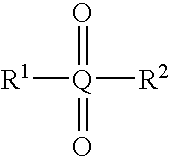Patents
Literature
97 results about "Chronic venous insufficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
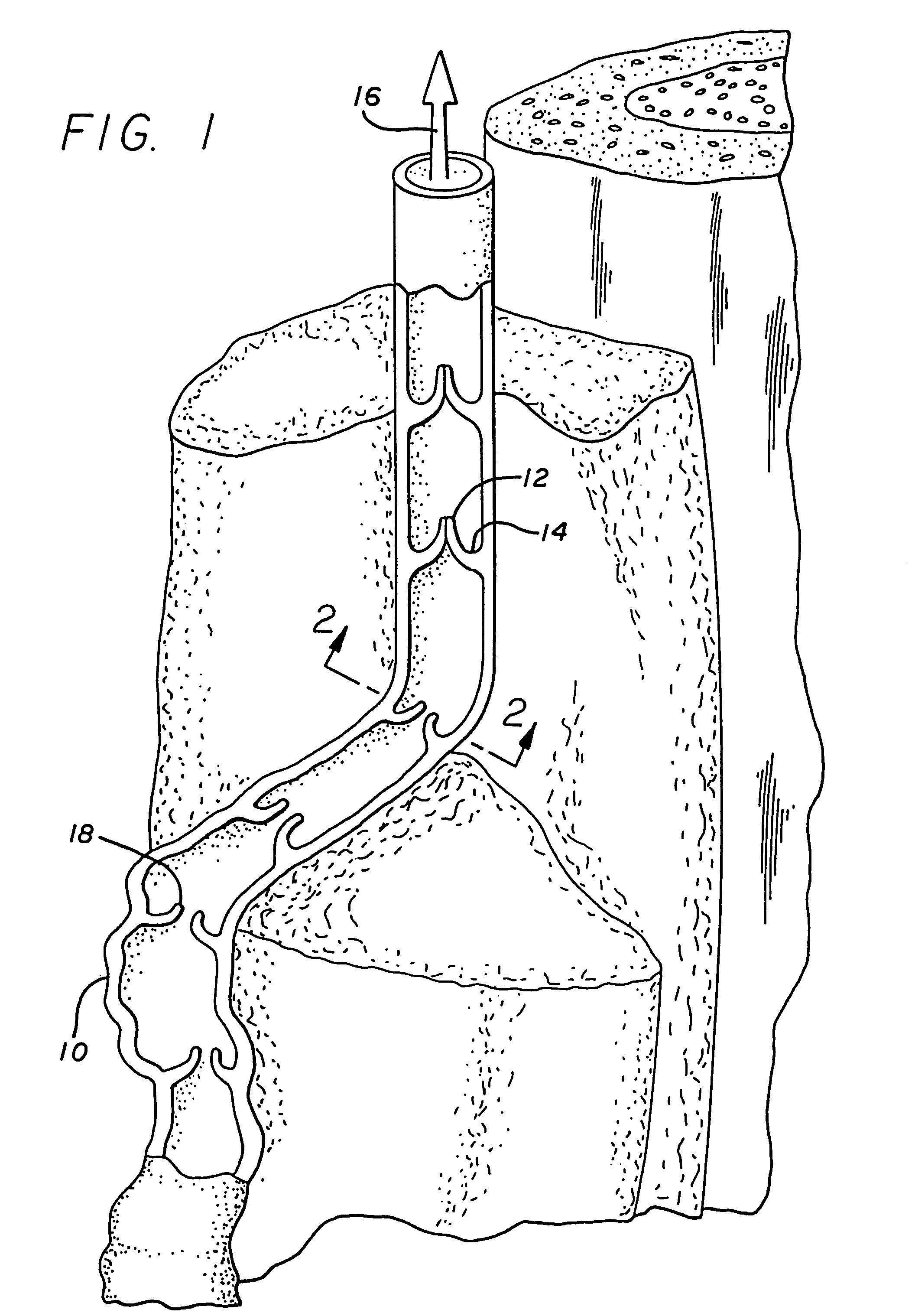
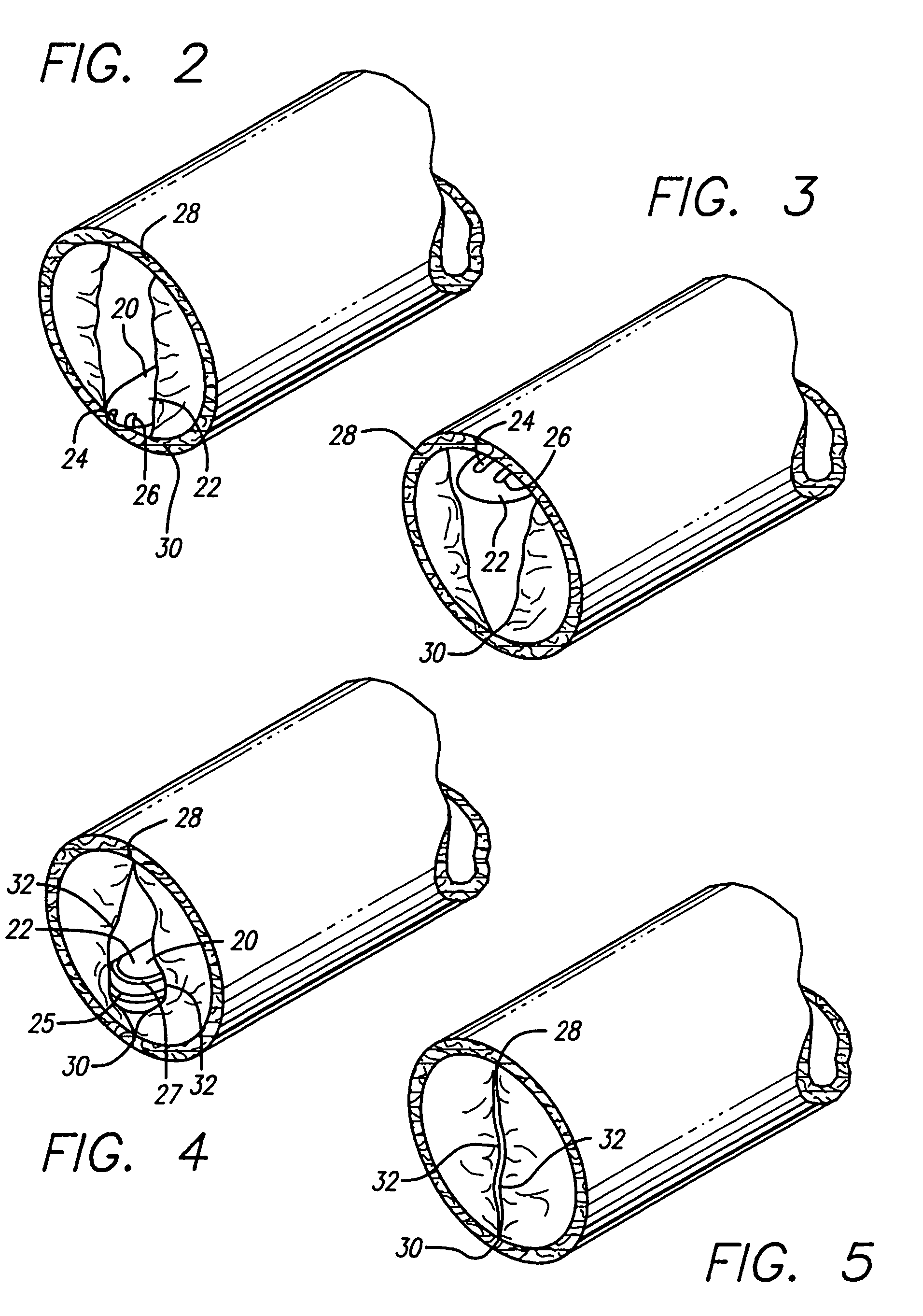
Malfunction of venous walls and/or valves in systemic circulation, especially in the legs, that result in peripheral pooling of blood known as stasis.
Soluble guanylate cyclase activators
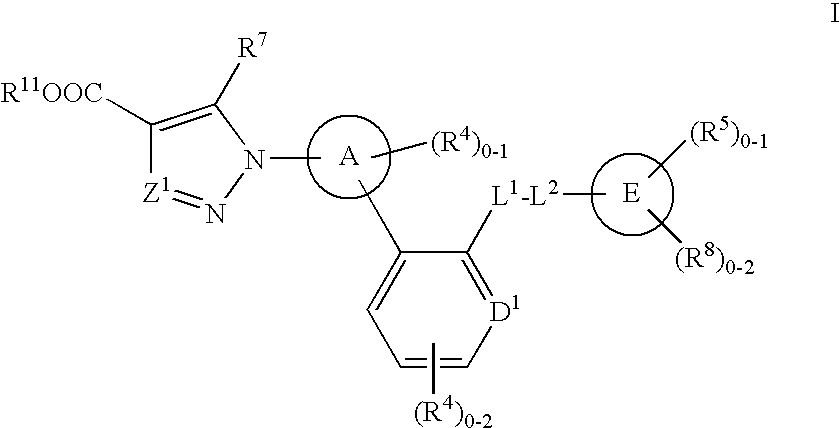
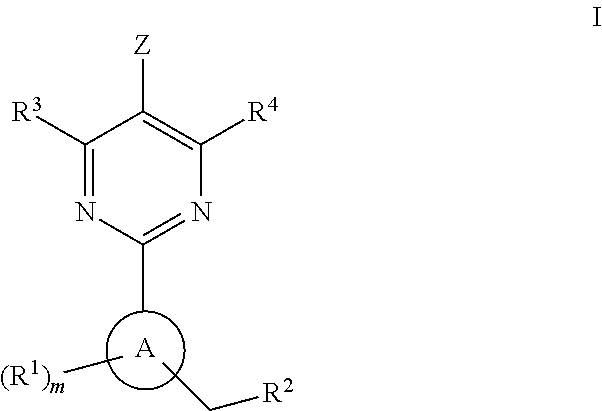
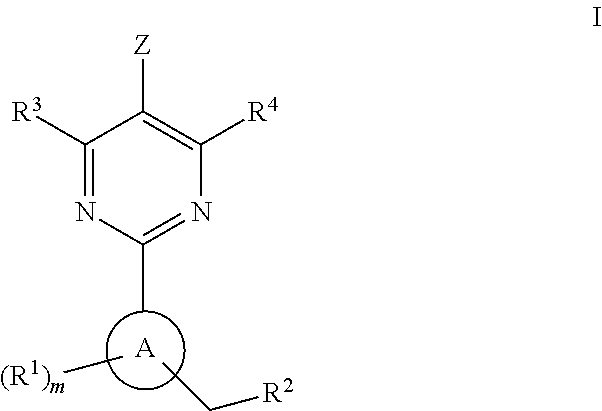
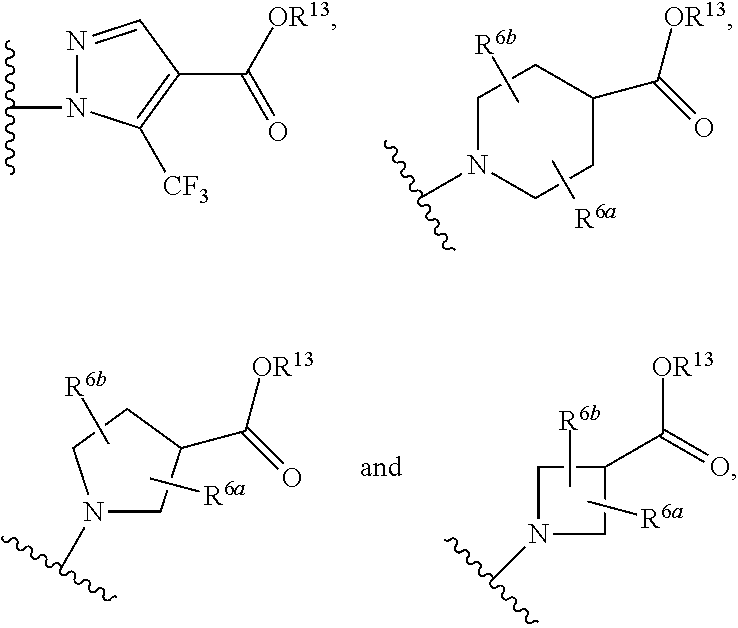
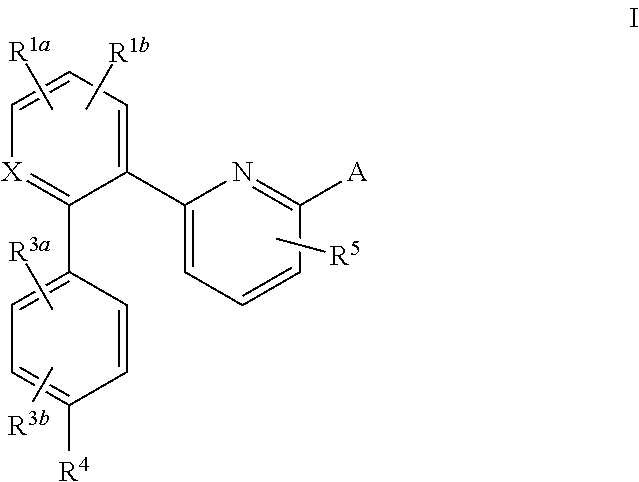
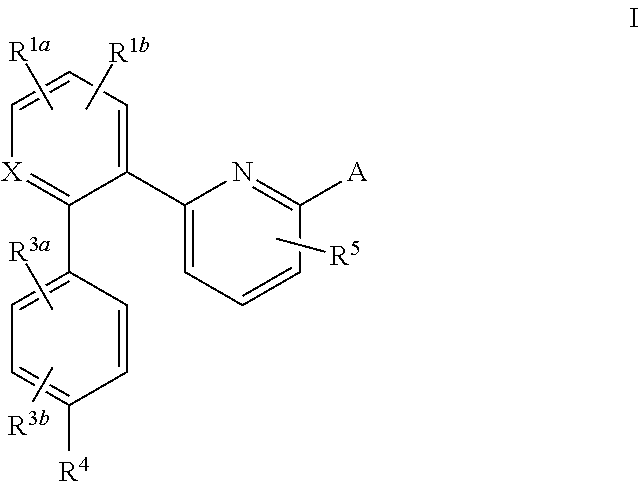
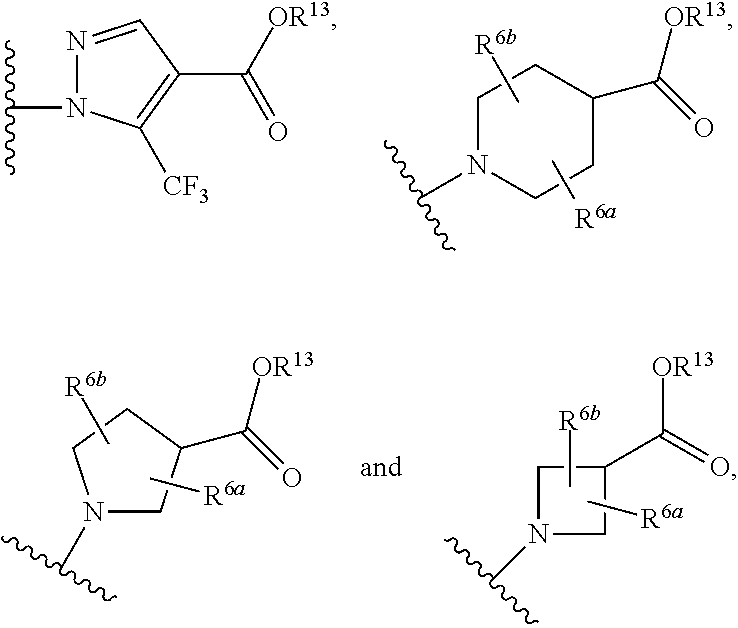
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME CORP
Soluble guanylate cyclase activators
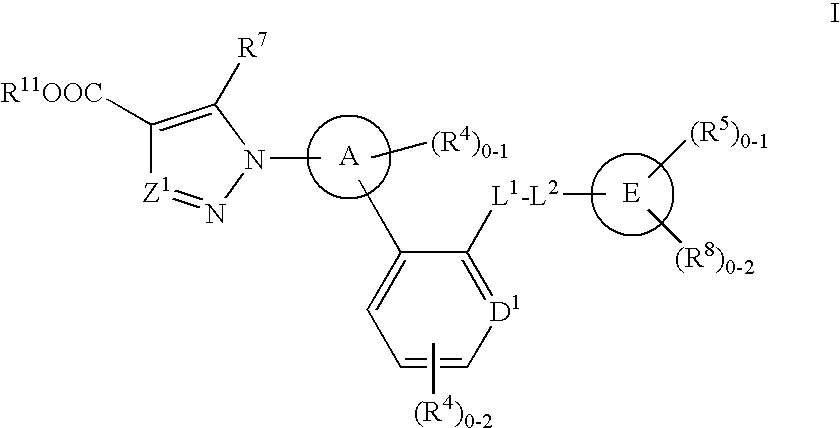
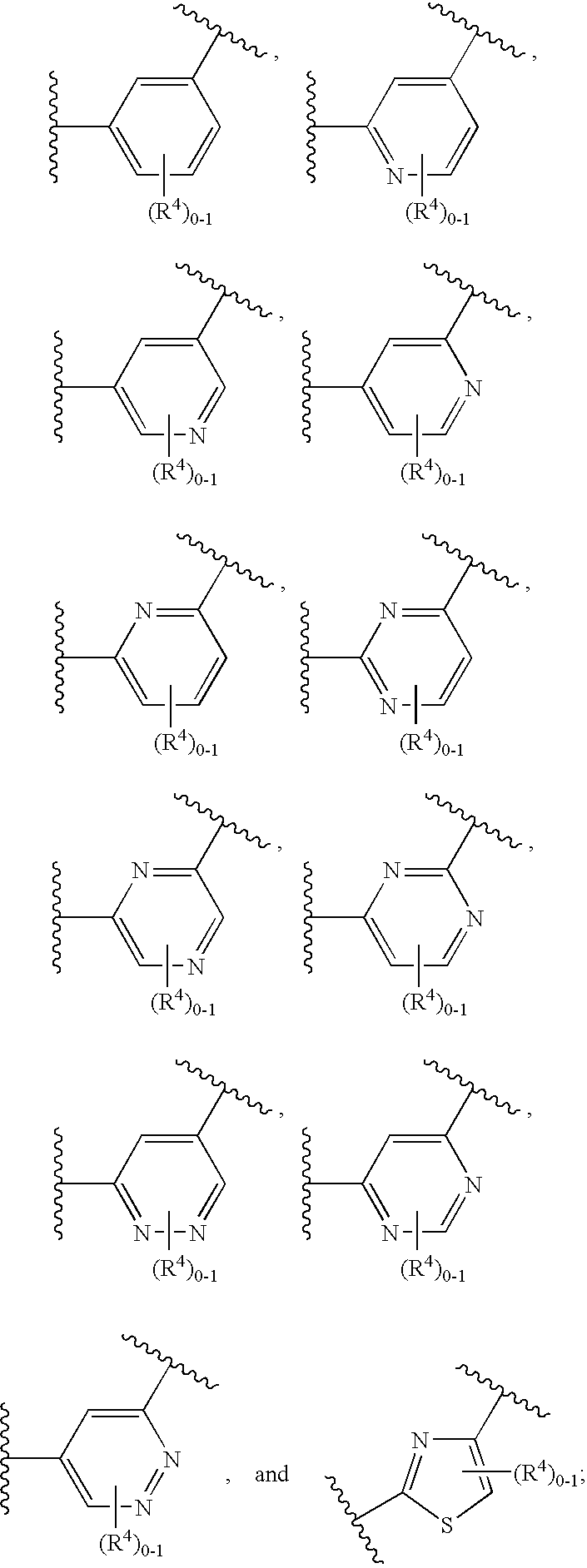
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
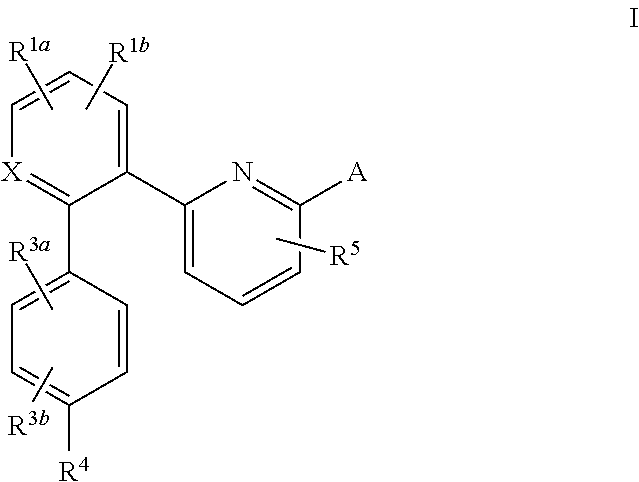
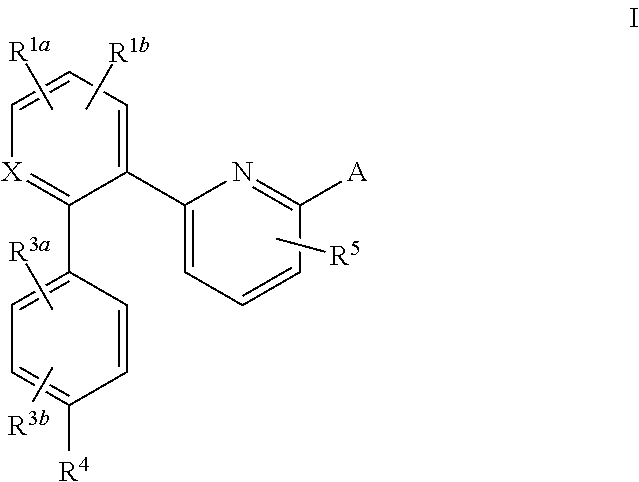
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
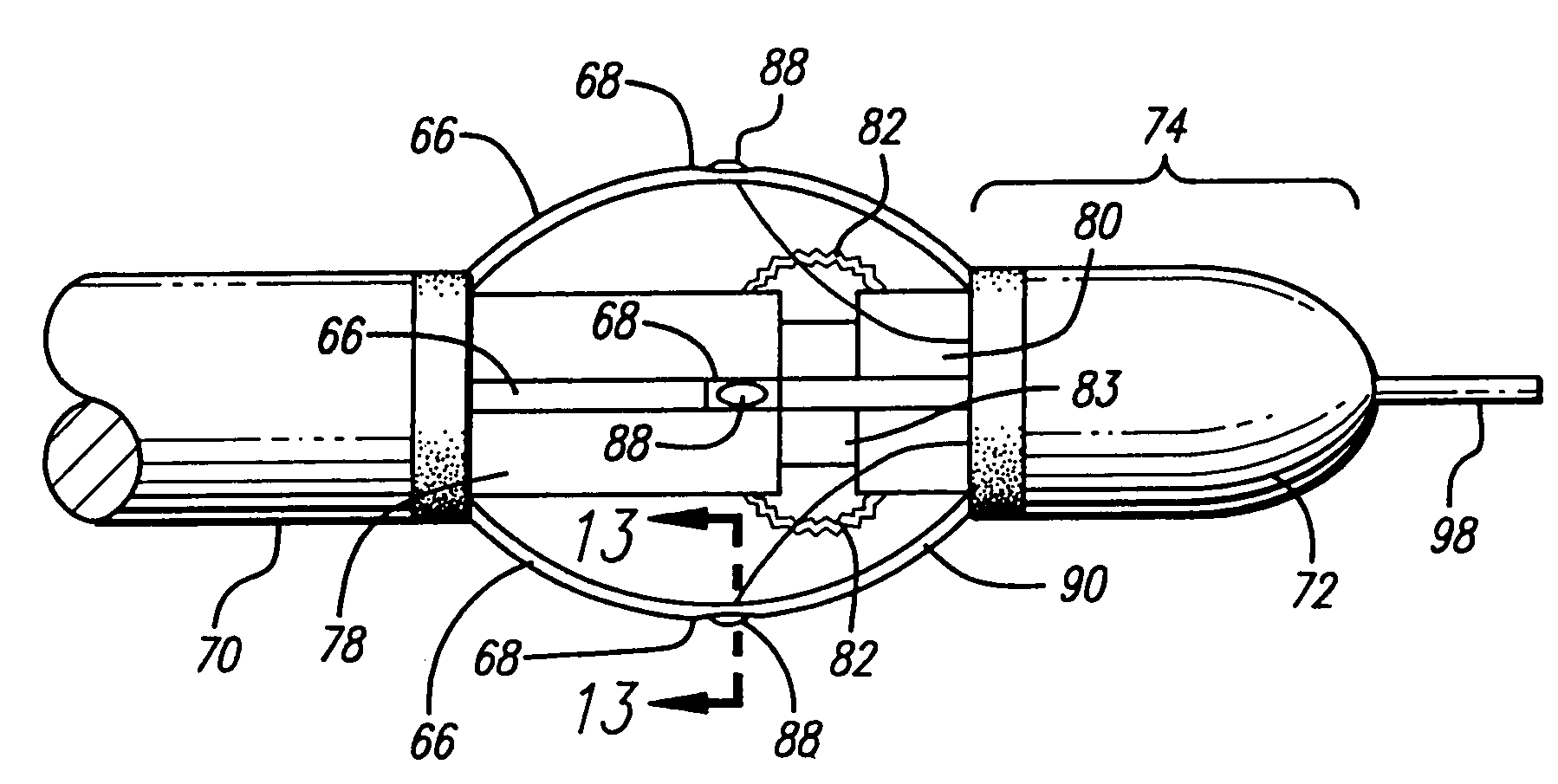
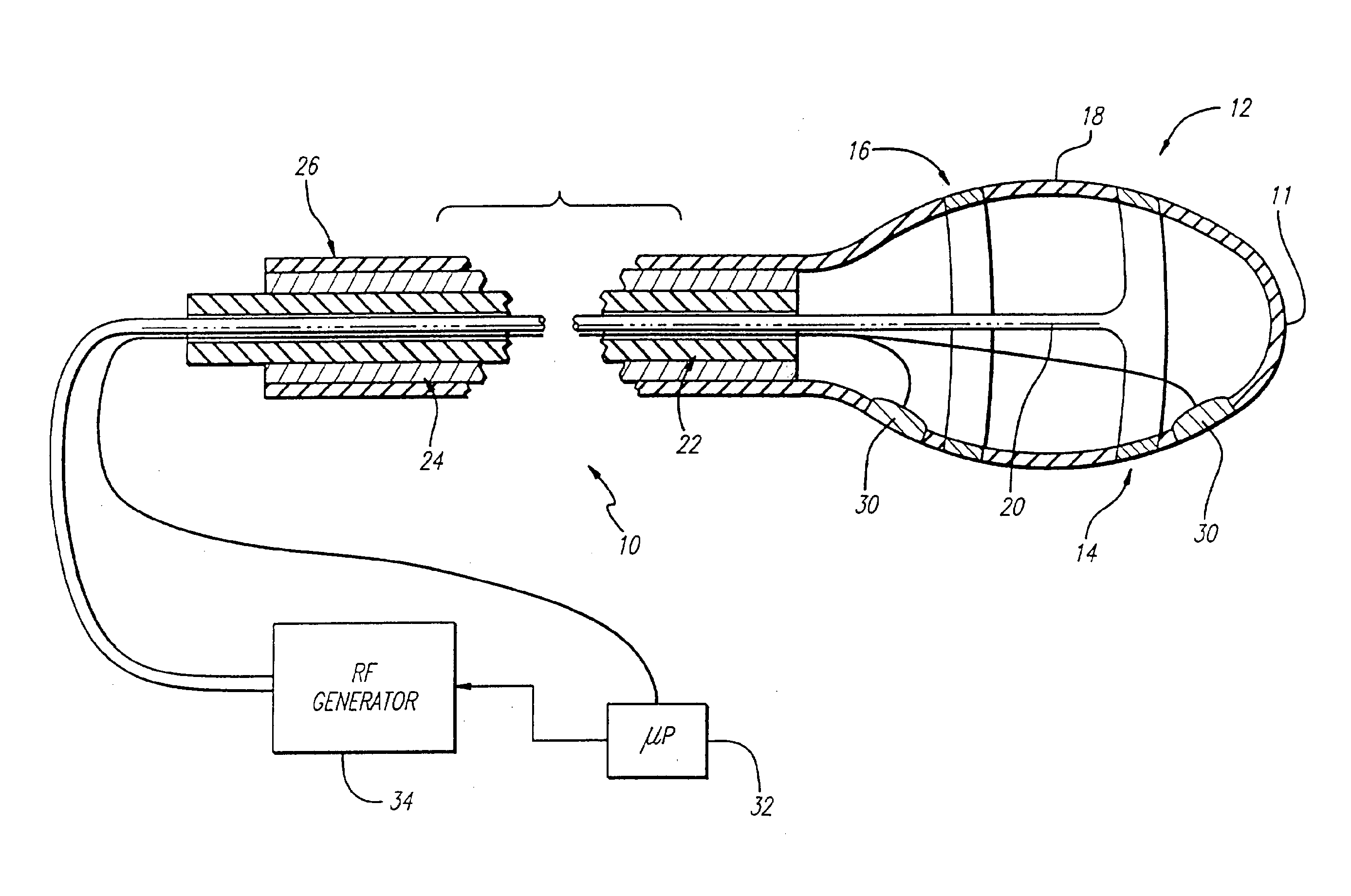
Apparatus for treating venous insufficiency using directionally applied energy
InactiveUS6981972B1Restore valvular competenceTreat multipleElectrotherapySurgical instruments for heatingVenous ValvesInvasive treatments
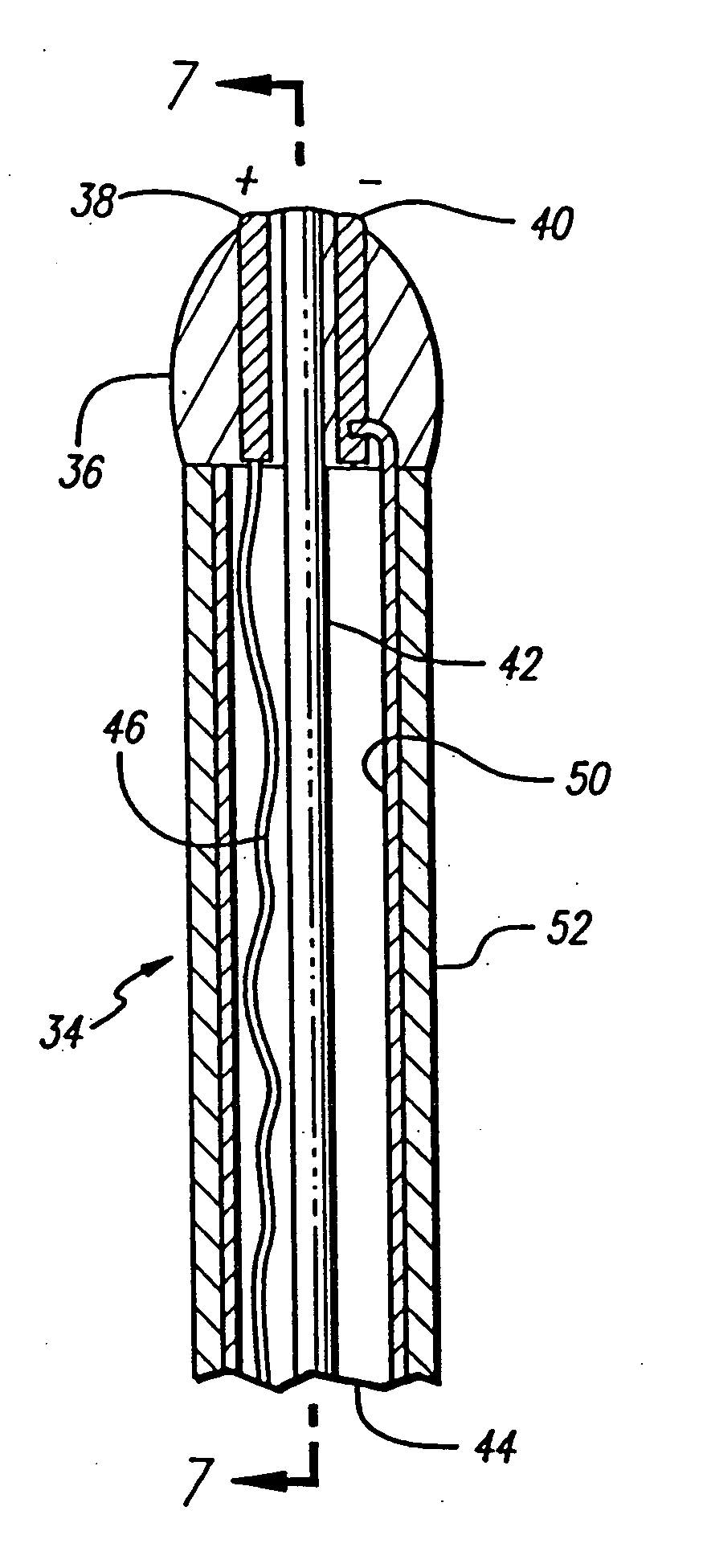
A catheter introduces electrodes in a vein for a minimally invasive treatment of venous insufficiency by the application of energy to cause selective heating of the vein. The catheter is positioned within the vein to be treated, and the electrodes on the catheter are moved toward one side of the vein. RF energy is applied in a directional manner from the electrodes at the working end of the catheter to cause localized