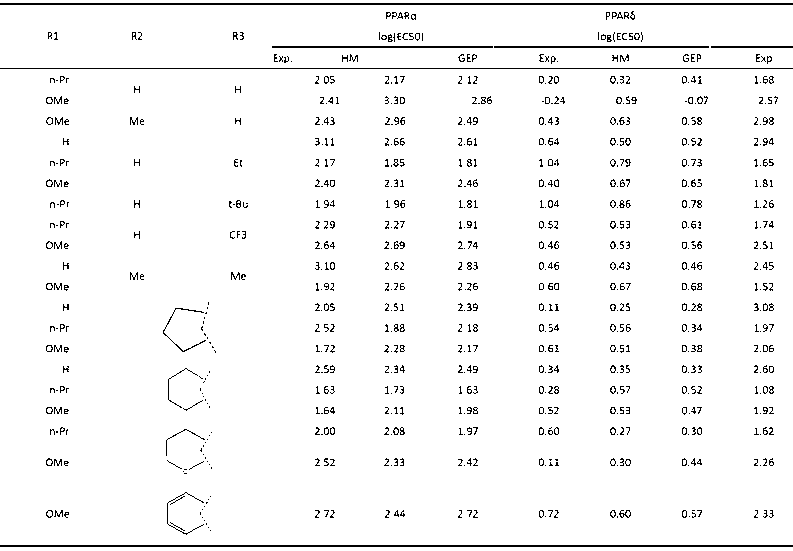
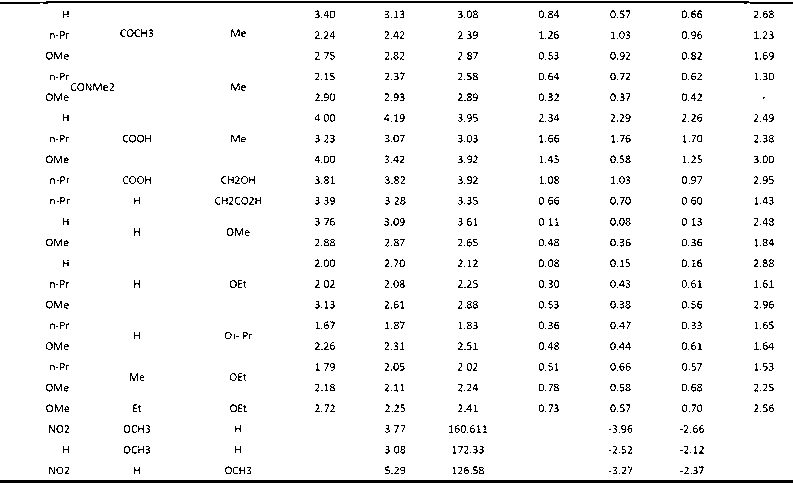
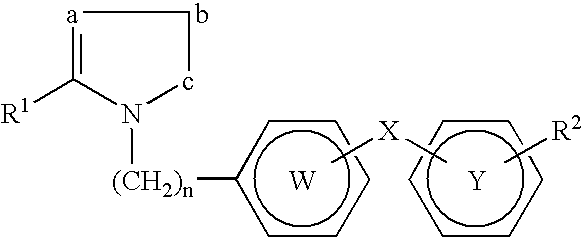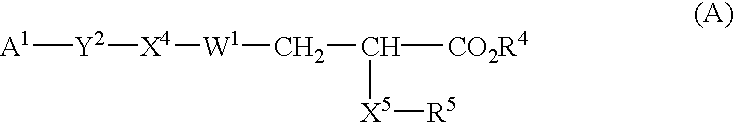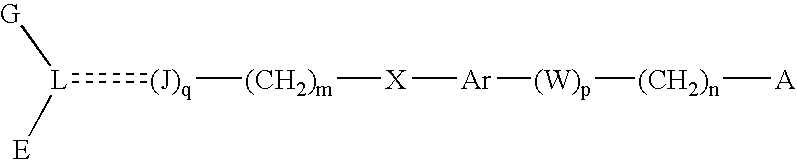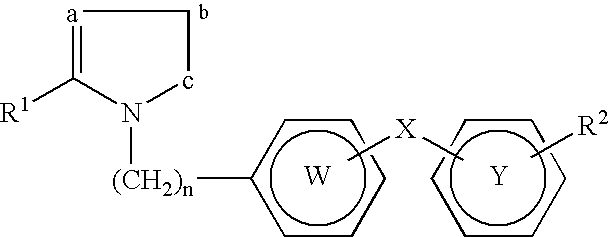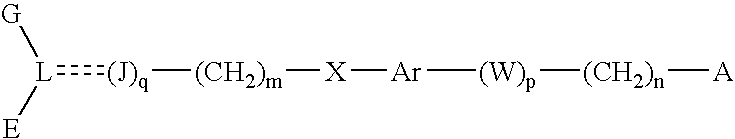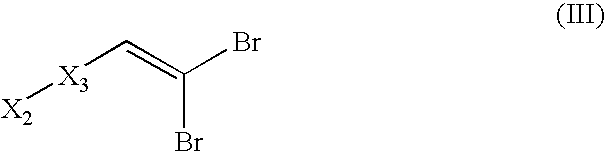Patents
Literature
160 results about "Peroxisome proliferator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
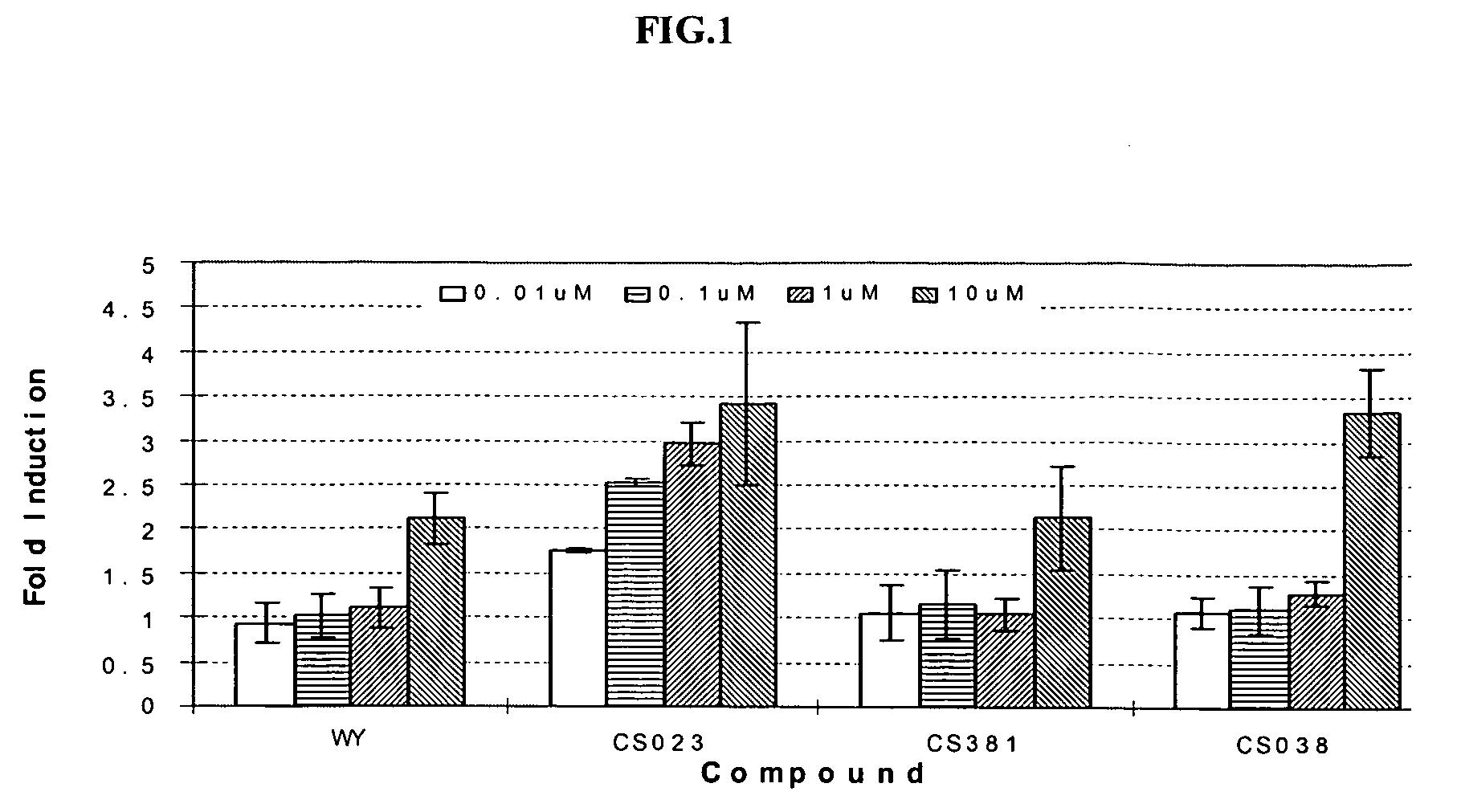
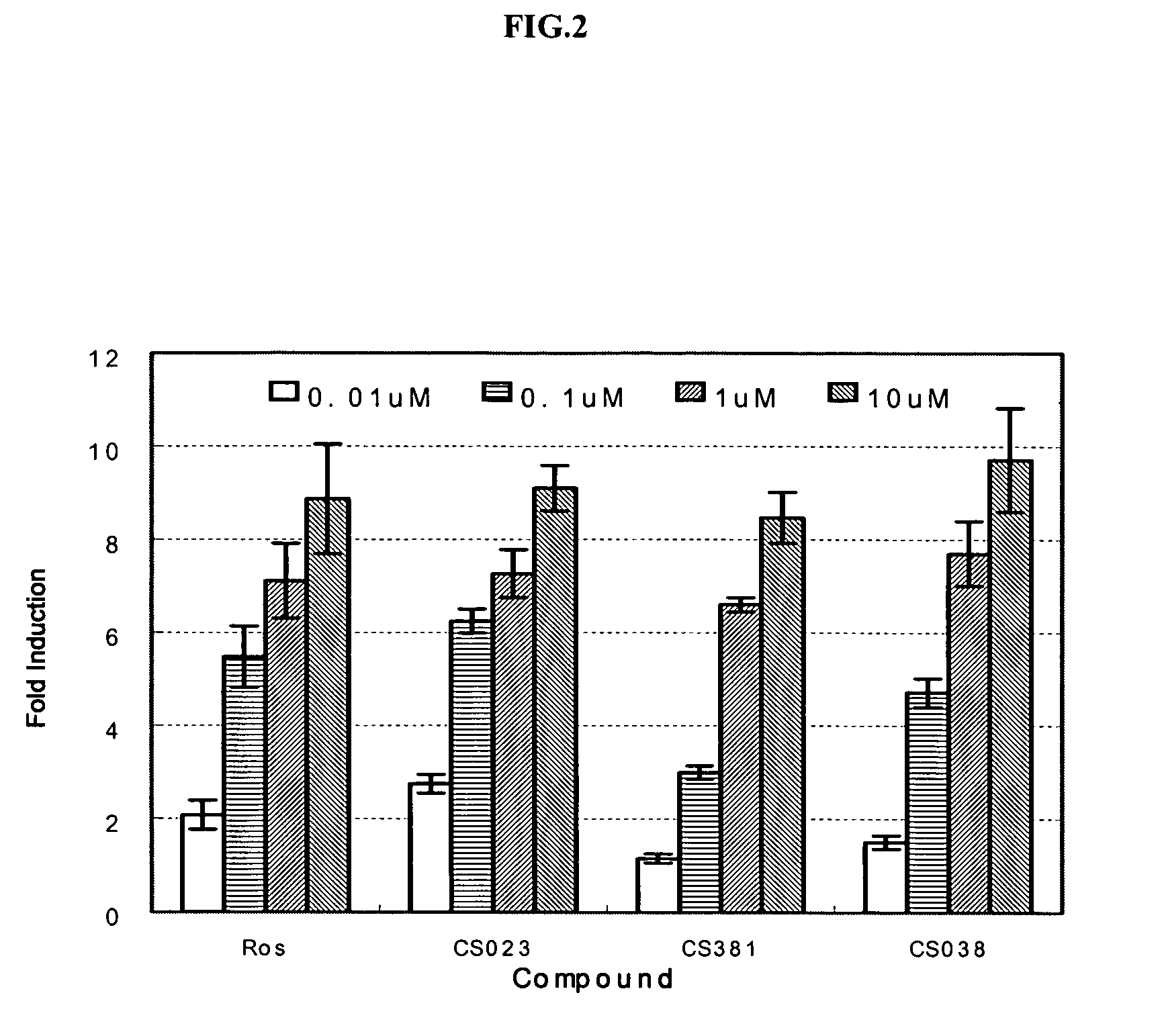
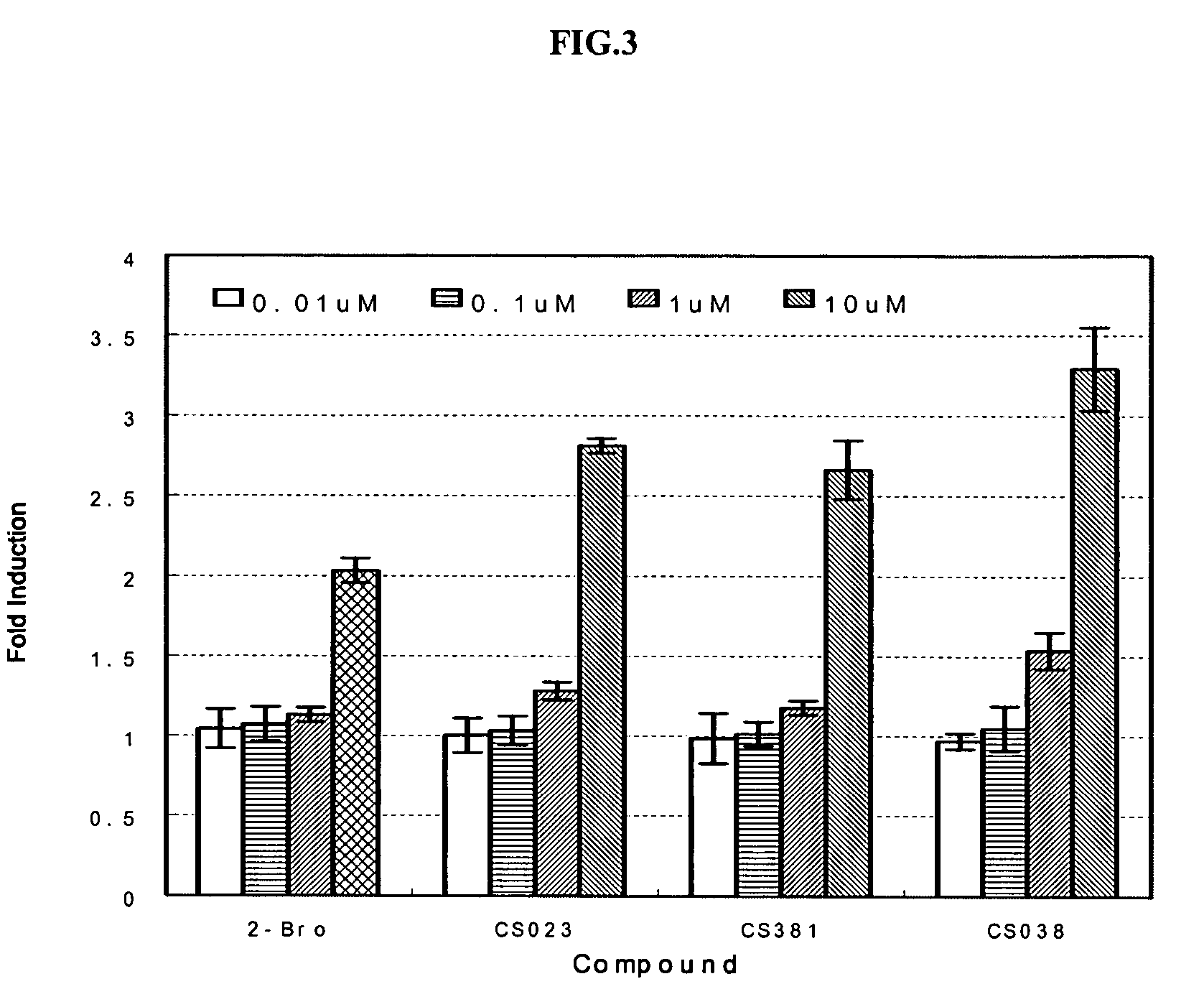
Peroxisome proliferator activated receptor modulators
