New sesquiterpene quinone compound from abelmoschus sagittifolius, as well as preparation method and applications thereof
A Wuzhishan ginseng and compound technology, applied in the field of new sesquiterpene quinone compounds and their preparation, to achieve good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 compound
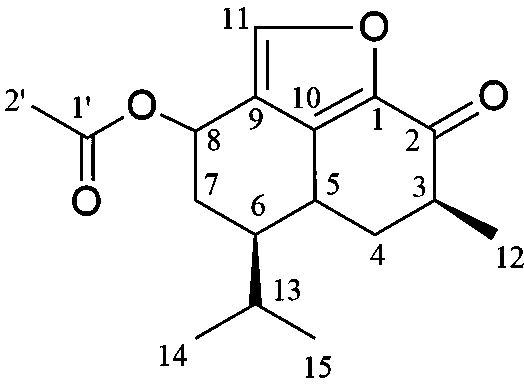
[0024] Collect Wuzhishan ginseng from Wuzhi Mountain in Hainan, dry it in the shade, slice and crush it, totaling 190g, extract it with acetone for 2 hours, let it stand still, take the acetone solution to concentrate, and obtain 9g extract. Take 20g of silica gel (100-200 mesh) for dry mixing, 40g of silica gel (300-400 mesh) for dry packing, and elute with petroleum ether-acetone system (1:0, 9:1, 4:1, 2: 1, 0:1), each 250ml is a fraction, concentrated in an ampoule, and a total of 26 fractions are obtained. After thin-layer chromatography, 7 parts were obtained, namely CDL-1-3, CDL-4-5, CDL-6-7, CDL-8-10, CDL-11-17, CDL-18-20, CDL -21-26. Among them, the CDL-4-5 part is semi-preparatively separated in liquid phase, methanol-water (72:28) is used as mobile