heating and corresponding shrinkage of the adjacent venous tissue, which may include commissures, leaflets and ostia. Fluoroscopy or ultrasound may be used to detect shrinkage of the vein. After treating one section of the vein, the catheter can be repositioned to place the electrodes to treat different sections of the vein until all desired venous valves are repaired and rendered functionally competent.
Owner:TYCO HEALTHCARE GRP LP
Phosphate binder with reduced pill burden
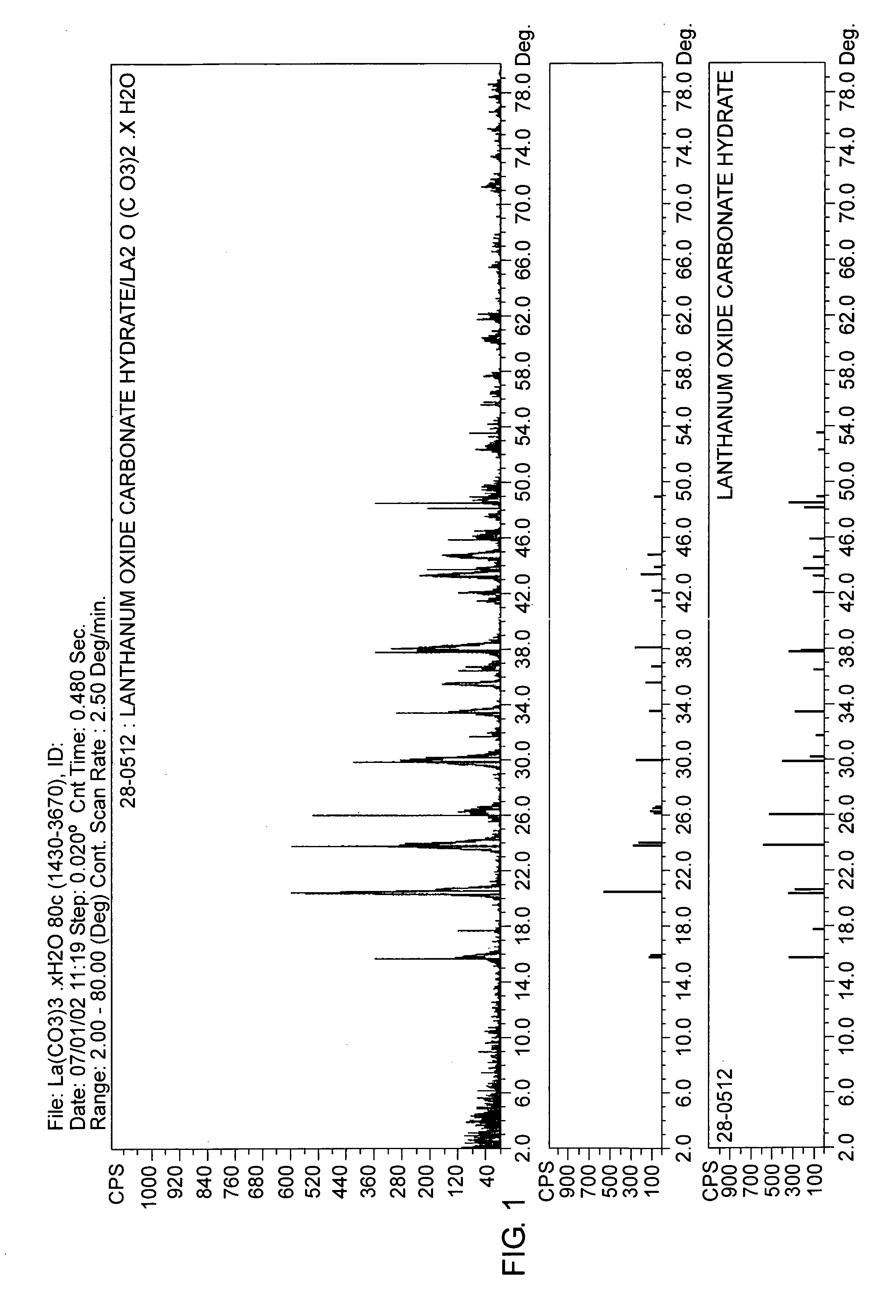
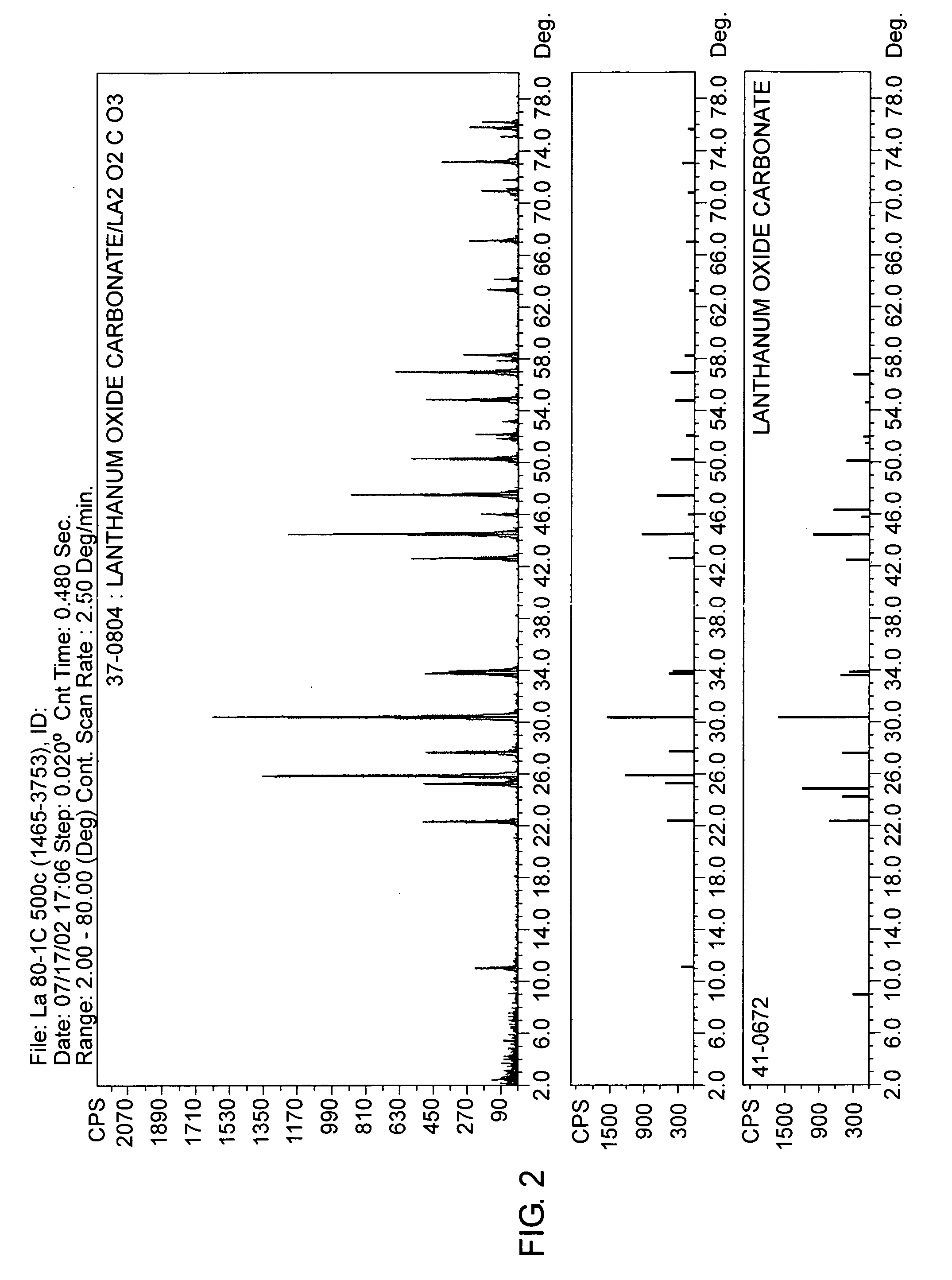
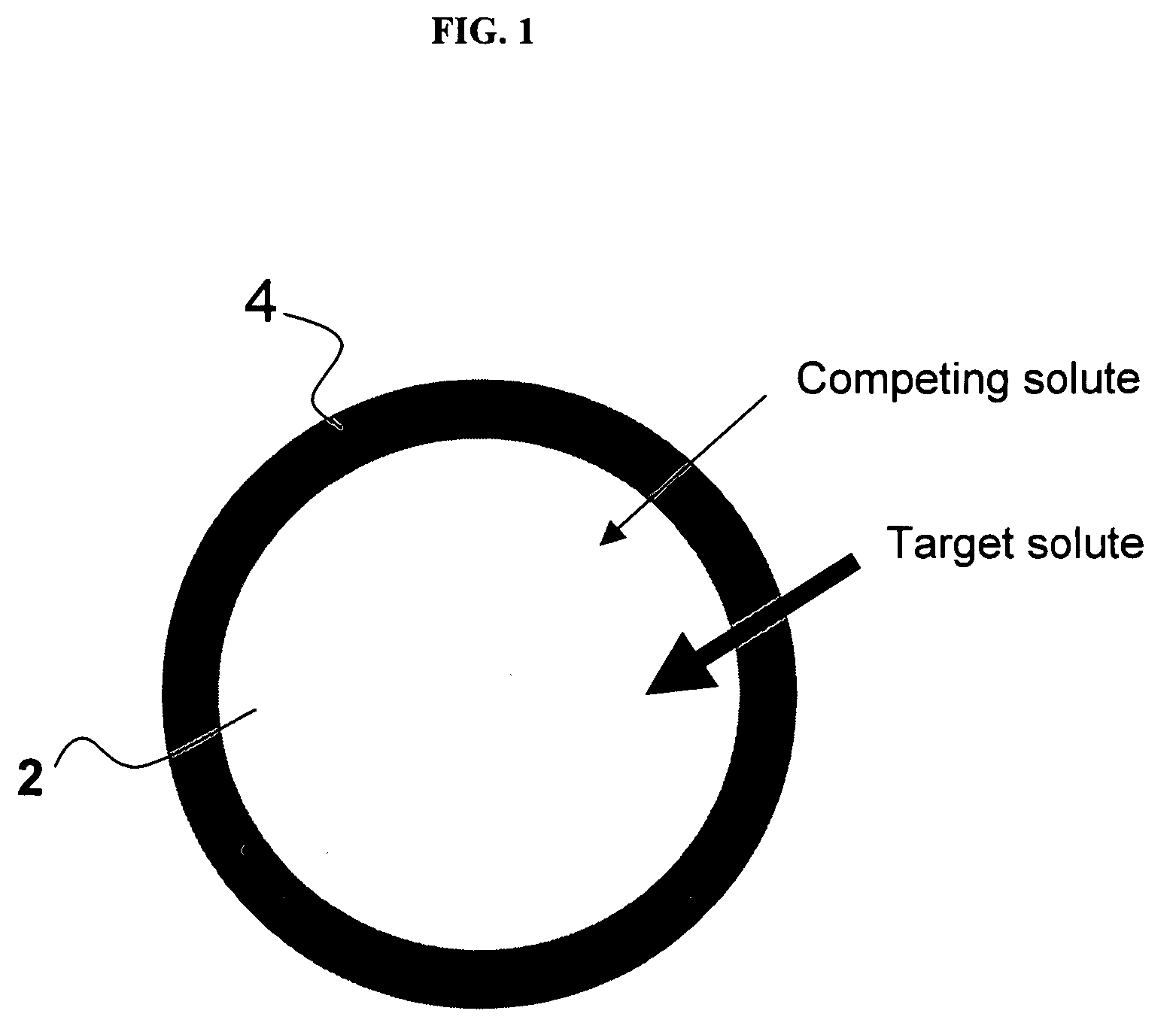
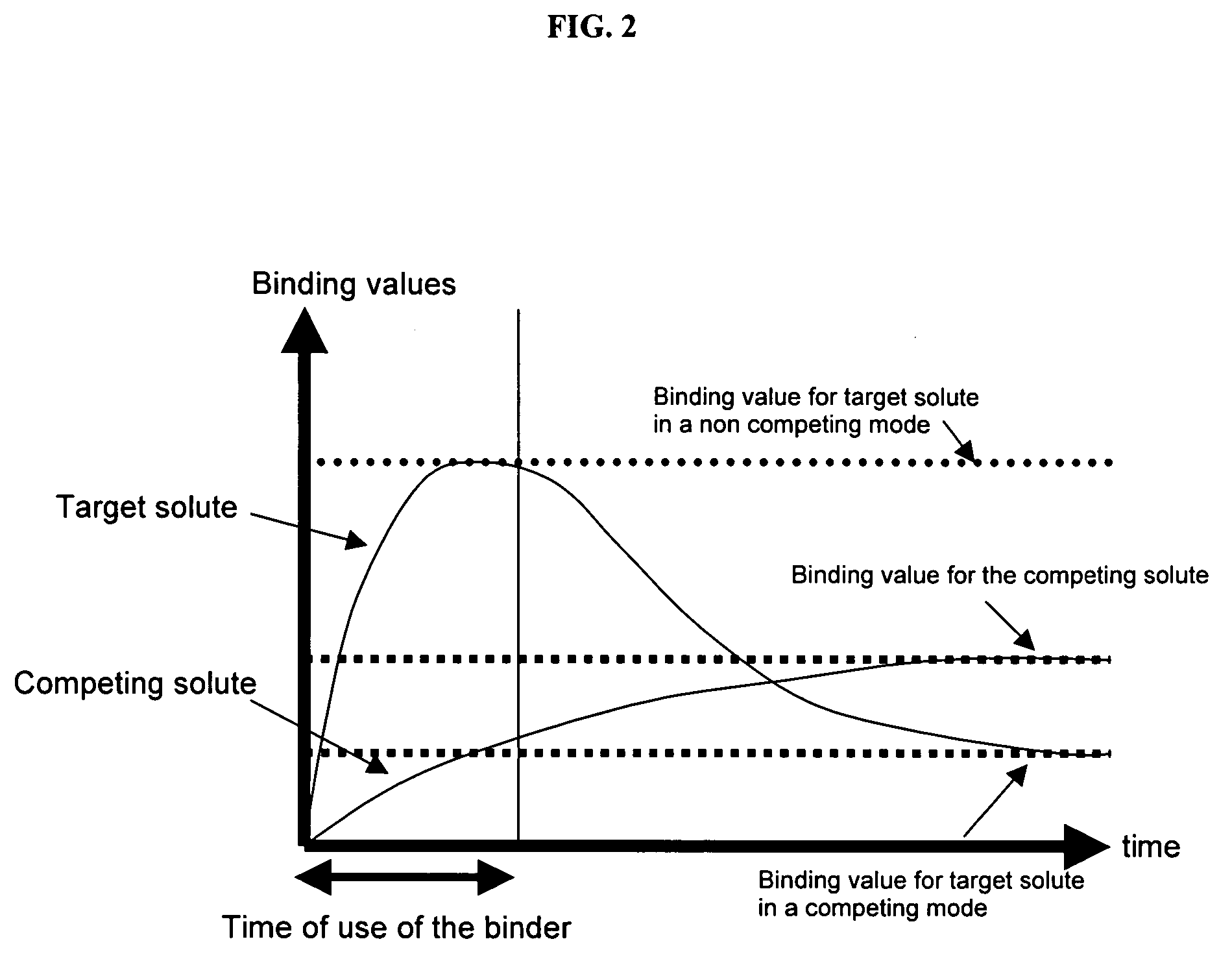
The present invention is generally directed to compositions and formulations that can be used for the treatment of diseases such as End Stage Renal Disease (“ESRD”) and Chronic Renal Insufficiency (“CRI”). Specifically, it is directed to lanthanum-based compounds that bind phosphate and that can be formulated to provide for a reduced pill burden relative to other phosphate binders. In a formulation aspect of the present invention, a formulation is provided the includes a lanthanum-based, phosphate binder. The formulation is typically characterized in that in may be swallowed without chewing. Formulations of the present invention, along with a lanthanum-based compound, may optionally include the following: mass diluting agents; binders; coatings; compression / encapsulation aids; disintegrants; lubricants; plasticizers; slip / anti-electrostatic agents; powder lubricants; and, sweeteners. Where the formulation is in the form of a tablet, it typically has a volume between 0.3 cm3 and 1.2 cm3, preferably between 0.35 cm3 and 0.50 cm3. Each tablet typically includes enough phosphate binder such that only 3 or less tablets need to be ingested each day for a patient suffering from ESRD.
