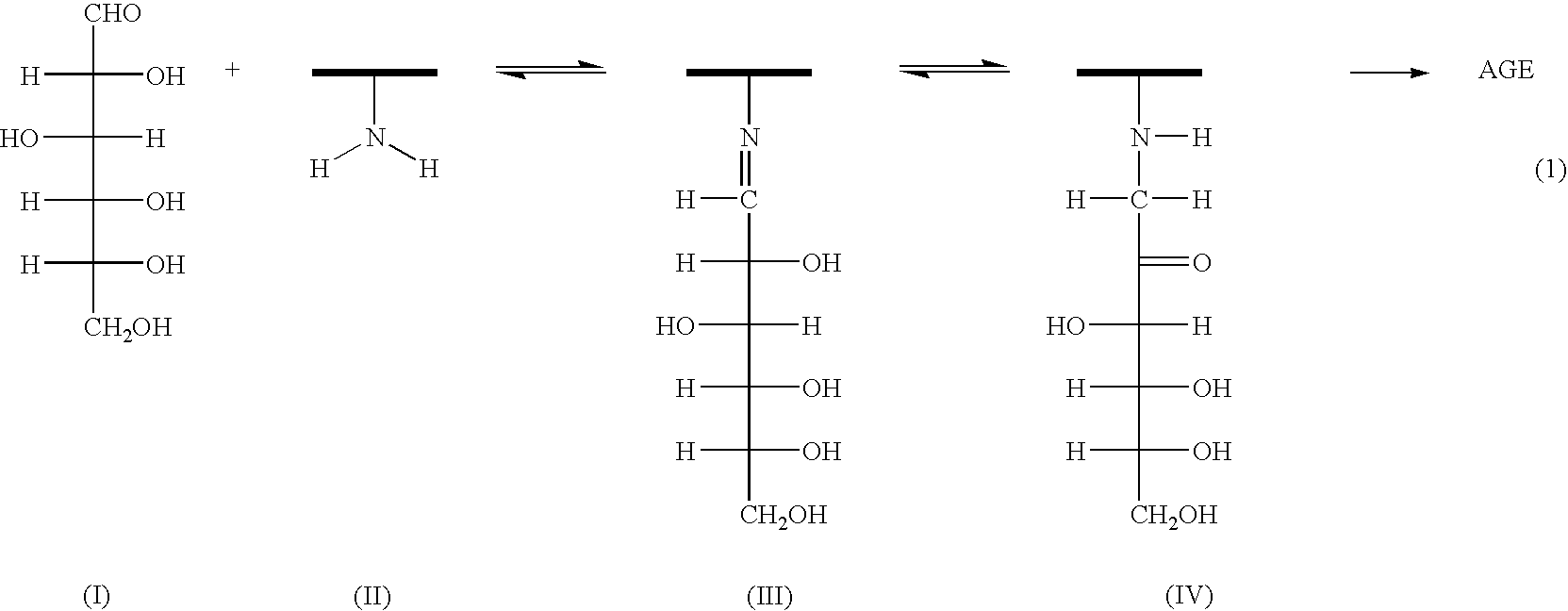Patents
Literature
109 results about "End stage renal disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
End stage renal disease (ESRD) is the last stage (stage five) of chronic kidney disease (CKD). This means kidneys are only functioning at 10 to 15 percent of their normal capacity.