phase, chromatographic column SB-Phenyl (5μm, 9.4×250mm), flow rate 2ml / min, and ultraviolet detector at the same time Set 4 detection wavelengths, 210, 230, 254, 270n...
Embodiment 2
[0025] Identification of Example 2 Compounds
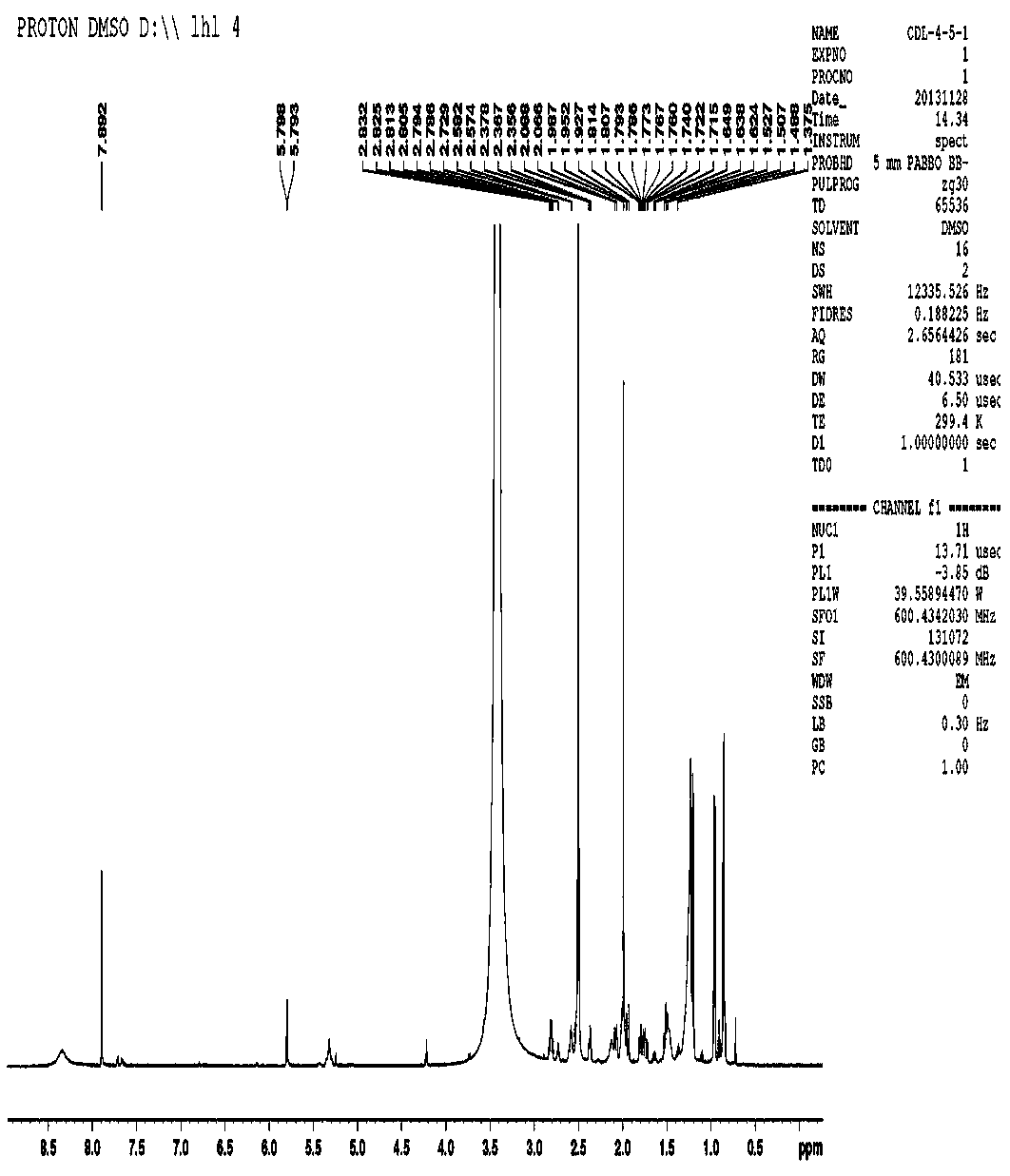
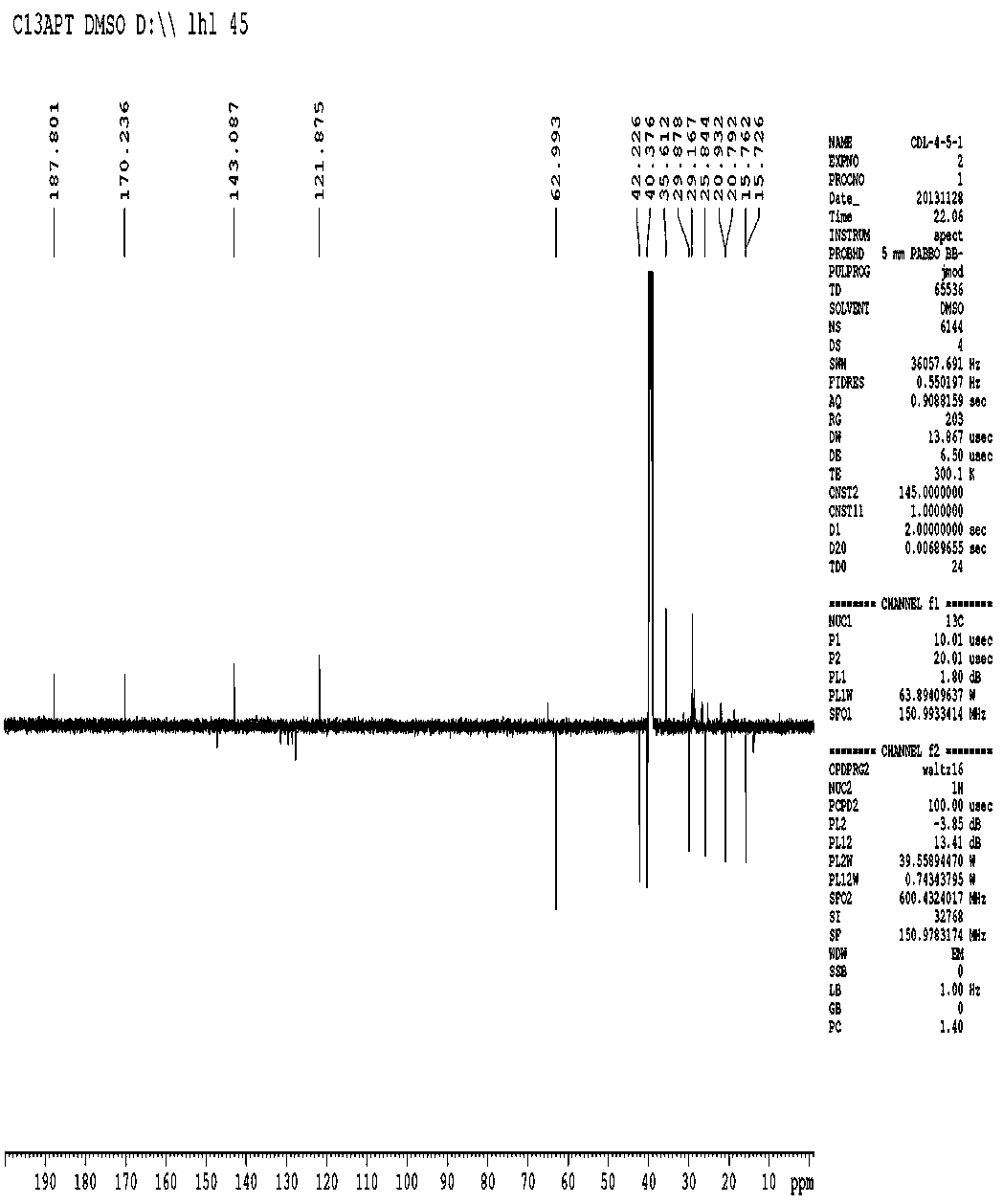
[0026] The new compound is a colorless crystal, m.p. 125 o C, [ α ]23.5 D + 3 o ( c 1.15, CHCl 3 ), high-resolution HR-ESI-MS gives m / z 290.1582 [M] + (calculated value 290.1579), determine its molecular formula as C 17 h 22 o 4 . The infrared spectrum shows that the molecule contains a carbonyl group (1640 cm -1 ) and furan ring (1530, 870 cm -1 ). according to 1 The H-NMR spectrum can be judged, in δ H There is a methyl group at 1.98(s, H-2’), at δ H 1.20 (d, J =7.8 Hz, H-12), 0.96(d, J =7.2 Hz, H-14), 0.86(d, J =6.6 Hz, H-15) with 3 methine groups. At the same time, in δ H 5.80 (1H, d, J = 3.0 Hz, H-8), 7.89 (1H, s, H-11) with 2 oxygen atom signals. according to 13 It can be judged from the C-NMR spectrum that the molecule contains 17 carbon signals, including 4 methyl signals ( δ C 15.7, 20.9, 15.9, 187.8), 2 methylene signals ( δ C 35.6, 29.2), 6 methine signals ( δ C 42.2, 29...
Embodiment 3
[0030] Example 3 Test of antitumor activity
[0031] Hela (human cervical cancer cell line) and HepG-2 (human liver cancer cell line) were selected and cultured in DMEM medium containing 10% calf serum at 37°C and 5% CO 2 Cultured in an incubator until the cells grew to a certain density (1×10 6 ). Digest with 0.25% trypsin, pipette into a single cell suspension, and inoculate the cell suspension in a 96-well plate (0.8×10 4 / well), cultured for 24 h. The compound was dissolved in DMSO and added to a 96-well plate. After 48 h of incubation, 100 μl of MTT / well (concentration of 5 mg / ml) was added and incubated at 37°C for 4 h. Remove the supernatant, add 200 μl DMSO to each well and shake gently to completely dissolve the formazan, measure the absorbance (OD) value at a wavelength of 490 nm with a microplate reader, and calculate the cell death rate as IC 50 Values represent the cytotoxic activity of the compounds.
[0032] The results showed that the new compound had IC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com