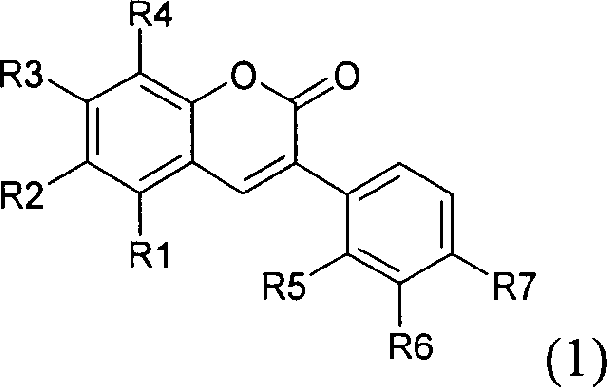
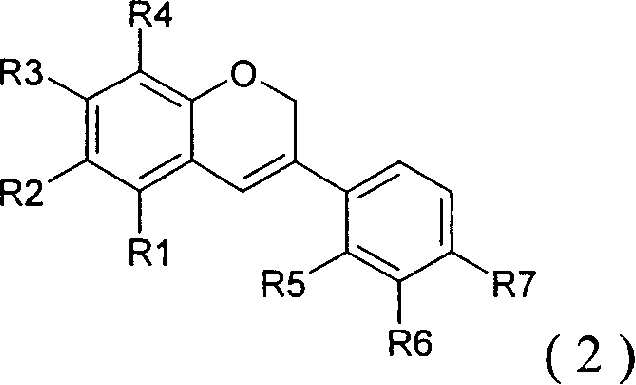
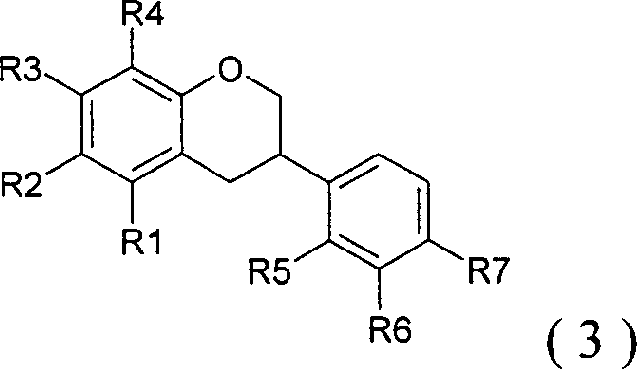
Peroxisome proliferator activated receptor ligands and process for producing the same
A technology of peroxisome, proliferator, applied in improving insulin resistance or field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] (Embodiment 1) from Radix Glycyrrhizae extract, isolate compound (1)
[0113]Soak 1.2 kg of finely ground licorice (G. ウラレンシス: Tohoku licorice) in 5.5 L of ethyl acetate, extract at room temperature in the dark for 7 days, and obtain an extract by filtration. The extract was concentrated under reduced pressure to remove the solvent to obtain 74.0 g of an extract. The extract was applied to a silica gel chromatography column (1500 ml), and eluted with chloroform:methanol=19:1 (v / v) to obtain fractions. The eluted fraction was concentrated under reduced pressure to remove the solvent to obtain 55.4 g of a crude fraction. Through repeated silica gel column chromatography, ODS silica gel column chromatography, high performance liquid chromatography with ODS column, gel filtration chromatography, and phase-separation thin-layer chromatography, the obtained crude fraction was purified to obtain compound 1 (225 mg), compound 2 (80.7mg), Compound 3 (19.6mg), Compound 4 (22.2m...
Embodiment 2
[0123] (Embodiment 2) from Radix Glycyrrhiza Extract, isolate compound (2)
[0124] In the same manner as in Example 1, ethanol extraction was carried out from a finely pulverized product of Glycyrrhiza licorice (G. ulalensis), and various chromatography was performed to obtain compounds 13-17. As a result of structural analysis, it was identified that compound 13 is isoliquiritigenin, compound 14 is kaempferol 3-O-methylester, and compound 15 is licorice flavonol ( licoflavonol), compound 16 is トパゾリン (topazolin), and compound 17 is licorice isoflavone.
Embodiment 3
[0125] (Embodiment 3) from Radix Glycyrrhiza extract, isolate compound (3)
[0126] In the same manner as in Example 1 or Example 2, ethanol extraction was carried out from the finely ground product of Glycyrrhizae (G. 24-28. As a result of structural analysis, it was identified that compound 18 was glabridin, compound 19 was glabrene, compound 20 was hispa glabridin B (hispa glabridin B), and compound 21 was 4'-O-methyl grabridin (4'- O-methylglabridin), compound 22 is 3 hydroxy-4'-O-methyl glabridin (3'-hydroxy-4'-O-methylglabridin), compound 23 is glabrol (glabrol), compound 24 is licochalcone A (licochalcone A), compound 25 is licochalcone B (licochalcone B), compound 26 is licochalcone C (licochalcone C), compound 27 is glycyrdione A (glycyrdione A), compound 28 is licochalcone C (glycyrdione C).
[0127] The structural formulas of compounds 1-28 are shown in Tables 10-13.
[0128] Table 10
[0129]
[0130] Table 11
[0131]
[0132] Table 12
[0133]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com