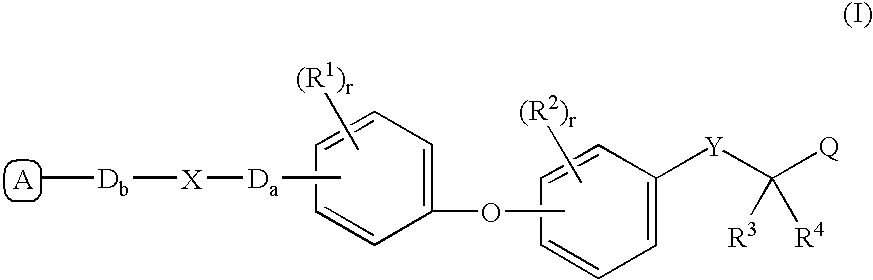
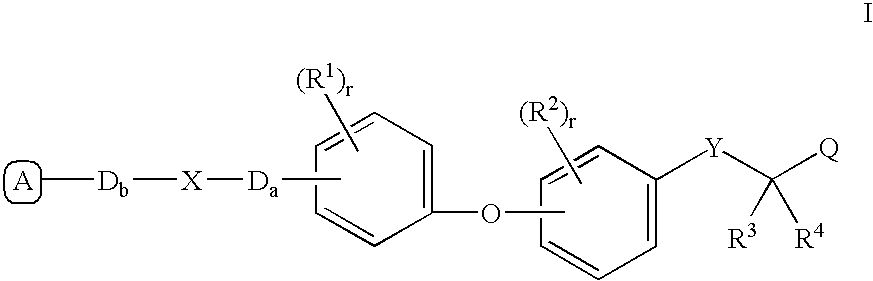
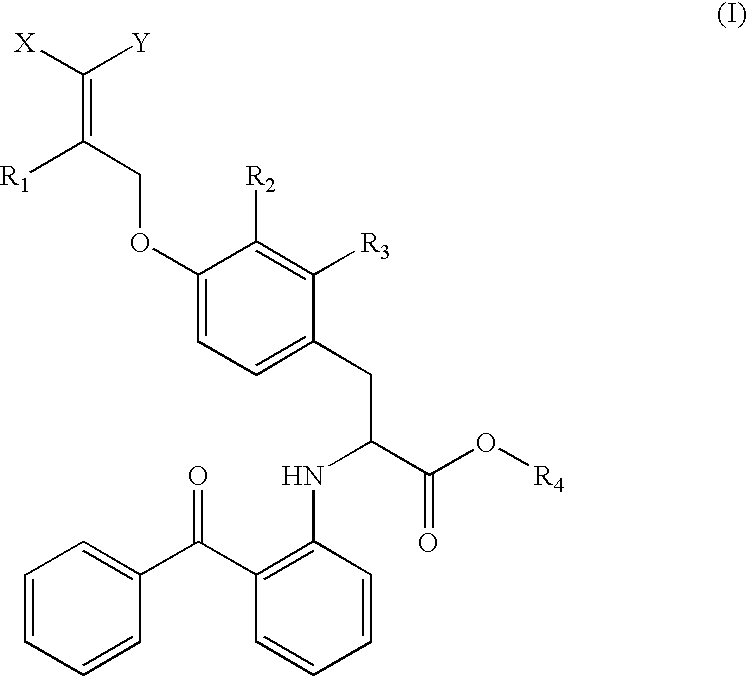
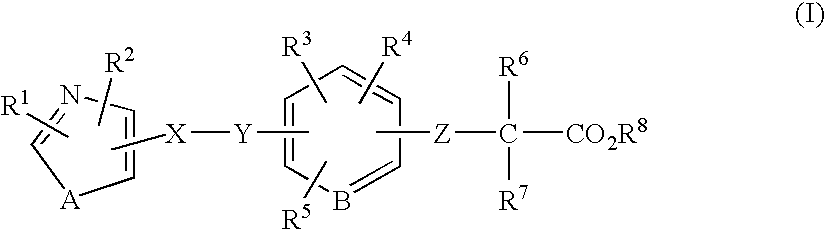
The present invention is directed to a compound of formula (I), or a pharmaceutically acceptable salt, solvate hydrate or stereoisomer thereof, which is useful in treating or preventing disorders mediated by a peroxisome proliferator activated receptor (PPAR) such as syndrome X, type II diabetes, hyperglycemia, hyperlipidemia, obesity, coagaulopathy, hypertension, arteriosclerosis, and other disorders related to syndrome X and cardiovascular diseases.