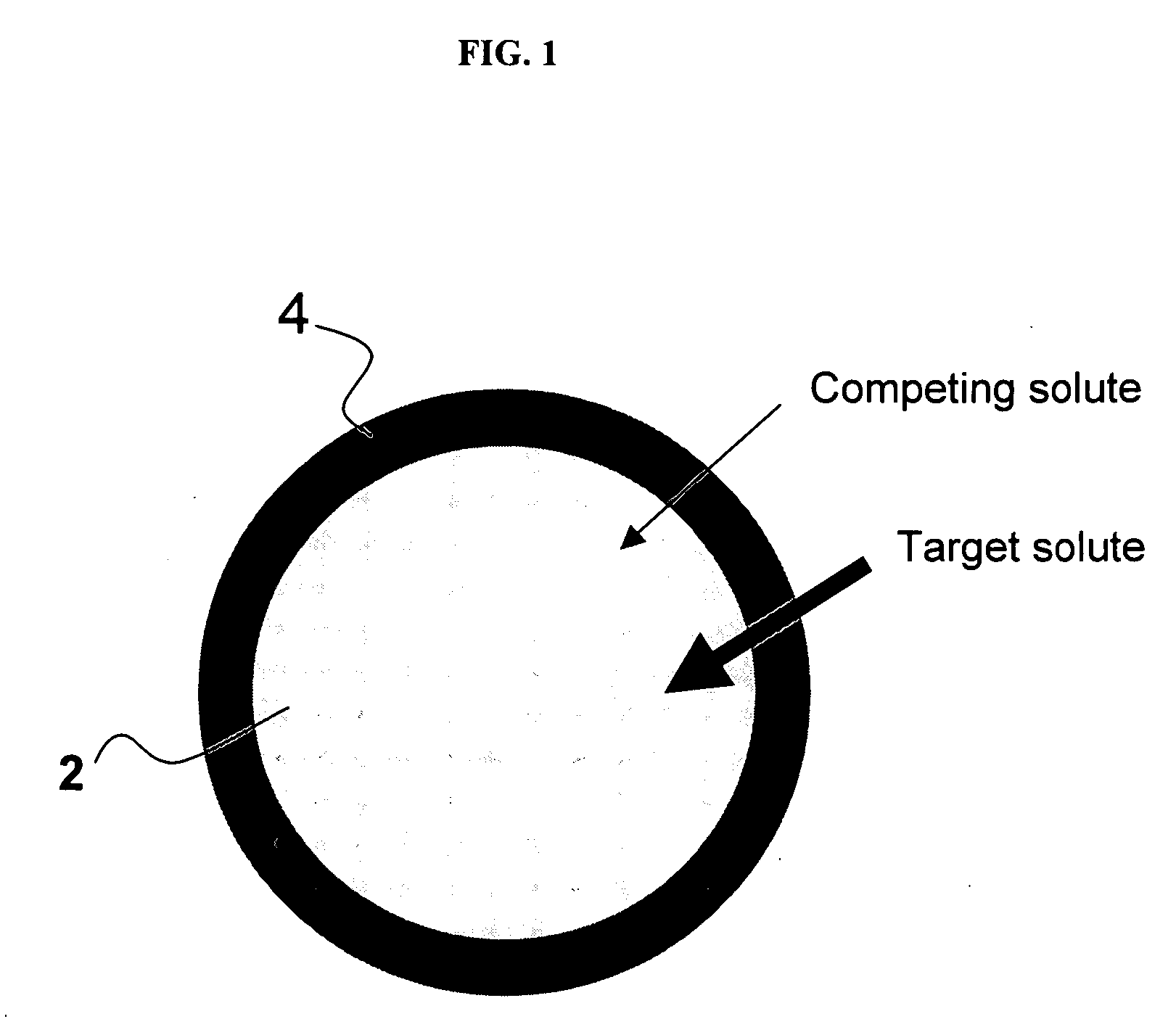
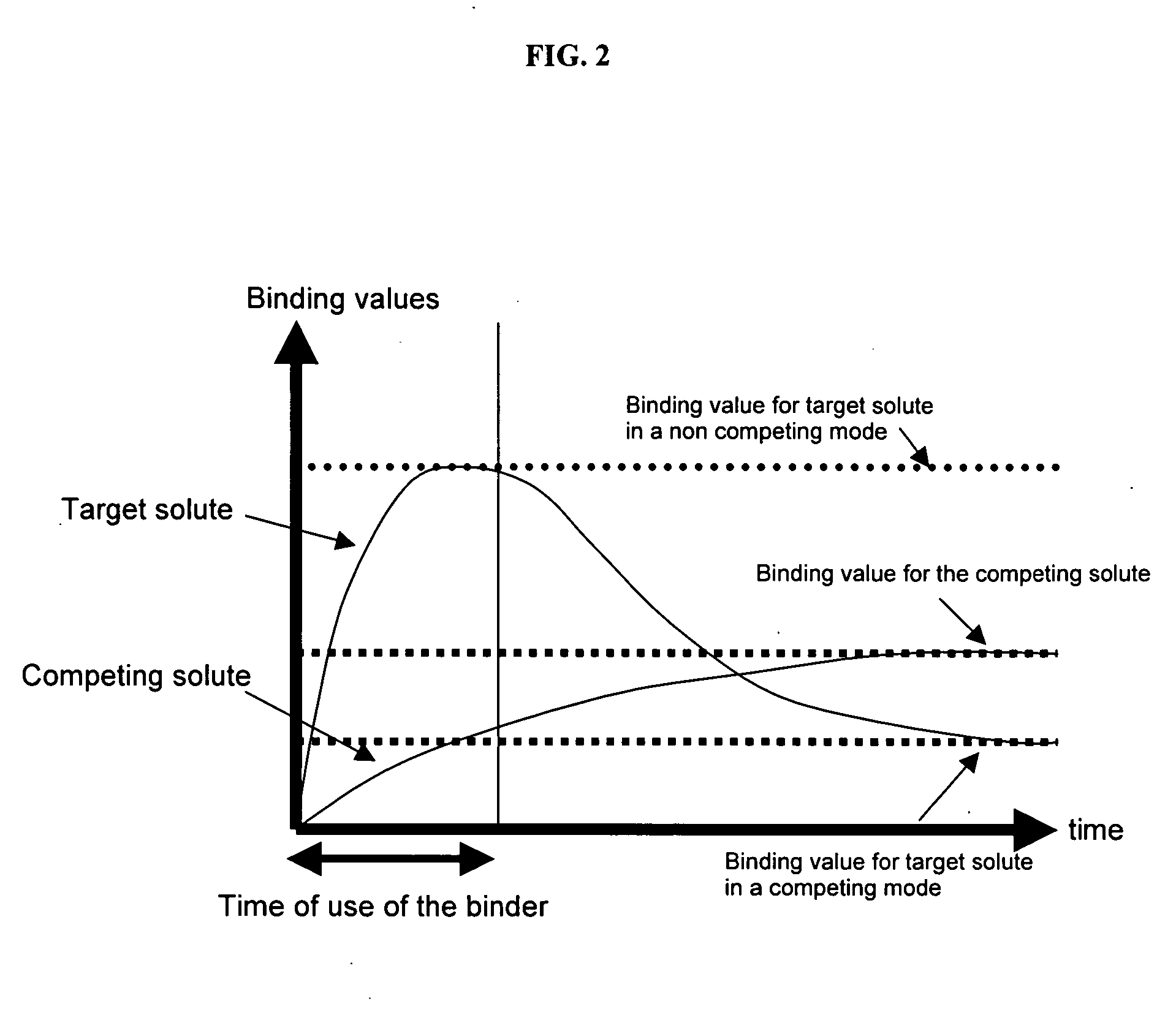
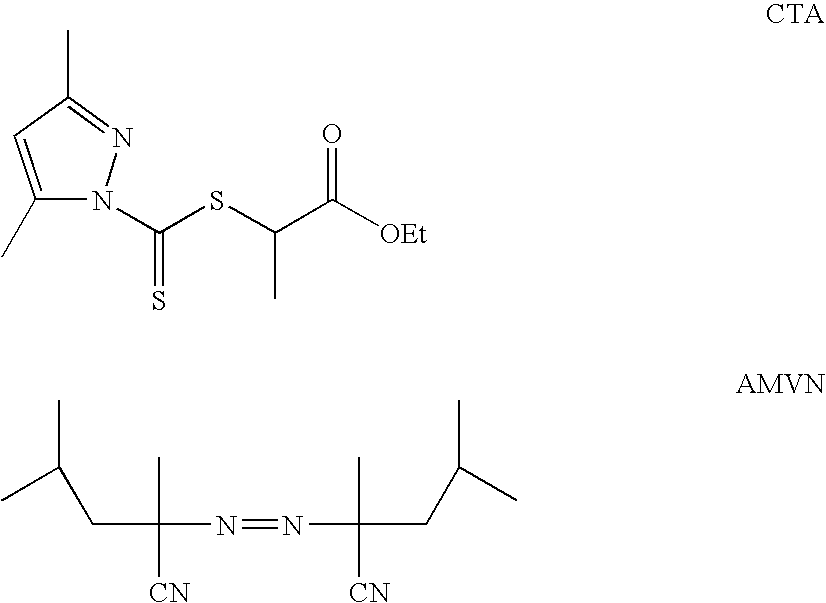
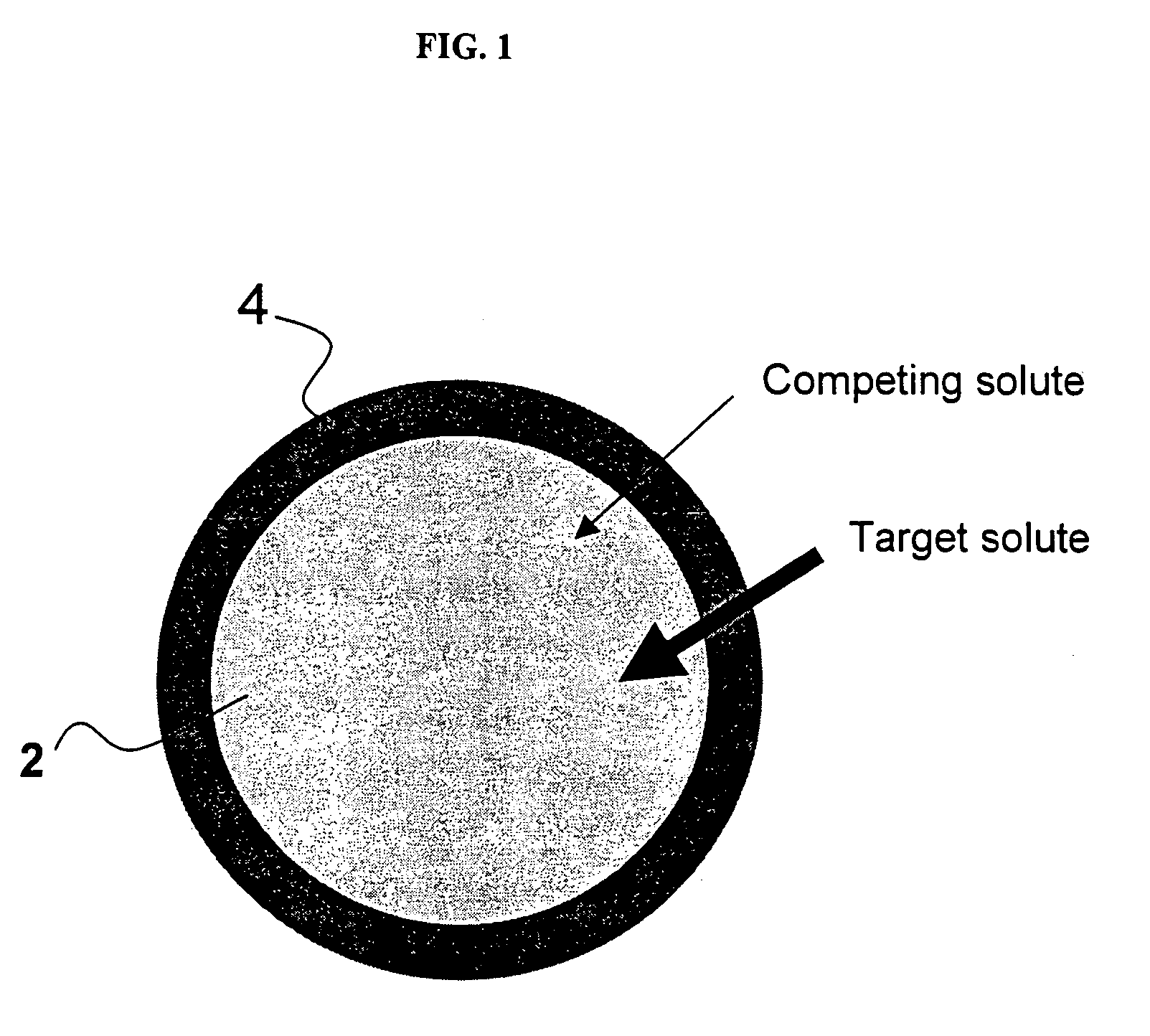
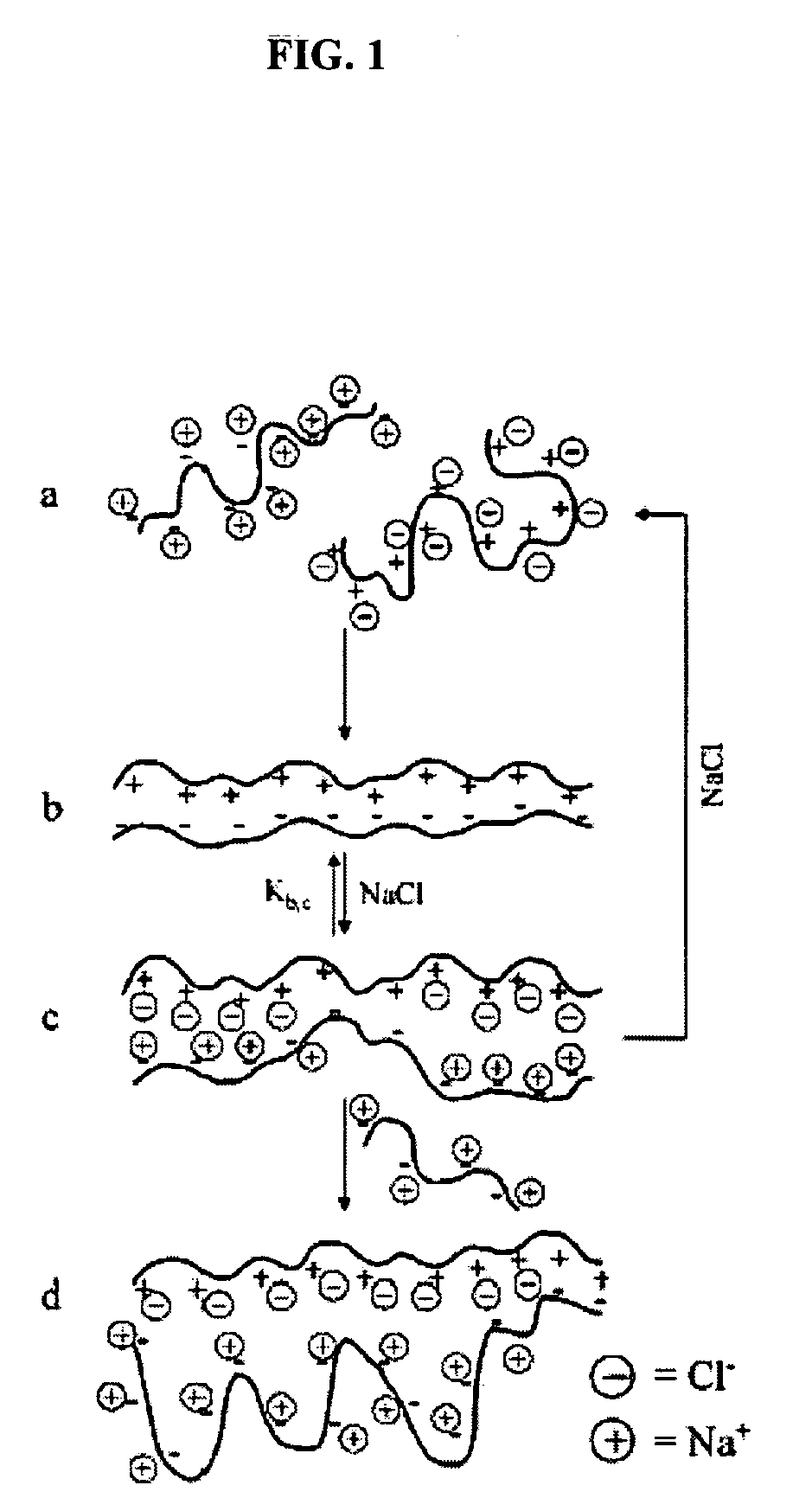
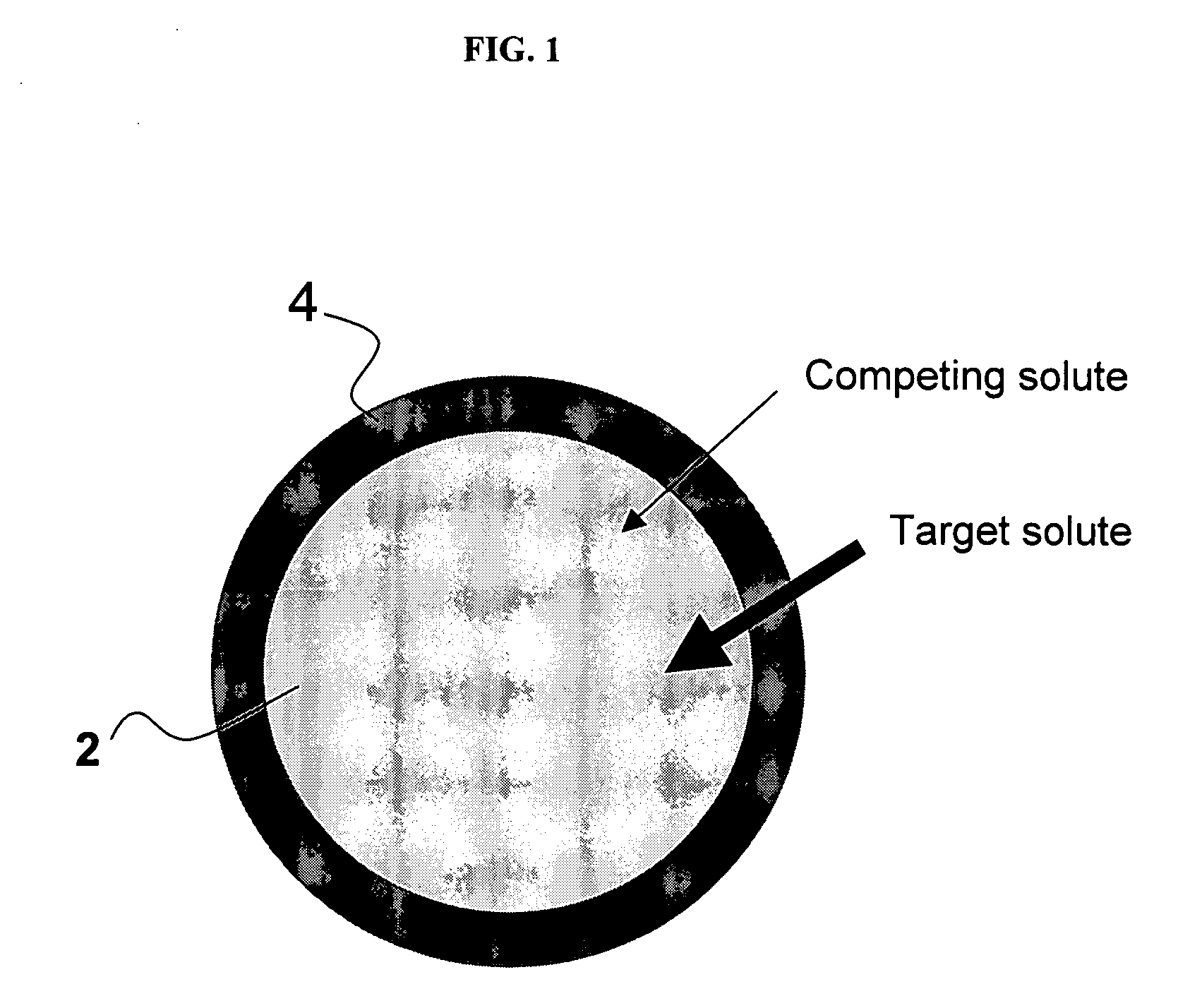
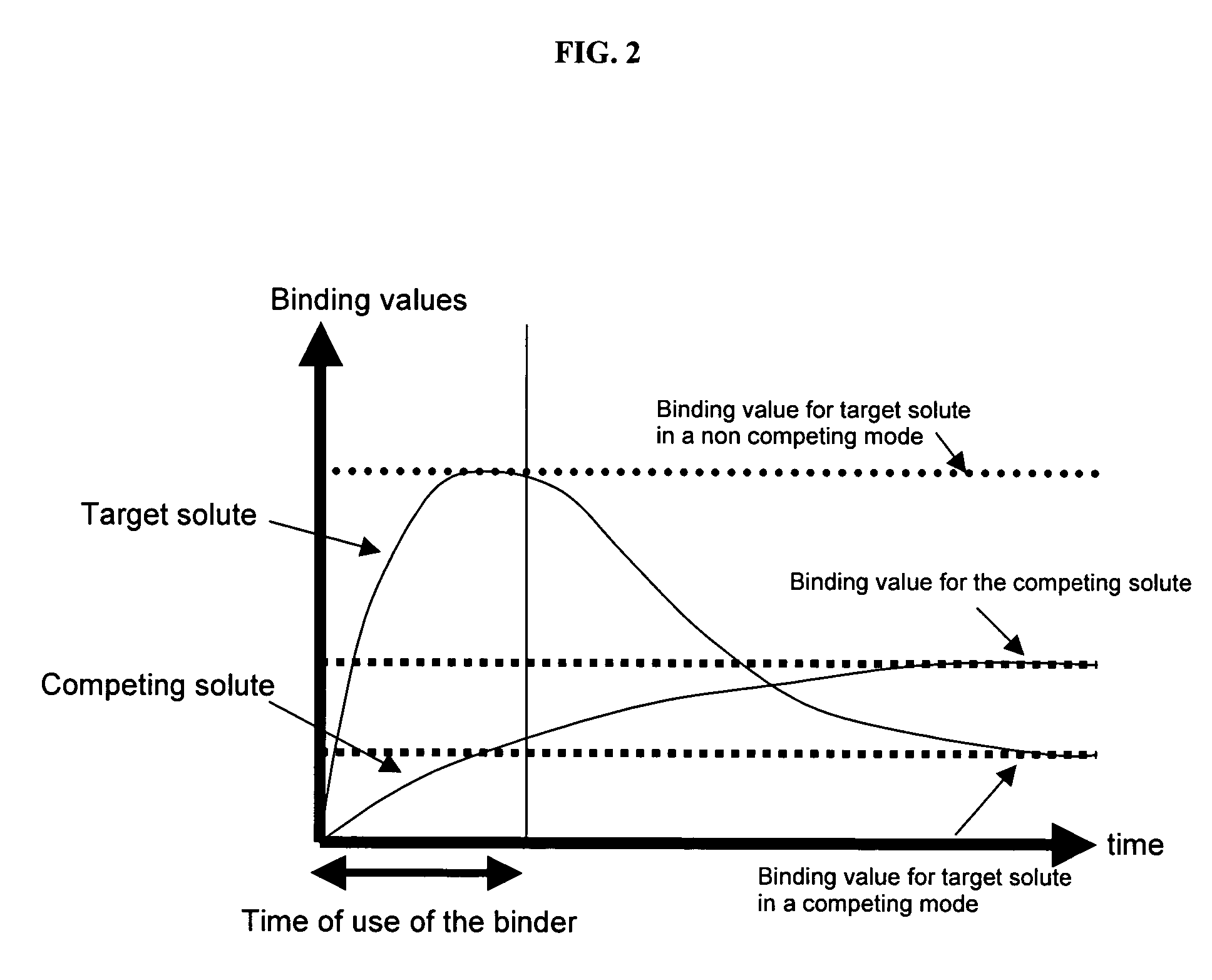