Owner:SPECTRUM PHARMA INC
Method for treating venous insufficiency using directionally applied energy
InactiveUS20060069417A1Restore valvular competenceTreat multipleSurgical instruments for heatingLight therapyVenous ValvesInvasive treatments
A catheter introduces electrodes in a vein for a minimally invasive treatment of venous insufficiency by the application of energy to cause selective heating of the vein. The catheter is positioned within the vein to be treated, and the electrodes on the catheter are moved toward one side of the vein. RF energy is applied in a directional manner from the electrodes at the working end of the catheter to cause localized heating and corresponding shrinkage of the adjacent venous tissue, which may include commissures, leaflets and ostia. Fluoroscopy or ultrasound may be used to detect shrinkage of the vein. After treating one section of the vein, the catheter can be repositioned to place the electrodes to treat different sections of the vein until all desired venous valves are repaired and rendered functionally competent.
Owner:COVIDIEN LP
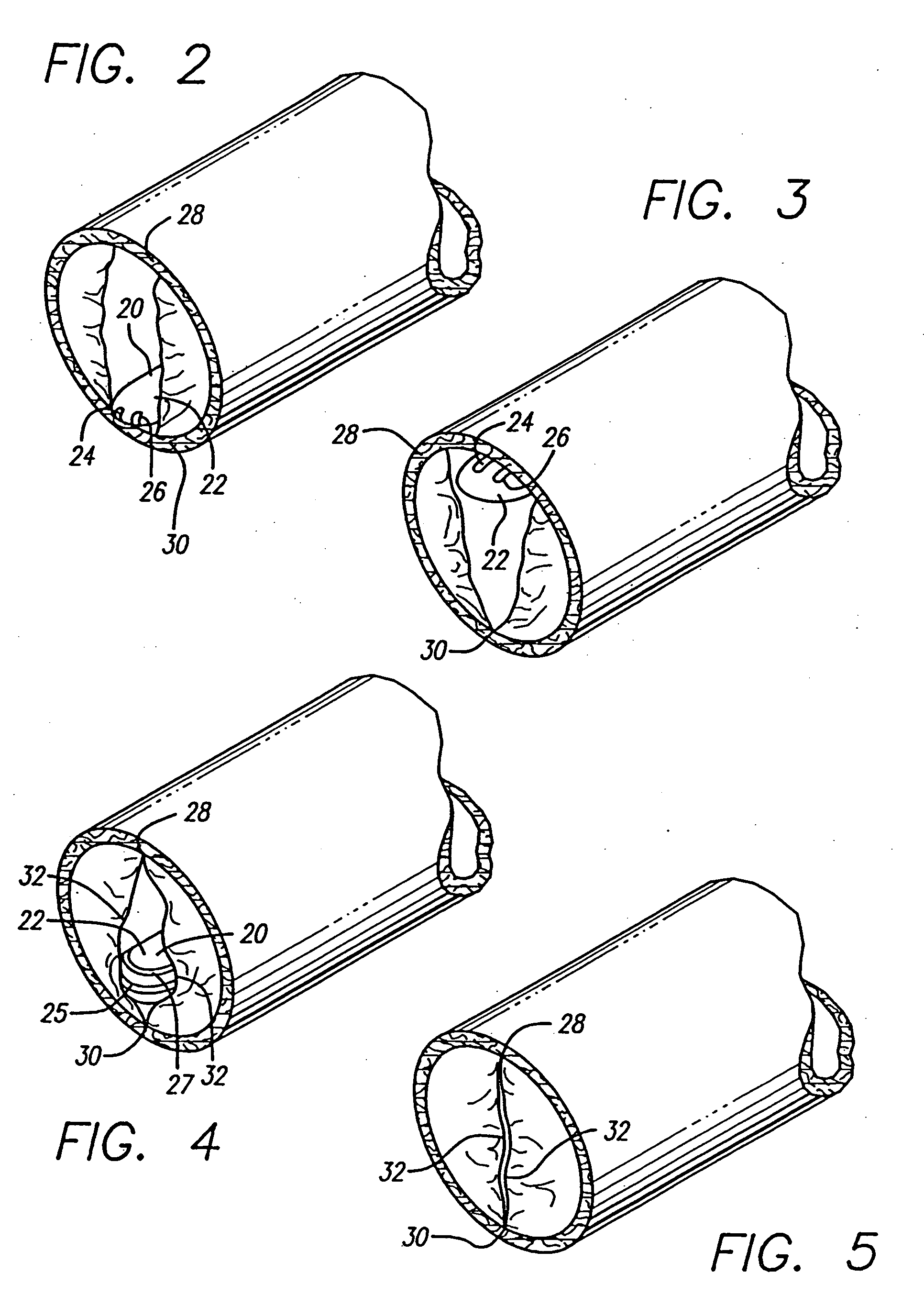
Valved stent for chronic venous insufficiency
InactiveUS20090254175A1Minimize turbulenceReduce molecular weightVenous valvesBlood vesselsChemical treatmentCoupling
The invention discloses a valved stent and process of manufacture for treating chronic venous insufficiency having the geometry of the supporting frame and its coupling to the membrane of a specific geometry that provides the valvular mechanism for optimal function. The membrane may comprise a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts and crosslinked.
Owner:QUIJANO RODOLFO C +1
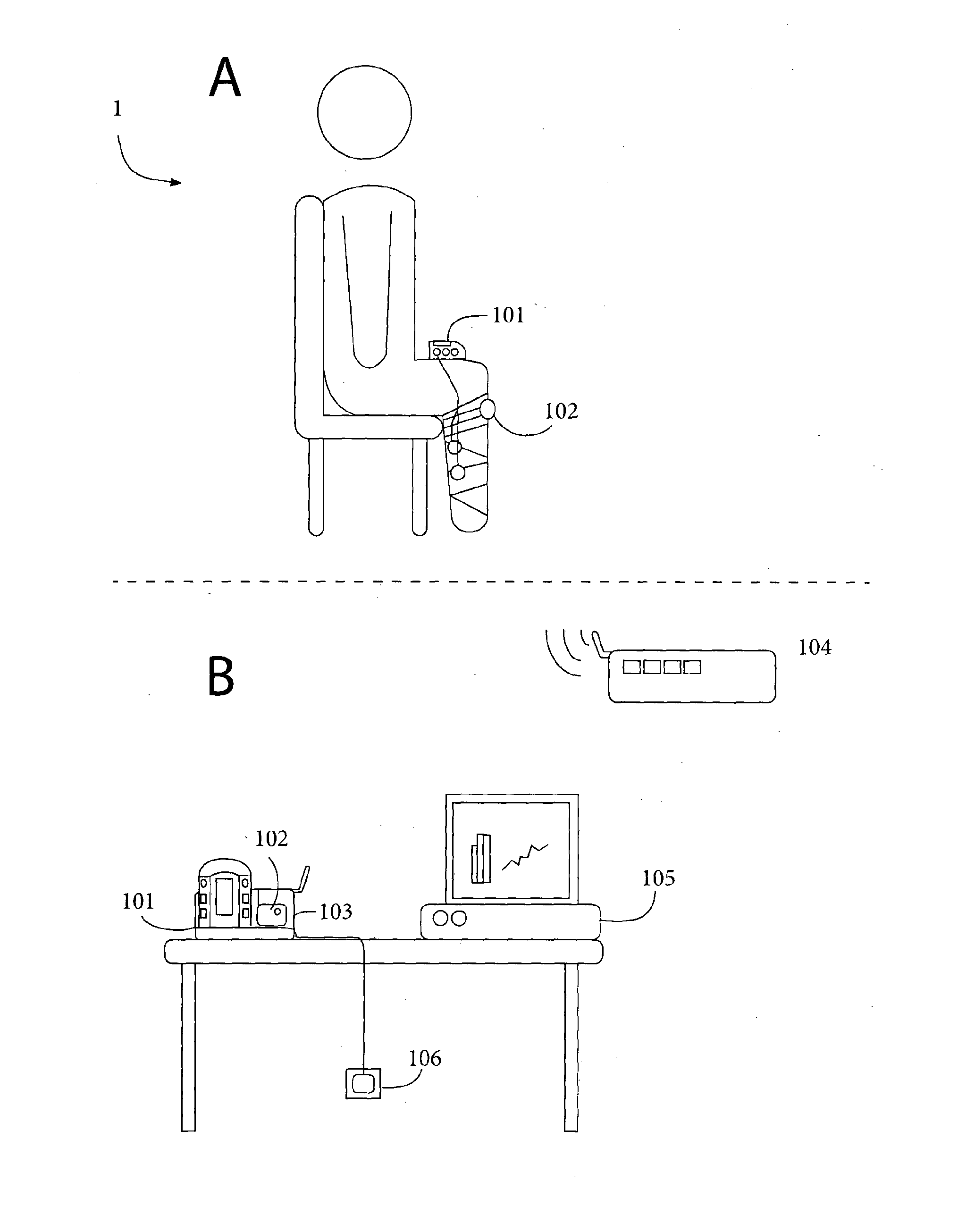
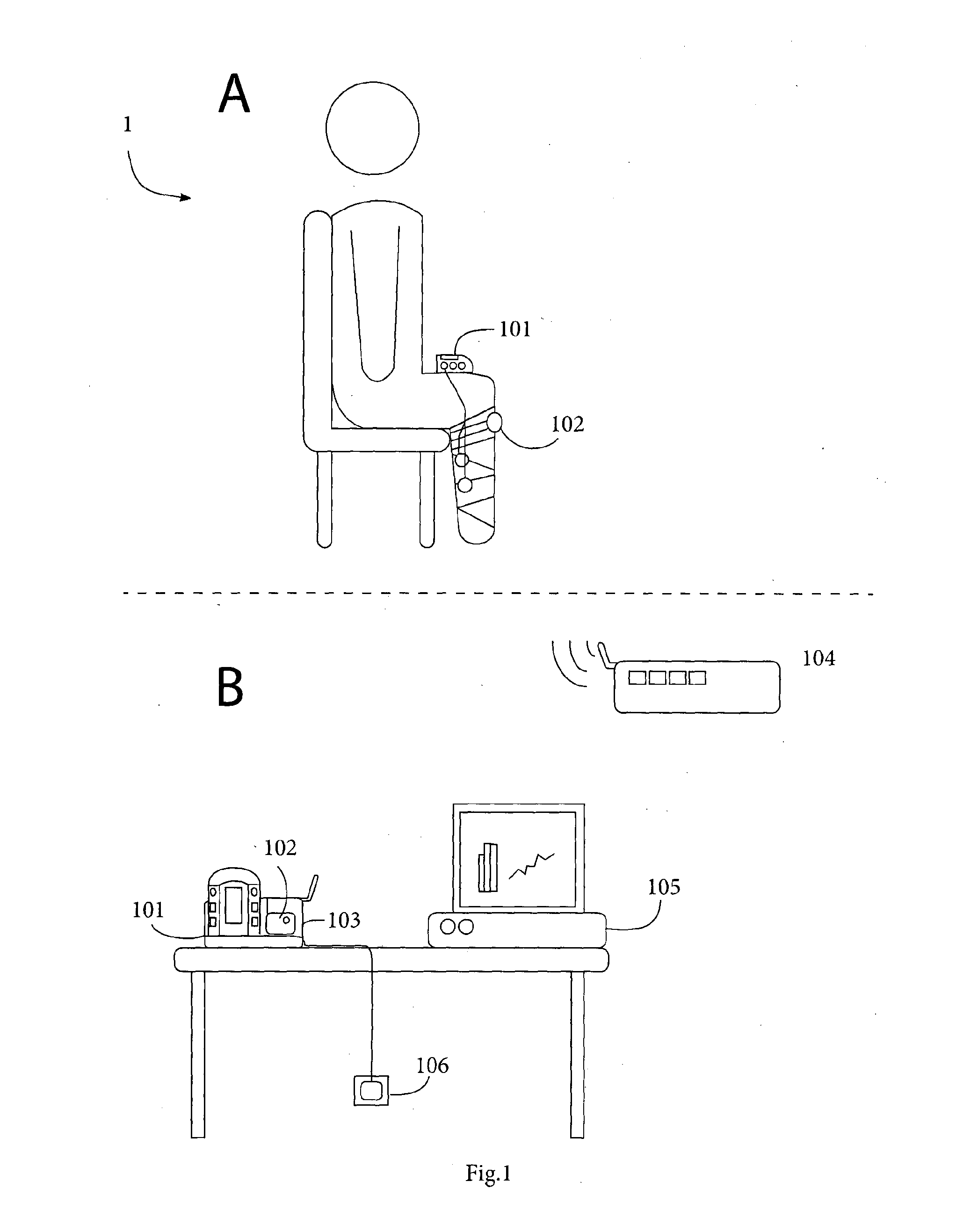
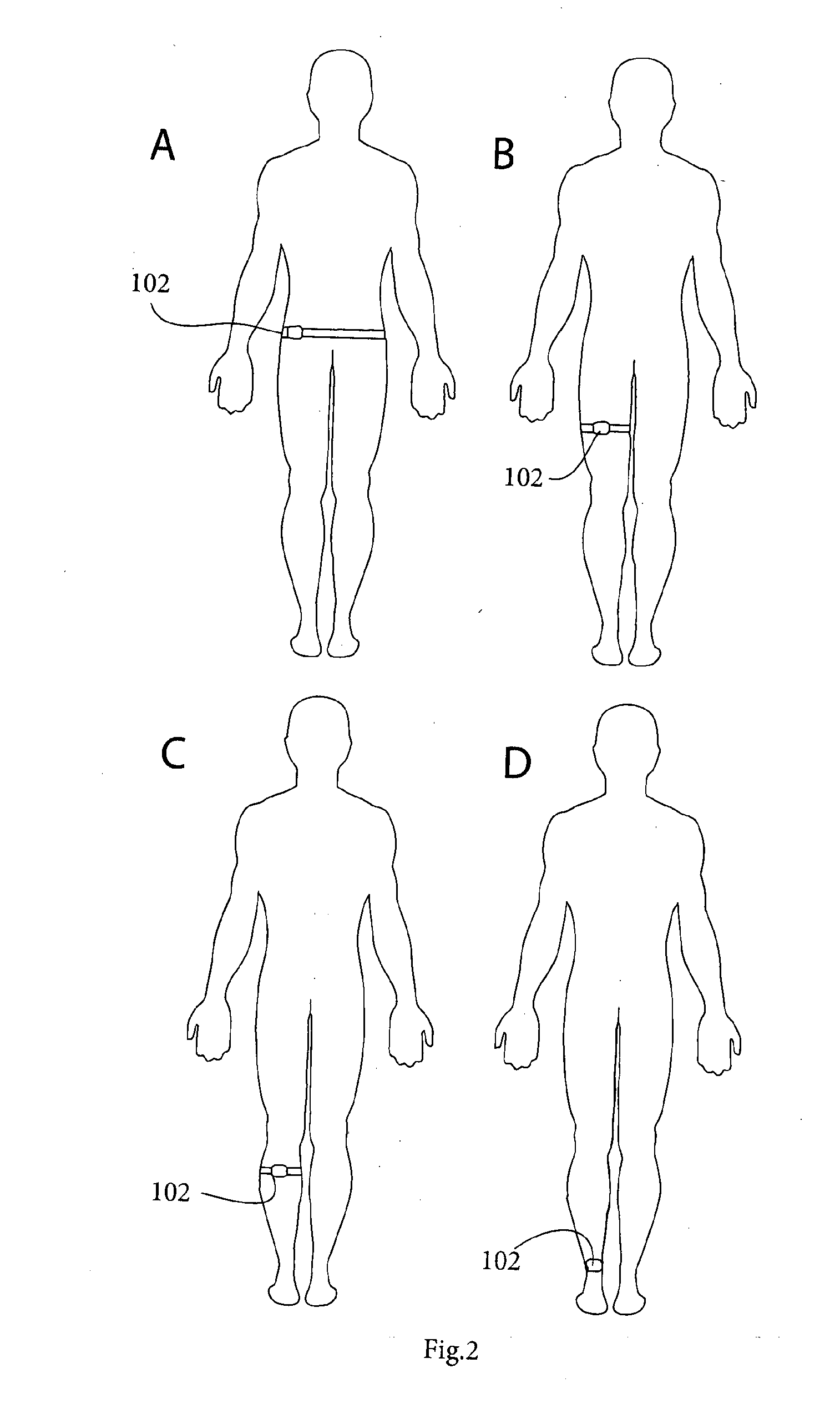
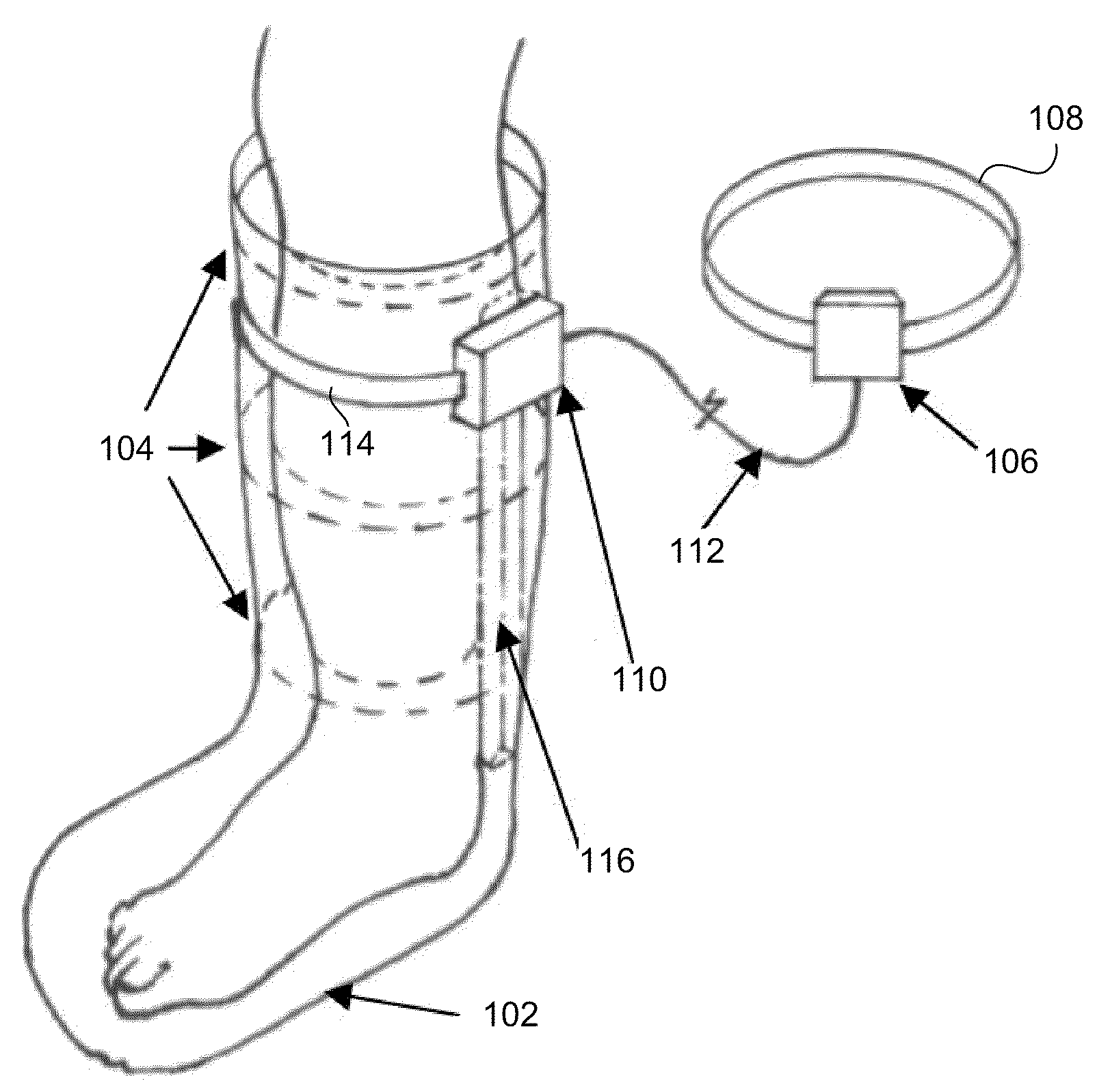
System for management and prevention of venous pooling
A monitoring system comprises sensors adapted to be worn by a user, and, a processor linked with the sensor. The processor receives sensor data and processes this data to determine user posture data including data indicative of vertical distance between level of the user's heart and ankle. Based on the posture data together with a value for degree of user chronic venous insufficiency and / or blood density, generate an estimate of user static venous pressure while the user is static, without calf muscle pump activity. The processor also processes the sensor data to determine if there is calf muscle pump activity, and generates an estimate of user active venous pressure according to the static venous pressure estimate, rate of calf muscle activity, and a value for degree of user chronic venous insufficiency. The processor may generate the venous pressure estimate in real time, and may control an NMES device accordingly.
