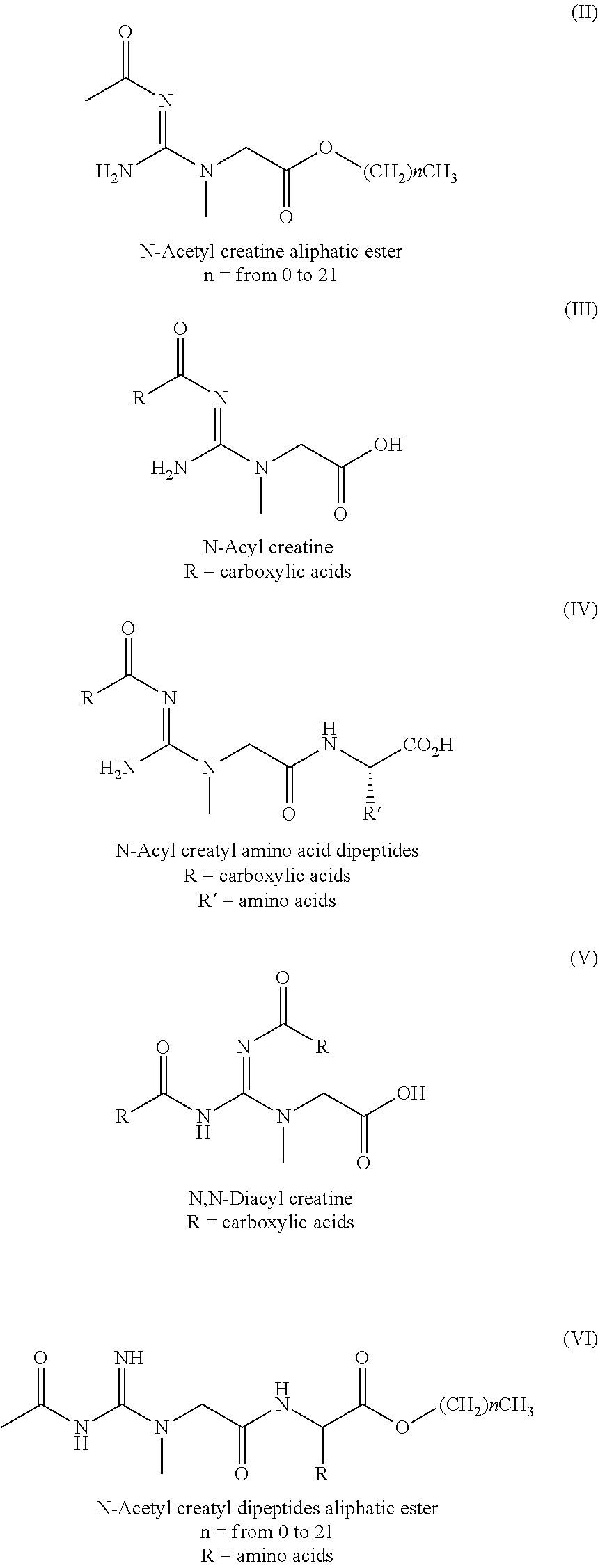
Bio-available n-acetyl creatine species and compositions thereof
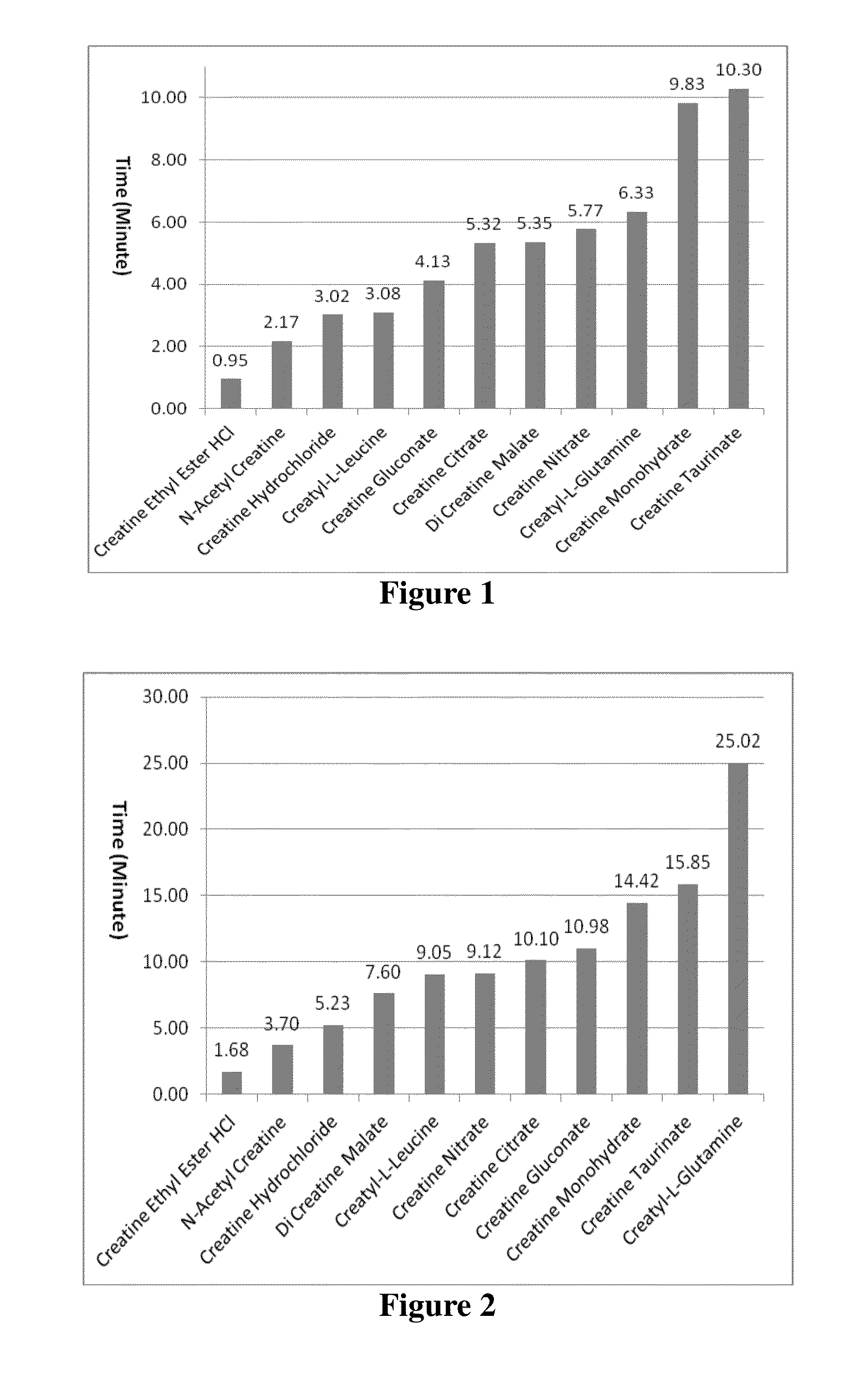
ActiveUS20150238453A1Improve solubilityImprove bioavailabilityPowder deliveryOrganic active ingredientsSolubilityBioavailability
Creatine derivatives and compositions containing a [one or more?] bioactive form[s] of creatine. The new chemical entity comprises an acetyl creatine, N-acyl creatines, N,N-diacyl creatines or any N-acetyl creatine species with enhanced solubility and bioavailability. Also provided by this invention are various methods for providing several beneficial effects that comprise administering compositions comprising N-acetyl creatine, N-acyl creatines, N,N-diacyl creatines or any N-acetyl creatine species to a mammalian subject, either chronically or acutely.
Owner:JHO INTPROP HLDG
Corticosteroid linked beta-agonist compounds for use in therapy
InactiveUS20090318396A1Minimizing systemic absorptionHigh affinityBiocideOrganic active ingredientsPhosphorylationMedicine
Owner:GILEAD SCI INC
5-lipoxygenase inhibitor and preparation method, medical composite and application thereof
InactiveCN101684098ALarge doseOrganic active ingredientsOrganic chemistryDisease5-Lipoxygenase Inhibitor
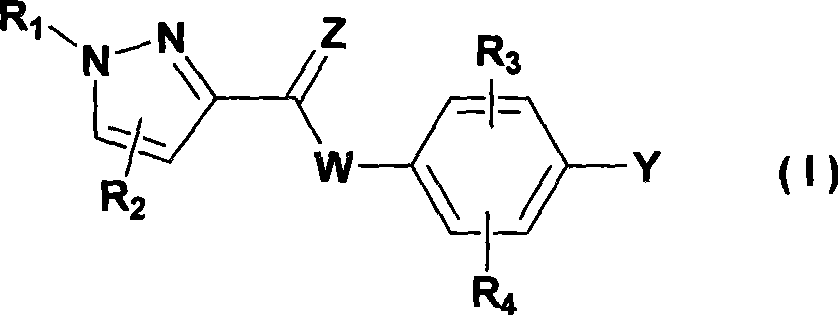
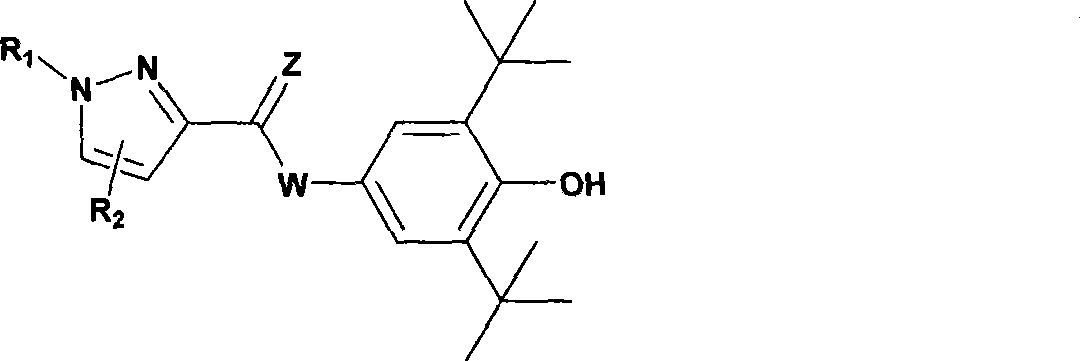
The invention provides a 5-lipoxygenase inhibitor and a preparation method, a medical composite and an application thereof. More specifically, the invention provides a compound shown in general formula (I) or acceptable salt, solvate or hydrate thereof in pharmacy. The invention also relates to a preparation method, medical composite and application of the compound. The biological activity experiment shows that the compound of the invention is effective 5-LOX small molecule inhibitor with novel structure. Therefore the compound is expected to be developed to be new powerful chemical entity forcuring diseases related to leukotriene.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Calmangafodipir, a new chemical entity, and other mixed metal complexes, methods of preparation, compositions, and methods of treatment
ActiveUS9187509B2Improvement in preparation and useImproved treatment of and protectionOrganic active ingredientsNervous disorderCompound (substance)Oxygen
Owner:EGETIS THERAPEUTICS AB
Pharmaceutically active compositions comprising oxidative stress modulators (OSM), new chemical entities, compositions and uses
Described herein are pharmaceutical compositions and medicaments, and methods of using such pharmaceutical compositions and medicaments in the treatment of inflammation and cancer.