Owner:ELI LILLY & CO
Composition and method of treating facial skin defect
InactiveUS20090291986A1Increase differentiationReduce appearance problemsCosmetic preparationsBiocideFacial skinAgonist
This invention relates to a subcutaneous deliverable composition containing an agonist of the peroxisome proliferator-activated receptor-gamma, and a method for treating facial skin defects in a mammalian subject using the subcutaneous deliverable composition.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Heterocyclic modulators of PPAR
InactiveUS20070191371A1Modulate activityUseful PPAR modulating activityBiocideOrganic chemistryDiseasePeroxisome proliferator
The present invention relates to compounds and methods useful as modulators of Peroxisome Proliferator-Activated Receptors (PPARs) for treatment or prevention of disease.
Owner:KALYPSYS INC
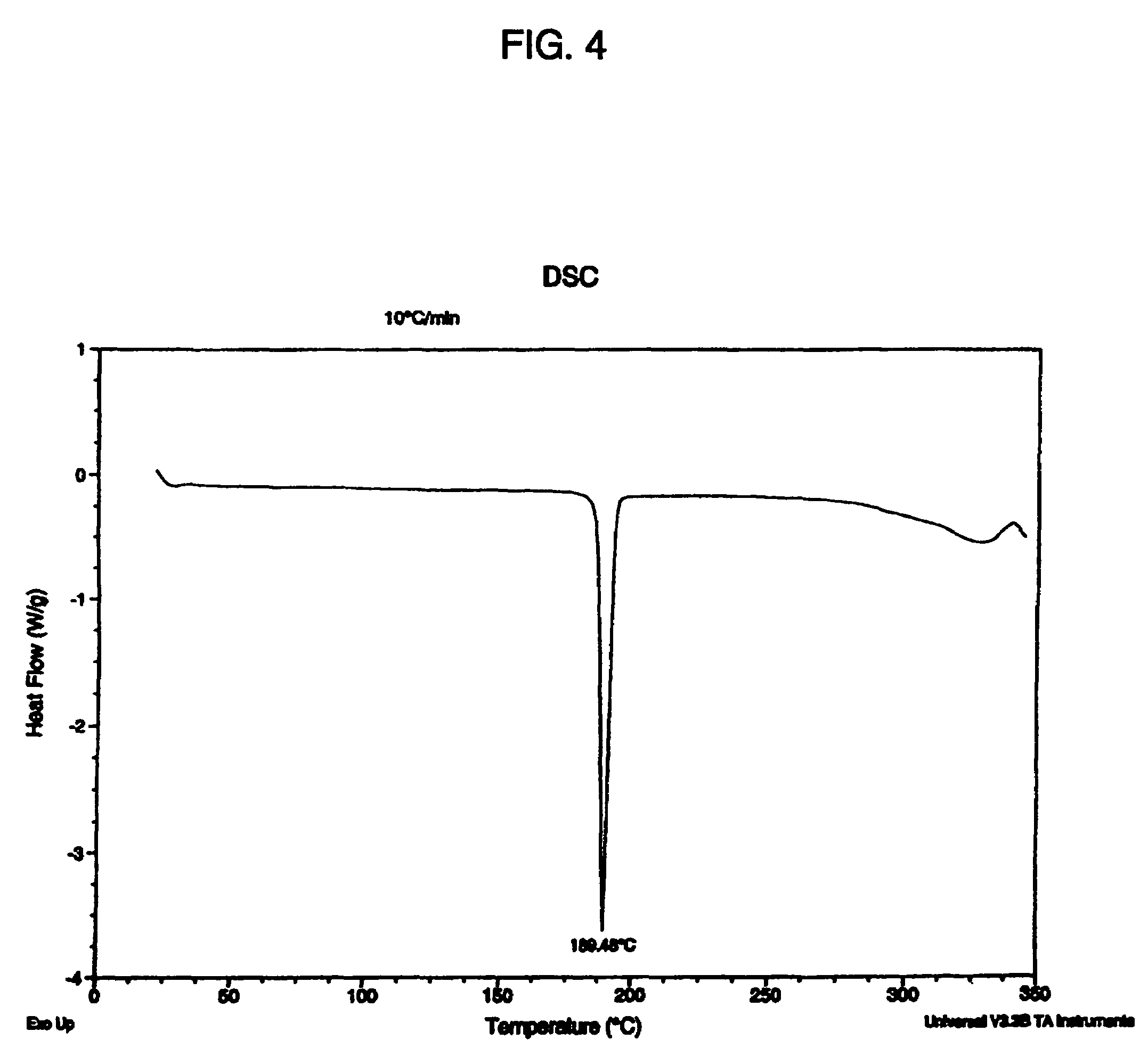
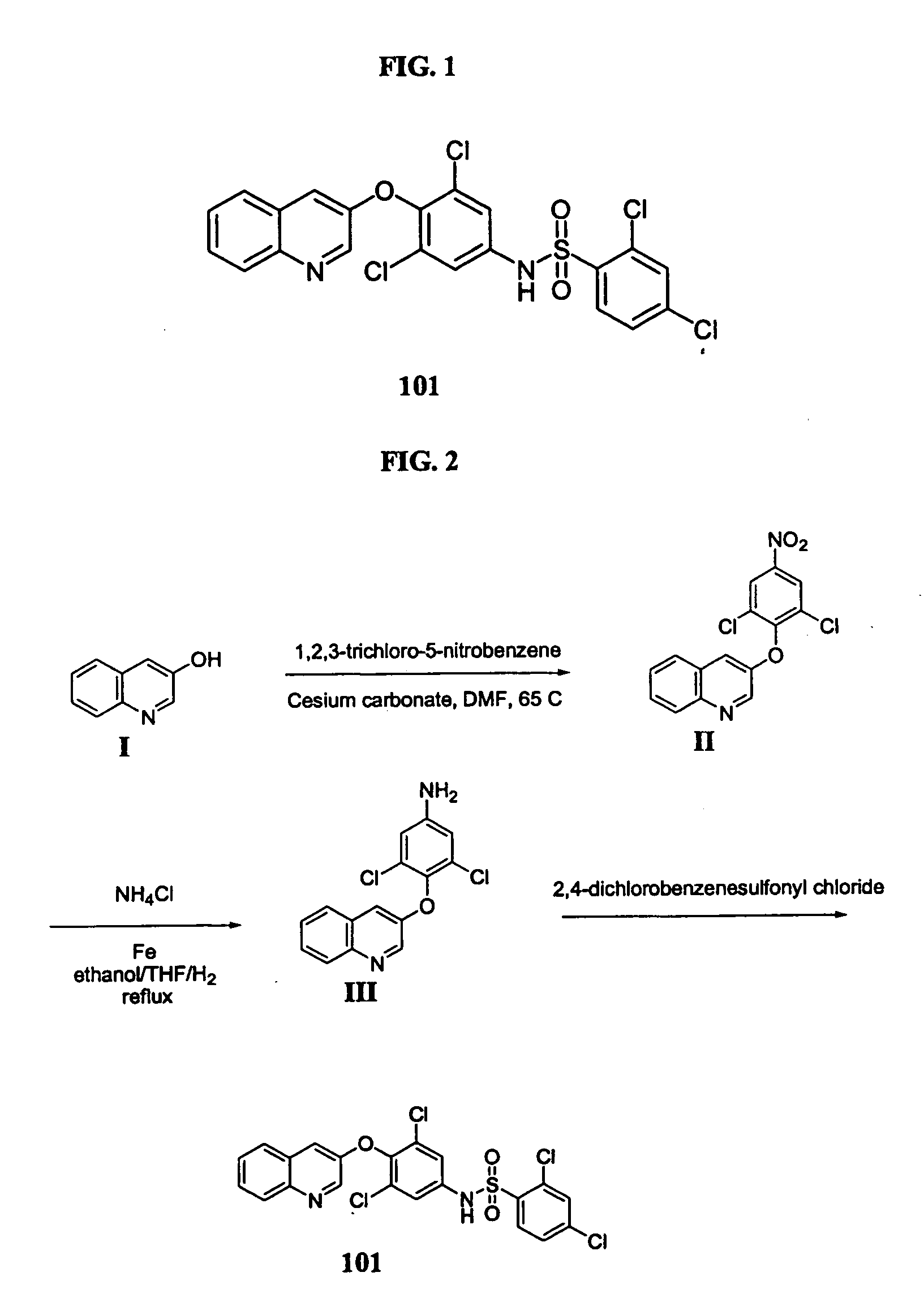
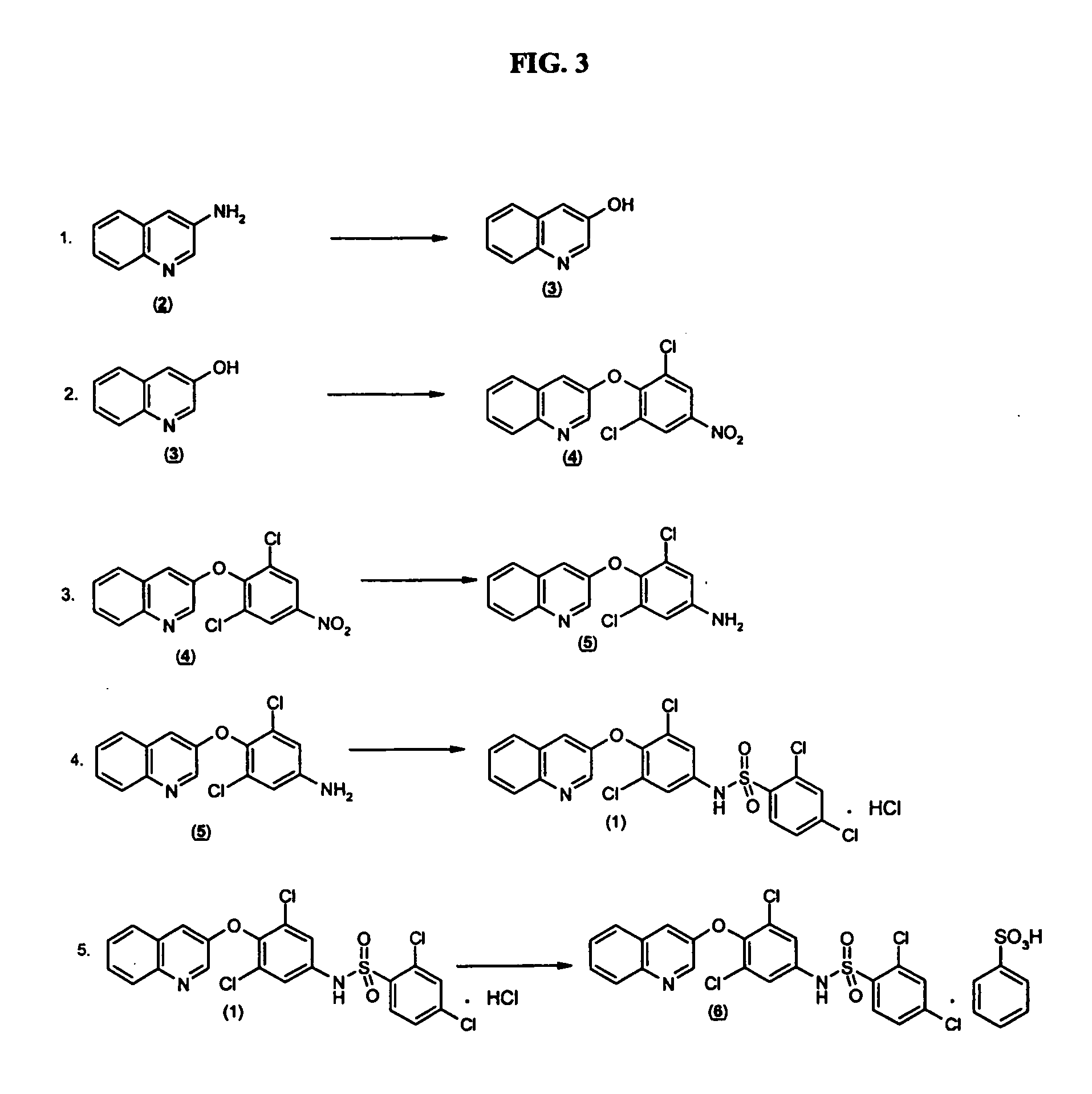
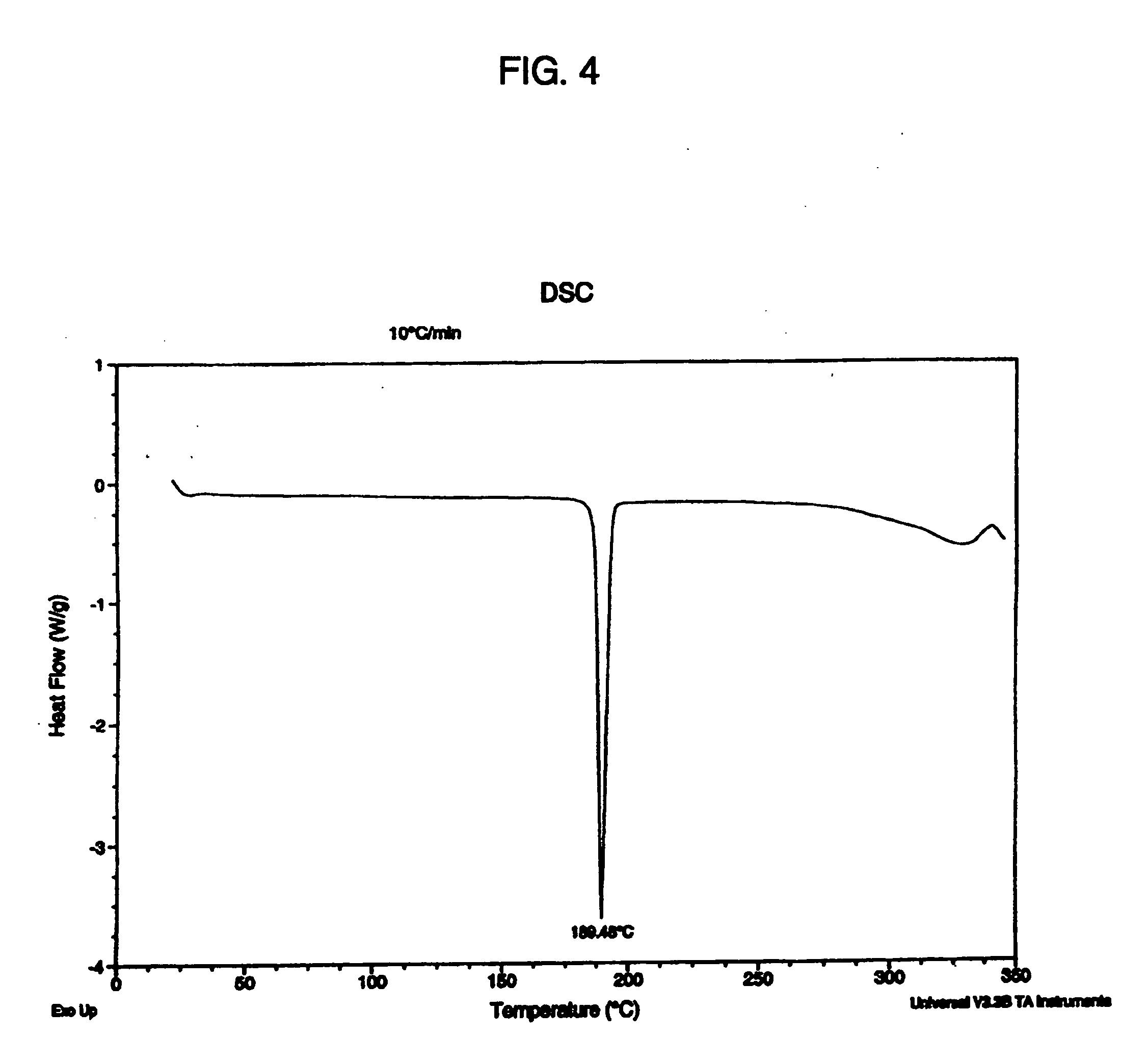
Salts and polymorphs of a potent antidiabetic compound
ActiveUS7223761B2BiocideOrganic chemistryEnergy homeostasisPeroxisome proliferator-activated receptor
Salts and polymorphs of a compound useful in the treatment of inflammatory and metabolic conditions and diseases are provided herein. In particular, the invention provides salts and polymorphs of a compound which modulates the expression and / or function of a peroxisome proliferator-activated receptor. The salts and polymorphs are useful for the treatment or prevention of conditions and disorders associated with energy homeostasis such as type II diabetes, lipid metabolism, adipocyte differentiation and inflammation.
Owner:AMGEN INC
Pharmacological method for treatment of neuropathic pain
InactiveUS20070249561A1Inhibiting and relieving neuropathic painBiocideElcosanoid active ingredientsMedicinePeroxisome proliferator
Disclosed are methods and compositions useful for treatment of neuropathic pain. In particular, the present invention provides methods of activating gamma-subtype peroxisome proliferator-activated receptors (PPARγ) to inhibit, relieve, or treat neuropathic pain.