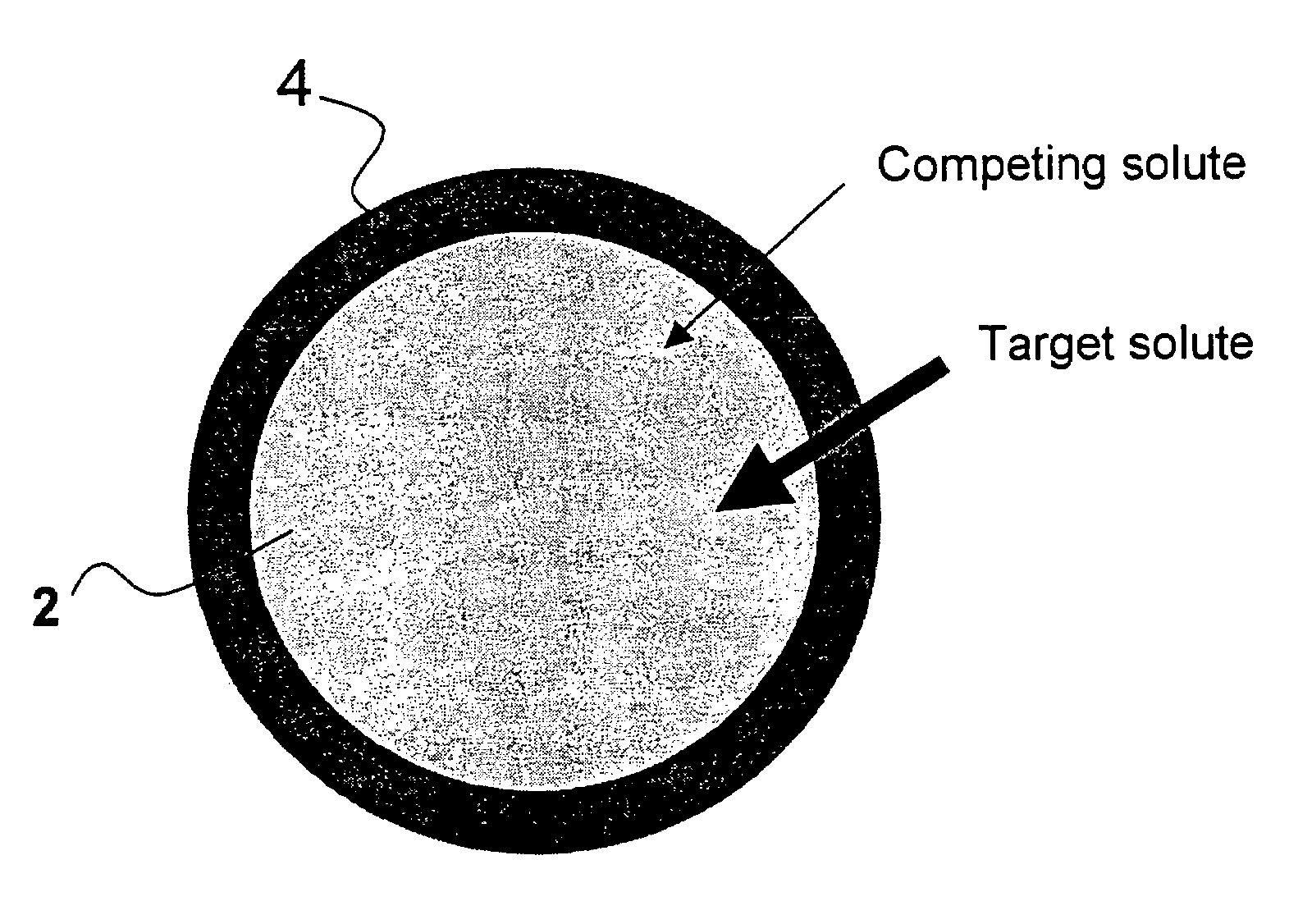
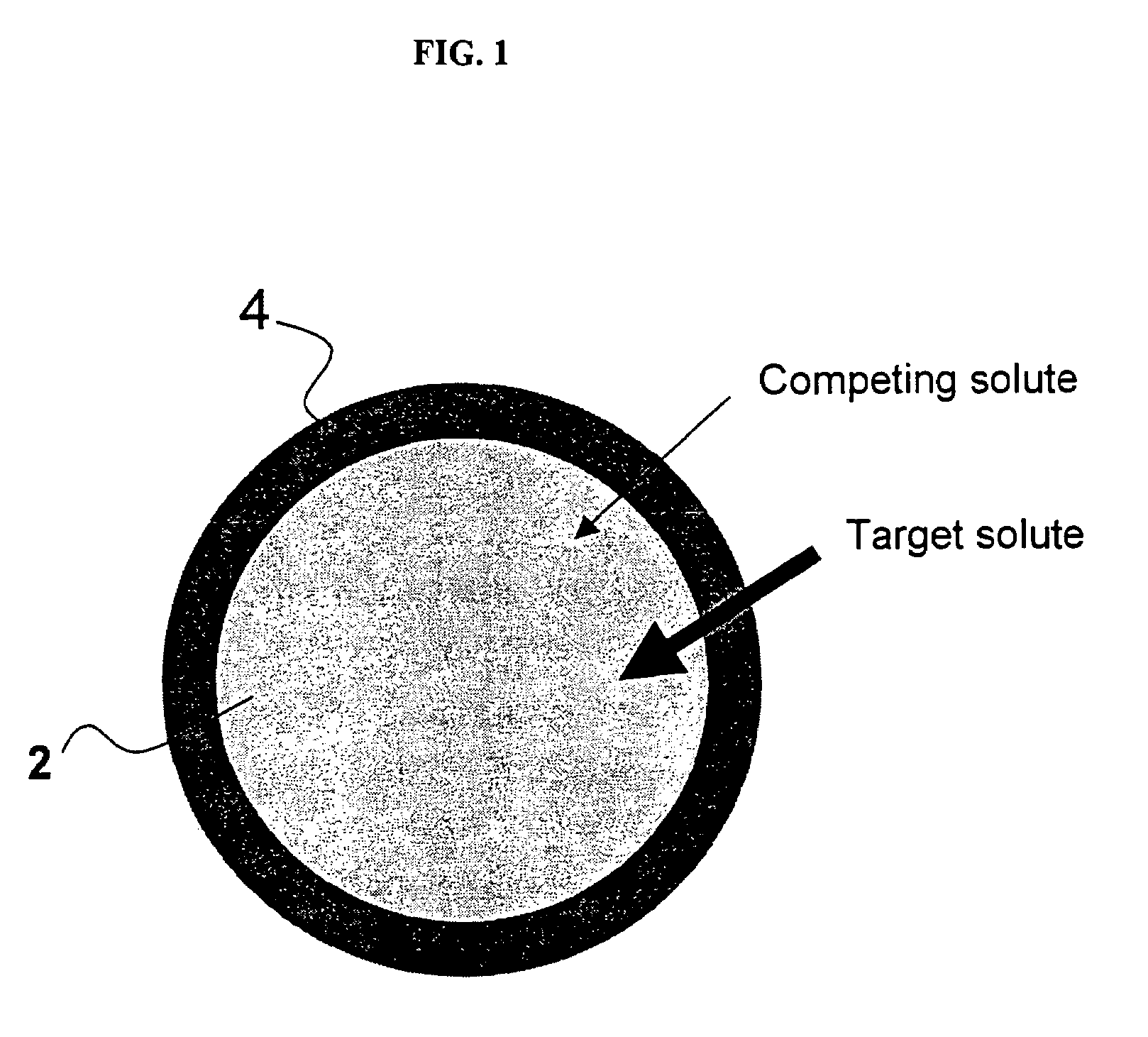
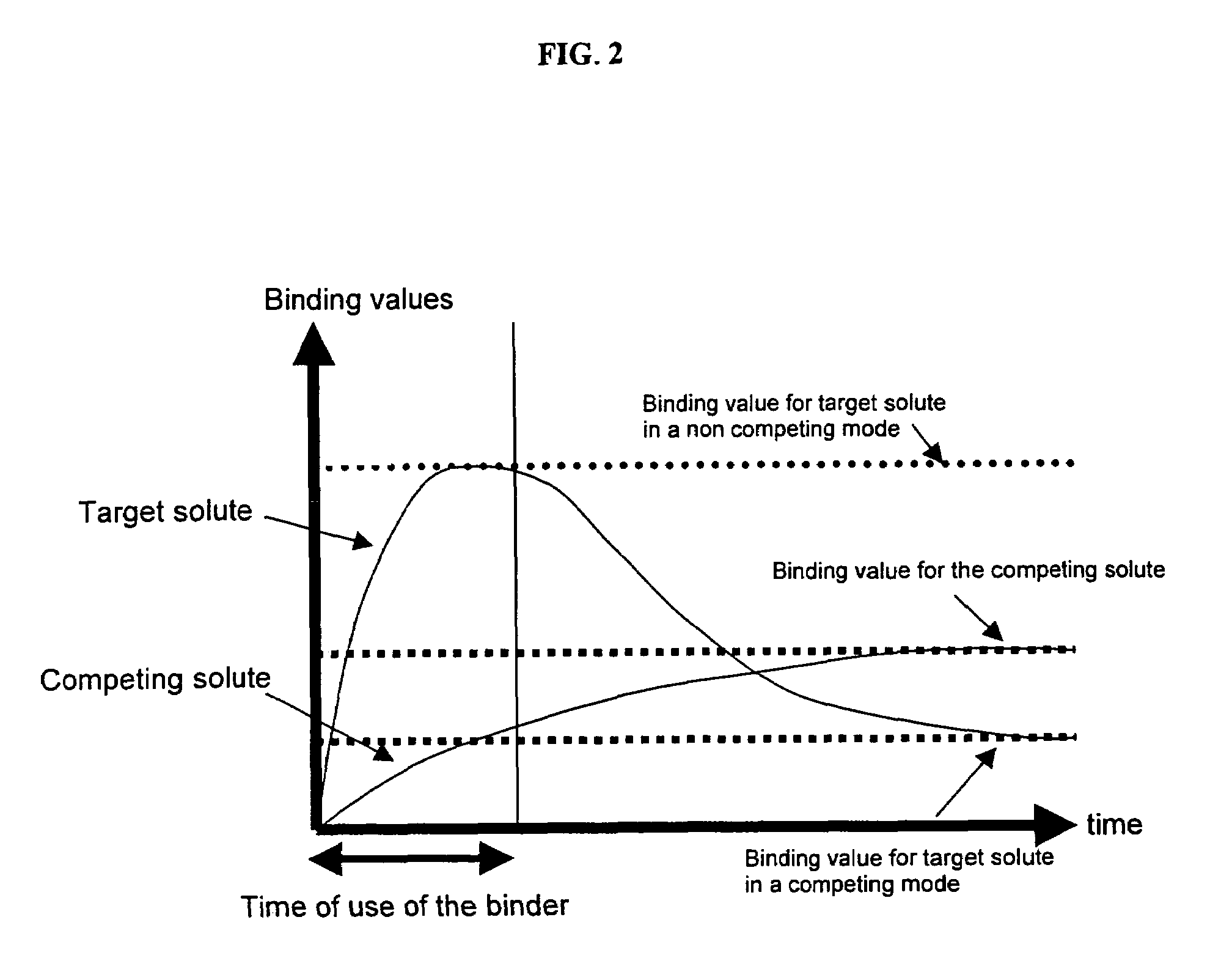
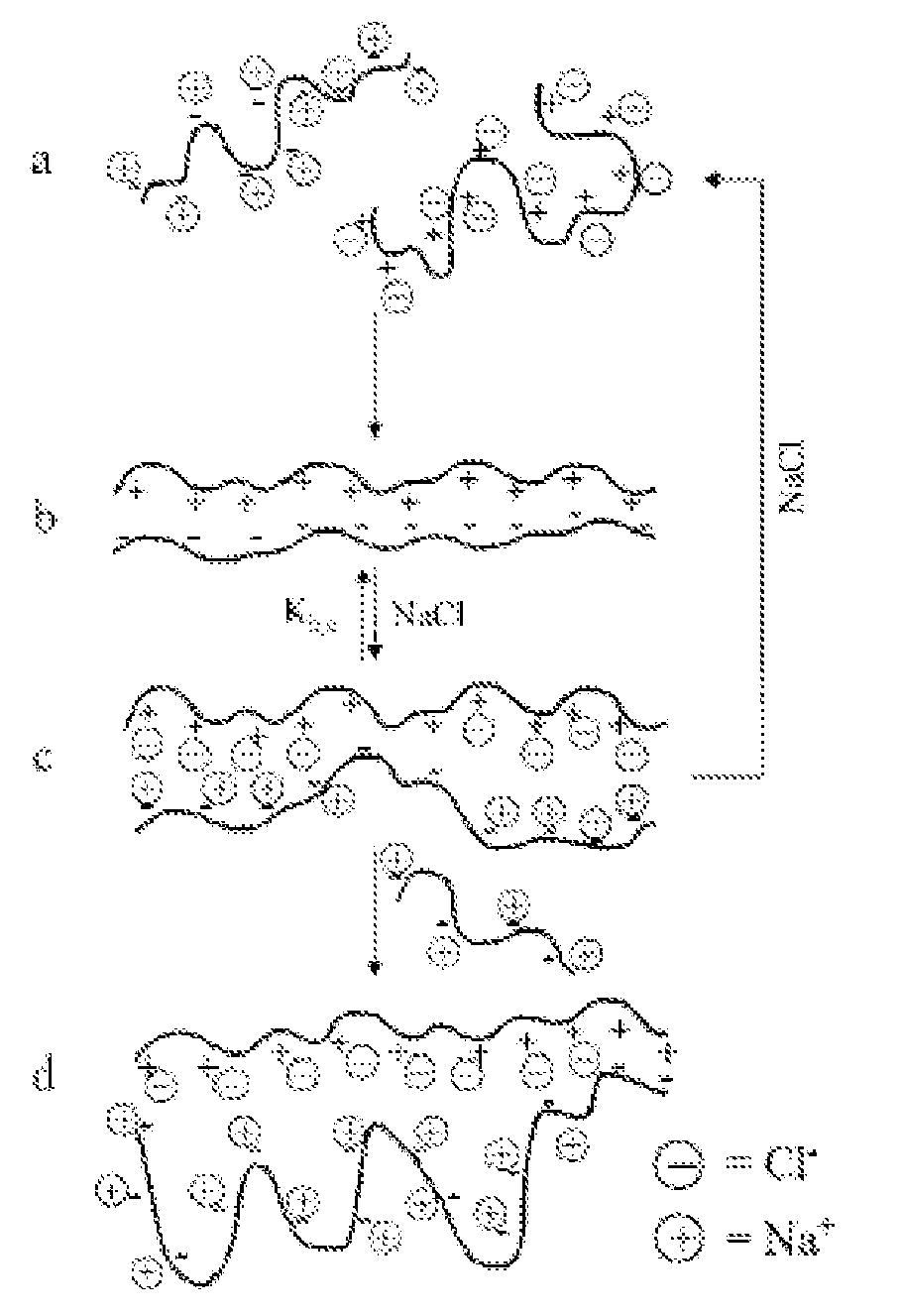
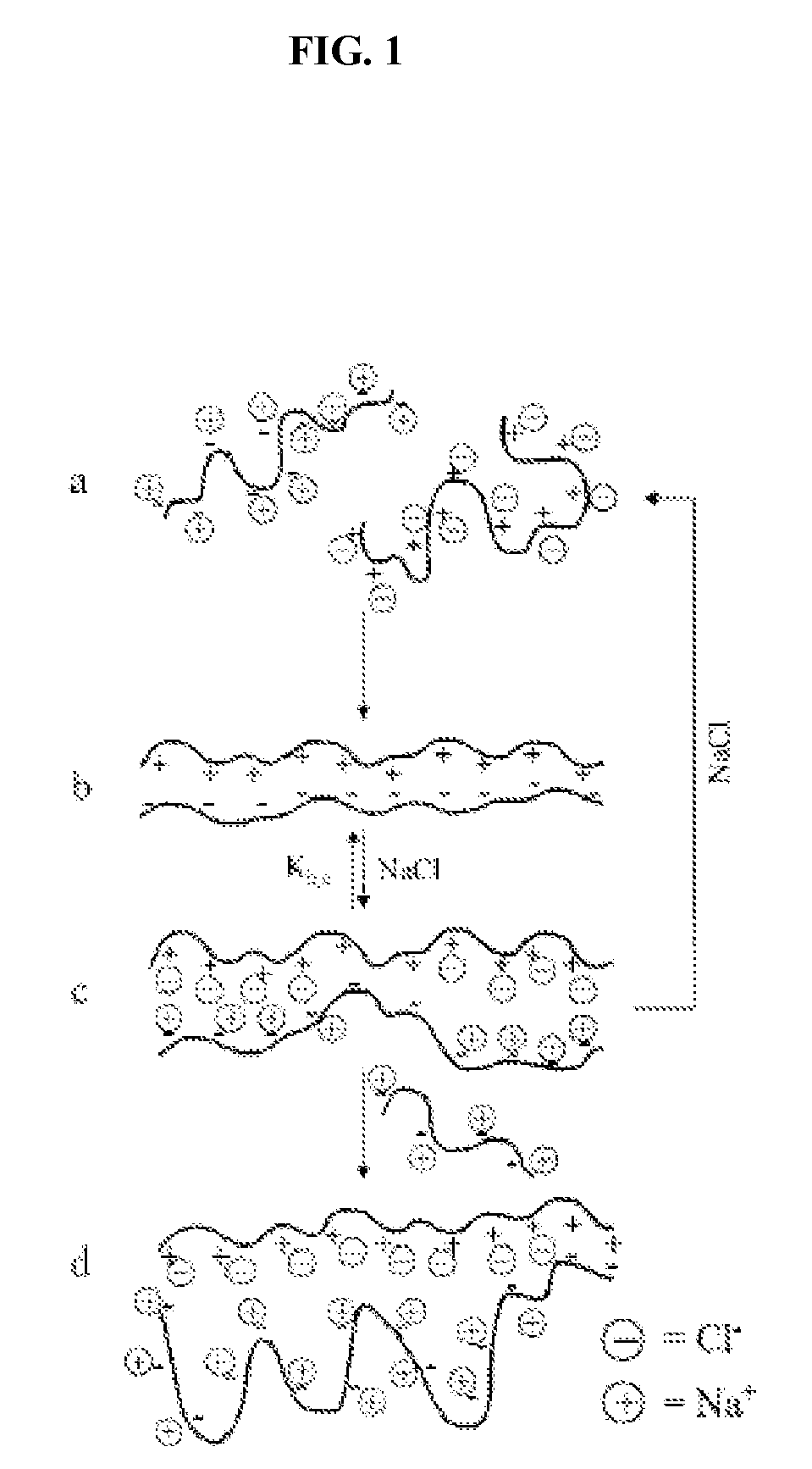
Ion binding compositions
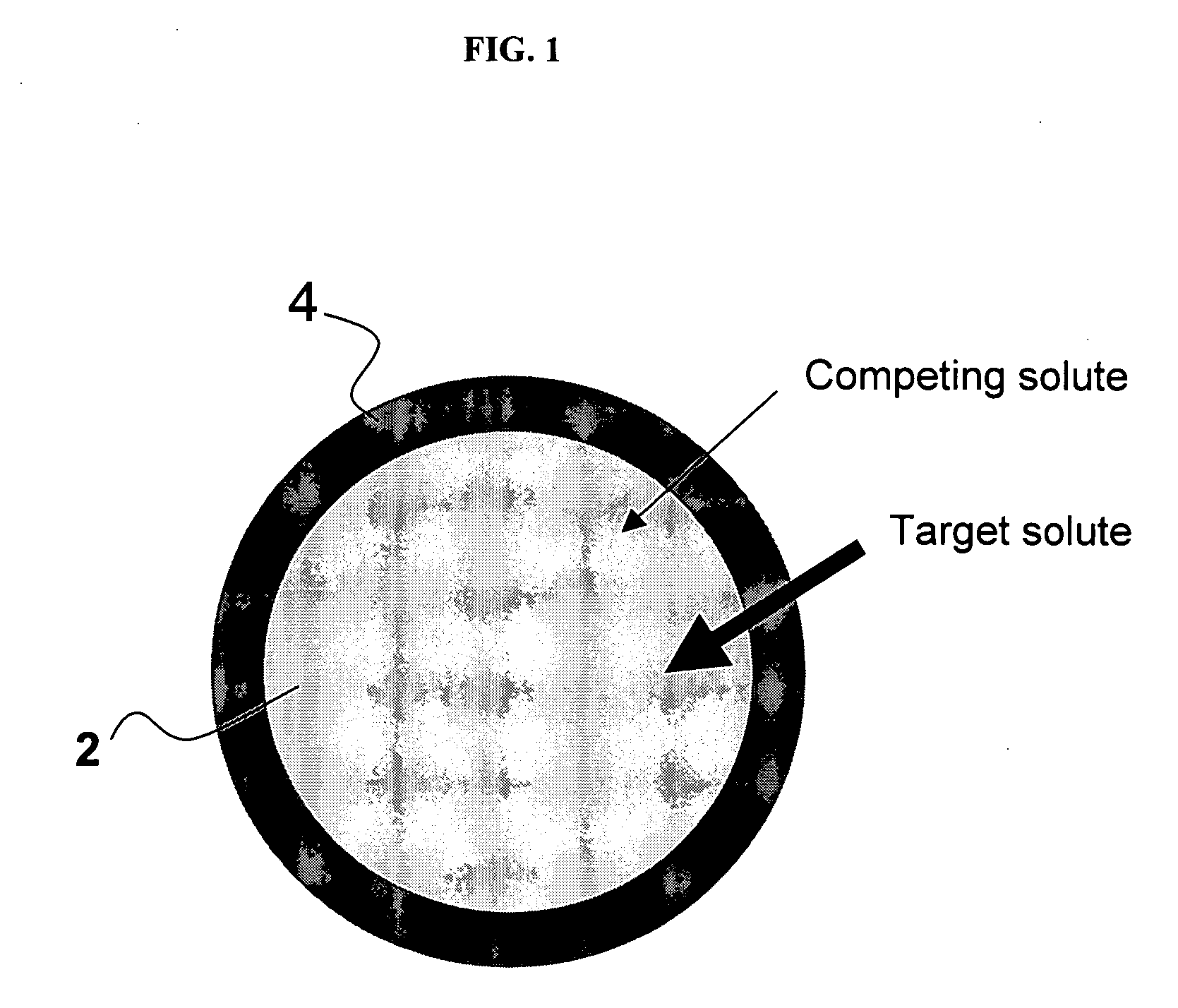
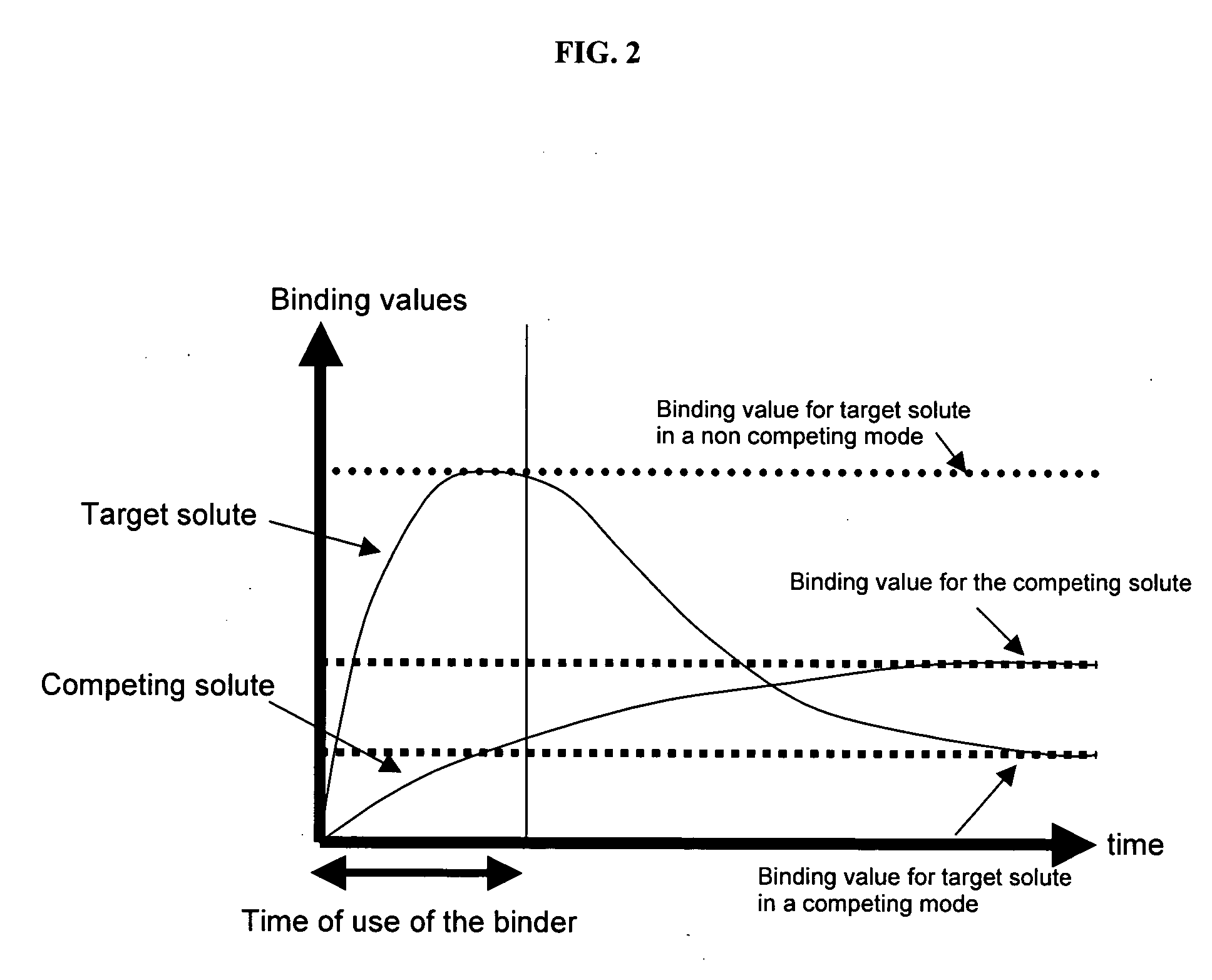