Owner:COLBY PHARMA CO
Novel pyrazole 5-lipoxygenase small molecule inhibitors, preparation method, pharmaceutical composition and application thereof
The invention relates to novel pyrazole 5-lipoxygenase small molecule inhibitors shown in a general formula (I), enantiomers, diastereoisomers, racemic modifications, mixtures, and pharmaceutically acceptable salts thereof. The invention also relates to a method for preparing the pyrazole compounds. In addition, the pyrazole compounds have better prevention and treatment effect on diseases related to leukotriene produced by an experimental 5-lipoxygenase (5-LOX) metabolic pathway. The biological experimental activity shows that the compounds are effective 5-LOX small molecule inhibitors with novel structures. Therefore, the compounds expect to be developed into novel powerful chemical entities for treating diseases such as inflammation, cancer, asthma and atherosclerosis related to 5-LOX and the leukotriene.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Reagents and methods for the determination of pk/adme-tox characteristics of new chemical entities and of drug candidates
InactiveUS20090029391A1Easy to castAnalysis by subjecting material to chemical reactionBiological testingConductive polymer compositeNanoparticle
Methods using colloidal conductive polymers, noble metal nanoparticles or of stable metal / conductive polymer composite colloids as reagents for at least the in vitro prediction of, if not the determination of, PK / ADME-tox properties of new chemical entities (NCEs) are provided. Also provided are kits that include the subject methods and reagents for application thereof. The salient characteristics of these methods and reagents pertaining to the present invention reside in the fact that the reactions in which they are involved are homogeneous, rapid, and proceed as “mix and read” processes.
Owner:PHARMA DIAGNOSTICS
New sesquiterpene quinone compound from abelmoschus sagittifolius, as well as preparation method and applications thereof
ActiveCN104341376AGood anti-tumorHas antitumor activityOrganic chemistryAntineoplastic agentsFood materialMtt method
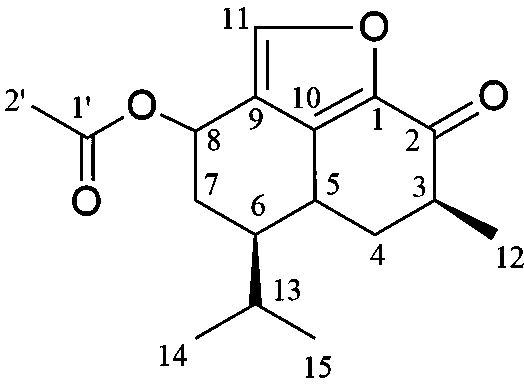
The invention discloses a sesquiterpene quinone compound separated and purified from abelmoschus sagittifolius, as well as a preparation method and applications thereof, relating to the technical field of medicines. The new sesquiterpene quinone compound with antitumor activity is named as Acylhisbiscone B, with the molecular formula of C17H22O4 and the structural formula shown in the specification. According to the preparation method, the detection on the antitumor activity of the new compound is carried out by adopting an MTT method through combination of column chromatography and preparative liquid chromatography purification methods. In vitro experiment results show that the new sesquiterpene quinone compound has activity on significantly inhibiting the growth of Hela (human cervical carcinoma cells) and HepG-2 (human hepatoma carcinoma cells). The new sesquiterpene quinone compound can provide new chemical entity or lead compound for research of antitumor medicaments, and can also be used as food raw materials for preparation of health food.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI HAINAN BRANCH
Reagents and methods for the determination of pk/adme-tox characteristics of new chemical entities and of drug candidates
Methods using colloidal conductive polymers, noble metal nanoparticles or of stable metal / conductive polymer composite colloids as reagents for at least the in vitro prediction of, if not the determination of, PK / ADME-tox properties of new chemical entities (NCEs) are provided. Also provided are kits that include the subject methods and reagents for application thereof. The salient characteristicsof these methods and reagents pertaining to the present invention reside in the fact that the reactions in which they are involved are homogeneous, rapid, and proceed as 'mix and read' processes.