Owner:TULANE EDUCATIONAL FUND
Novel PPAR ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
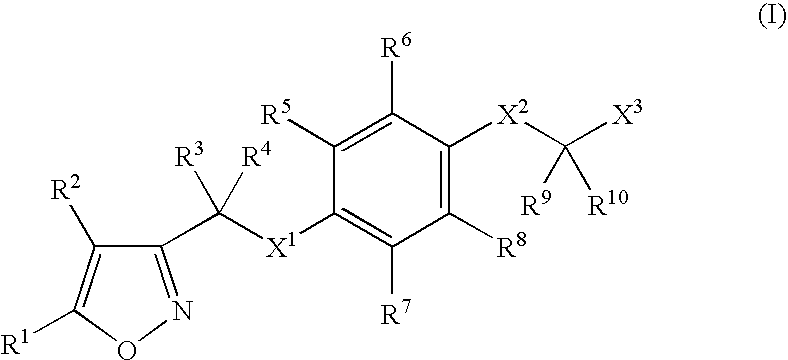
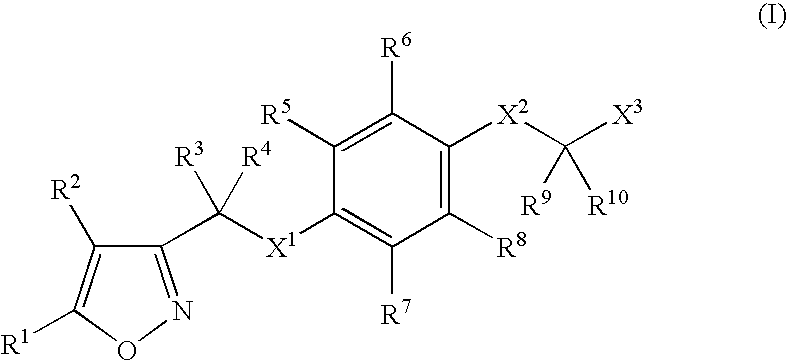
Isoxazole derivatives as peroxisome proliferator-activated receptors agonists
InactiveUS20070054902A1Treatment and prevention for hyperlipidemiaBiocideNervous disorderHalogenChemical compound
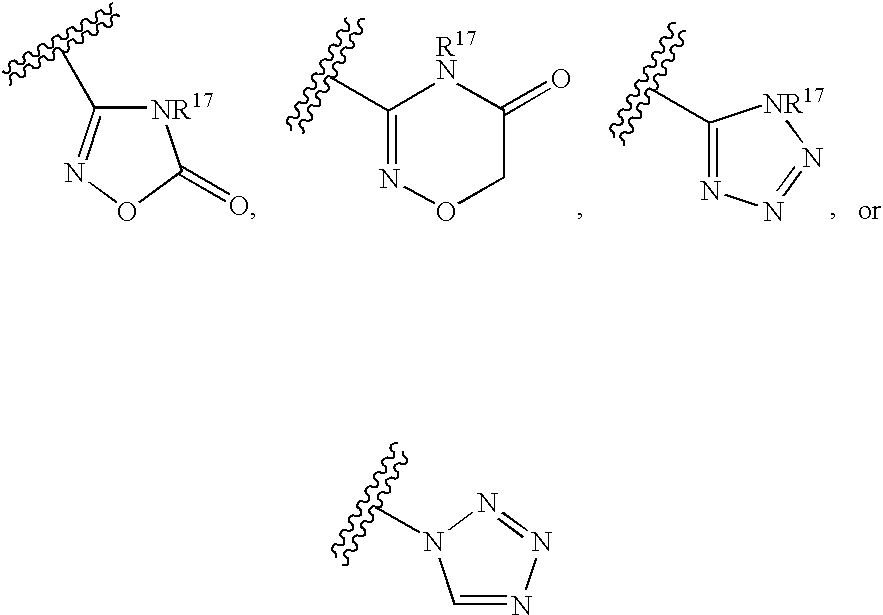
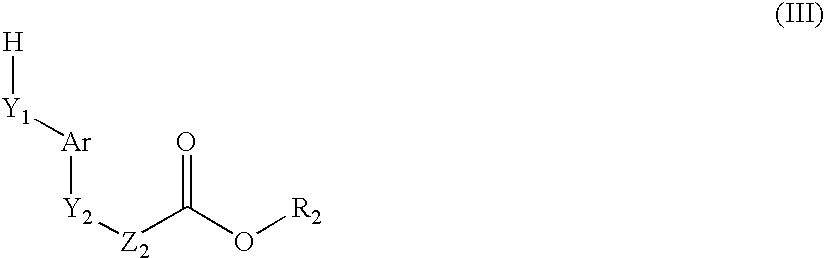
A compound of formula (I): (wherein R1-R10 are each independently hydrogen, halogen, optionally substituted lower alkyl or the like, X1 is —O—, —S—, —NR11— (wherein R11 is hydrogen, lower alkyl or the like), —CR12R13CO—, —(CR12R13)mO—, —O(CR12R13)m- (wherein R12 and R13 are each independently hydrogen or lower alkyl and m is a integer between 1 and 3) or the like, X2 is a bond, —O—, —S—, —NR14— (wherein R14 is hydrogen, lower alkyl or the like, R14 and R6 can be taken together with the neighboring atom to form a ring) or —CR15R16— (wherein R15 and R16 are each independently hydrogen or lower alkyl, R15 and R6 or R10 can be taken together with the neighboring carbon atom to form a ring, R16 and R9 can be joined together to form a bond), X3 is COOR17, C(═NR17)NR18OR19 or the like), a pharmaceutically acceptable salt or a solvate thereof.
Owner:SHIONOGI & CO LTD
Compounds, their preparation and use
Novel compounds of general formula (I), the use of these compounds as medicaments, pharmaceuticaly compositions comprising the compounds and methods of treatment employing these compounds and compostiones. The present compounds may be useful in the treatment and / or prevention of conditions mediated by nuclear receptors, in particular the Peroxisome Proliferator-Activated Receptors (PPAR). The compounds exert their effects by modulating the PPARγ response in a partial agonist manner.
Owner:HIGH POINT PHARMA
Peroxisome proliferator activated receptor ligands and process for producing the same
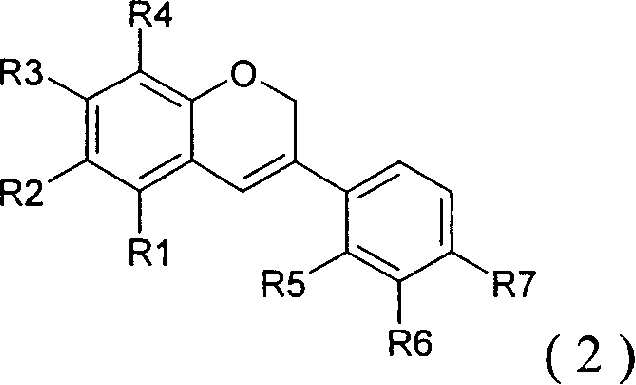
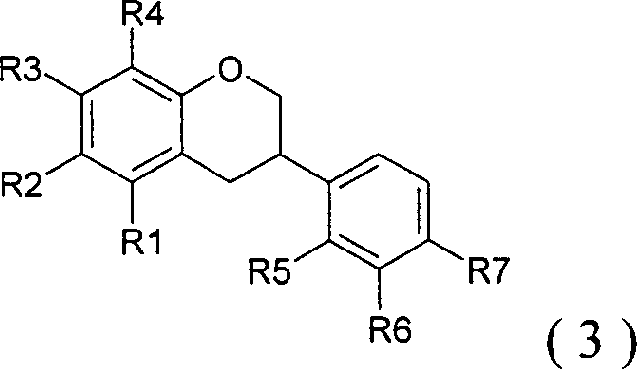
The present invention easily and efficiently provides a peroxisome proliferator-activated receptor ligand, and a composition for amelioration of insulin resistance or for prevention and / or amelioration of the insulin resistance syndrome containing the same, as an active ingredient. The present invention relates to a peroxisome proliferator-activated receptor ligand which comprises a prenylflavonoid, a chalcone derivative exclusive of prenyl flavonoids, a flavonol derivative exclusive of prenylflavonoids, and a salt, a glycoside and / or an esterified substance thereof acceptable as a pharmaceutical preparation or a food or a beverage; a composition containing the above ligand; a plant-derived extract containing the above ligand; and a process for producing the above extract.
Owner:KANEKA CORP
Substituted phenylpropionic acid derivatives as agonists to human peroxisome proliferator-activated receptor (PPAR) alpha
InactiveUS6506797B1Improve securityHigh binding activityBiocideOrganic chemistry3-phenylpropanoic acidAcid derivative
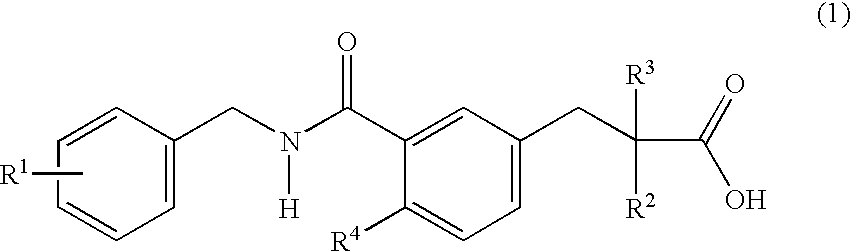
The invention provides novel substituted phenylpropanoic acid derivatives that activate by binding to receptor as ligands of human peroxisome preliferant-activated receptor alpha (PPARalpha), and exhibit potent decreasing action on lipids in blood (cholesterol and triglyceride).It relates to a substituted phenylpropanoic acid derivatives represented by a general formula (1),their pharmaceutically acceptable salts and their hydrates, and processes for preparing them.
Owner:KYORIN PHARMA CO LTD
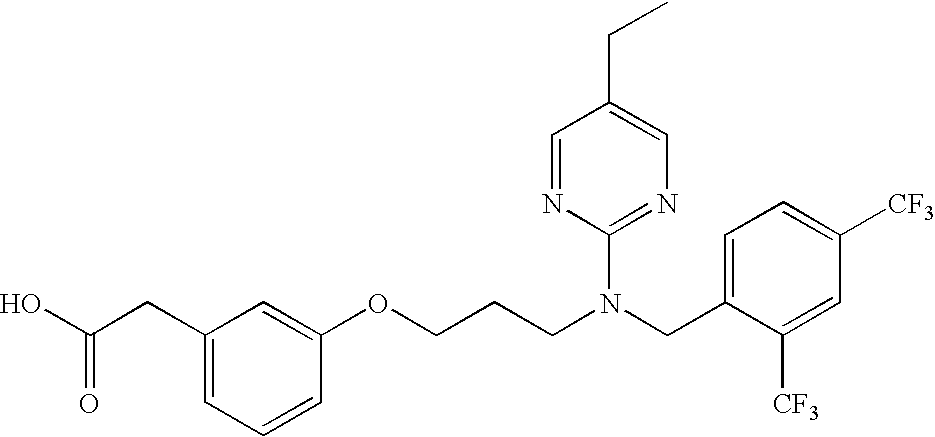
Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
ActiveUS20120263670A1Reduce interdialytic weight gainMore palatable dietBiocideSynthetic polymeric active ingredientsIntestinal tract diseasesDisease
The present disclosure is directed to compounds and methods for the treatment of disorders associated with fluid retention or salt overload, such as heart failure (in particular, congestive heart failure), chronic kidney disease, end-stage renal disease, liver disease, and peroxisome proliferator-activated receptor (PPAR) gamma agonist-induced fluid retention. The present disclosure is also directed to compounds and methods for the treatment of hypertension. The present disclosure is also directed to compounds and methods for the treatment of gastrointestinal tract disorders, including the treatment or reduction of pain associated with gastrointestinal tract disorders.
Owner:ARDELYX
Compounds, their preparation and use
InactiveUS7091245B2BiocideCarbamic acid derivatives preparationPeroxisome proliferatorCombinatorial chemistry
Novel compounds of the general formula (I), the use of these compounds as pharmaceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and / or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ suptype.