Owner:THE NAT UNIV OF IRELAND GALWAY +1
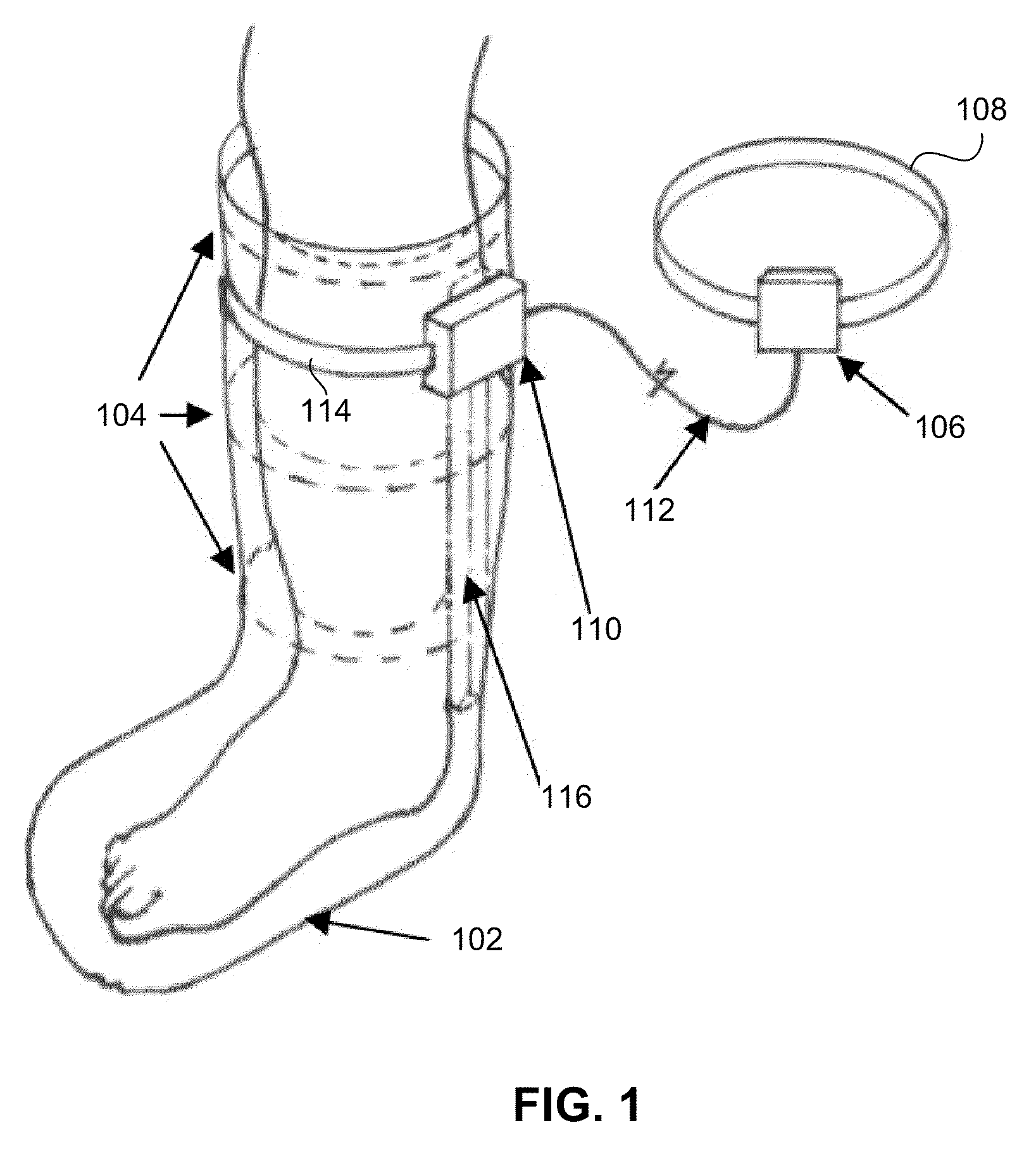
Ambulatory negative pressure therapeutical compression device
InactiveUS20110288458A1High in nutrientsIncrease oxygenUltrasound therapyPneumatic massageAmbulatoryCompression device
Embodiments of the present invention relate to medical devices and treatments for chronic venous insufficiency, open ulceration and related medical conditions, and more particularly to a device and treatment incorporating negative pressure compression to the foot and lower leg or other appendage of a patient.
Owner:MEDEFFICIENCY
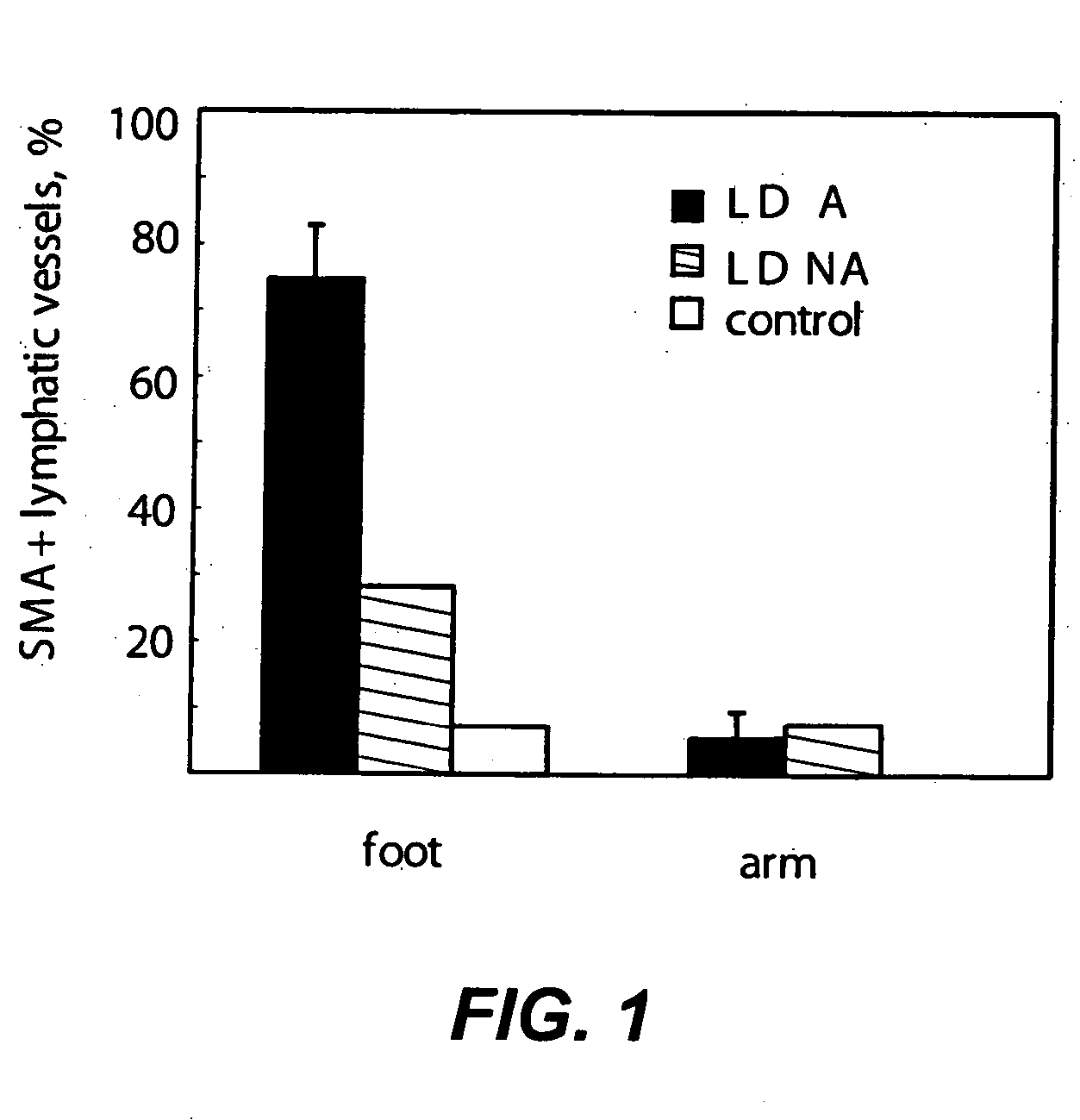
Compositions and methods for treatment of lymphatic and venous vessel arterialization
InactiveUS20060025338A1Promote healingReduce edemaOrganic active ingredientsVirusesSmooth muscleLymphatic vessel
The present invention is directed to methods and compositions that may be used in disrupting the association of smooth muscle cells with lymphatic endothelial cells and in correcting the valvular dysfunction in veins and lymphatic vessels. Such compositions are useful for therapeutic and prophylactic treatment of impaired lymphatic and venous function, particularly for the treatment of lymphedema distichiasis or chronic venous insufficiency.
Owner:VEGENICS PTY LTD
Methods, Devices and Systems for Treating Venous Insufficiency
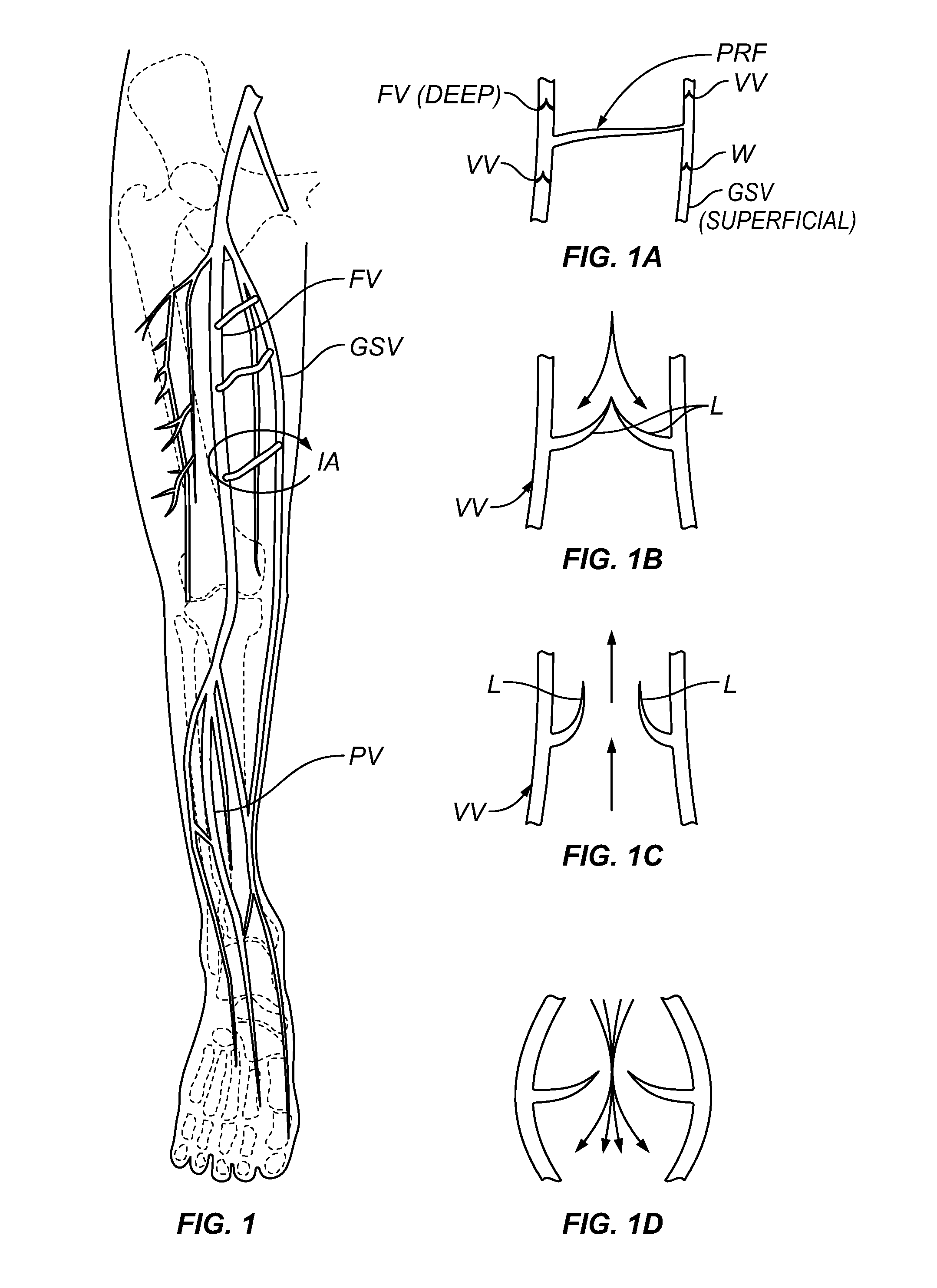
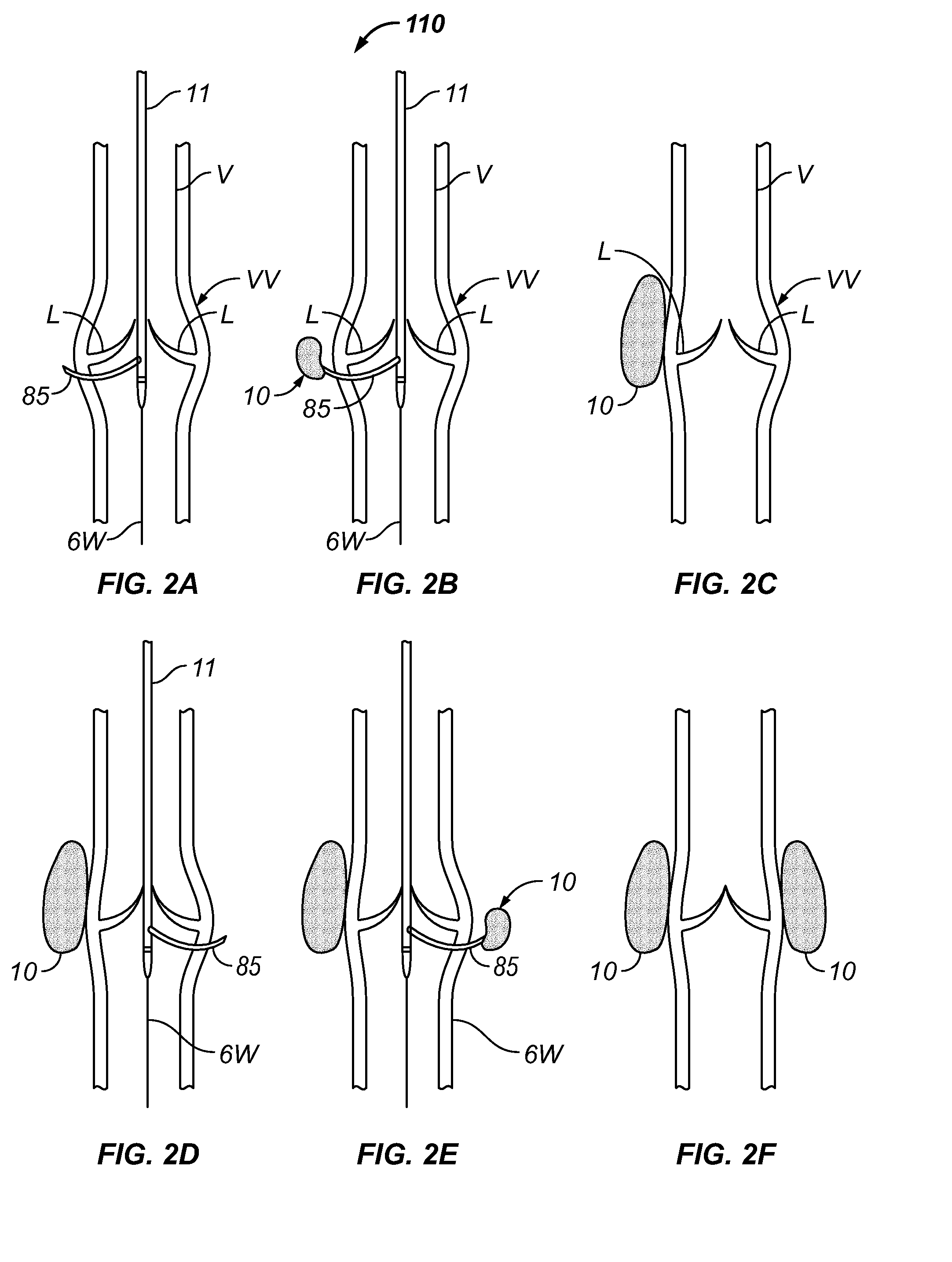
Methods and systems for improving the competency of a venous valve wherein one or more compressor(s) (e.g., space occupying material(s) or implantable device(s)) is / are delivered to one or more location(s) adjacent to a venous valve to compress the venous valve in a manner that causes one or both leaflets of the valve to move toward the other, thereby improving closure or coaptation of the valve leaflets. The compressor(s) may be delivered by an open surgical approach, by a direct percutaneous approach or by a transluminal catheter-based approach.