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
Ion binding compositions
ActiveUS20060024336A1Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic renal insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
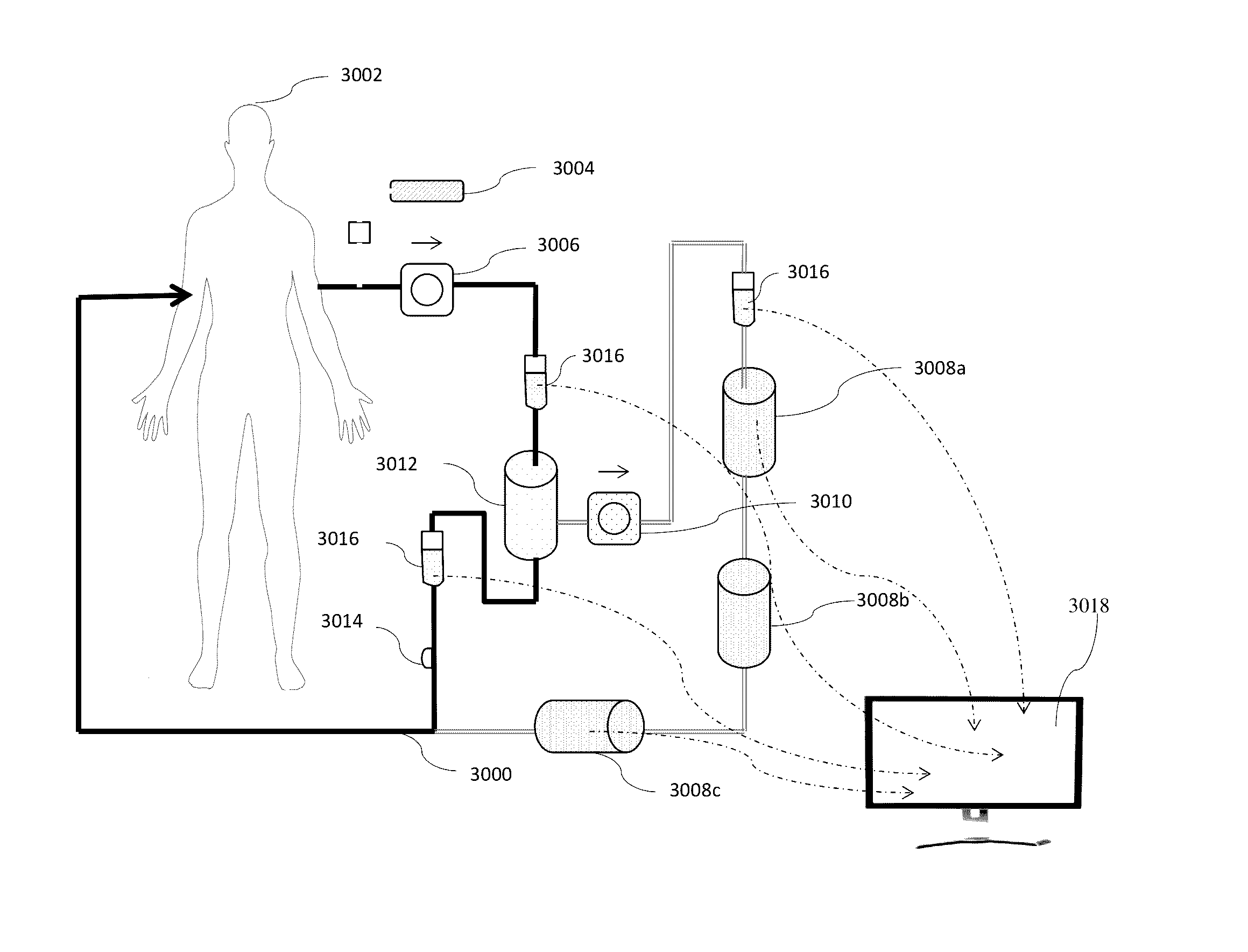
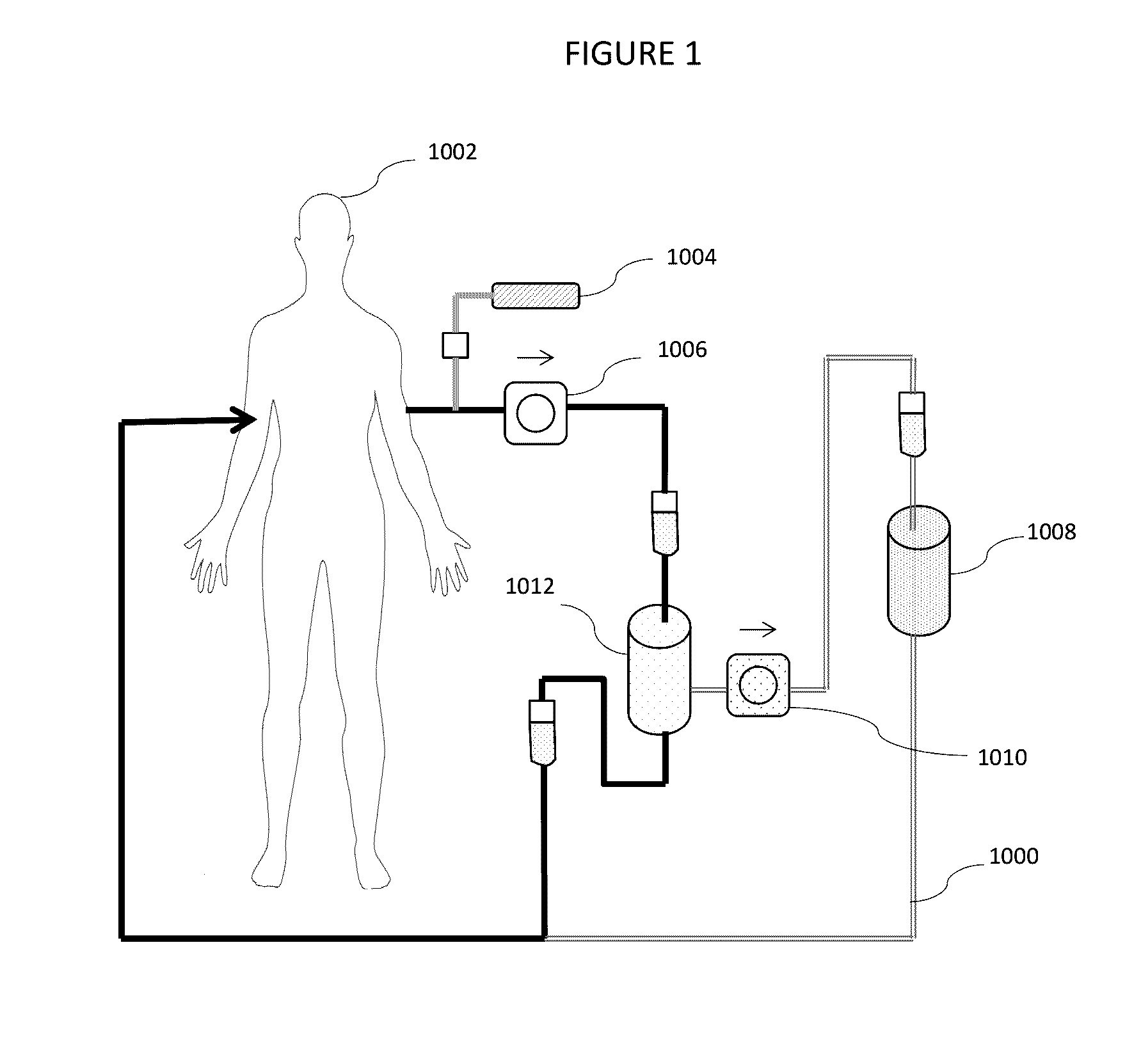
