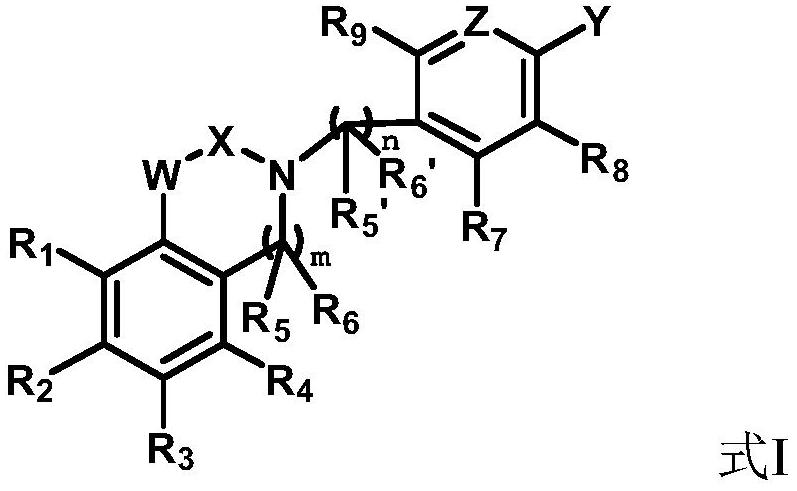
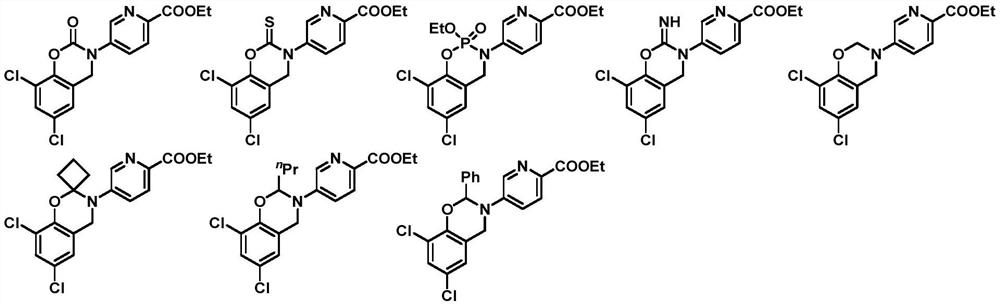
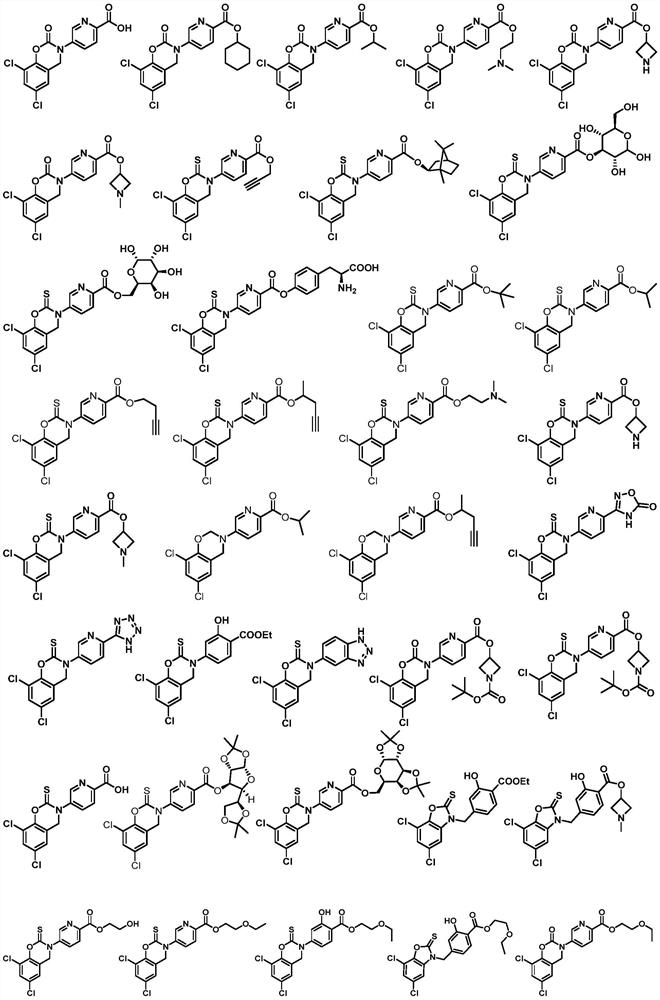
Compounds with neuroprotective effect, and preparation method and application thereof
A compound and unsaturated technology, applied in the field of biomedicine, can solve problems such as ineffective neuroprotective agents and complex pathogenesis of stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] The preparation of embodiment 1 compound 04013
[0180]
[0181] Step 1, Compound 2
[0182]To a solution of compound 1 (2.0 g, 14.5 mmol) in ethanol (20 mL) was slowly added dropwise thionyl chloride (20 mL) at 25°C. The reaction system was heated to 60°C and stirred overnight. Concentrated under reduced pressure, the residue was extracted with water (50mL) and ethyl acetate (50mL×2), the combined organic phases were dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain white solid compound 2 (0.90g, yield: 37.5%)
[0183] Step 2. Compound 04006-1
[0184] A methanol (20 mL) solution of compound 2 (0.90 g, 5.4 mmol) and compound 3 (1.0 g, 5.0 mmol) was stirred at 25° C. for 1 hour, sodium cyanoborohydride (0.38 g, 6.0 mmol) was added, and stirred overnight. The reaction was quenched with aqueous sodium bicarbonate (100 mL), concentrated under reduced pressure to remove methanol, extracted with ethyl acetate (100 mL X 3), the combi...
Embodiment 2
[0190] The preparation of embodiment 2 compound 04015
[0191]
[0192] Step 1, compound 04015