Owner:MEDTRONIC VASCULAR INC
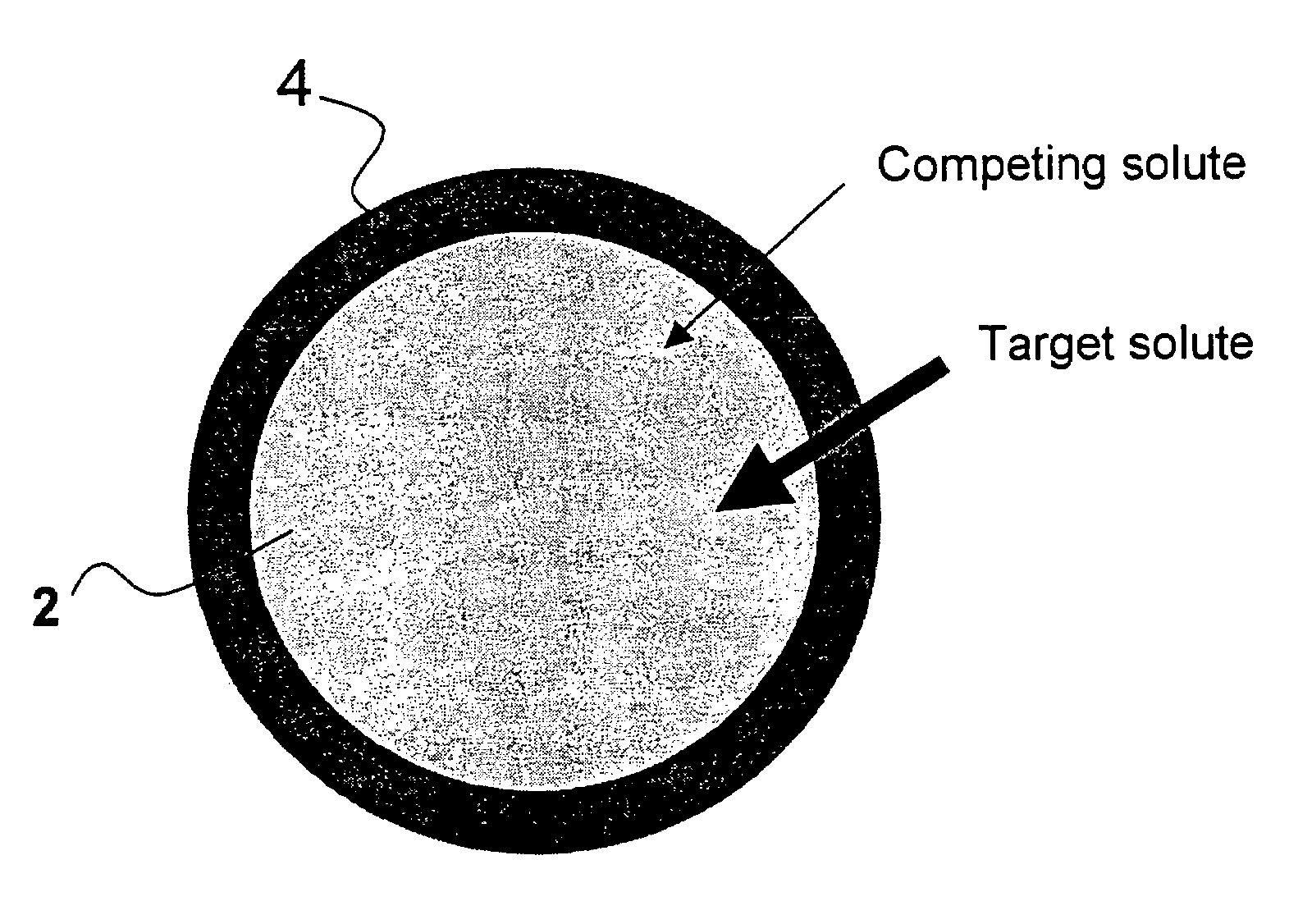
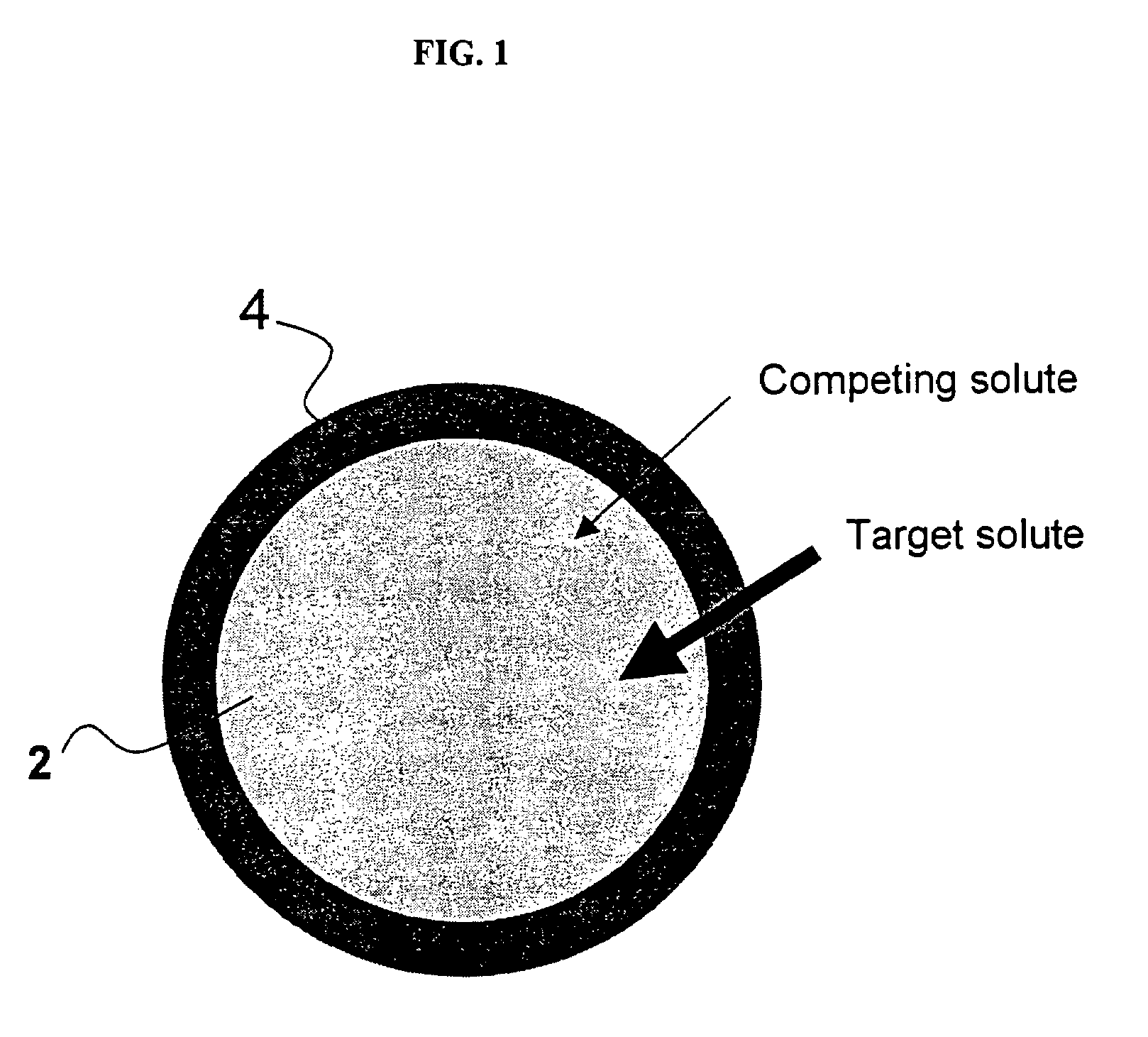
Ion binding compositions
ActiveUS8192758B2Powder deliveryGranular deliveryEnd stage renal diseaseChronic venous insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR (INT) AG
Composition comprising an aqueous extract of red vine leaves and a diuretic
InactiveUS20050142236A1Preventing and alleviating discomfortMinimum and adverse reactionBiocideUnknown materialsMedicineChronic venous insufficiency
This invention relates to a new composition containing the effective dosage of an aqueous extract of red vine leaves (1) and a diuretic (2) for preventing or alleviating the discomfort associated with mild-to-moderate chronic venous insufficiency of the legs. The compositions according to this invention may also contain pharmaceutically or dietetically acceptable additives.
Owner:BOEHRINGER INGELHEIM INT GMBH
Ion binding compositions
ActiveUS8282960B2Powder deliveryGranular deliveryEnd stage renal diseaseChronic venous insufficiency
Owner:VIFOR (INT) AG
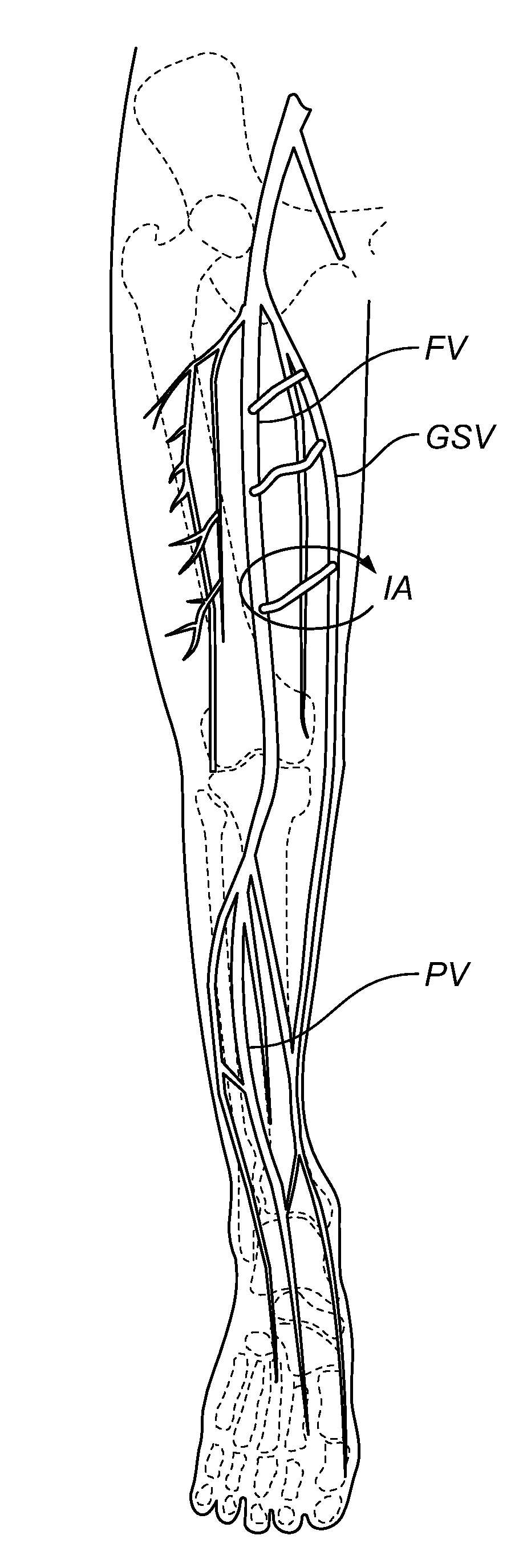
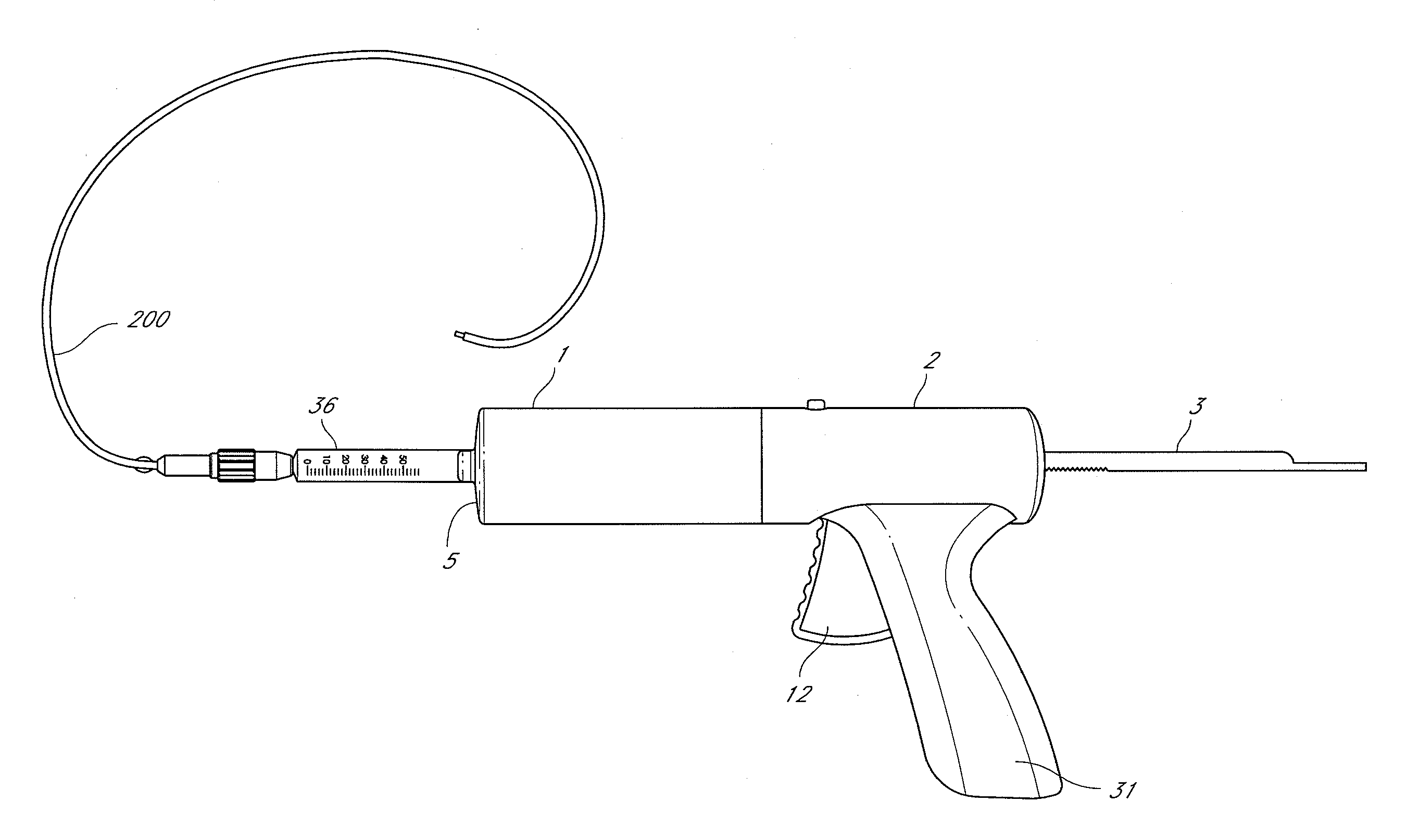
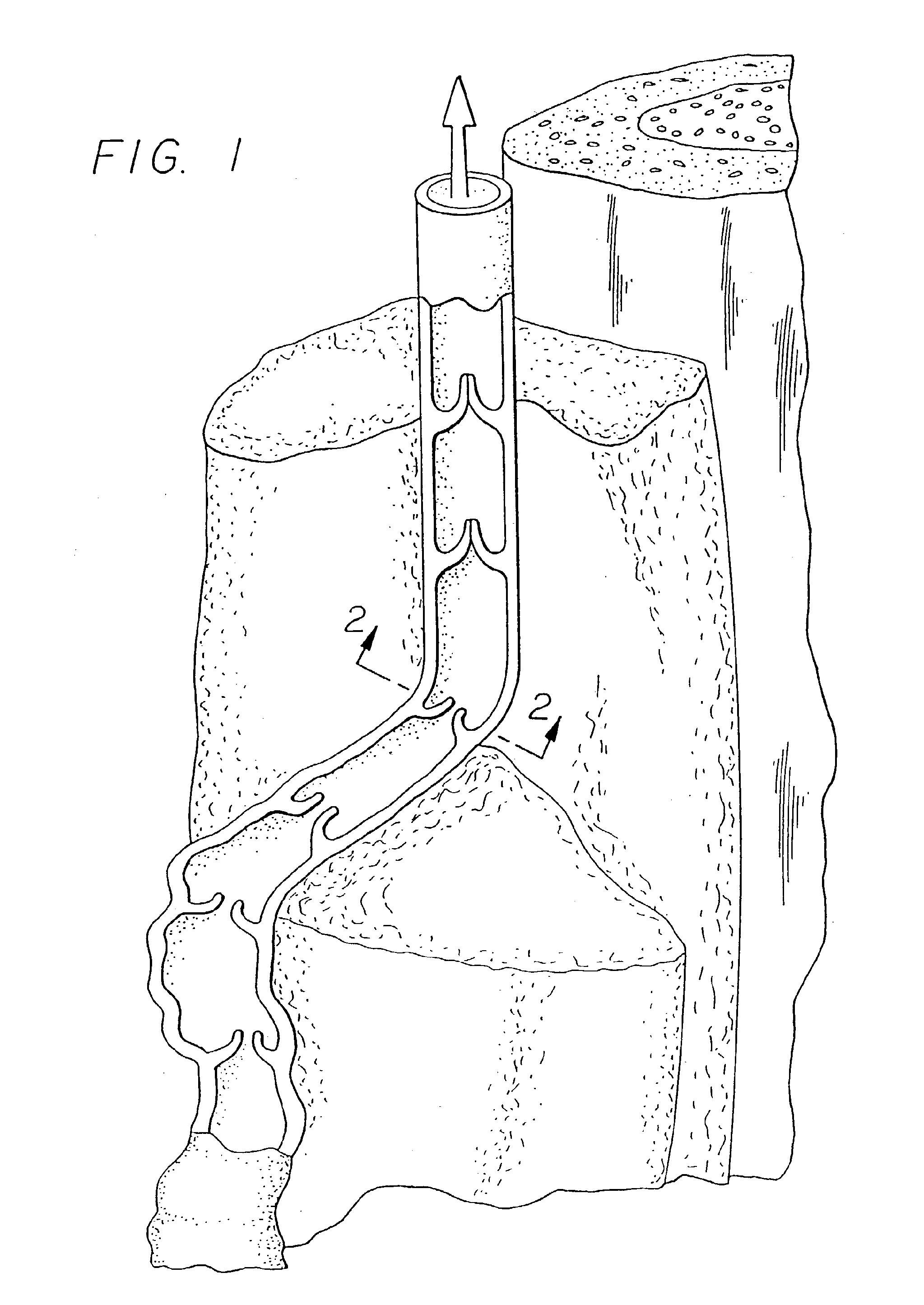
Systems and methods for treatment of perforator veins for venous insufficiency
Systems and methods for the treatment of perforator veins for venous insufficiency are described. The systems can include a catheter assembly comprising a proximal hub, a spin lock on the proximal hub, a elongate body overmolded to the proximal hub, and a distal end, the catheter, the elongate body configured to be placed within a perforator vein; an extension tubing having a proximal female hub, a distal male hub, and an elongate body therebetween, the distal male hub having a spin lock thereon, the distal male hub configured to be attached to the proximal hub of the catheter assembly; a syringe filled with a volume of media including cyanoacrylate; and an injector configured to automatically dispense a bolus of the media sufficient to coapt the perforator vein from the syringe upon actuation of a control on the injector. Methods are also disclosed.
Owner:TYCO HEALTHCARE GRP LP
Composition comprising an aqueous extract of red vine leaves and a blood circulation-improving agent
InactiveUS20050202110A1Preventing and alleviating discomfortMinimum and adverse reactionBiocideUnknown materialsMedicineChronic venous insufficiency
This invention relates to a new composition containing the effective dosage of an aqueous extract of red vine leaves (1) and a blood circulation-improving agent (2) for preventing or alleviating the discomfort associated with mild-to-moderate chronic venous insufficiency of the legs. The compositions according to this invention may also contain pharmaceutically or dietetically acceptable additives.