Owner:PHARMA DIAGNOSTICS NV
Design and synthesis of optimized ligands for PPAR
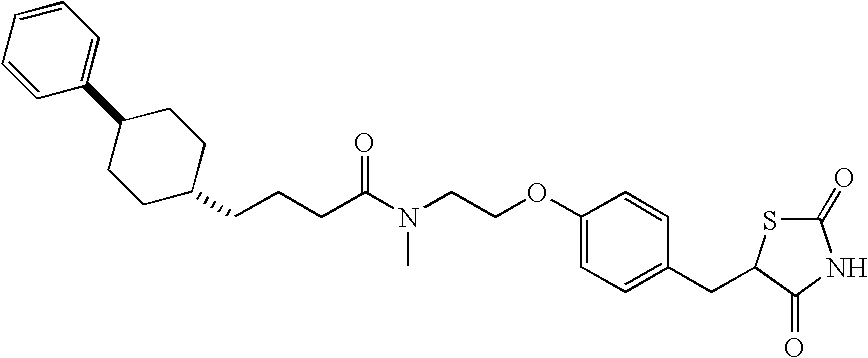
This invention provides new chemical entities useful for treating a variety of clinical disorders including those that are influenced by the activity of peroxisome proliferator activated receptors (PPAR). The structures of the compounds and methods to design, make and use the compounds are provided. Compounds and methods for administering therapeutic compositions comprising the compounds in cases of the disease psoriasis are provided. An exemplary compound having the formula compound is 5adamantan-2-yl-pentanoic acid {2-[4-(2,4-dioxo-thiazolidin-5-yl-methyl)-phenoxy]-ethyl}-methyl-amide is provided.
Owner:BETHESDA PHARMA +1
Chrysotoxine bibenzyl derivative and preparation method and application thereof
InactiveCN105175262ANeuroprotectiveMild reaction conditionsNervous disorderOrganic compound preparationChrysotoxineNeuroprotective Drugs