Owner:HIGH POINT PHARMA
Drug screening method
ActiveCN103294933ASimple structureSpecial data processing applicationsChemical compoundPeroxisome proliferator
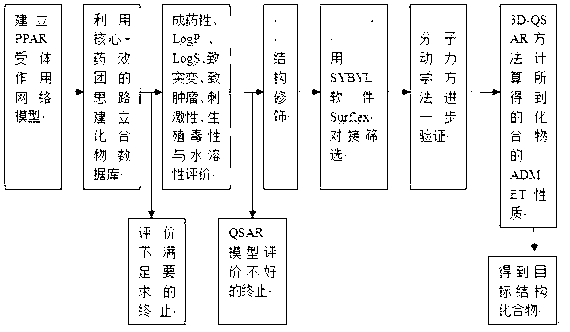
A drug screening method includes the steps: A, screening PPARs (peroxisome proliferator-activated receptors) and building a PPAR action model; B, building a compound database; C, evaluating druggability, LogP, LogS, mutagenicity, tumorigenicity, irritation, reproductive toxicity and water solubility of compounds in the database, and building two-dimensional and three-dimensional compound databases; D, performing structural modification on compounds in the two-dimensional and three-dimensional compound databases; E, performing docking computation on novel molecular structural compounds, and screening; F, verifying the compounds by the aid of a molecular dynamics method, and selecting and determining molecules; and G, obtaining ADMET (absorption, distribution, metabolism, excretion and toxicity) properties of the verified compounds obtained by computation so as to obtain target compounds finally. By the scheme, structures of the compounds are further optimized while structure-function relationship of the compounds is researched, and novel leading compounds with medicinal value are sought.
Owner:司宏宗
PPAR Ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
InactiveUS20110129546A1Improve antioxidant capacityGood anti-inflammatory effectBiocideAntimycoticsDiseaseIsoflavones
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a base anti-inflammatory agent, such as indometacin; one or more optional active ingredients selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol™, L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF-α) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:UMBERT MILL IGNACIO
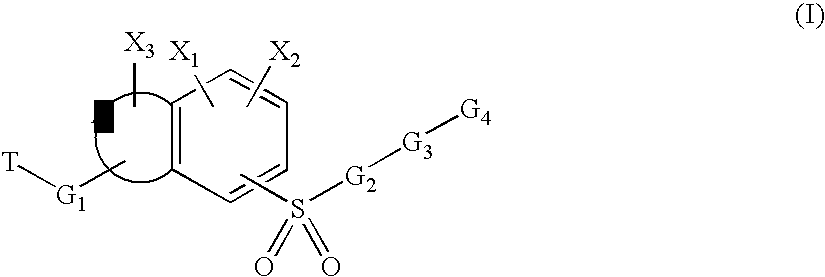
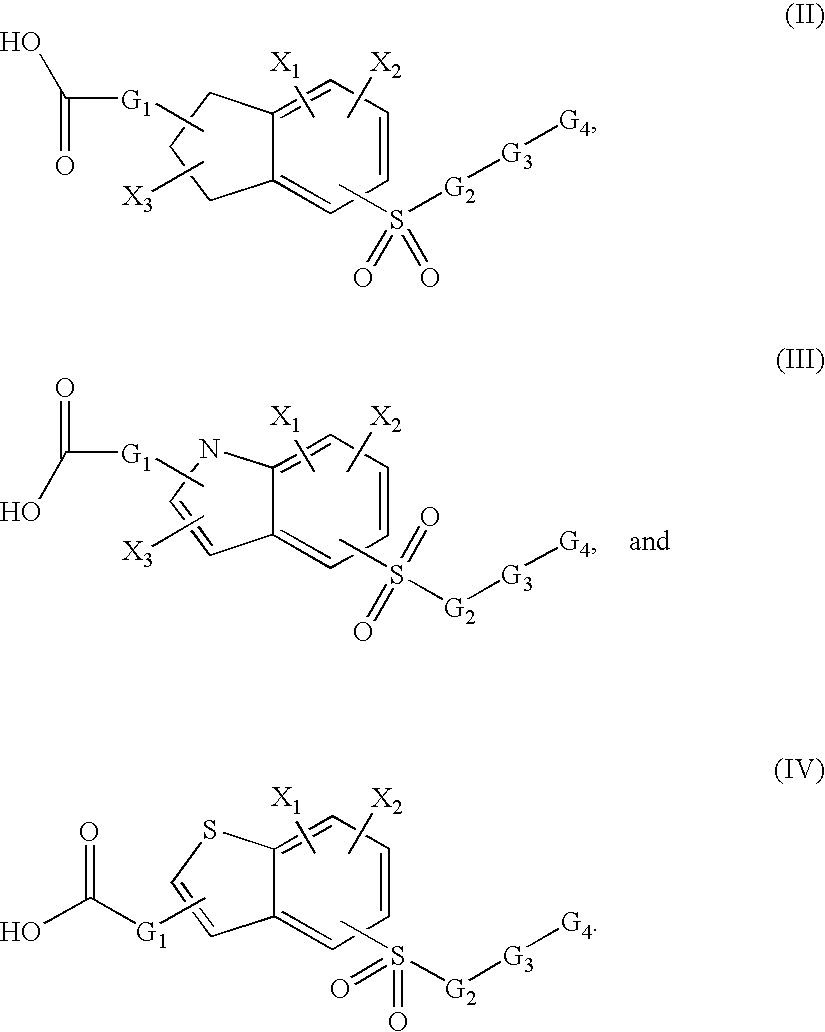
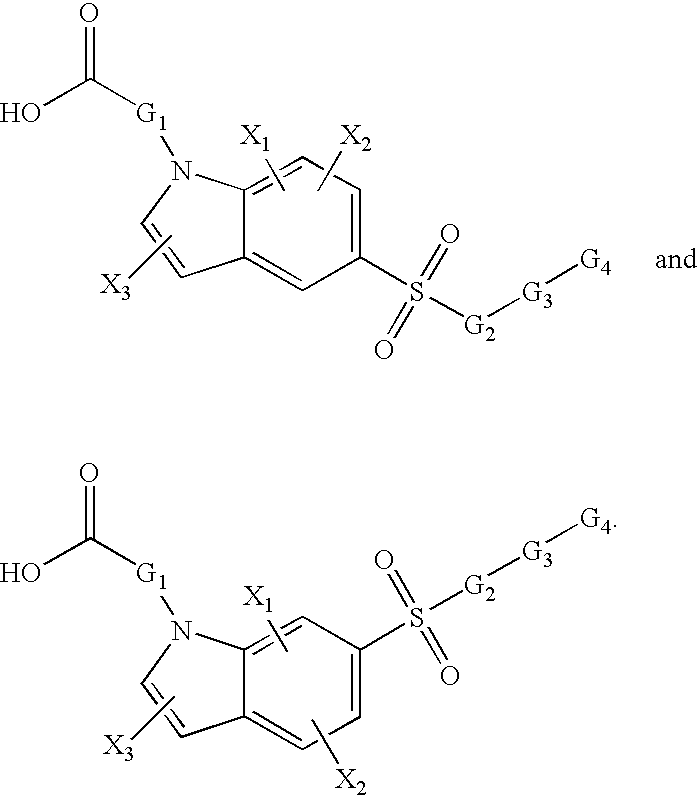
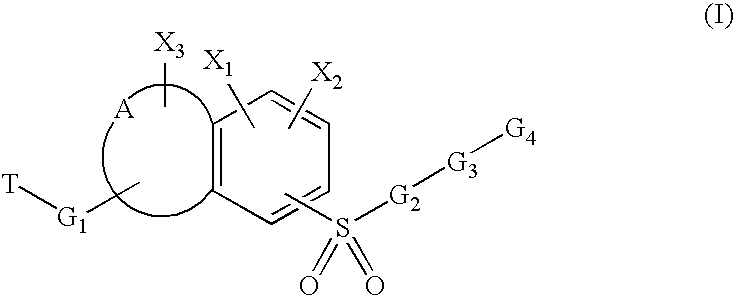
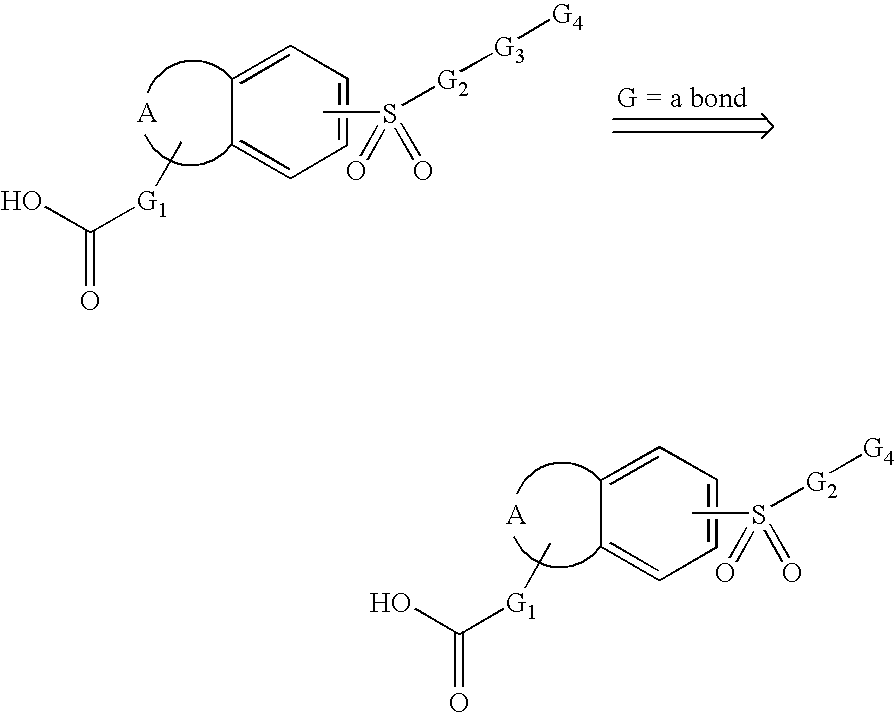
Sulfonyl-substituted bicyclic compounds as modulators of PPAR
Compounds as modulators of peroxisome proliferator activated receptors, pharmaceutical compositions comprising the same, and methods of treating disease using the same are disclosed.
Owner:KALYPSYS INC
Compositions comprising novel PPAR ligands and anti-hyperlipemic agents
InactiveUS20070054949A1Convenient treatmentPrevent goodBiocideAntipyreticDyslipidemiaTG - Triglyceride
Methods are provided for treating or preventing conditions comprising hypertension and dyslipidemia using antihyperlipemic agents and compounds that antagonize the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs) and lower triglycerides or elevate blood HDL-cholesterol. Compositions are provided for treating or preventing conditions comprising hypertension and dyslipidemia, comprising antihyperlipemic agents which lower triglycerides and inhibit cholesterol synthesis such as statins, and compounds that antagonize or block the angiotensin II type 1 (AT1) receptor, activate PPARs and lower triglycerides or elevate blood HDL-cholesterol such as sartans.
