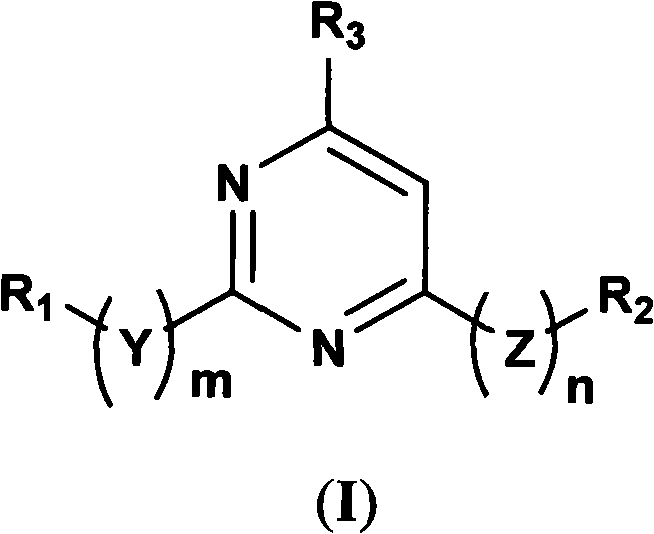
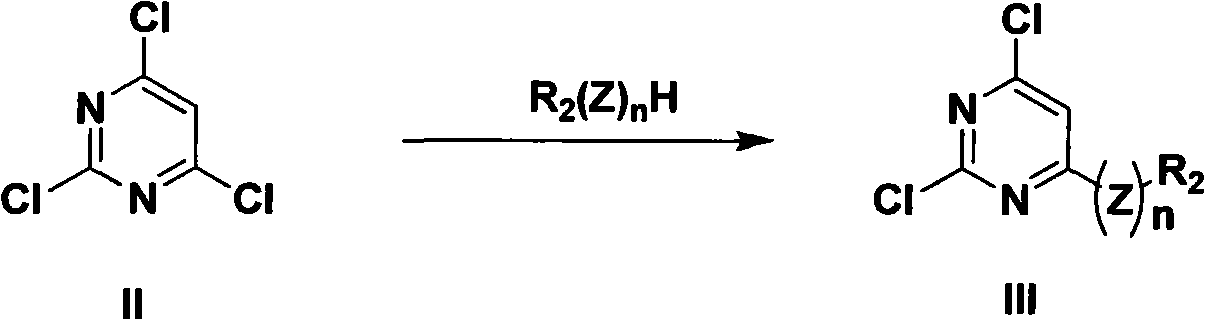
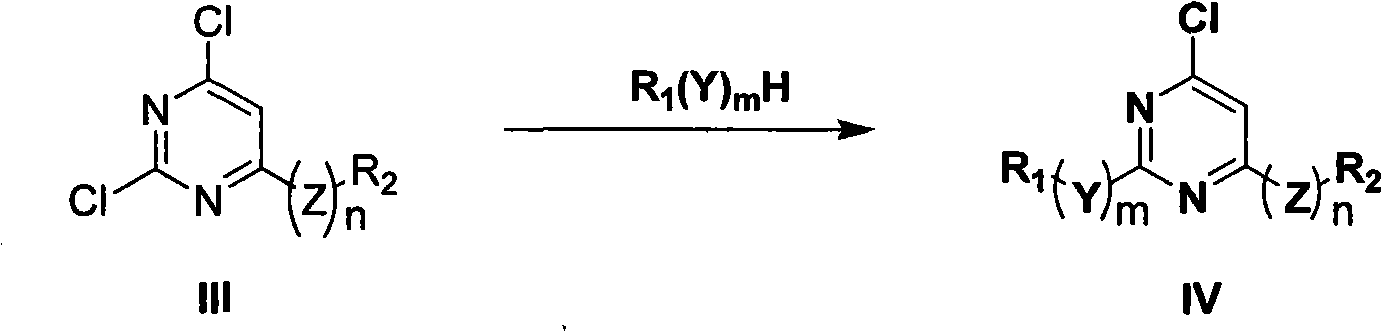
5-lipoxygenase inhibitor and preparation method, medical composite and application thereof
A solvate, compound technology, applied in 5-lipoxygenase inhibitor, application in medicine, compound of general formula and the field of preparation thereof, can solve the problems of low bioavailability, methemoglobin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0089] The preparation of embodiment 12,6-dichloro-N-(4-methoxyphenyl) pyrimidin-4-amine
[0090] Dissolve 1g (5.5mmol) of 2,4,6-trichloropyrimidine in 25mL of absolute ethanol, add 0.64g (6.0mmol) of anhydrous sodium carbonate and 0.62g (6.6mmol) of p-methoxyaniline, stir and heat Reflux for 1.5 hours. After cooling to room temperature, 25 mL of water was added to precipitate a precipitate, which was washed with a large amount of water and dried in vacuum overnight to obtain a gray powder product. Pure product was obtained by recrystallization from absolute ethanol. Yield 90%. Mp 160-161°C; 1 H NMR (CDCl 3 ): δ3.84 (s, 3H), 6.36 (s, 1H), 6.96 (d, 2H), 7.2 (d, 2H), 7.37 (br, 1H); LRMS (EI) m / z 269 (M + ).
Embodiment 2
[0091] Example 2 6-Chloro-N 2 , N 4 - Preparation of two (4-methoxyphenyl) pyrimidine-2,4-diamine
[0092] Dissolve 0.27g (1mmol) of 2,6-dichloro-N-(4-methoxyphenyl)pyrimidin-4-amine in 25mL of dioxane solution, add 0.22g (2mmol) of anhydrous sodium carbonate and 3.3 mmol of p-methoxyaniline was heated to reflux for 48 hours, and the reaction was detected by TLC. Cool to room temperature, add water, extract with ethyl acetate (20mL×3), combine organic layers, MgSO 4 Dry, filter, and evaporate to dryness to obtain a residue, which is separated on a silica gel column to obtain the product.
Embodiment 3
[0093] Example 3 N 2 , N 4 - Preparation of bis(4-methoxyphenyl)-6-(4-methylpiperazin-1-yl)pyrimidine-2,4-diamine (1a)
[0094] Add 0.2g 6-chloro-N to a 50mL eggplant-shaped bottle 2 , N 4 - Bis(4-methoxyphenyl)pyrimidine-2,4-diamine and 10 mL of N-methylpiperazine, heated to reflux overnight. Cool to room temperature, add water, extract with ethyl acetate (20mL×3), combine the organic layers, and wash with water (20mL×1), saturated NaCl aqueous solution (20mL×1), MgSO 4 Dry, filter, and evaporate to dryness to obtain a residue. Esco flash preparative separation chromatography (PE / EA=4 / 1) purifies, obtains final product N 2 , N 4 - bis(4-methoxyphenyl)-6-(4-methylpiperazin-1-yl)pyrimidine-2,4-diamine. Yield: 83%. Mp 156-157°C; 1 H NMR (300Hz, DMSO-d 6 ): δ2.20(s, 3H), 3.36(m, 4H), 3.44(m, 4H), 3.71(s, 3H), 3.73(s, 3H), 5.39(s, 1H), 6.82(d, 2H), 6.85(d, 2H), 7.48(d, 2H), 7.58(d, 2H); LRMS(EI) m / z 420(M + ); HRMS(EI) m / z calculated value C 23 h 28 N 6 o 2 (M + )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com