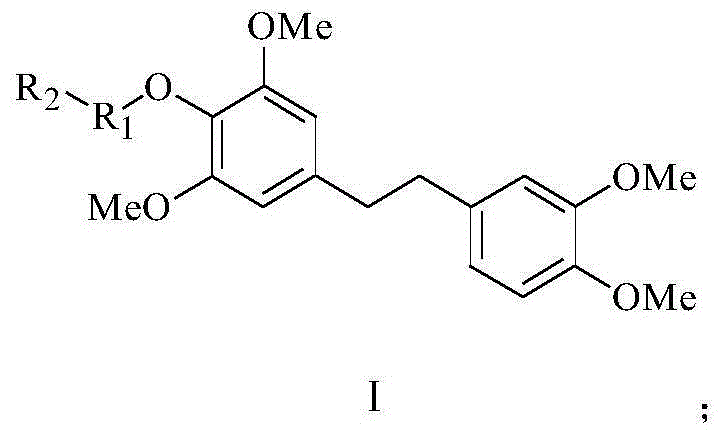
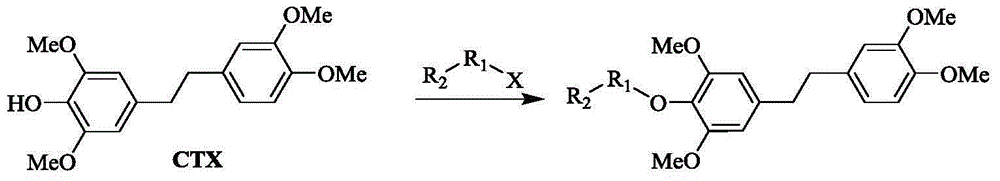Chrysotoxine bibenzyl derivative and preparation method and application thereof
A technology of Dendrobium Dendrobium and Dendrobium bibenzyl, which is applied in the field of Dendrobium Dendrobium bibenzyl derivatives and preparation thereof, can solve the problems of expensive raw material source, low bioavailability, weak protective effect and the like, and achieves the Easily reproducible, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
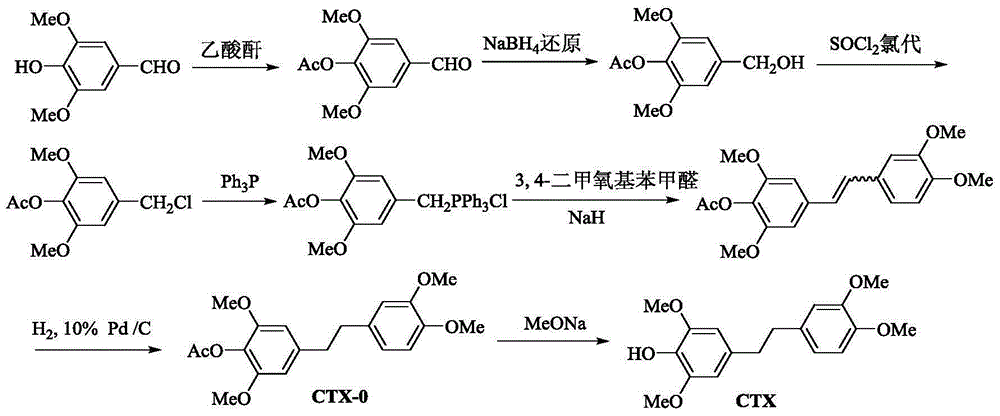
[0035] Preparation of Example 14-(3,4-dimethoxyphenethyl)-2,6-dimethoxyphenyl acetate (CTX-0) and Dendrobium tympanodin (CTX)
[0036] a. 4-formyl 2,6-dimethoxyphenyl acetate
[0037]
[0038] Add 8.33 g (0.046 mol) of syringaldehyde powder, 10 mL of dichloromethane, and catalytically metered DMAP (4-dimethylaminopyridine) into a 100 mL single-necked bottle, and stir to fully dissolve. Slowly add 4.75 mL (0.051 mol) of acetic anhydride dropwise, and react at room temperature for 1 h. Add water to wash away DMAP and acetic anhydride, wash 3 times in total, each time with 10 mL of water. Anhydrous Na 2 SO 4 The organic layer was dried and concentrated to recover dichloromethane to obtain 10.19 g of white crystals with a yield of 99.8%. Identified as 4-formyl 2,6-dimethoxyphenyl acetate.
[0039] ESI-MSm / z:225[M+H] + , The relative molecular mass is 224.
[0040] 1 H-NMR (300MHz, CDCl 3 )δ: 9.93 (1H, s, -CHO), 7.17 (2H, s, H-2, H-6), 3.92 (6H, s, 2×-OCH 3 ),2.38(3H,s...
Embodiment 2
[0076] Preparation of Example 24-(3,4-dimethoxyphenethyl)-2,6-dimethoxyphenylpropionate (CTX-1)
[0077]
[0078] Add 30 mg (0.094 mmol) of Dendrobium tympanodin, 27 mg (0.141 mmol) of EDC·HCl, 11 μL (0.141 mmol) of propionic acid, a catalyst amount of DMAP, and 3 mL of dichloromethane into a 50 mL single-necked bottle. Stir well and react at room temperature for 48h. Cool, extract with water to remove water-soluble impurities, and use anhydrous Na for the organic layer 2 SO 4 Dry and concentrate under reduced pressure to obtain the mixture, which is purified by silica gel column chromatography (PE-EA20:1-8:1) to obtain 22.1 mg of white solid with a yield of 63.4%. It was identified as 4-(3,4-dimethoxyphenethyl)-2,6-dimethoxyphenylpropionate.
[0079] ESI-MSm / z:392.3[M+NH 4 ] + ,375.2[M+H] + , The relative molecular mass is 374.
[0080] 1 H-NMR (300MHz, CDCl 3 )δ: 6.82 (1H, d, J = 8.1Hz, H-5'), 6.74 (1H, dd, J = 1.8, 8.1Hz, H-6'), 6.67 (1H, d, J = 1.8Hz, H-2'),6....
Embodiment 3
[0081] Preparation of Example 34-(3,4-dimethoxyphenethyl)-2,6-dimethoxyphenylbutyrate (CTX-2)
[0082]
[0083] Referring to the synthesis method of CTX-1, 30 mg (0.094 mmol) of Dendrobium tympani was added, 27 mg (0.141 mmol) of EDC·HCl, 14 μL (0.150 mmol) of butyric acid, DMAP as catalyst, and 3 mL of dichloromethane were added. Stir well and react at room temperature for 48h. Cool, extract with water to remove water-soluble impurities, and use anhydrous Na for the organic layer 2 SO 4 Dry and concentrate under reduced pressure to obtain the mixture, which is purified by silica gel column chromatography (PE-EA 20:1-8:1) to obtain 14 mg of light yellow oil with a yield of 38.3%. It was identified as 4-(3,4-dimethoxyphenethyl)-2,6-dimethoxyphenylbutyrate.
[0084] ESI-MSm / z:406.3[M+NH 4 ] + ,389.2[M+H] + , The relative molecular mass is 388.
[0085] 1 H-NMR (300MHz, CDCl 3 )δ: 6.82 (1H, d, J = 8.1Hz, H-5'), 6.74 (1H, dd, J = 1.8, 8.1Hz, H-6'), 6.67 (1H, d, J = 1.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com