[0193] To a solution of compound 04006-1 (0.2 g, 0.58 mmol) in ethyl acetate (10 mL) was added TCDI (thiocarbonyldiimidazole) (118 mg, 0.6 mmol) at 25°C, and stirred for 16 hours. The reaction solution was washed with water, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was purified with a preparative plate to obtain compound 04015 (22 mg, yield: 10%).
[0194] MS(ESI): Calcd.for C 16 h 12 Cl 2 N 2 o 3 S 382; Found 383 [M+H] + .
[0195] HNMR (400MHz, CD 3 OD): δ8.83(s,1H),8.28(d,J=8.0Hz,1H),7.91(d,J=8.0Hz,1H),7.48(s,1H),7.07(s,1H), 4.83(s, 2H), 4.52(q, J=6.0Hz, 2H), 1.47(t, J=6.0Hz, 3H).
Embodiment 3
[0196] The preparation of embodiment 3 compound 04016
[0197]
[0198] Step 1, compound 04016
[0199] At 25°C, phosphorus oxychloride (0.55mL) was added to a solution of compound 04006-1 (200mg, 0.59mmol) and triethylamine (178mg, 1.76mmol) in toluene (10mL), and the reaction system was heated to React at 70°C for 16 hours. Concentrate under reduced pressure, add ethanol (20 mL) to the residue, and stir for half an hour. It was concentrated under reduced pressure, and the residue was purified by preparative chromatography to obtain 04016 (49.7 mg, yield: 19.6%).
[0200] MS(ESI): Calcd.for C 17 h 17 Cl 2 N 2 o 5 P 430; Found 431 [M+H] + .
[0201] HNMR (400MHz, CDCl 3 ): δ8.70(d, J=2.4Hz, 1H), 8.13-8.10(d, J=8.8Hz, 1H), 7.87-7.80(dd, d, J=8.8Hz, 2.0Hz, 1H), 7.43 (s,1H),7.15(d,J=2.4Hz,1H),4.88-4.70(m,2H),4.47(q,J=7.2Hz,2H),4.35-4.24(m,2H),1.47- 1.42(t,J=7.2Hz,3H),1.38-1.32(t,J=7.2Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com