Owner:BETHESDA PHARMA
Activator of peroxisome proliferator-activated re-ceptor delta
A compound represented by the following general formula (I):(wherein R1 represents phenyl, etc. which can have substituents selected from the group consisting of C1-8 alkyl, C1-8 alkyl having halogen, halogen, hydroxyl, etc.; R2 represents C1-8 alkyl, etc.; A represents oxygen, sulfur, etc.; X represents C1-8 alkylene chain, etc.; Y represents C(═O), CH═CH, etc.; R3, R4, and R5 each represents hydrogen, C1-8 alkyl, etc.; B represents CH or nitrogen; Z represents oxygen or sulfur; R6 and R7 each represents hydrogen, C1-8 alkyl, etc.; and R8 represents hydrogen or C1-8 alkyl; provided that at least one of R3, R4, and R5 is not hydrogen) or a salt of the compound; and a PPAR-δ activator which contains the compound or salt as the active ingredient.
Owner:NIPPON CHEMIPHAR CO LTD
Methods for the selective modulation of PPAR
InactiveUS20070190079A1BiocideSulfur/selenium/tellurium active ingredientsDiseaseSelective modulation
The present invention relates to methods of selective modulation of peroxisome proliferator activated receptors (PPARs) over G-protein coupled receptor 40 (GPR40), and the use of therapeutically effective amounts of compounds and pharmaceutical compositions which selectively modulate PPAR over GPR40 for the treatment of diseases in patients in need thereof. The methods disclosed herein are exceptionally useful in treating metabolic diseases whilst avoiding certain side effects common to modulators of PPAR previously disclosed in the art.
Owner:KALYPSYS INC
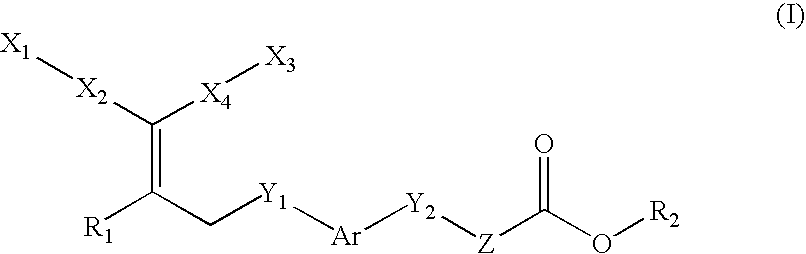
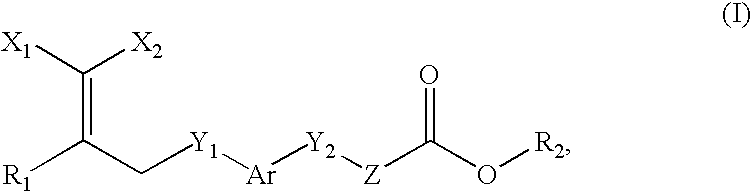
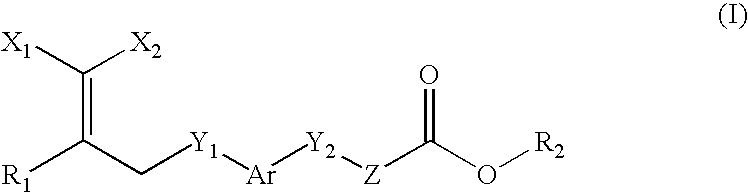
Peroxisome proliferator activated receptor-active arylene acetic acid derivatives
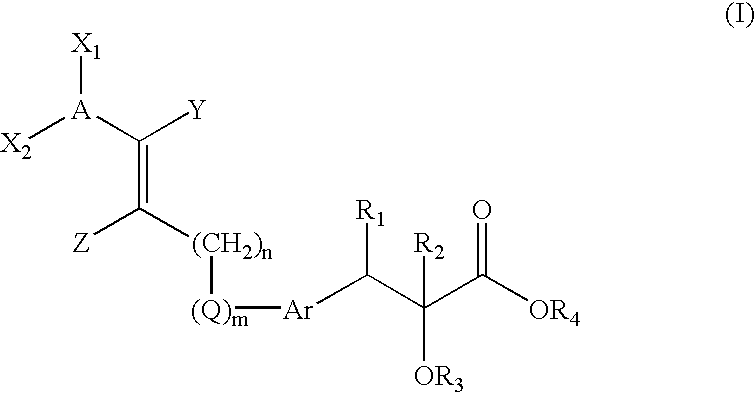
Novel compounds of the general formula (I),wherein Ar, R1, R2, X1, X2, Y1, Y, and Z are as defined in the specification, the use of these compounds as pharmaceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and / or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ subtype.
Owner:VTV THERAPEUTICS LLC
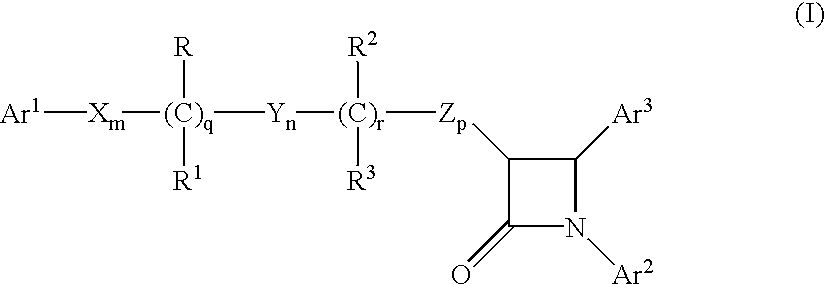
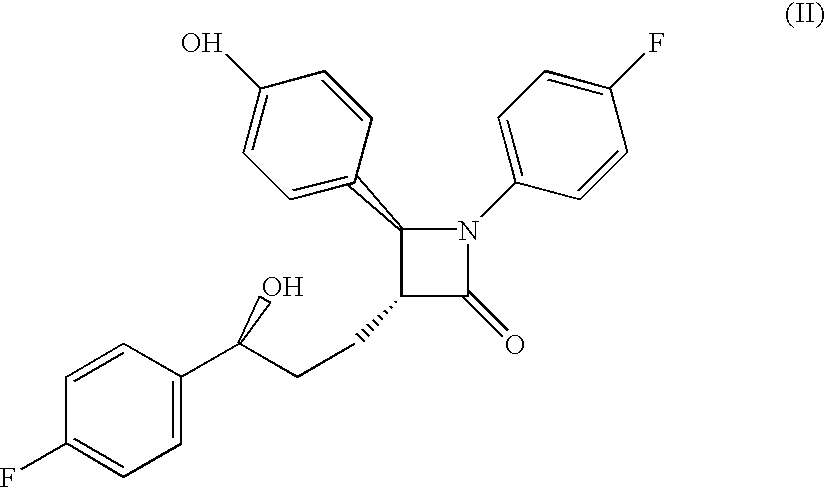
Sterol absorption inhibitor compositions
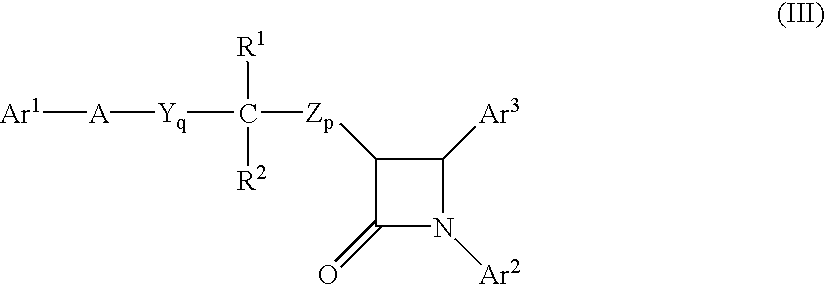
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one peroxisome proliferator-activated receptor activator; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity and lowering plasma levels of sterols.
Owner:ORGANON LLC
Compounds, their preparation and use
InactiveUS6972294B1Great potentialBiocideOrganic chemistryPeroxisome proliferatorCombinatorial chemistry
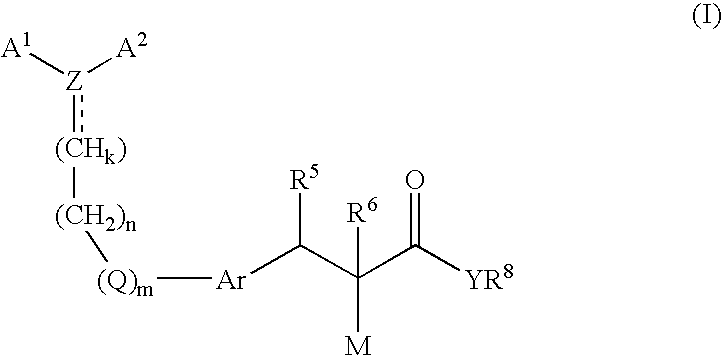
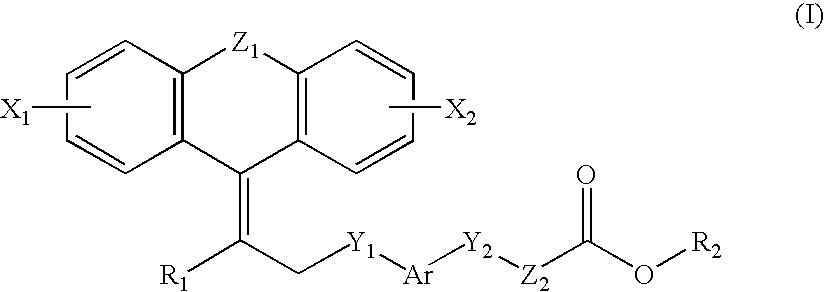
Disclosed are novel compounds of formula (I) wherein A1, A2, Ar, R5, R6, R8, M, Q, Y, Z, k, m and n are as defined in the specification. These compounds are useful in the treatment and / or prevention of conditions mediated by nuclear receptors, in particular the Peroxisome Proliferator-Activated Receptors (PPAR). Such conditions include diabetes and obesity.