Owner:BOEHRINGER INGELHEIM INT GMBH
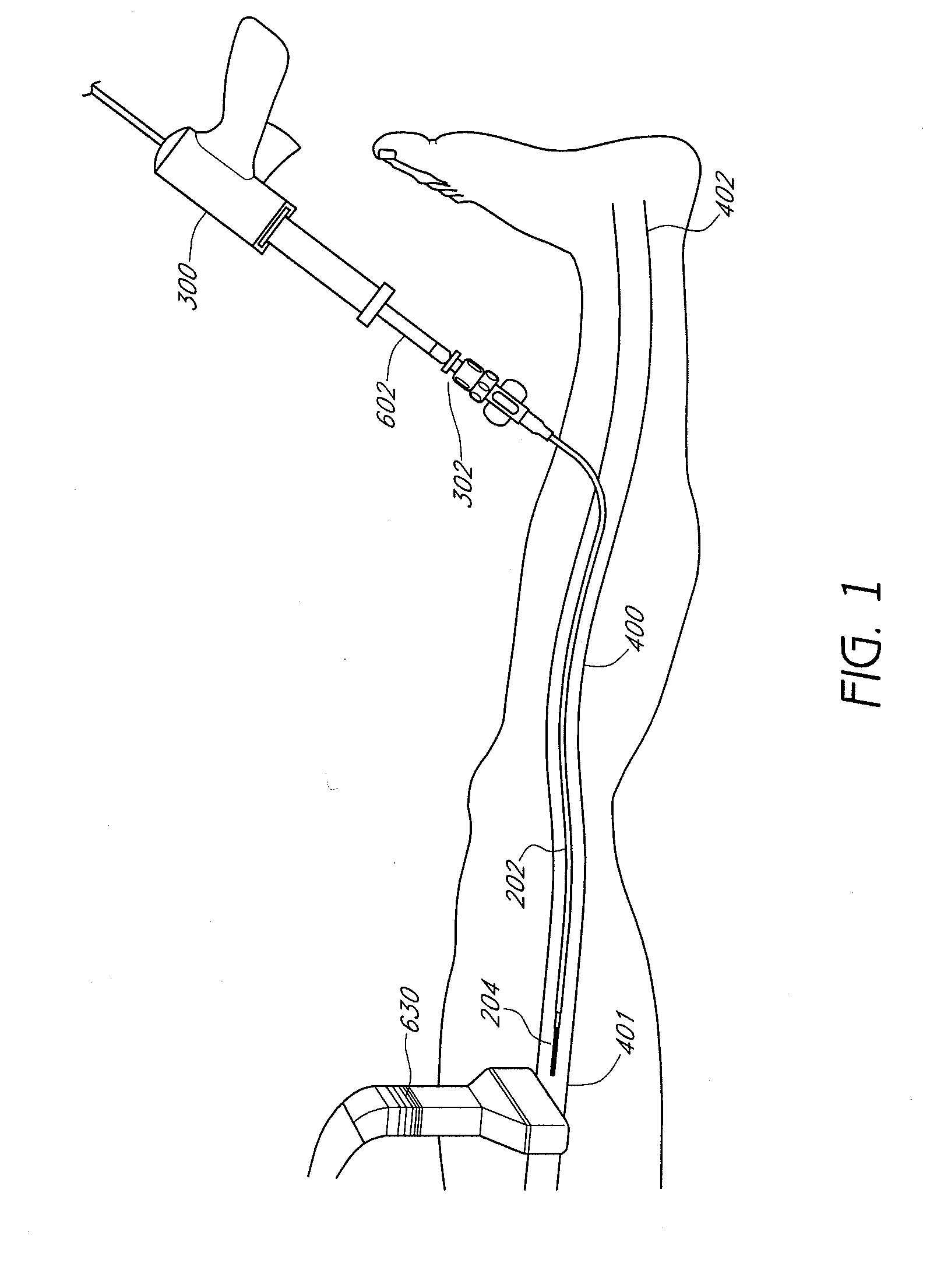
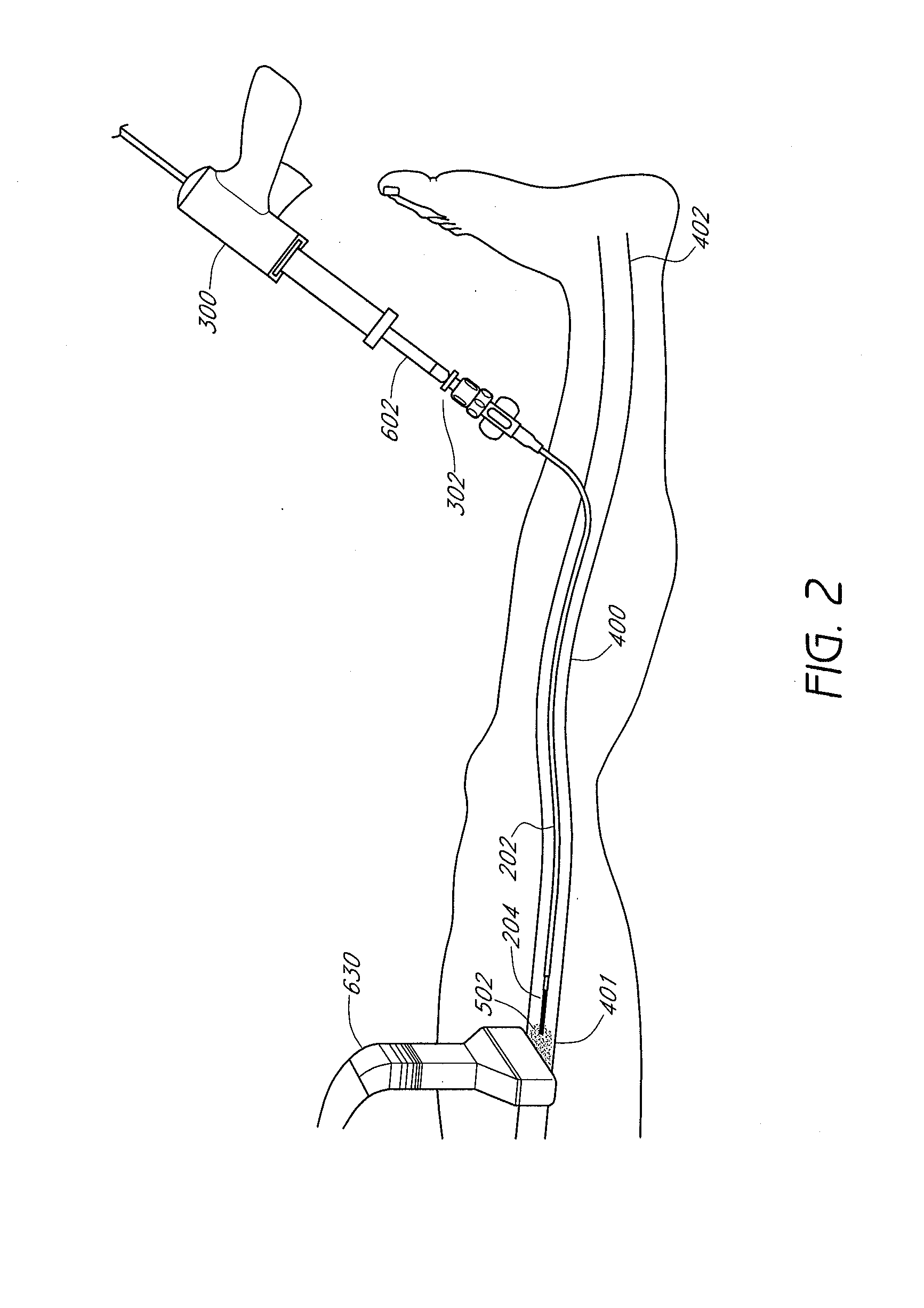
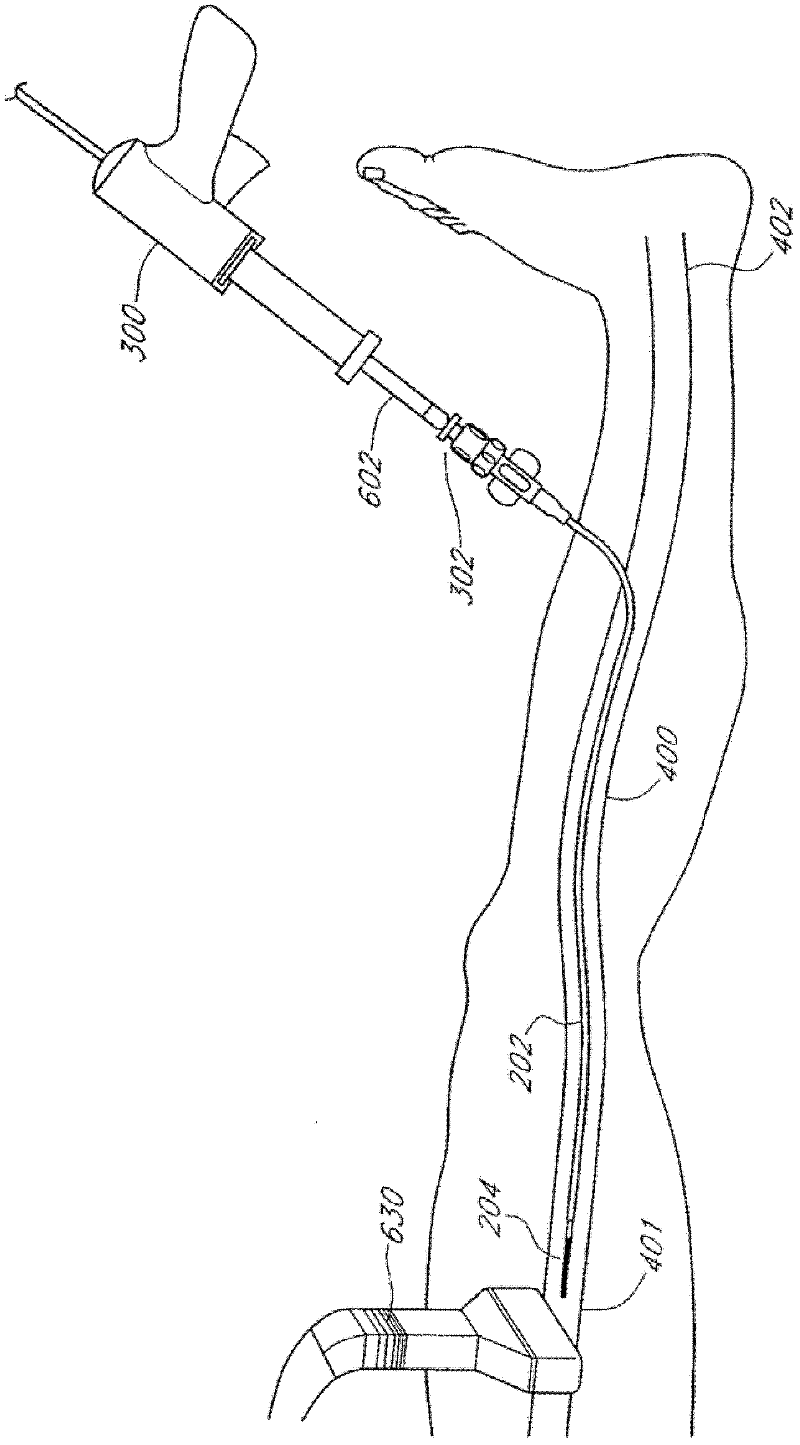
Methods and devices for venous occlusion for treatment of venous insufficiency
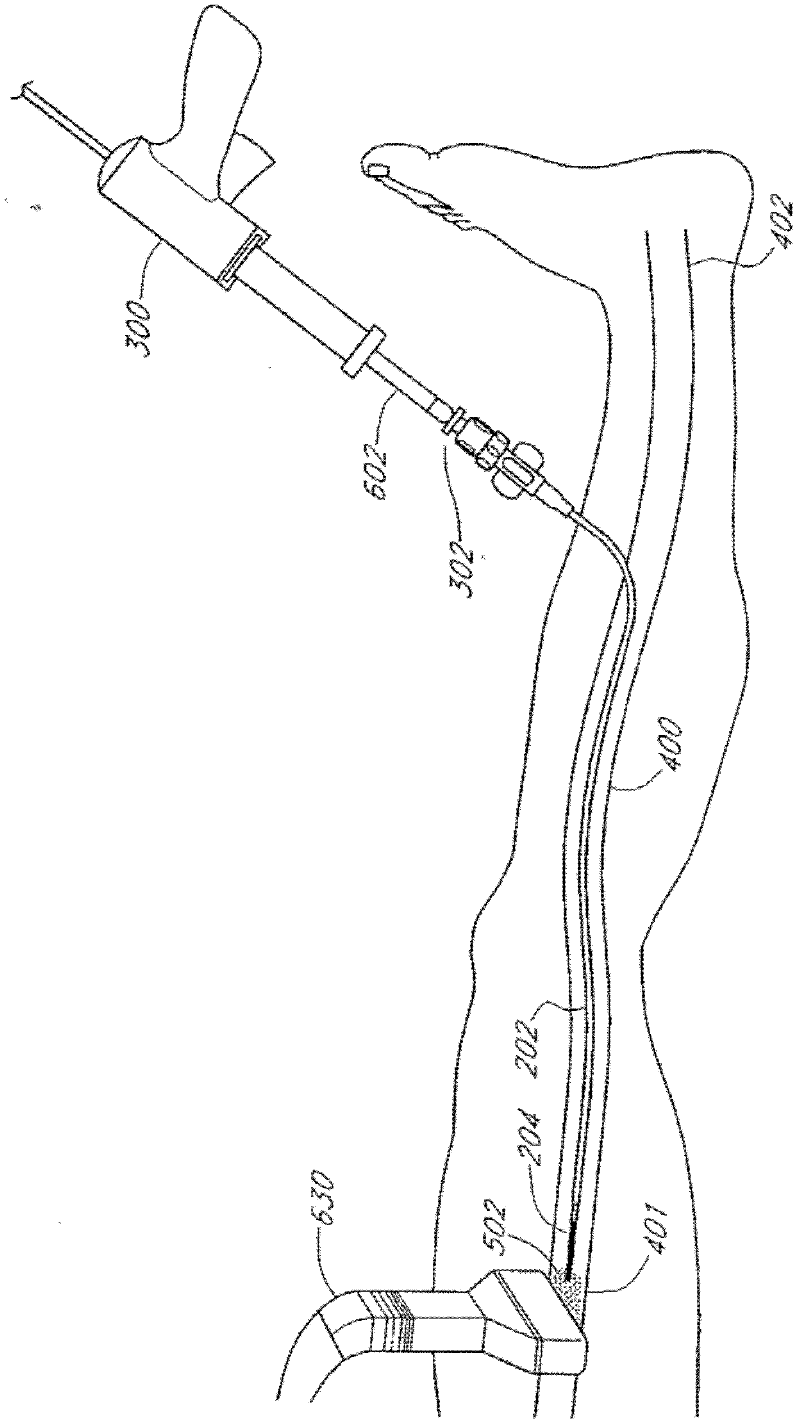
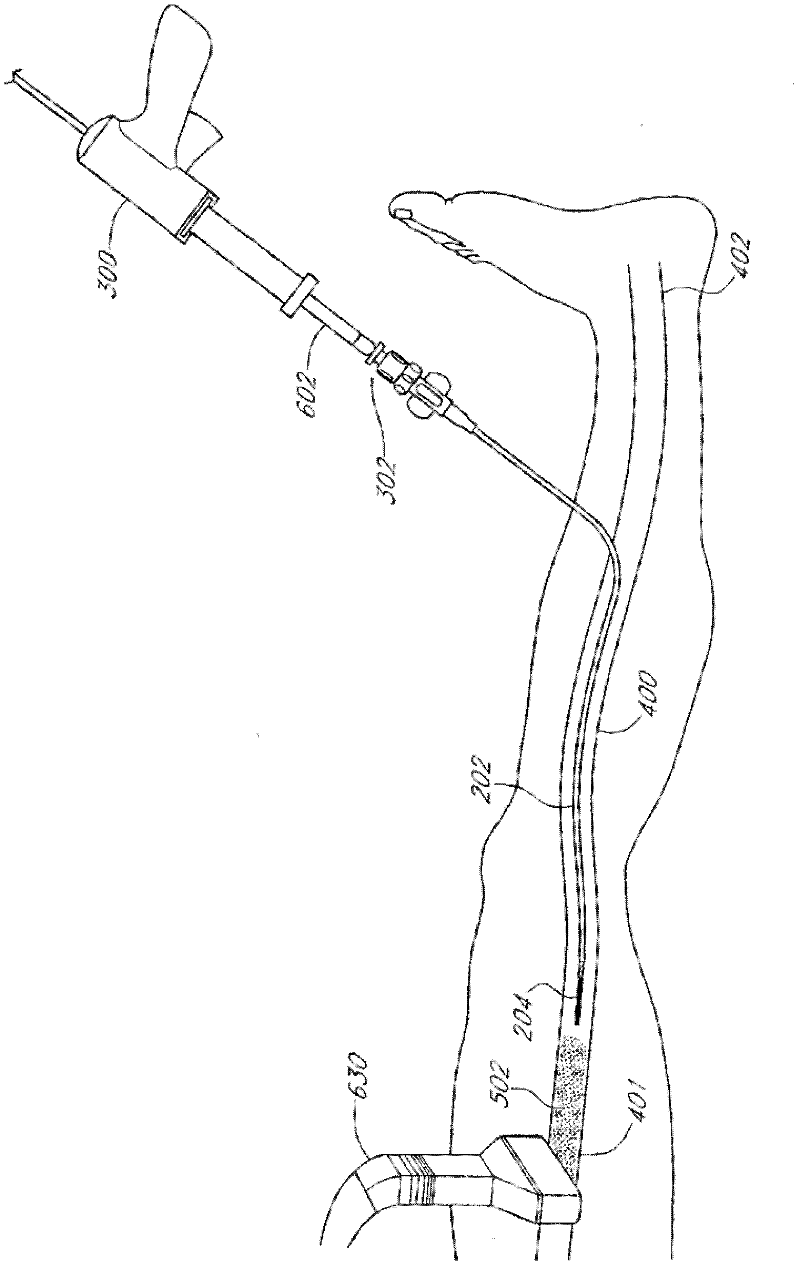
Methods, devices and systems are described for treating venous insufficiency in which the vein is compressed at least partially along a treatment zone. A system can be provided including an injection device (300), such as a glue gun, that is operably connected to a delivery catheter (202) that can be advanced across a treatment zone in the vein (400). The delivery catheter can be used to deliver one, two, or more boluses of media (V2, V2') (e.g., cyanoacrylate) to occlude the vein (400) along different spaced-apart sections of the treatment zone. External compression can also be applied to the vein (400) by a compression element, such as a hand (640) or multifunctional ultrasound transducer (630), to occlude portions of the vein (400) along the treatment zone prior to or during the introduction of the boluses of media (V2. V2').
Owner:TYCO HEALTHCARE GRP LP
Ion binding compositions
ActiveUS7429394B2Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic venous insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR (INT) AG
External composition comprising an aqueous extract of red vine leaves and an ant-inflammatory agent
ActiveUS20050271757A1Potentiate of relaxationPreventing and relaxing chronic venousBiocideUnknown materialsMedicineChronic venous insufficiency
This invention relates to a new composition containing the effective concentration of an aqueous extract of red vine leaves (1) and an anti-inflammatory agent (2) for preventing or alleviating the discomfort associated with mild-to-moderate chronic venous insufficiency of the legs. The compositions according to this invention may also contain pharmaceutically or cosmically acceptable additives.
Owner:BOEHRINGER INGELHEIM INT GMBH
Composition comprising an aqueous extract of red vine leaves and an antithrombotic agent
InactiveUS20050142235A1Preventing and alleviating discomfortEffective internal compositionBiocideSalicyclic acid active ingredientsAntithrombotic AgentMedicine
This invention relates to a new composition containing the effective dosage of an aqueous extract of red vine leaves (1) and an antithrombotic agent (2) for preventing or alleviating the discomfort associated with mild-to-moderate chronic venous insufficiency of the legs. The compositions according to this invention may also contain pharmaceutically or dietetically acceptable additives.
Owner:BOEHRINGER INGELHEIM INT GMBH
Chinese medicine for treating chronic kidney disease
InactiveCN101024053AGood treatment effectHeat-clearing and detoxifyingUrinary disorderPlant ingredientsDiseaseCooked Rehmannia Root
The present invention discloses a Chinese medicine for effectively curing chronic nephrosis with obvious therapeutic effect. Said Chinese medicine is made up by using 12 Chinese medicinal materials of cooked rehmannia root, Chinese yam, moutan root bark, alisma tuber, poria, hedyotis and others through a certain preparation process. Said Chinese medicine has the functions of improving renal function, regulating lipometabolism, reducing urinary protein and raising immunity of body, so that it can be effectively used for curing the diseases of chronic renal insufficiency and renal failure, etc.
Owner:郑红梅
Composition for treating chronic venous insufficiencies using an extract of red vine leaves
InactiveUS6991816B2Preventing and alleviating discomfortPreventing and alleviatingOrganic active ingredientsBiocideVeinDietary supplement
The invention relates to a dietary supplement consisting of an aqueous extract of red vine leaves and an acceptable carrier which prevents and reduces the discomfort relating to mild-to-moderate chronic venous insufficiency of the legs.
Owner:BOEHRINGER INGELHEIM INT GMBH
Soluble guanylate cyclase activators
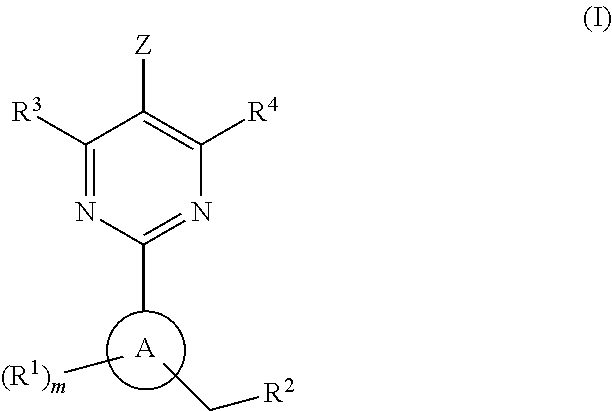
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Apparatus for treating venous insufficiency
InactiveUS20100106156A1Less invasiveEmit energyDilatorsSurgical instruments for heatingVeinInvasive treatments
A catheter delivers an electrode within a vein for a minimally invasive treatment of varicose veins and venous insufficiency using RF energy. The catheter is introduced into a patient and positioned within the section of the vein to be treated. The electrode radiates high frequency energy towards the vein, and the surrounding venous tissue becomes heated and begins to shrink. The catheter includes a controllable member for limiting the amount of shrinkage of the vein to the diameter of the member. The electrode remains active until there has been sufficient shrinkage of the vein. The extent of shrinkage of the vein may be detected by fluoroscopy. After treating one section of the vein, the catheter and the electrode can be repositioned intraluminally within the vein to treat different sections of the vein until all desired venous sections and valves are repaired and rendered functionally competent.
Owner:COVIDIEN LP
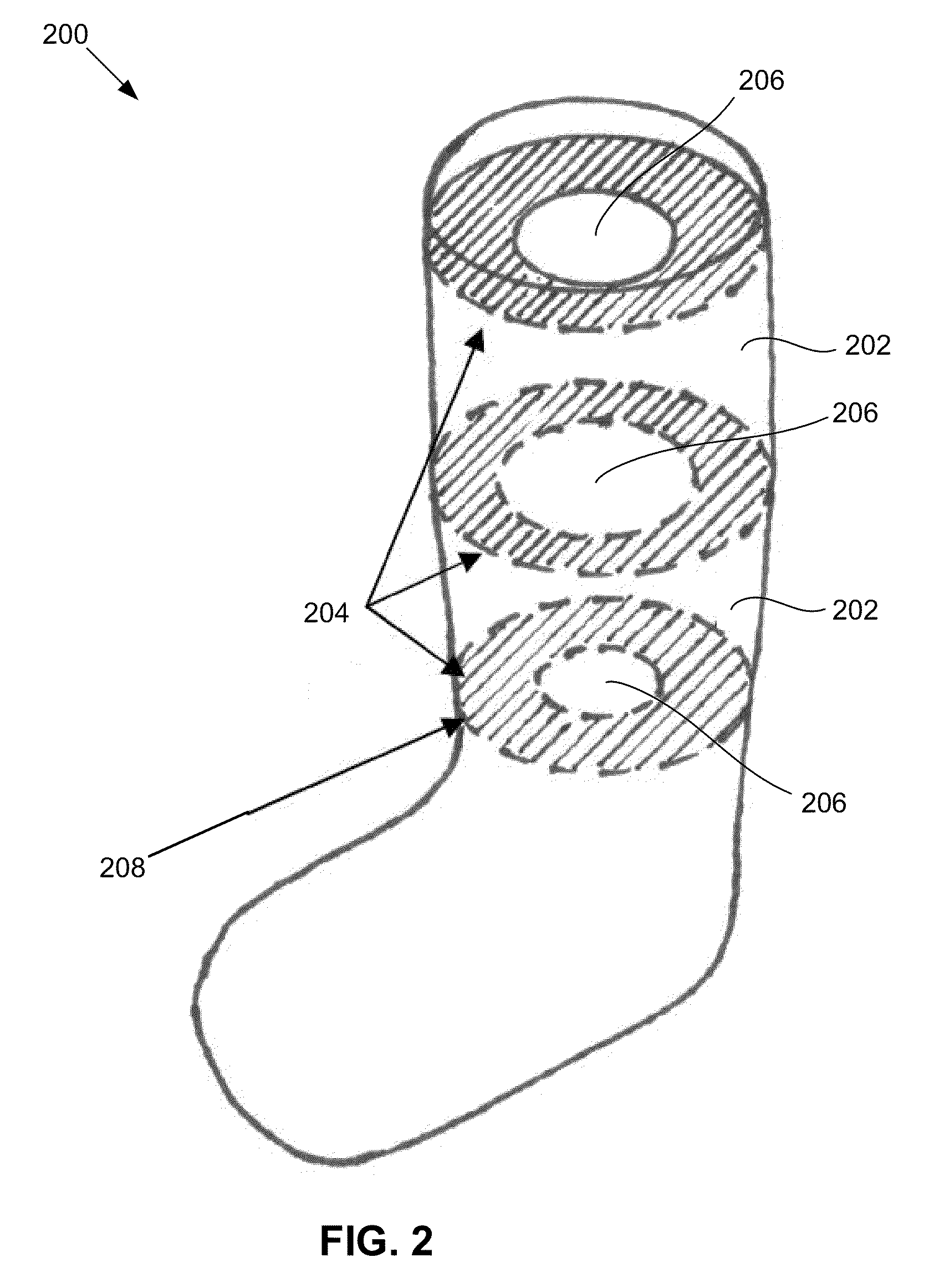
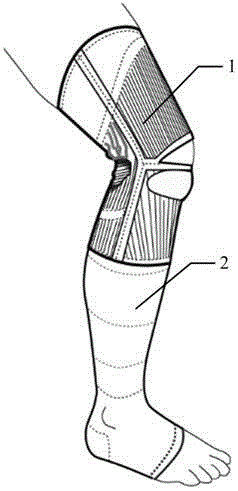
Pressure orthotics device
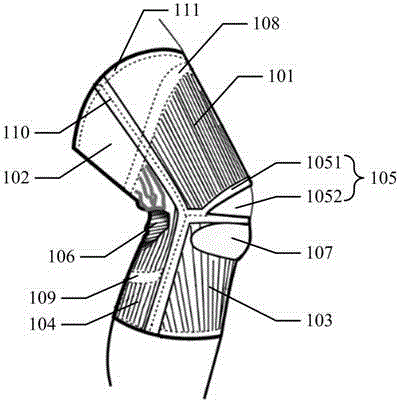
ActiveCN105996224AReduce tensionRelieve knee painProtective garmentPressure decreaseLower limb circumference
The embodiment of the invention discloses a pressure orthotics device. The pressure orthotics device comprises a knee guard part and a leg guard part, wherein the knee guard part and the leg guard part are of an integral type or a sleeving-wearing type; the knee guard part is woven by three-dimensional knitting, and forms a plurality of functional blocks which are differently stretched in a limb biaxial direction, apply support force or pressure on a knee joint and the periphery thereof, and are seamlessly connected; the leg guard part is woven by three-dimensional knitting, and forms a plurality of pressure blocks which produce pressures decreasing step by step from bottom to top in a lower limb circumference direction, and are seamlessly connected. The pressure orthotics device can be used for preventing and treating lower limb knee pain and chronic venous insufficiency; by means of systemic pressure management, the tensioned state of thigh muscles can be relieved, the knee joint and a flexion-extension angle thereof are controlled and stabilized, and lower limb blood circulation is promoted; besides, the woven blocks of the knee guard part and the leg guard part are respectively seamlessly connected, so the wearing comfort of the pressure orthotics device can be improved.