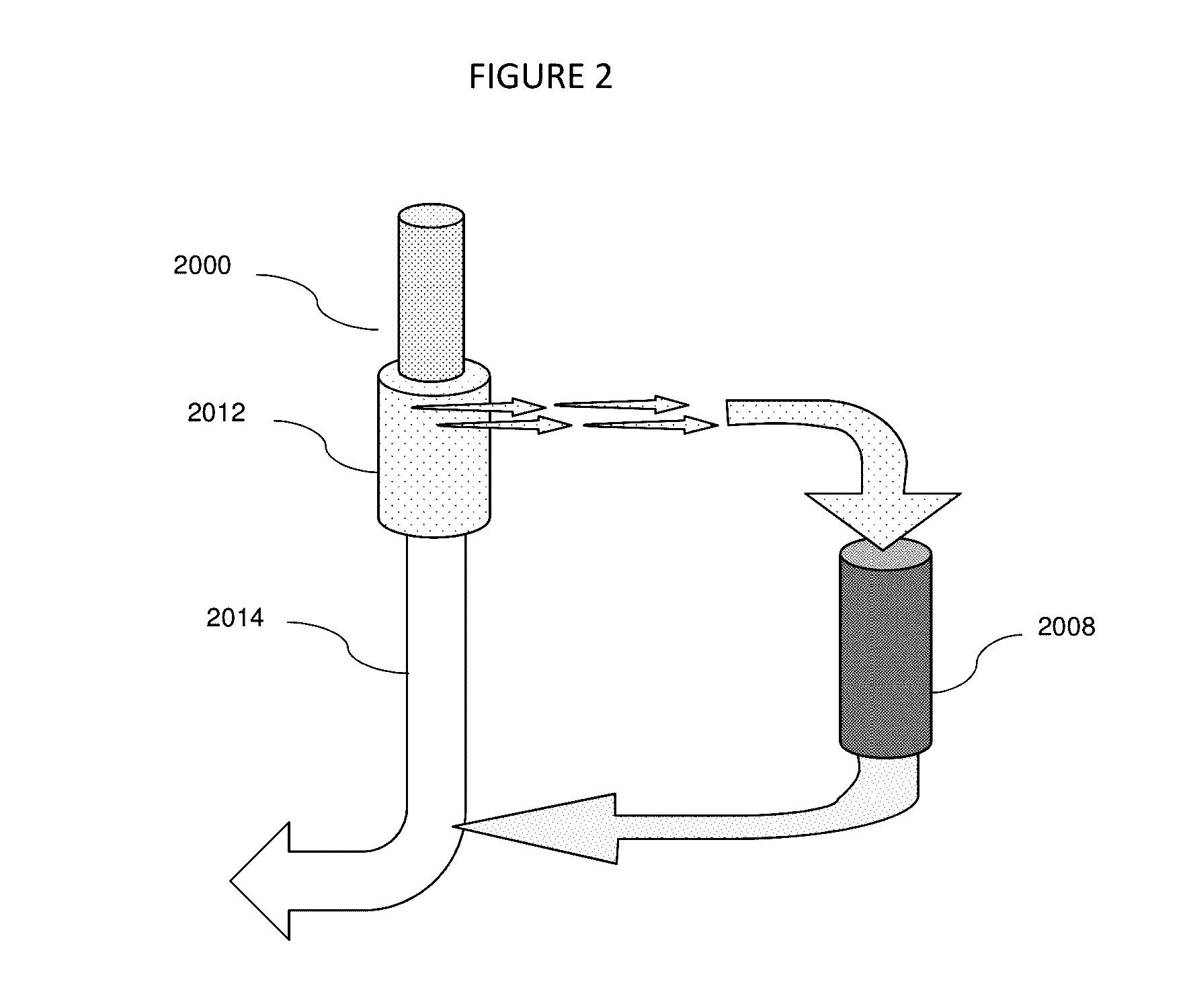
Fluid circuit for delivery of renal replacement therapies
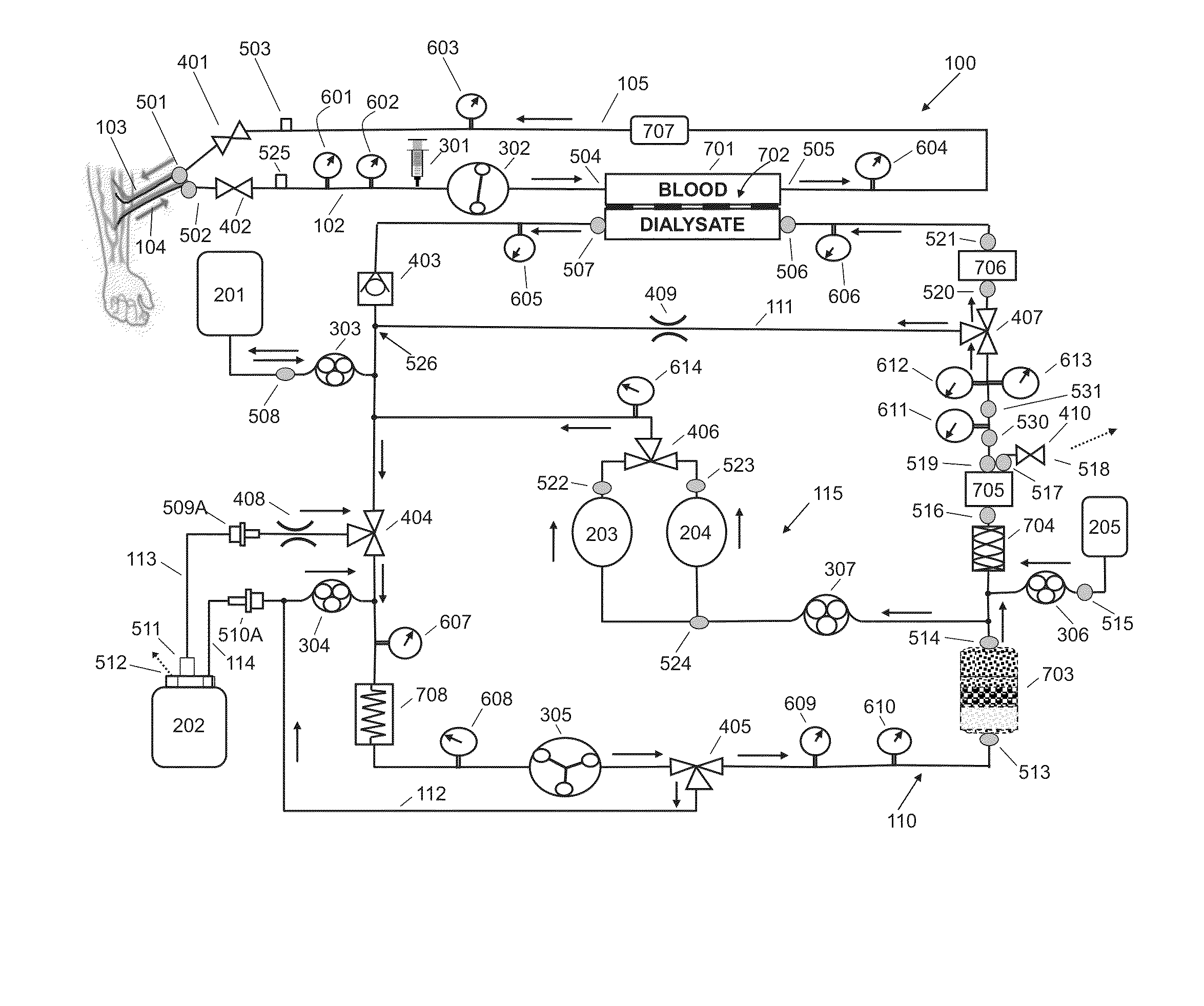
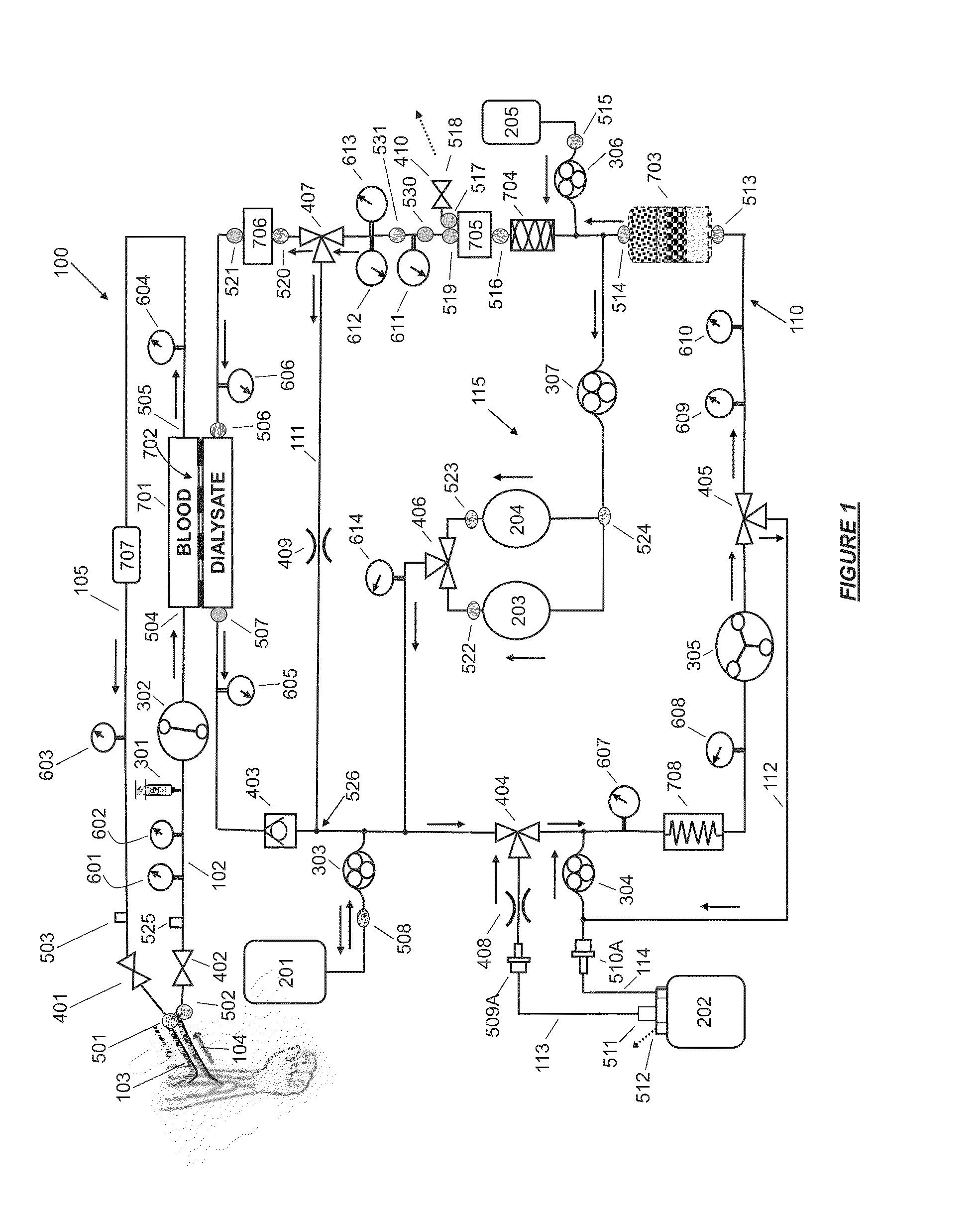
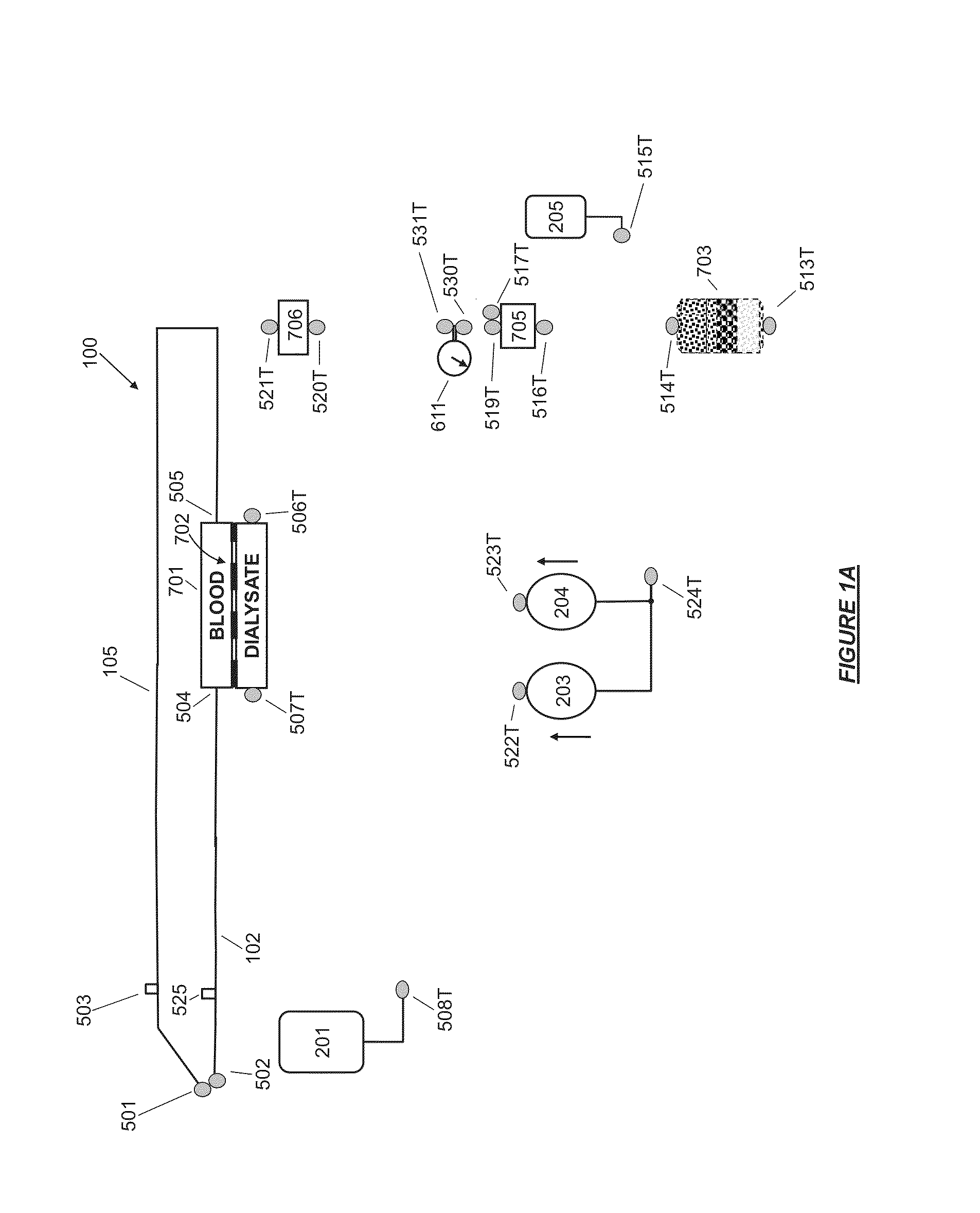
A flow loop for hemodialysis, hemodiafiltration and hemofiltration for the treatment of pathological conditions such as End Stage Renal Disease (ESRD) that has a controlled compliant flow path for preparing fluids required for a hemodialysis therapy session from water. The controlled compliant flow path modifies water into any one of a solution for priming a hemodialysis system, a physiologically compatible solution for contacting blood, a physiologically compatible solution for infusion to a subject, and a solution for blood rinse back to a subject. The controlled compliant flow path has a means for selectively metering in and metering out fluid from the flow path.
