Dendrobium officinale analogue and preparation method and application thereof
A technology of dendrobium officin and its analogues, which is applied in the field of dendrobium officin analogues and their preparation, and can solve the problems of low bioavailability, expensive source of raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
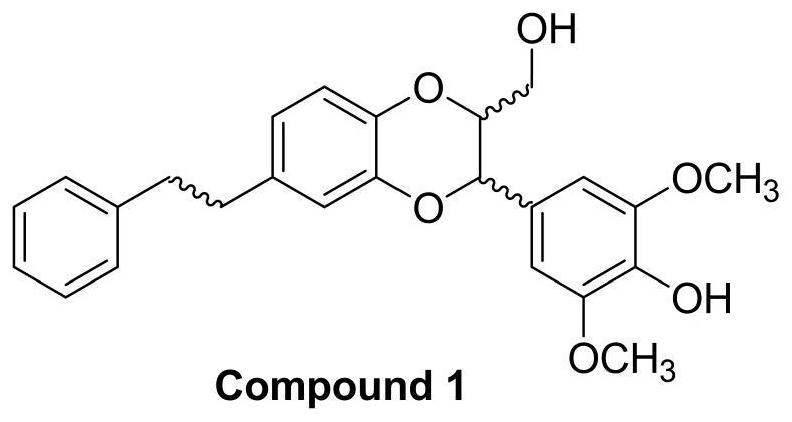
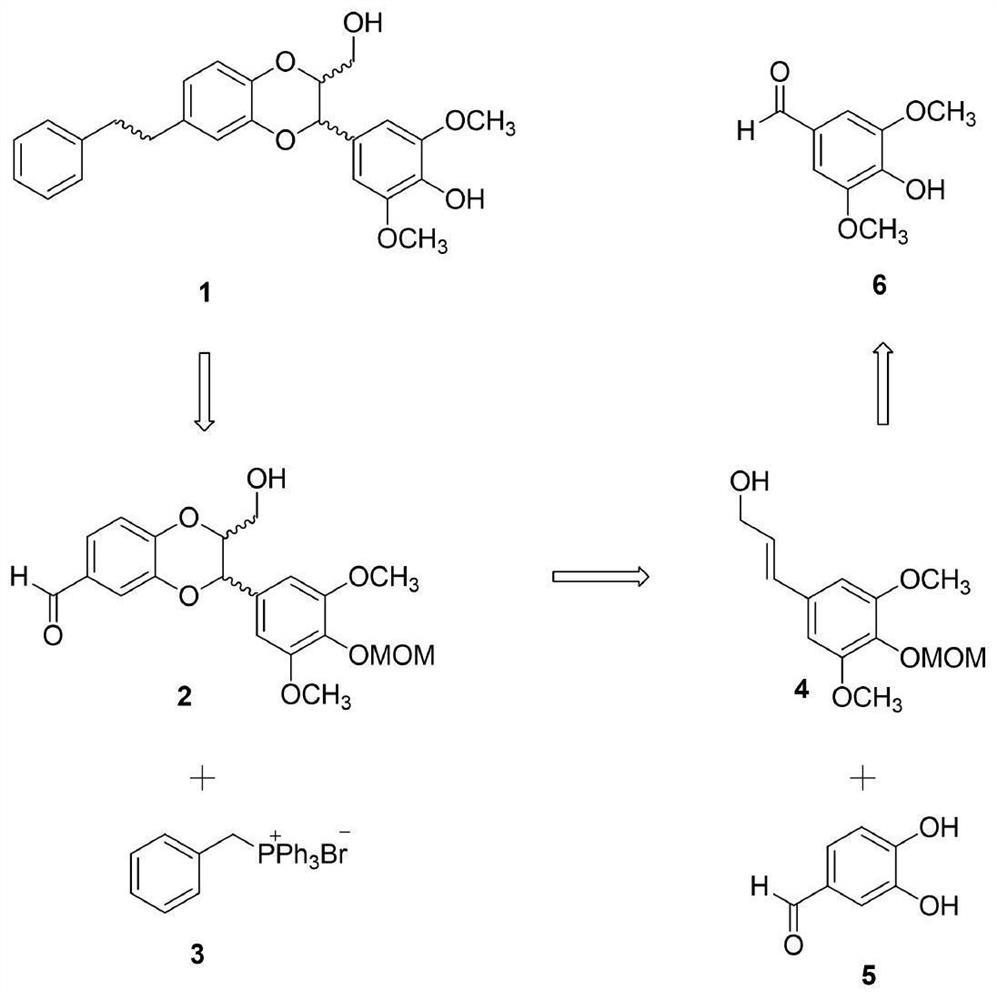
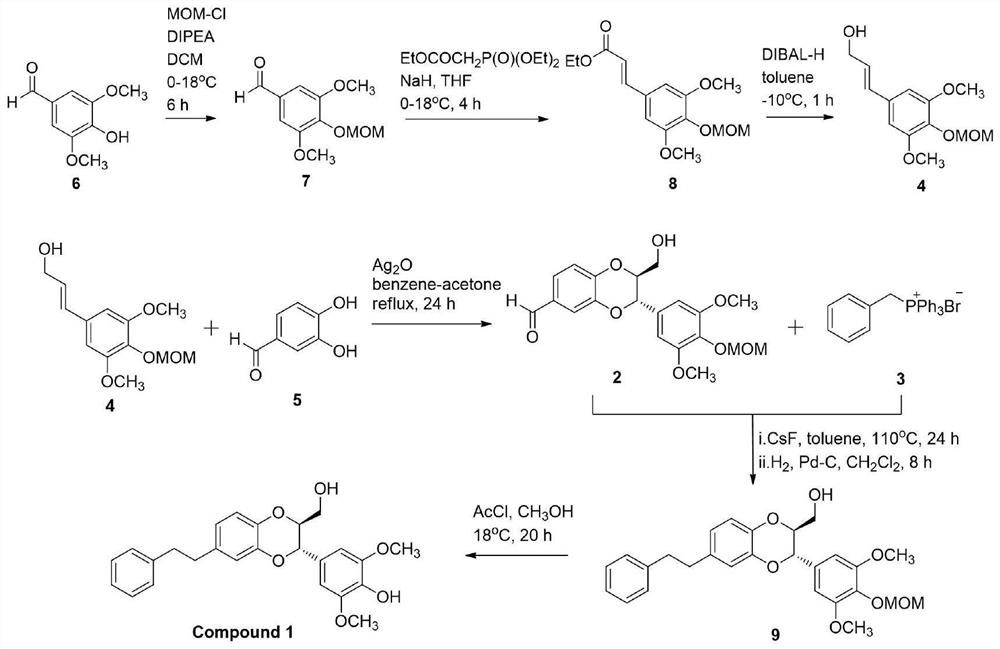
[0030] Embodiment 1: the synthesis of Dendrobium officinale analogue Compound 1
[0031] The synthesis of Dendrobium officinale analogue Compound 1 is as follows: image 3 shown.
[0032] Synthesis of compound 7: Add 3,5-dimethoxy-4-hydroxybenzaldehyde (9.1 g, 50 mmol), N,N-Diisopropylethylamine (14.0 mL, 80 mmol) into a 250 mL round-bottomed flask replaced with nitrogen and 4-dimethylaminopyridine (61 mg, 0.5 mmol) were added to 100 mL of dichloromethane to fully dissolve, and chloromethyl ether (4.6 mL, 60 mmol) was added dropwise at 0ºC, and the temperature was raised after the addition was completed After reacting at room temperature for 6 h, TLC showed that the reaction was complete. The reaction solution was diluted with water, added with cold HCl aqueous solution (50 mL, 0.1 M), and extracted to obtain an organic layer, which was extracted twice with 50 mL of dichloromethane, and the organic phases were combined. The organic phase was washed with water, washed with s...
Embodiment 2
[0038] Embodiment 2: In vitro inhibitory effect of dendrobium officinale analogues on cancer cells
[0039] 1. Experimental materials