Owner:CHEMTAX IND
Method and Topical Formulation for Treating Localized Edema
ActiveUS20100063152A1Increase volumeIncrease the rate at which the metal ion sequestrant penetratesBiocidePeptide/protein ingredientsChronic venous insufficiencyMethylsulfonylmethane
Methods and formulations are provided for the treatment of localized edema, particularly localized edema resulting from chronic venous insufficiency. A metal ion sequestrant is topically administered to a subject afflicted with localized edema in combination with a permeation enhancer selected from methylsulfonylmethane and a combination of methylsulfonylmethane and dimethylsulfoxide. Topically administrable formulations for use in the aforementioned method are also provided.
Owner:LIVIONEX
Composition for treating chronic venous insufficiencies using an extract of red vine leaves
InactiveUS20060068043A1Preventing and alleviating discomfortPreventing and alleviatingBiocideOrganic active ingredientsDietary supplementChronic venous insufficiency
Owner:BOEHRINGER INGELHEIM INT GMBH
Traditional Chinese medicine composition for improving renal functions and restraining renal fibrosis
InactiveCN102475765ASignificant clinical effectImprove kidney functionUrinary disorderPlant ingredientsCreatinine riseAdditive ingredient
The invention discloses a traditional Chinese medicine composition for improving renal functions and restraining renal fibrosis. Active pharmaceutical ingredients of the traditional Chinese medicine composition comprise, by weight, 10-15 parts of the root of red-rooted salvia, 10-60 parts of astragalus membranaceus, 10-30 parts of herba epimedii, 5-30 parts of sinensis and 3-30 parts of prepared rhubarb. Clinical researches show that 168 patients with chronic renal insufficiency are treated by the traditional Chinese medicine composition, a remarkable clinical effect is obtained, clinical symptoms are reduced, the clinical symptoms of some patients are basically eliminated, the renal functions of the patients can be remarkably improved, blood urea nitrogen (BUN) and creatinine (Scr) are obviously reduced, and total effective rate achieves 88.66%. Animal