Owner:VTV THERAPEUTICS LLC
Compounds, their preparation and use
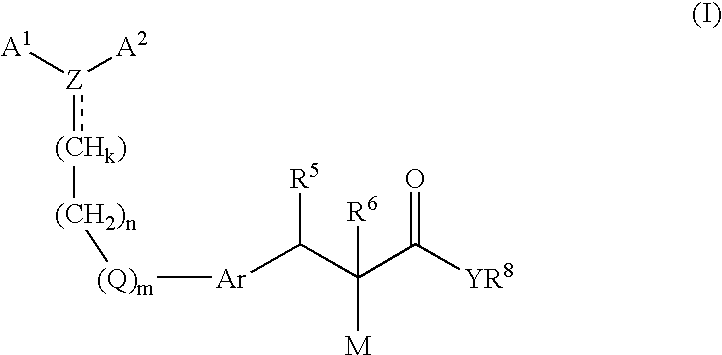
The present invention relates to compounds of the general formula (I) The compounds are useful in the treatment and / or prevention of conditions mediated by nuclear receptors, in particular the Peroxisome Proliferator-Activated Receptors (PPAR).
Owner:VTV THERAPEUTICS LLC
Novel Compounds, Their Preparation and Use
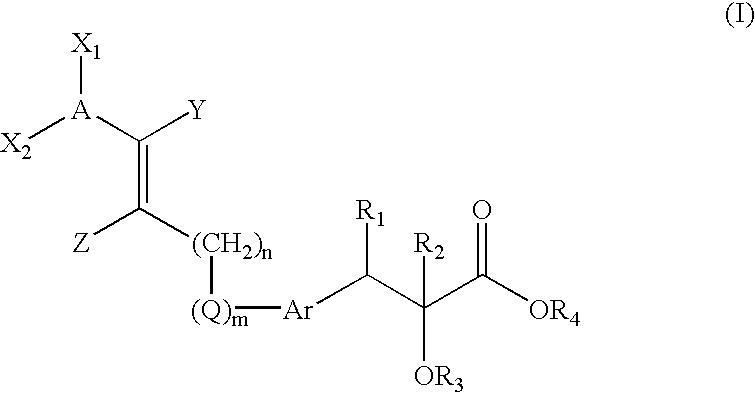
Novel compounds of the general formula (I), the use of these compounds as pharmaceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and / or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ subtype.
Owner:HIGH POINT PHARMA
Substituted arylalcanoic acid derivatives as PPAR pan agonists with potent antihyperglycemic and antihyperlipidemic activity
ActiveUS7268157B2Decrease hyperglycemiaDecrease hypertriglyceremiaBiocideOrganic chemistryAcute hyperglycaemiaCoronary artery disease
Disclosed is the preparation and pharmaceutical use of substituted arylalcanoic acid derivatives of Formula I, wherein ring A, ring B, R1, R2, R3, R4, R5, X, Alk1, Alk2, Ar1, and Ar2 are as defined in the specification. These compounds, as selective agonists activating peroxisome proliferator-activated receptors (PPAR), in particularly the RXR / PPARalpha, RXR / PPARgamma, and RXR / PPARdelta heterodimers, are useful in the treatment and / or prevention of type 2 diabetes and associated metabolic syndrome such as hypertension, obesity, insulin resistance, hyperlipidemia, hyperglycemia, hypercholesterolemia, atherosclerosis, coronary artery disease, and other cardiovascular disorders with improved side effects profile commonly associated with conventional PPARgamma agonists.
Owner:SHENZHEN CHIPSCREEN BIOSCIENCES CO LTD
Treatment of acne and other diseases
InactiveUS20070225301A1Enhanced sebum secretionAvoid problemsBiocideOrganic active ingredientsPemirolastDisease
The invention relates to compounds for the treatment of dermatological diseases where inflammation, matrix metalloproteinases (MMPs) and peroxisome proliferator-activated receptors (PPARs) play a role in mediating the disease, such as the treatment of acne with Pemirolast or a closely related compound thereof.
Owner:CARDOZ AB
Novel Compounds, Their Preparations and Use
Novel compounds of the general formula (I), the use of these compounds as pharmaceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and / or prevention of conditions mediated by Peroxisome proliferator-activated receptors (PPAR), in particular the PPARδ suptype.
Owner:HIGH POINT PHARMA
Composition and method of treating facial skin defect
InactiveUS20090291066A1Increase differentiationReduce appearance problemsCosmetic preparationsBiocideFacial skinAgonist
This invention relates to a subcutaneous deliverable composition containing an agonist of the peroxisome proliferator-activated receptor-gamma, and a method for treating facial skin defects in a mammalian subject using the subcutaneous deliverable composition.
Owner:PAPPAS APOSTOLOS +2
Modulators of peroxisome proliferator activated receptors
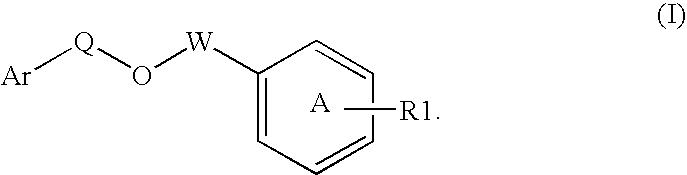
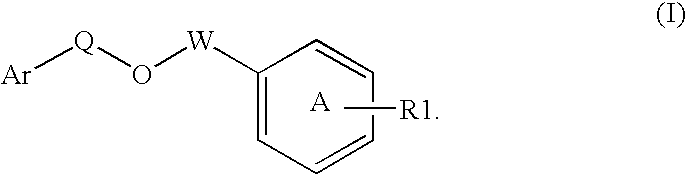
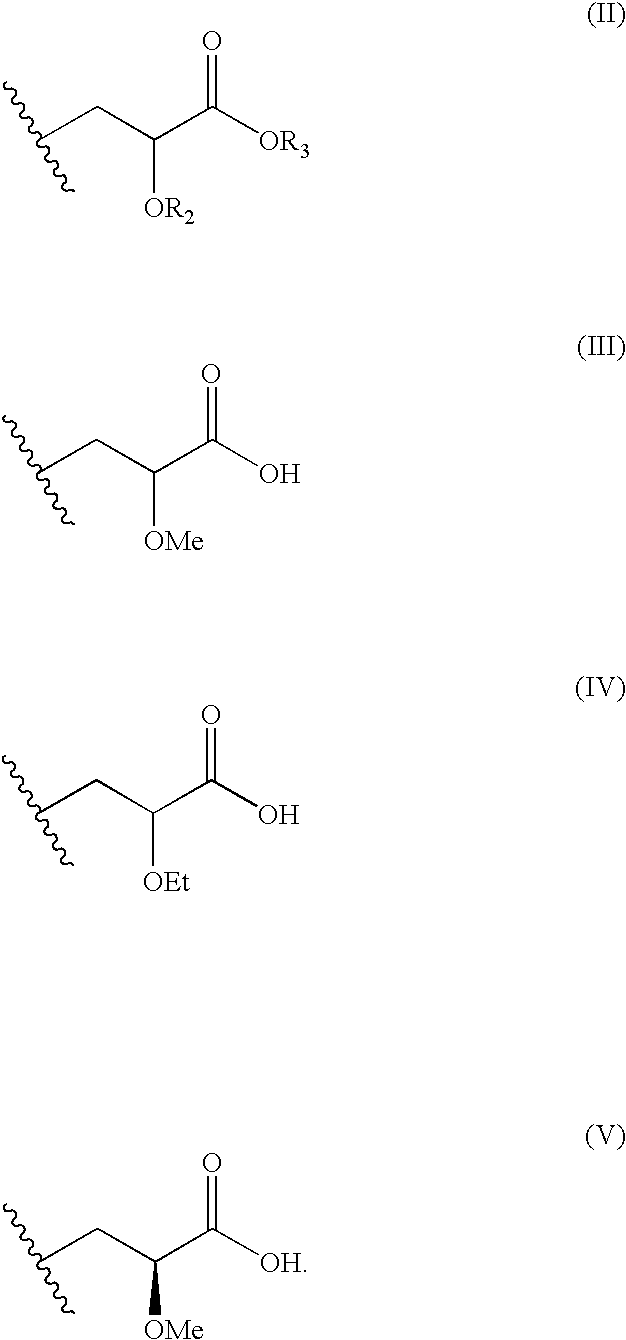
Disclosed is a compound represented by Structural Formula (I): Ar is a substituted or unsubstituted aromatic group. Q is a covalent bond, —CH2— or —CH2CH2—; W is a substituted or unsubstituted alkylene or a substituted or unsubstituted heteroalkylene linking group from two to ten atoms in length, preferably from two to seven atoms in length. Phenyl Ring A is optionally substituted with up to four substituents in addition to R1 and W, R1 is (CH2)n—CH(OR2)—(CH2)mE1, —(CH)═C(OR2)—(CH2)mE, —(CH2)n—CH(Y)—(CH2)mE or (CH)═C(Y)—(CH2)mE; wherein E is COOR3, C1–C3 alkylnitrile, carboxamide, sulfonamide, acylsulfonamide or tetrazole and wherein sulfonamide, acylsulfonamide and tetrazole are optionally substituted with one or more substituents independently selected from: C1–C6 alkyl, haloalkyl and aryl-C0-4-alkyl; R2 is H, an aliphatic group, a substituted aliphatic group, haloalkyl, an aromatic group, a substituted aromatic group, —COR4, —COOR4, —CONR5R6, —C(S)R4, —C(S)OR4 or C(S)NR5R6, R3 is H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. Y is O—, CH2—, —CH2CH2— or CH═CH— and is bonded to a carbon atom in Phenyl Ring A that is ortho to R1. R4–R6 are independently H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. n and m are independently 0, 1 or 2
Owner:ELI LILLY & CO +1
Salts and polymorphs of a potent antidiabetic compound
Salts and polymorphs of a compound useful in the treatment of inflammatory and metabolic conditions and diseases are provided herein. In particular, the invention provides salts and polymorphs of a compound which modulates the expression and / or function of a peroxisome proliferator-activated receptor. The salts and polymorphs are useful for the treatment or prevention of conditions and disorders associated with energy homeostasis such as type II diabetes, lipid metabolism, adipocyte differentiation and inflammation.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com