The present invention discloses a chrysotoxine bibenzyl derivative shown as a formula I. R1 represents C=O, CH2, NH, S=O or a covalent bond; R2 represents alkyl, phenyl, substituted phenyl, benzene ring substituted C1-3 alkyl, C5-C6 cycloalkyl, and a pentabasic or hexahydric unsaturated heterocyclic group containing one or more O, S and N. The invention also discloses a simple and efficient preparation method of the chrysotoxine bibenzyl derivative, and application of the derivative to the preparation of neuroprotective drugs and drugs for treating Alzheimer disease. The chrysotoxine 4-O-substituted bibenzyl derivative has significant protective effect on A beta-induced neuronal apoptosis, and is a potential novel chemical entity for the treatment of AD.
Owner:CHINA PHARM UNIV
Dendrobium officinale analogue and preparation method and application thereof
PendingCN112266377AGrowth inhibitionOrganic active ingredientsOrganic chemistryPharmaceutical drugPharmaceutical medicine
The invention discloses a dendrobium officinale analogue and pharmaceutically acceptable salt and hydrate thereof, and the analogue has anti-tumor activity, especially significantly inhibits the growth of colon cancer SW480 cells, and is a potential new chemical entity for treating colon cancer. The invention further discloses a simple and efficient preparation method of the dendrobium officinaleanalogue and application of the dendrobium officinale analogue in preparation of anti-cancer, anti-tumor and colorectal cancer treatment pharmaceutical compositions, and the dendrobium officinale analogue has important significance in research and development of anti-cancer or anti-colorectal cancer drugs.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
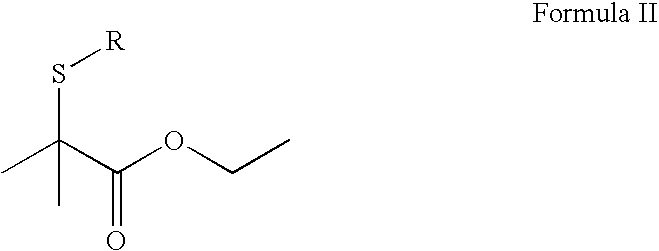
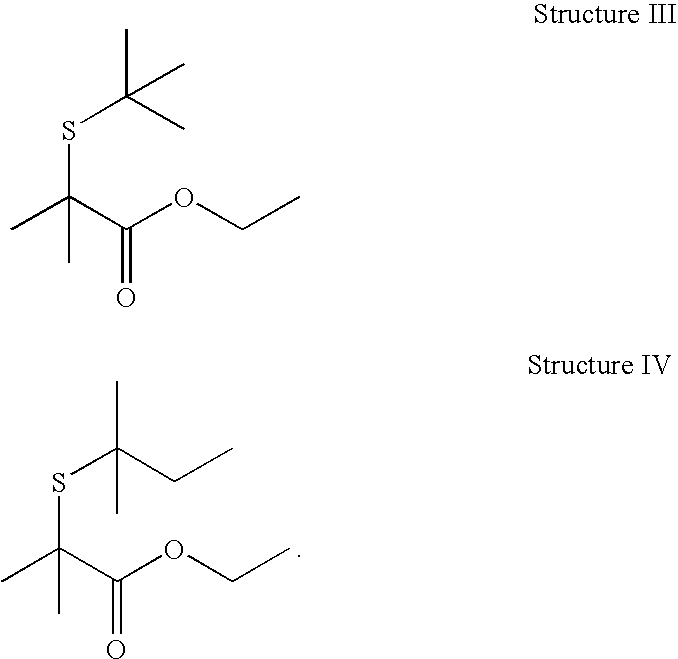
Thioester compounds and their use in fragrance or flavor applications
ActiveUS20090232747A1Increase the fragranceCosmetic preparationsBiocideCompound (substance)Food flavor
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Compositions of lithium salts and methods of use
ActiveUS9744189B1Powder deliveryInorganic active ingredientsNeuropsychiatric diseaseTherapeutic window
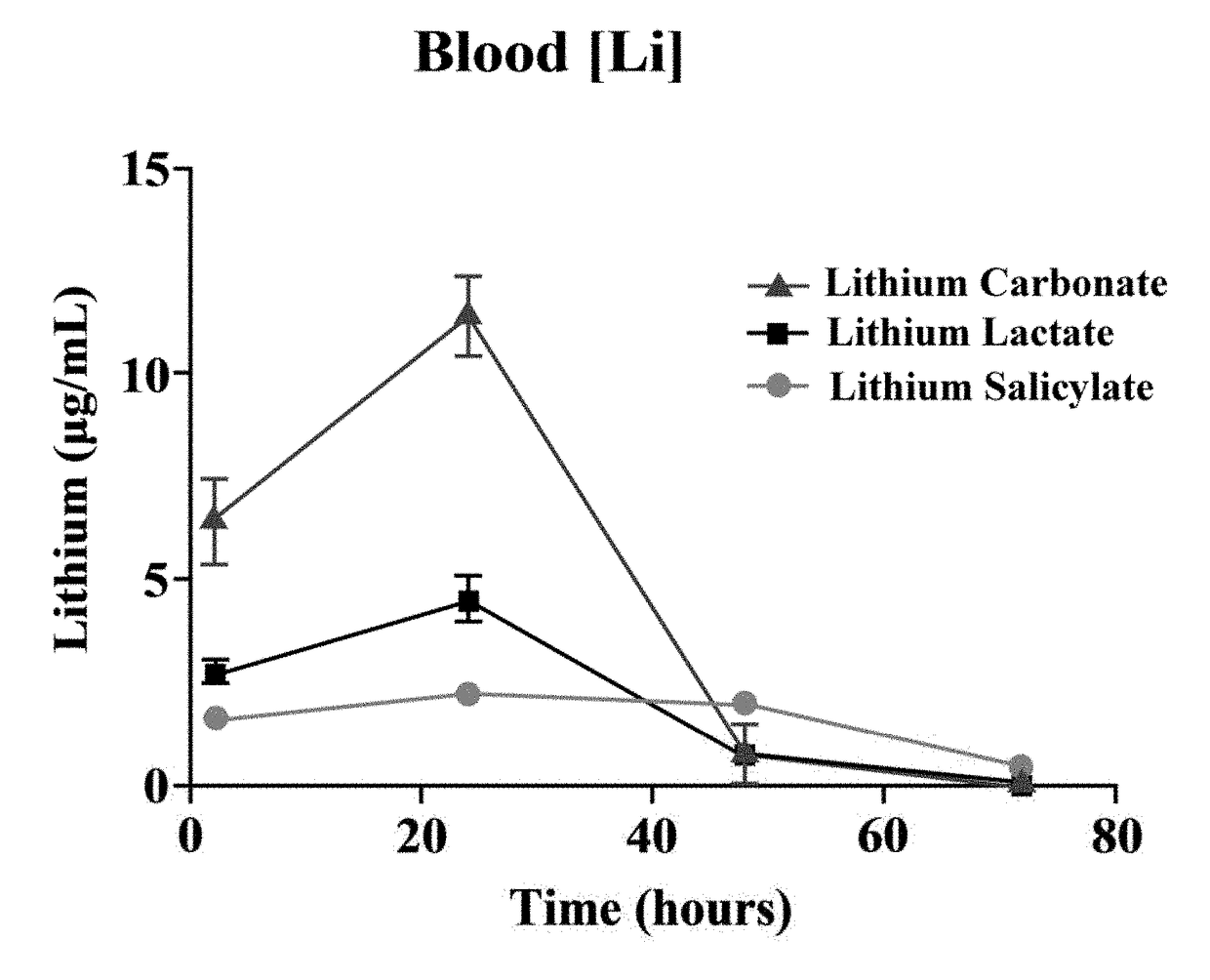
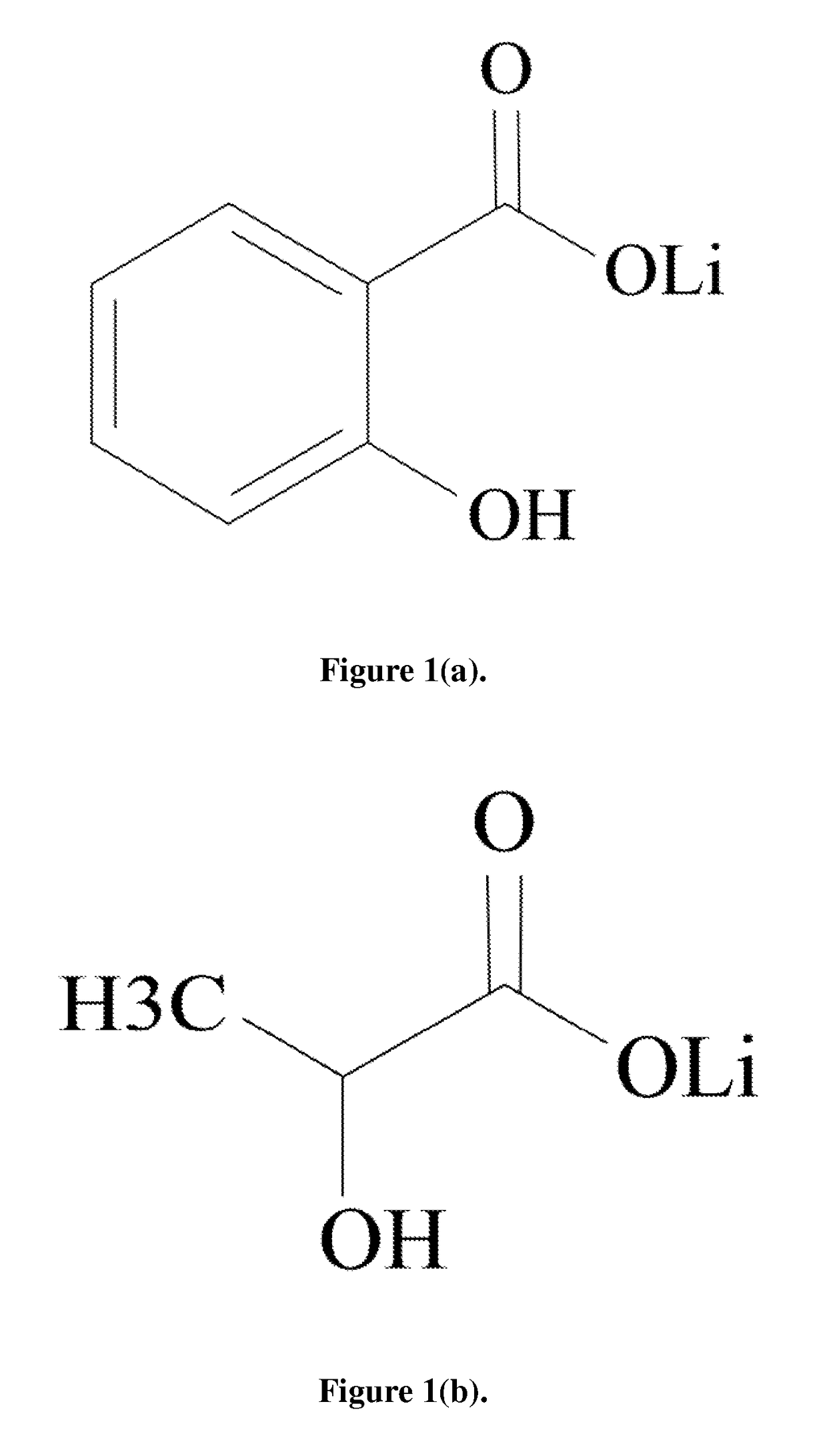