[0040] Cell lines: human lung cancer cell line A549, human breast cancer cell line MCF-7, human leukemia cell line HL-60, human colon cancer cell line SW480, human liver cancer cell line HepG2, human skin squamous cell line A431
[0041] Detection principle: MTT method to detect cell viability
[0042] 2. Test method
[0043] 1) Cell inoculation: Use RMP1640 or DMEM medium containing 10% fetal bovine serum to prepare a single cell suspension, and inoculate 10,000-20,000 cells per well into a 96-well plate with a volume of 100 µL per well.
[0044] 2) After 12 hours, the cells were replaced with 1% penicillin-streptomycin DMEM or RMP1640 medium, and the compound solution to be tested was added (fixed concentration 40µM for primary screening, at which concentration the compound that inhibited tumor cell growth at around 50% was entered at 5 co...
Embodiment 3
[0050] Example 3: In vitro inhibitory effect of dendrobium officinale analogs on colon cancer SW480 cells
[0051] Take the cells in the logarithmic growth phase to adjust the concentration of the cell suspension, and the seeding volume of the cells is 1000 / well. Set blank wells and positive control wells at the same time. Incubate for 24 h at 37°C in an incubator with 5% carbon dioxide. Add the compound to be tested in 1640 medium with 1% penicillin and streptomycin, after 24 hours of treatment, change back to 10% fetal bovine serum medium, and continue to culture for 14 days.
[0052] The result is as Figure 5 As shown, Dendrobium officinale analogs can significantly inhibit the growth of colon cancer SW480 cells in a concentration-dependent manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com