researches show that the traditional Chinese medicine composition can delay development of chronic renal failure, and the mechanism of the traditional Chinese medicine composition is relative to effects of effectively restraining precipitation of renal tissue collagen and secretion of TGF (transforming growth factor)-beta, accordingly restraining renal fibrosis, and the like. The traditional Chinese medicine composition is low in clinical application cost and excellent in curative effect, and is safe and reliable.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
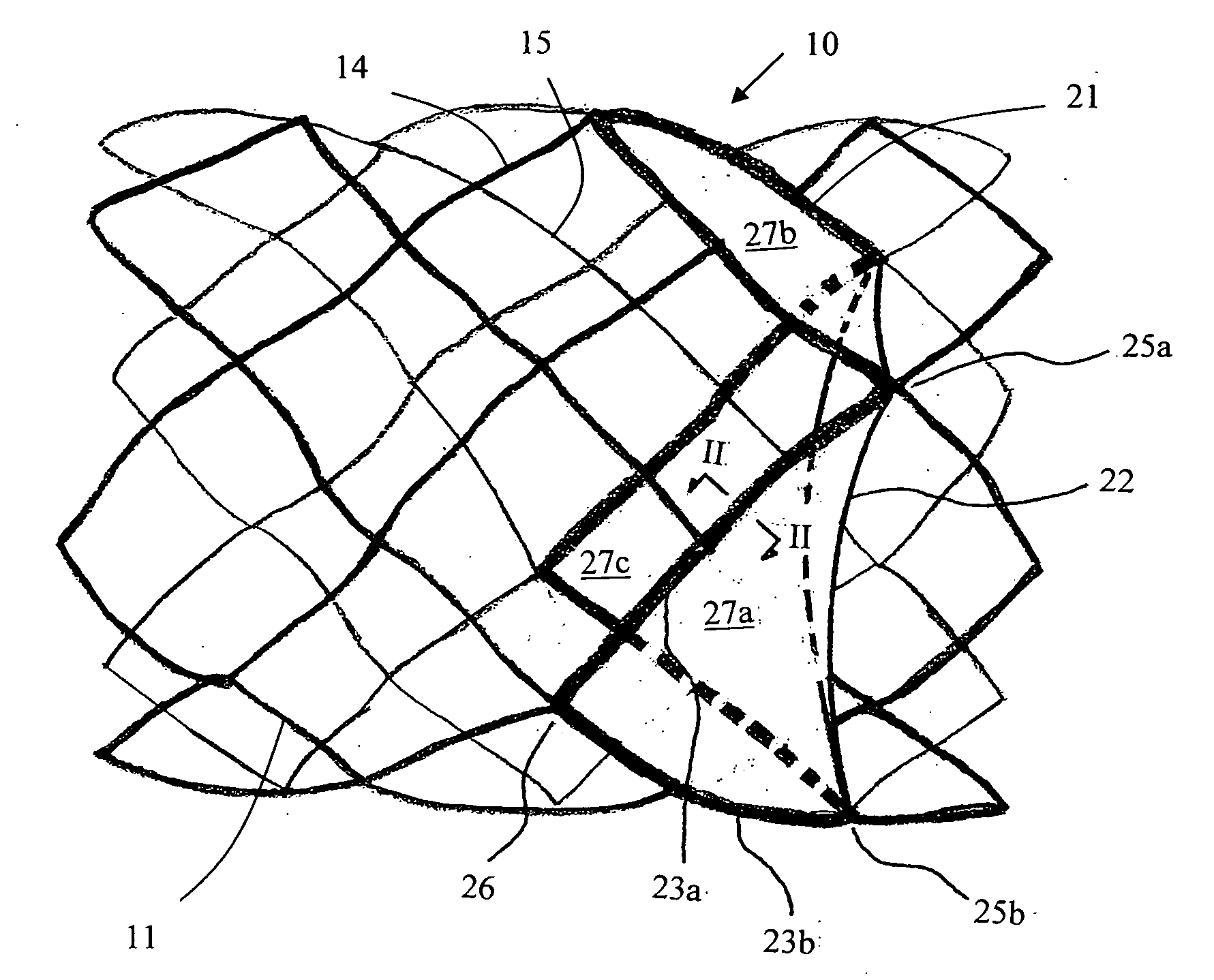
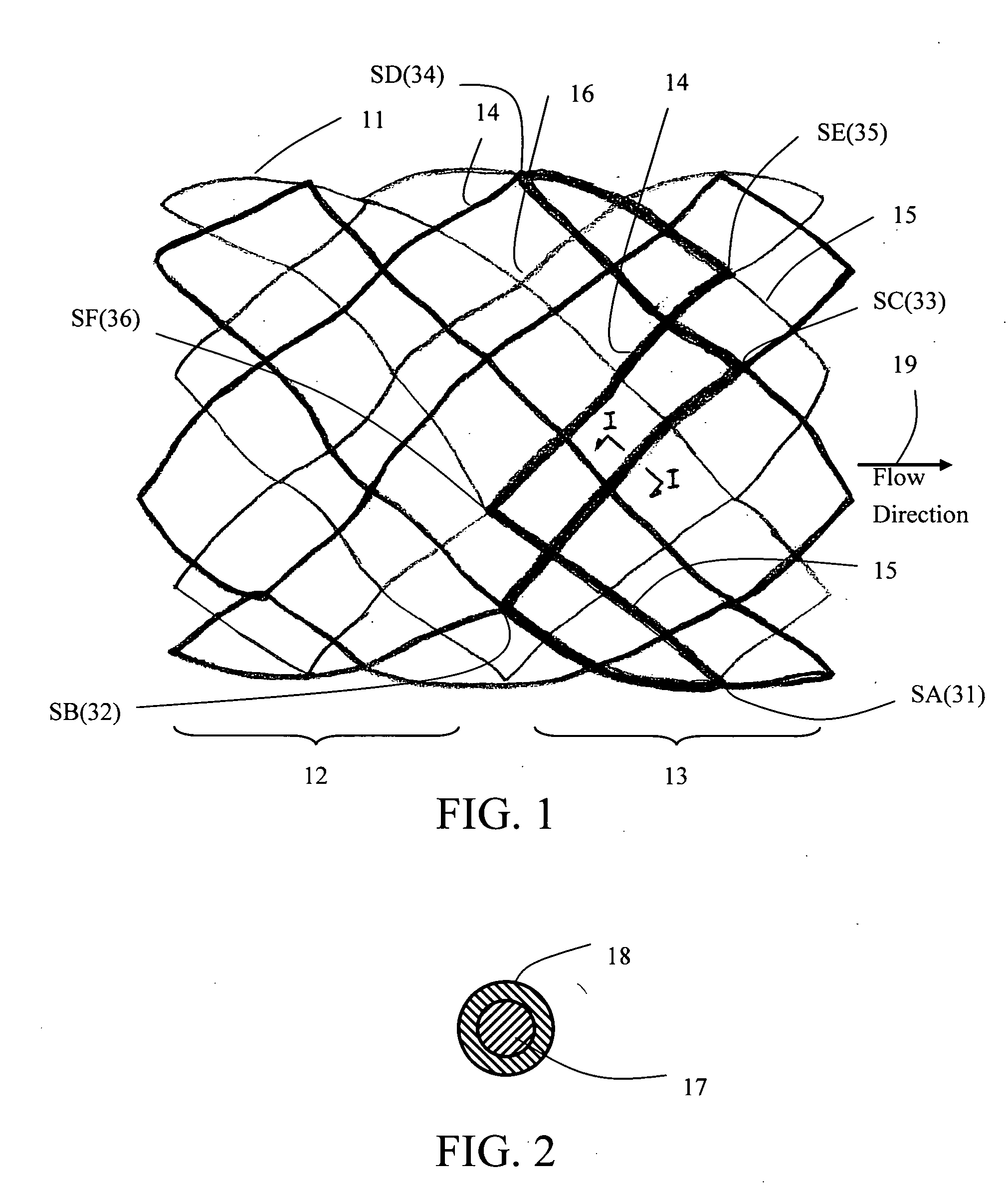
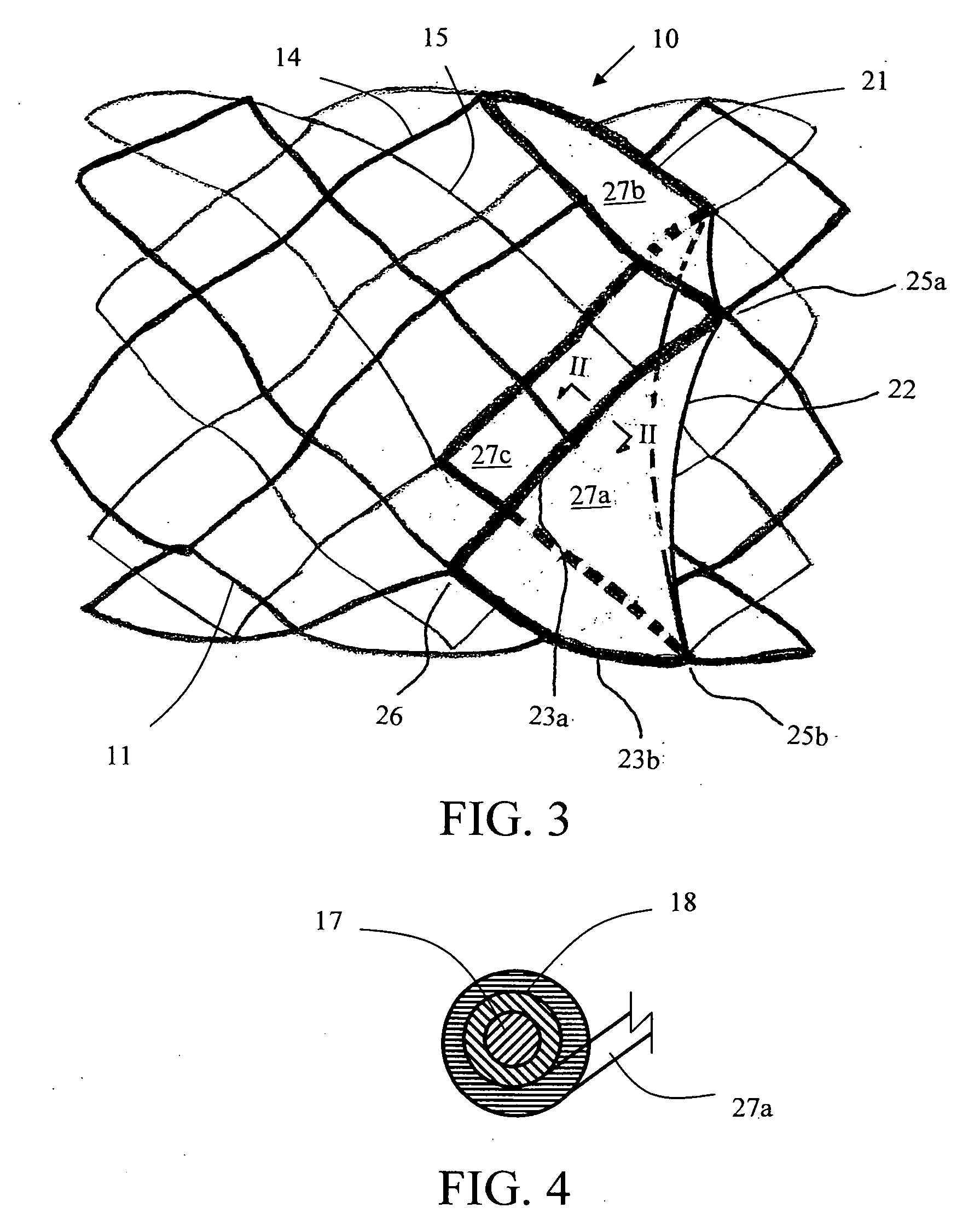
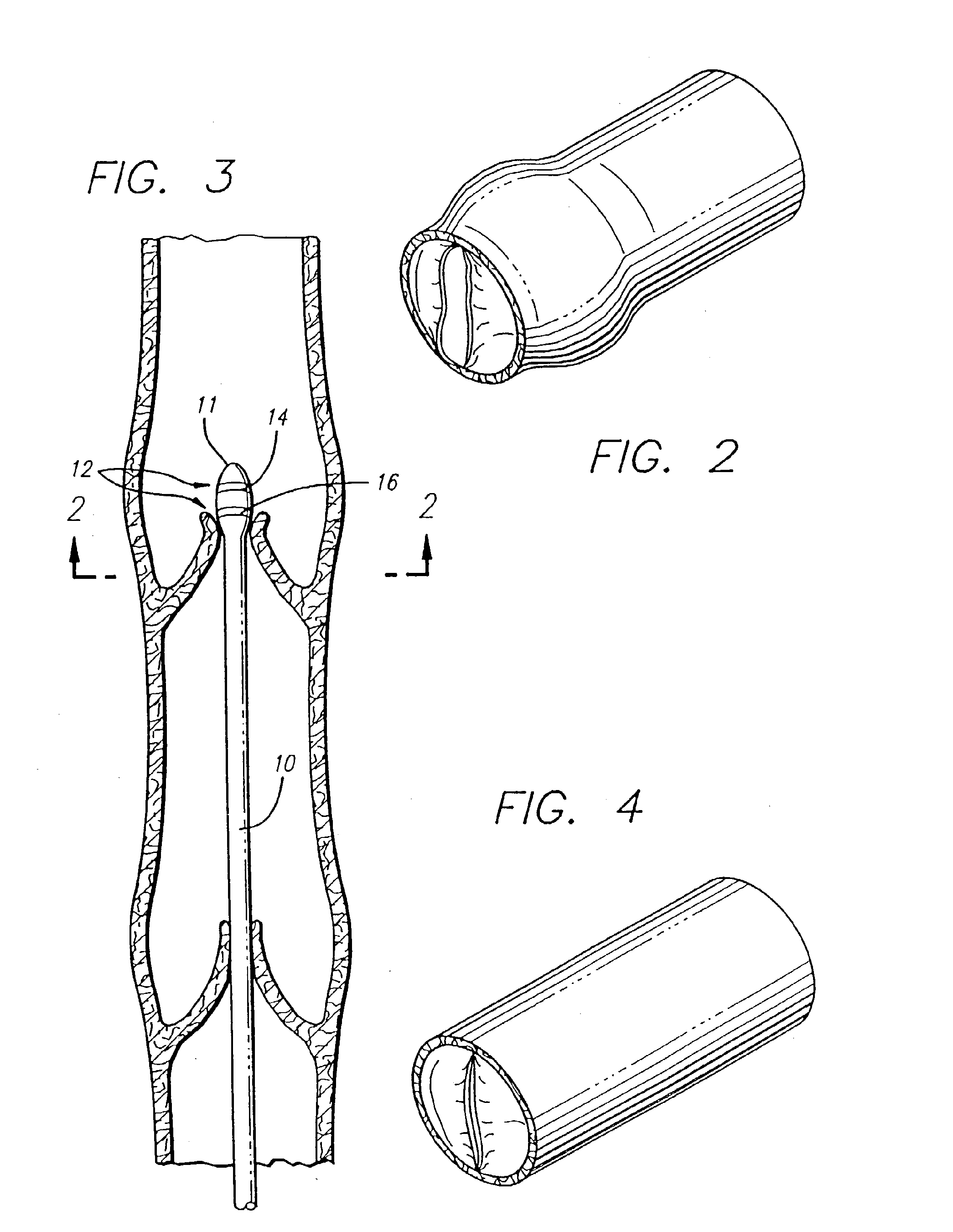
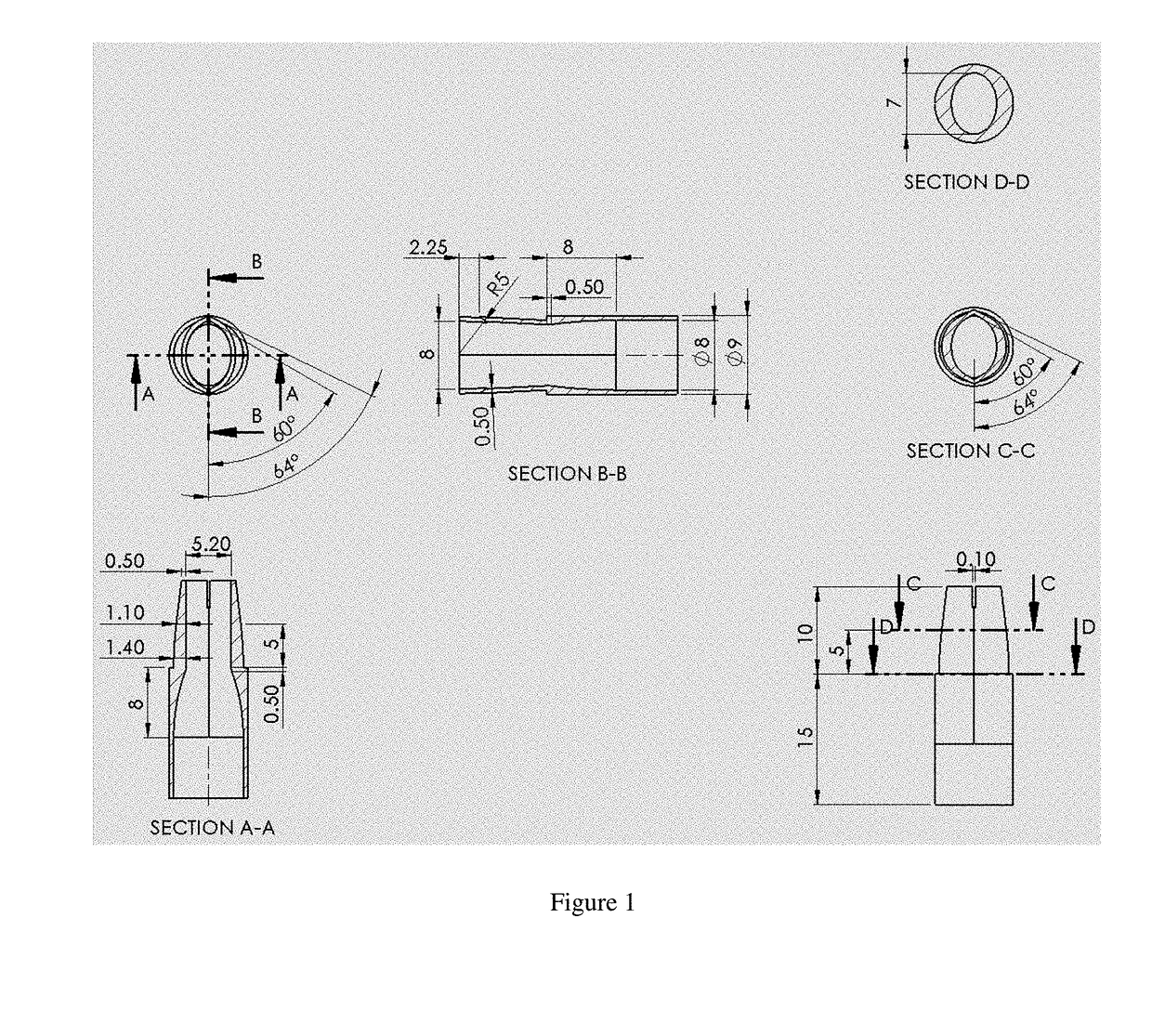
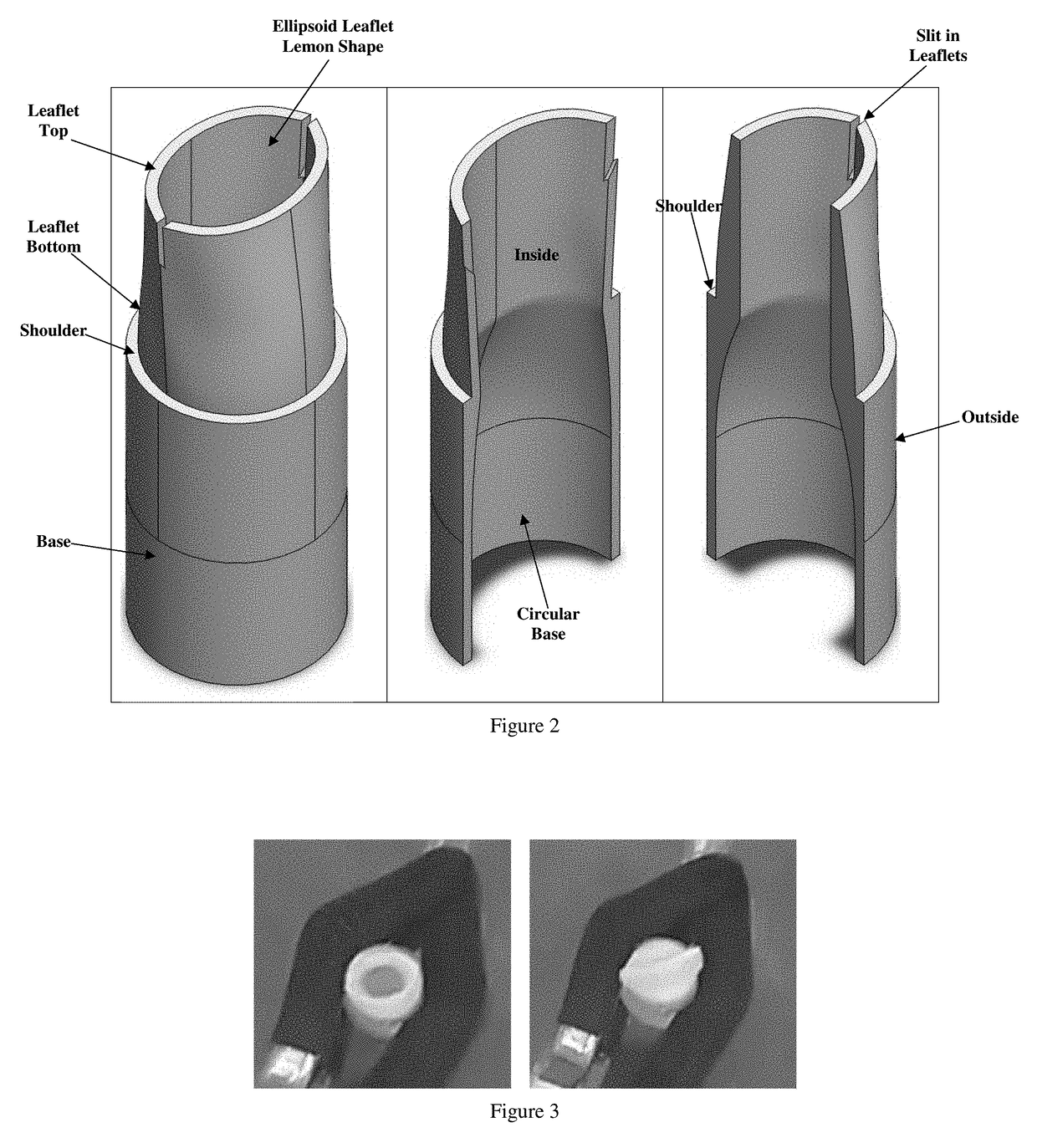
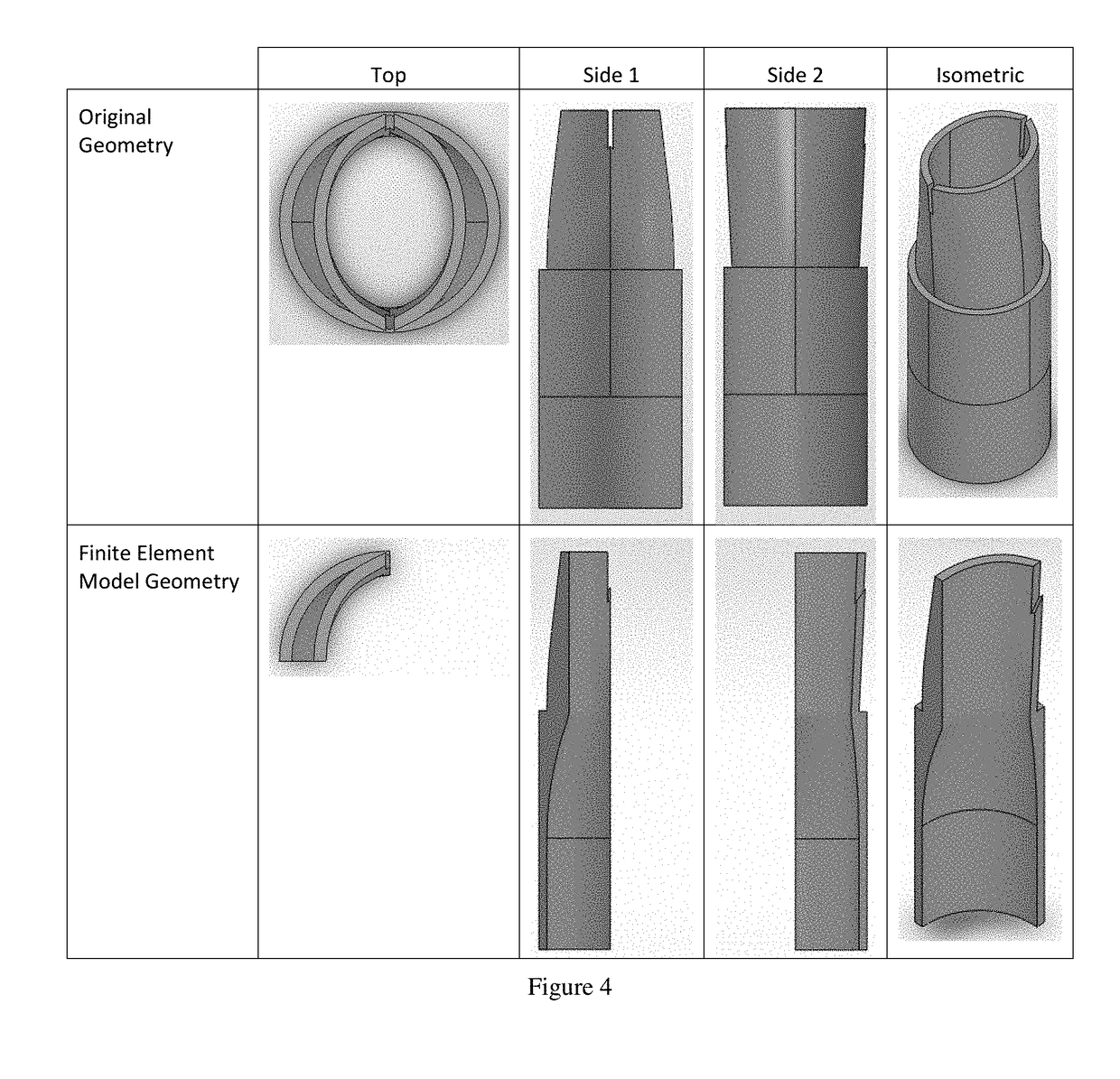
Implantable Open Vein Valve
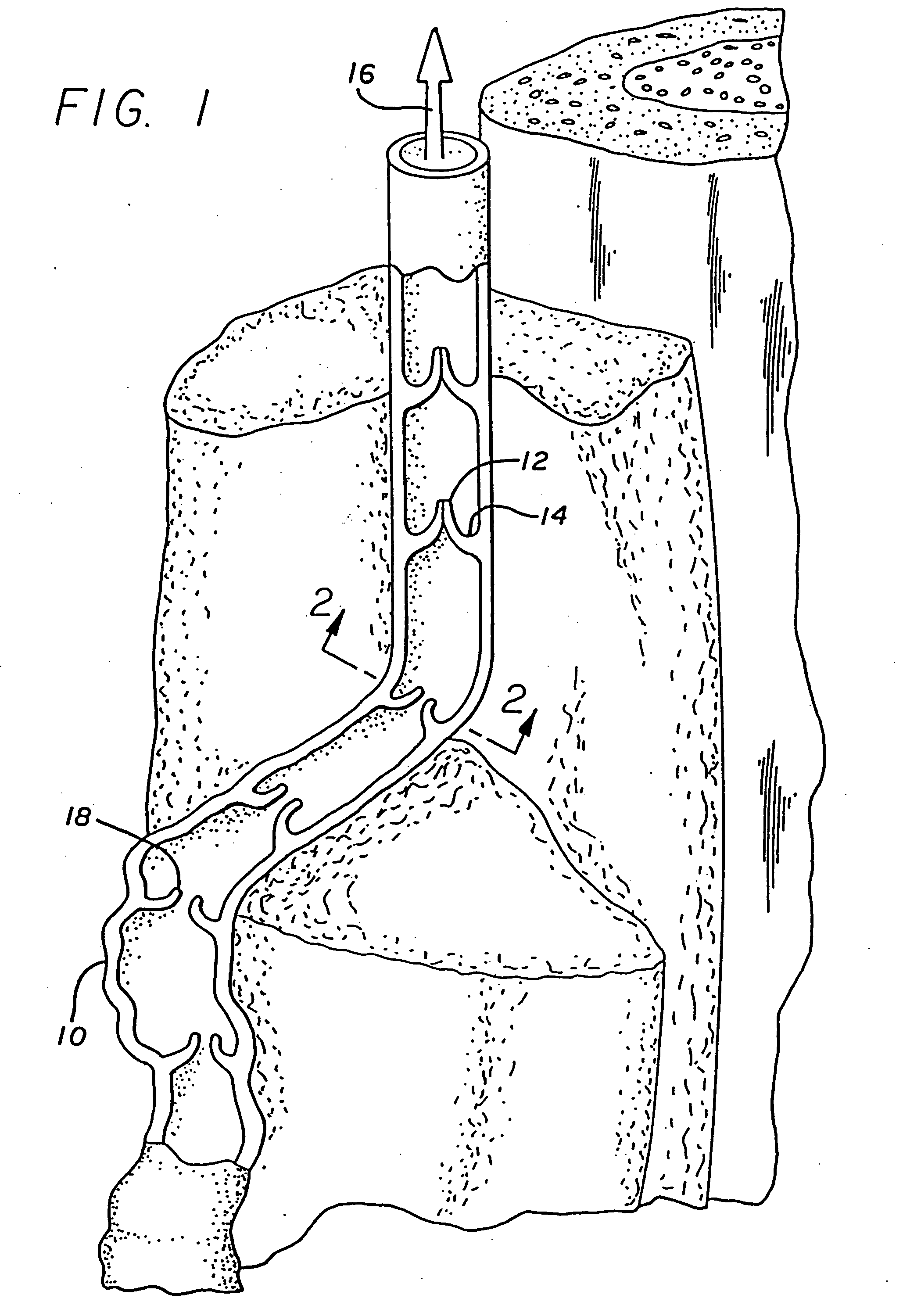
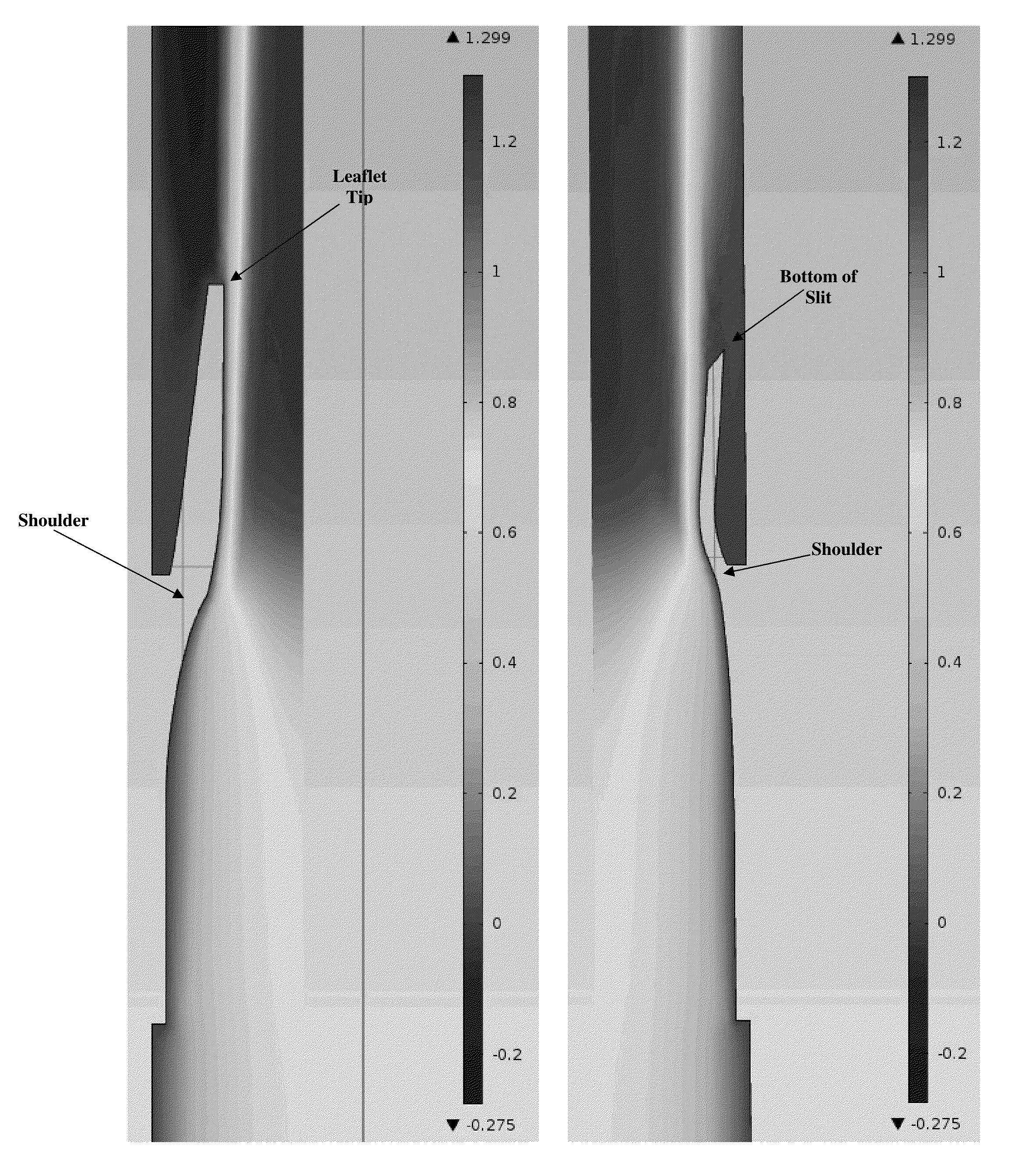
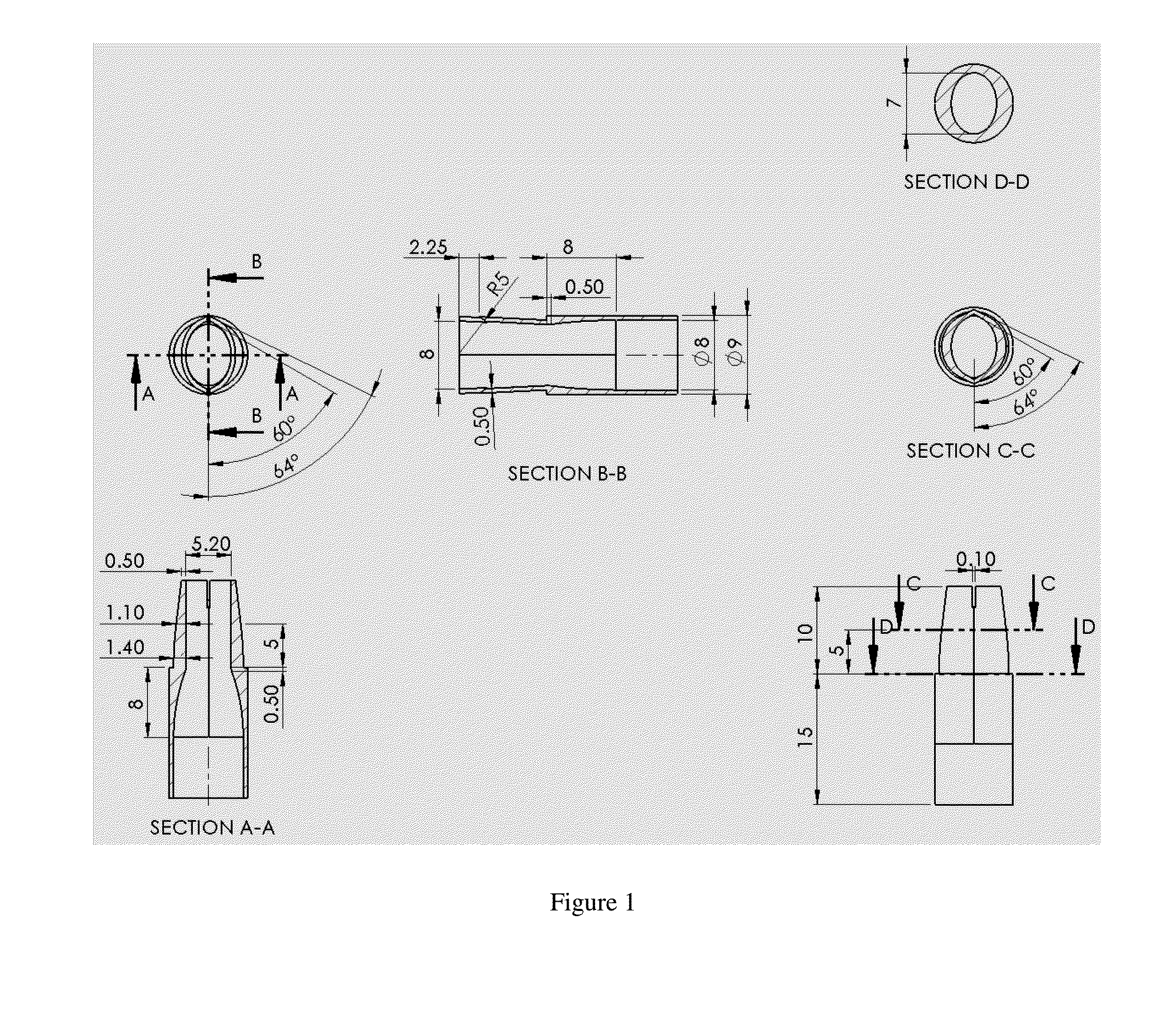
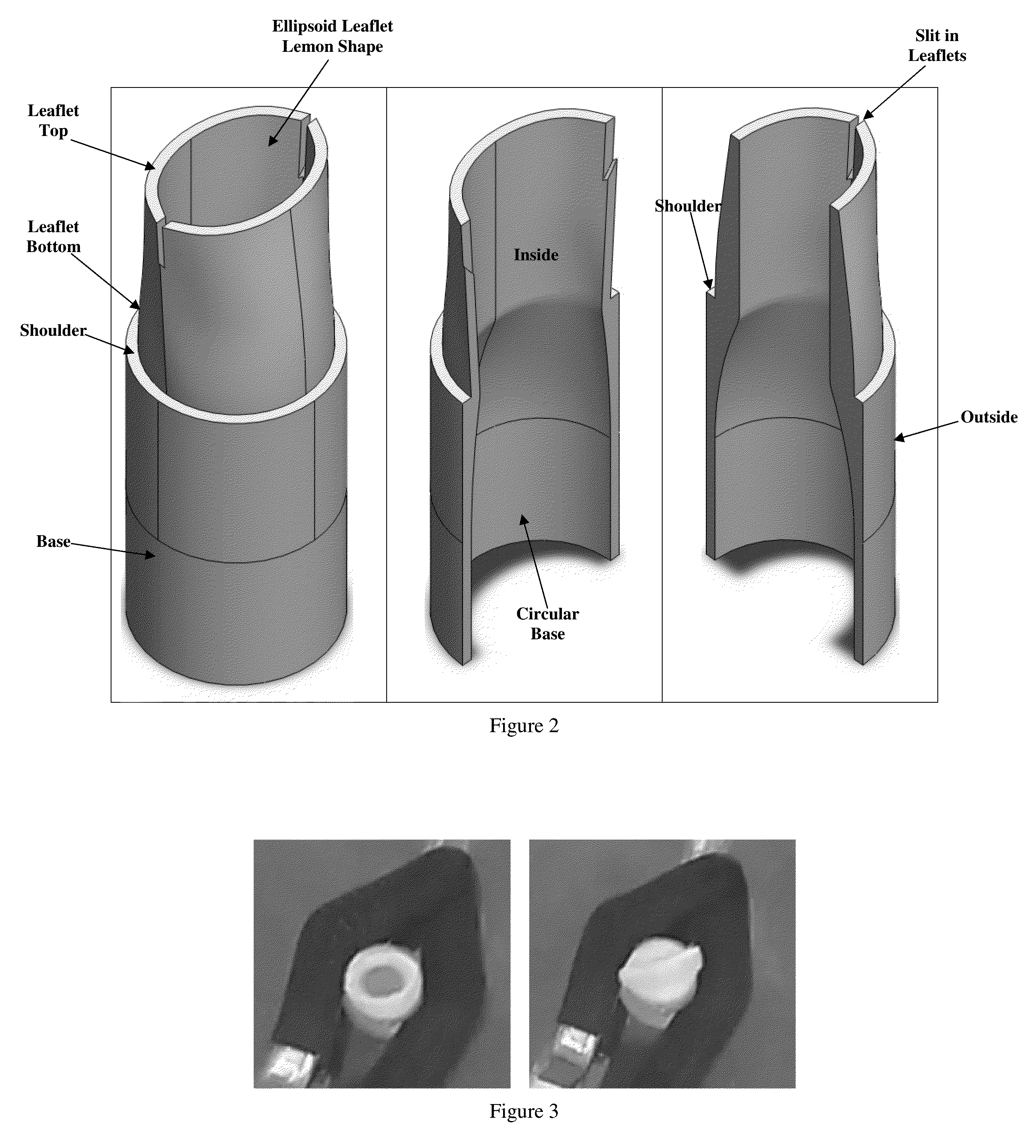
The invention provides prosthetic venous valves, and method of use thereof, for the effective treatment of individuals with venous reflux in chronic venous insufficiency (CVI). The development of such prosthetic venous valves in the areas of valve design, design specifications, verification and / or validation testing, computational analysis, valve placement and clinician guidance and procedure are provided. Manufacturing the prosthetic venous valves of the invention is also provided.
Owner:GEORGIA TECH RES CORP
Implantable open vein valve
The invention provides prosthetic venous valves, and method of use thereof, for the effective treatment of individuals with venous reflux in chronic venous insufficiency (CVI). The development of such prosthetic venous valves in the areas of valve design, design specifications, verification and / or validation testing, computational analysis, valve placement and clinician guidance and procedure are provided. Manufacturing the prosthetic venous valves of the invention is also provided.
Owner:GEORGIA TECH RES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com