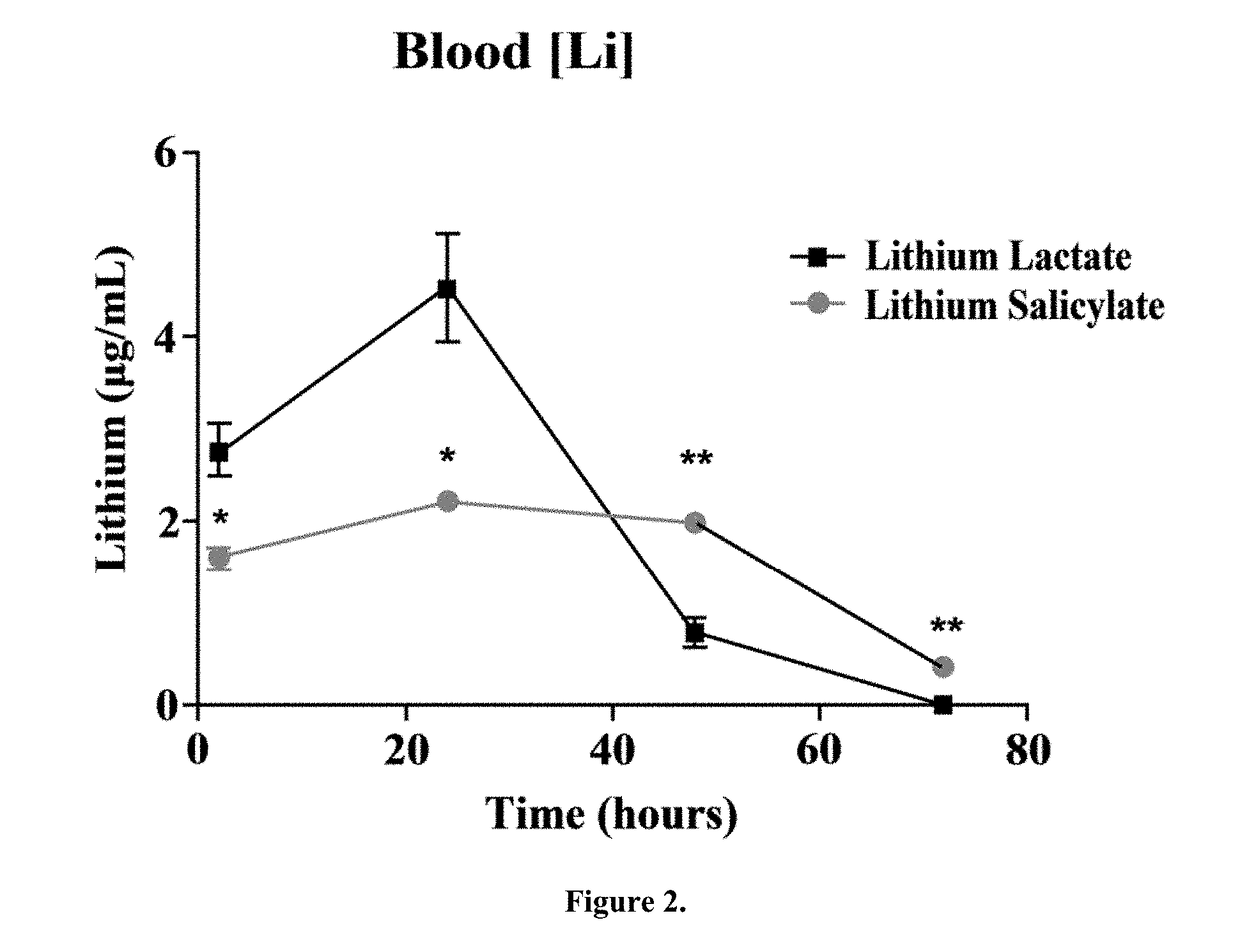
Lithium is regarded as the gold standard comparator and benchmark treatment for mania. One of the problems associated with Lithium is its narrow therapeutic window. Recent attempts to find new drugs with similar therapeutic activities have yielded new chemical entities. However, these potential new drugs have yet to match the many bioactivities attributable to lithium's efficacy for the treatment of neuropsychiatric diseases. Consequently, an intense effort for re-engineering lithium therapeutics using cocrystallization is currently underway. The evaluation of pharmacokinetics of previously unexplored lithium salts with organic anions (lithium salicylate and lithium lactate) has found that these lithium salts exhibit profoundly different pharmacokinetics compared to the more common FDA approved salt, lithium carbonate, in rats. Remarkably, lithium salicylate produced elevated blood and brain levels of lithium beyond 48 hours post-dose without the sharp peak that contributes to the toxicity problems of current lithium therapeutics.
Owner:UNIV OF SOUTH FLORIDA
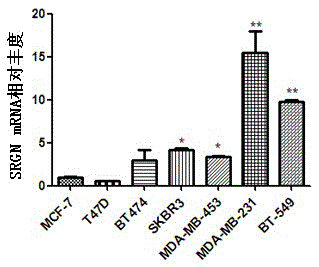
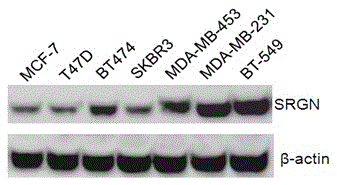
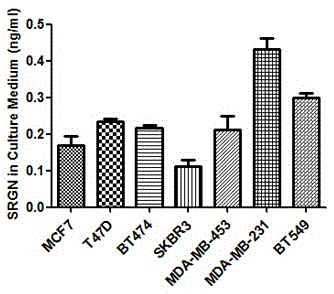
Application of SRGN gene in serving as serum marker for detecting triple-negative breast cancer
InactiveCN105586430AGood curative effectRapid Molecular TypingMicrobiological testing/measurementBiological material analysisSerum markersOncology
The invention discloses application of an SRGN gene and polypeptide thereof in serving as a detection marker for breast cancer molecular typing, in particular to application of the SRGN gene and the polypeptide thereof in serving as a serum marker for detecting a triple-negative breast cancer or in preparation of a detection reagent for the triple-negative breast cancer, and provides a novel breast cancer serum marker, that is, the SRGN gene and the polypeptide thereof. It is proved that expression of the SRGN gene in breast cancer serum is enhanced, and expression of the SRGN gene in serum of a triple-negative breast cancer patient is mainly and significantly increased compared with expression of the SRGN gene in serum of a breast cancer patient with the other molecular types. Accordingly, not only is a new target supplied to breast cancer serology detection, but also new data and a quick serology detection method are supplied to breast cancer molecular typing; a new chemical entity is designed at the gene level and the protein level by taking the gene as the target, breast cancer molecular typing can be quickly, conveniently and effectively performed, and therefore the breast cancer treatment effect is improved.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Methods to enhance bioavailability of organic small molecules and deposited films made therefrom
PendingCN107847458ALiquid surface applicatorsTetracycline active ingredientsSolubilityOrganic compound
Solid films and articles having a surface with discrete regions patterned with a deposited low molecular weight organic compound, such as pharmaceutical actives and new chemical entities, are provided. The organic compound may be present at >= about 99 mass % in the one or more discrete regions and may be crystalline or amorphous. The deposited organic compound may be deposited as a film having high surface area. The deposited organic compound exhibits enhanced solubility and bioavailability, by way of non-limiting example. Methods of organic vapor jet printing deposition method of such a lowmolecular weight organic compound in an inert gas stream are also provided.
Owner:RGT UNIV OF MICHIGAN
In Vitro Cell-based Methods for Biological Validation and Pharmacological Screening of Chemical Entities and Biologicals
This patent describes a novel in vitro cell-based method for biological validation and pharmacological screening of drugs, new chemical entities (NCEs) and biologics, which is predictive of in vivo testing for efficacy and adverse events in patients, as occurs in clinical trials. The same method can be used to create an in vitro cell-based assay to identify the ‘right marketed medication for the right patient’ (personalized medicine), and to identify responders and non-responders in ongoing clinical trials with NCEs.
Owner:MOHANLAL RAMON W
Ion Channel Inhibitory Compounds, Pharmaceutical Formulations, and Uses
The present invention is directed towards new chemical entities which primarily inhibit the human T-type calcium channels and differentially modulate other key ion channels to control cell excitability, and abnormal neuronal activity particularly involved in the development and maintenance of persistent or chronic pain, and / or neurological disorders. These novel compounds are useful in the treatment and prevention of neurological and psychiatric disorders and diseases in which these ion channels are involved. The invention is also directed towards pharmaceutical formulations comprising these compounds and the uses of these compounds.
Owner:AFASCI
Pharmaceutically active compositions comprising oxidative stress modulators (osm), new chemical entities, compositions and uses
InactiveCN102438615APeptide/protein ingredientsPharmaceutical delivery mechanismOxidative stressPharmacology
Owner:COLBY PHARMA CO
Flavonoid new chemical entity, composition and use
PendingCN105837646AEasy to slideEasy to makeAntibacterial agentsOrganic active ingredientsArthritisMicroangiopathy
The invention provides a flavonoid new chemical entity, which has little moisture absorption and good storage stability, and is applicable to preparation of food or health food or drugs for reduction of capillary fragility, treatment or prevention of chronic venous insufficiency, hemorrhoid, scurvy, venous lower limb ulcer, premenstrual syndrome, diabetics' microangiopathy and secondary upper limb lymphedema, cardiovascular diseases, blood glucose and lipid regulation, antisepsis and inflammation reduction, virus resisting, cancer resisting, immunity adjustment, radiation protection, liver protection, rheumatoid arthritis or arthritis resisting, ulcer resisting, phlegm eliminating, asthma relieving, black spots, freckles, free radical resistance, and oxidation resistance.
Owner:刘力
Calmangafodipir, a New Chemical Entity, and Other Mixed Metal Complexes, Methods of Preparation, Compositions, and Methods of Treatment
ActiveUS20150005259A1Low toxicityImprove protectionBiocideOrganic active ingredientsOxygenMixed metal
A mixed metal complex of a compound of Formula I, or a salt thereof, wherein the mixed metals comprise a Group III-XII transition metal and a Group II metal: (Formula I) (I) wherein X, R1, R2, R3, and R4 are as defined herein, is produced in a one step crystallization from a solution of the Group III-XII transition metal, the Group II metal, and a compound of Formula I. Methods for treatment of a pathological condition in a patient, for example, a pathological condition caused by the presence of oxygen-derived free radicals, comprises administering the mixed metal complex to the patient.
Owner:EGETIS THERAPEUTICS AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com