Owner:MOZARC MEDICAL US LLC
Ion binding compositions
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
Chemical ablation and method of treatment for various diseases
ActiveUS20160310200A1Improve treatment safetyImprove efficacyUltrasonic/sonic/infrasonic diagnosticsBalloon catheterAbnormal tissue growthDamages tissue
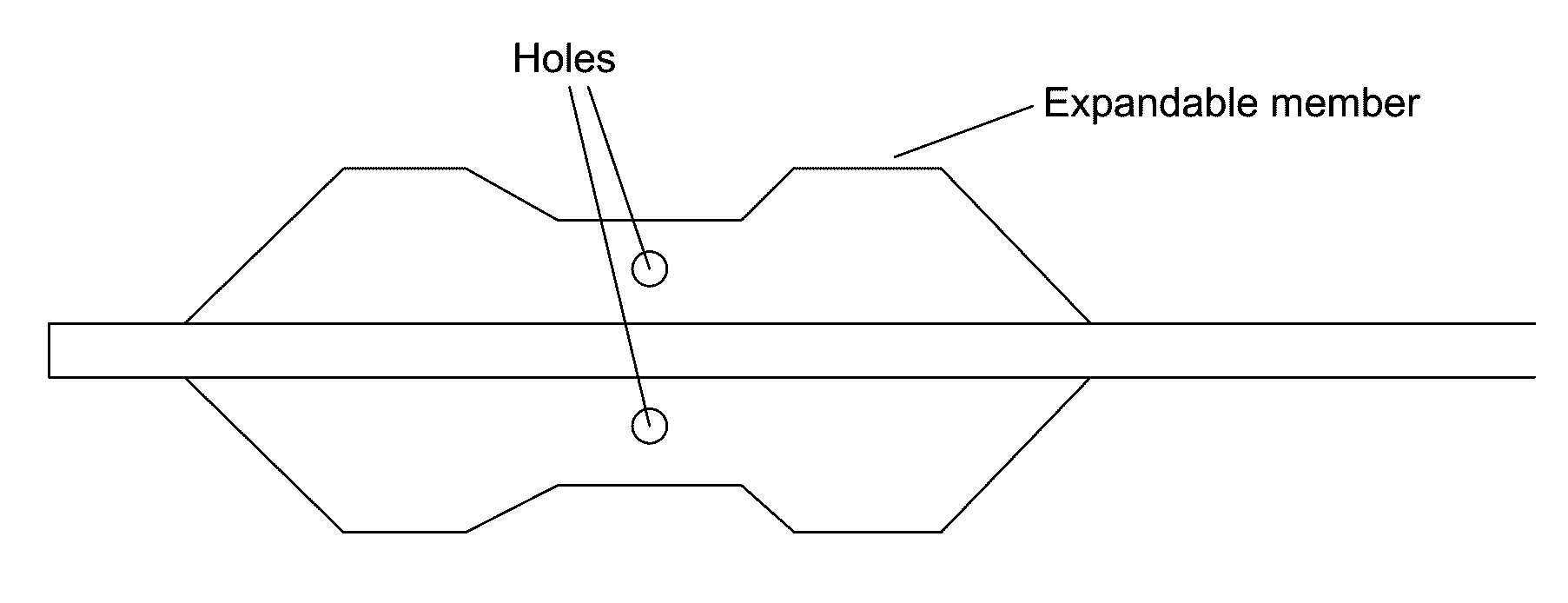
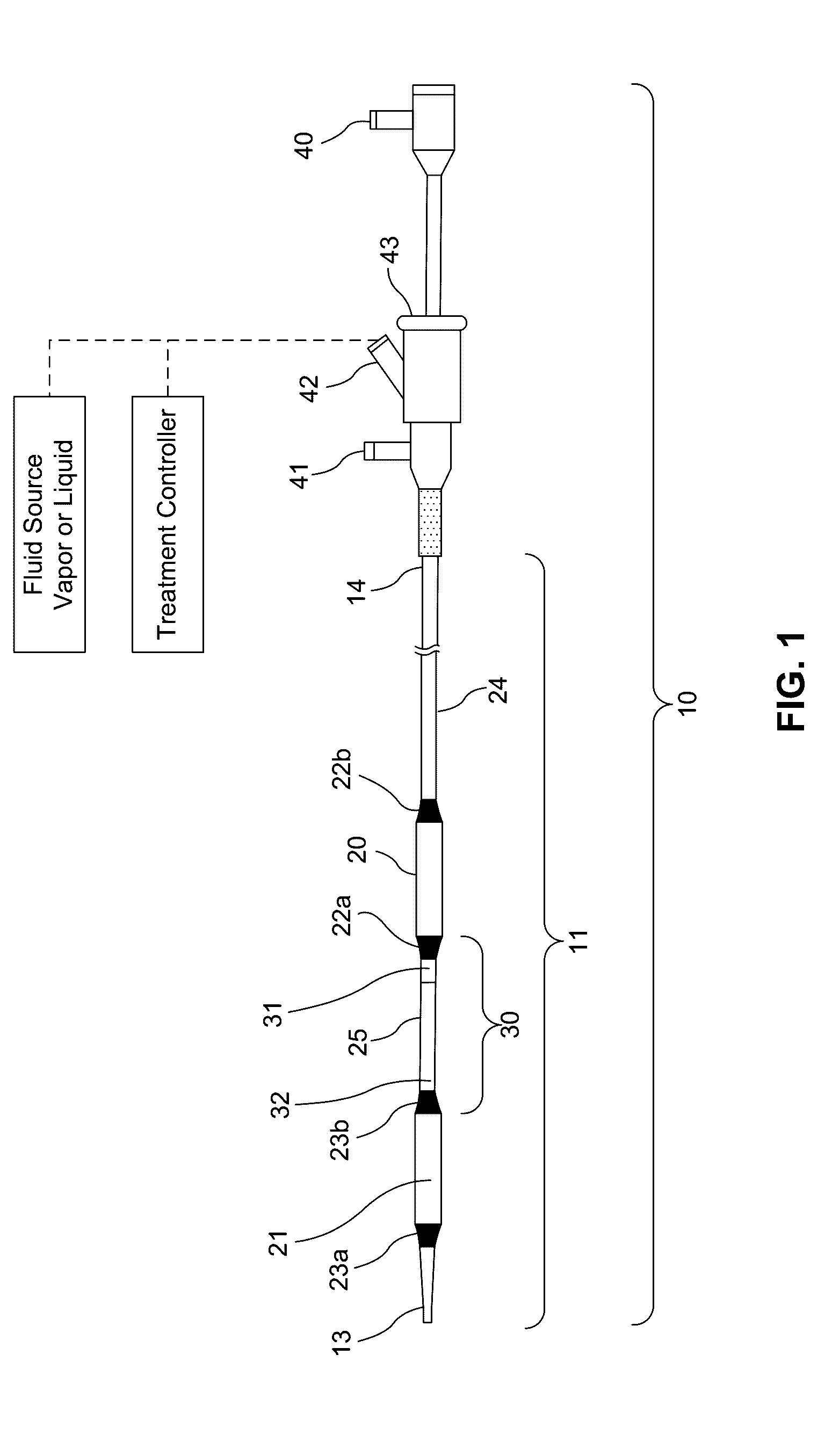
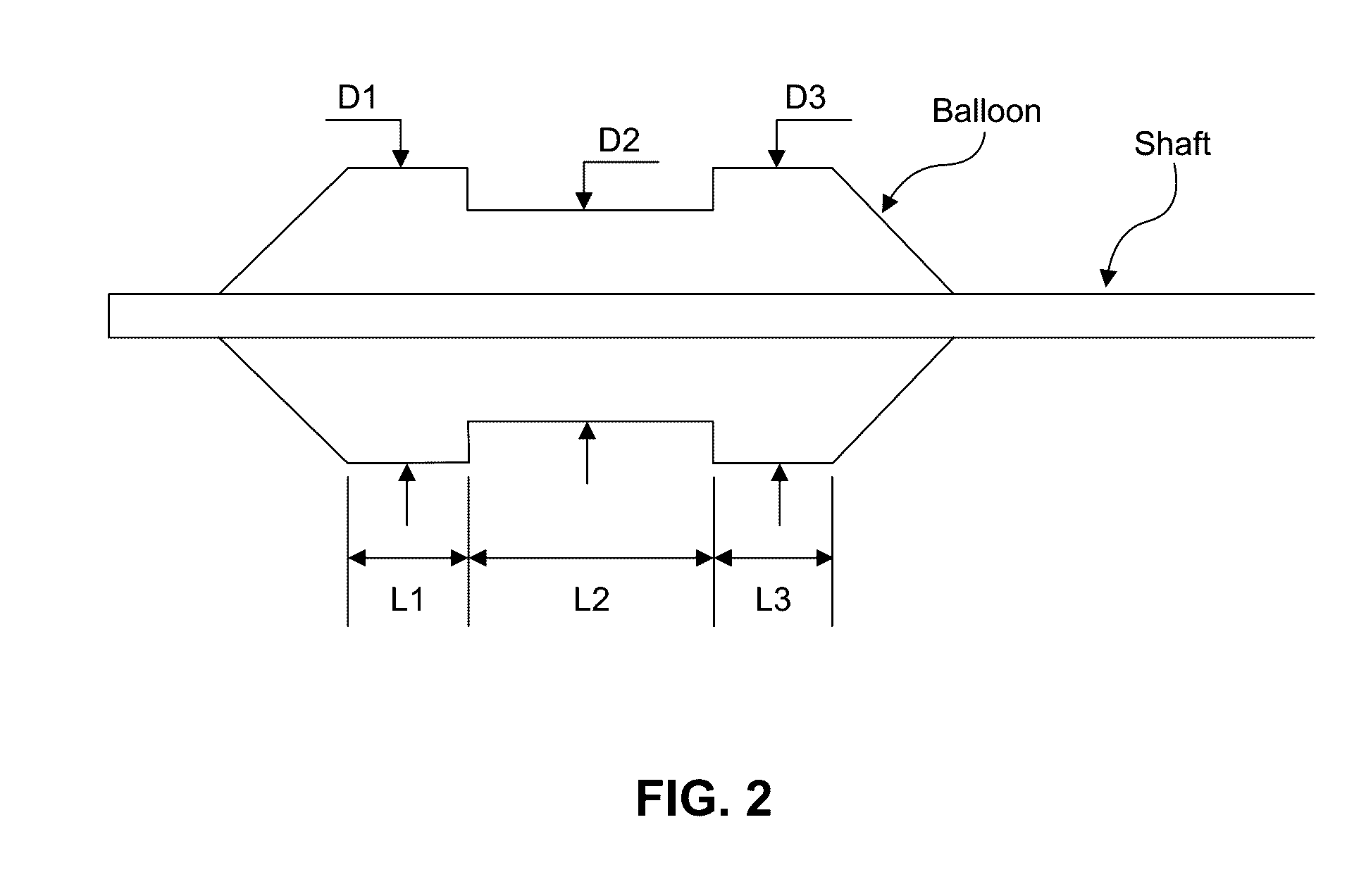
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Methods and compositions for treatment of ion imbalances
InactiveUS20050220750A1Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic renal insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides compositions comprising sodium-binding polymers and pharmaceutical compositions thereof. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
Compositions and methods of treating chronic kidney disease
InactiveUS20080317725A1Treat and prevent skeletal muscle wastingHigh in proteinBiocideHeavy metal active ingredientsNutritional deficiencyChronic renal disease
The invention relates to nutritional compositions and methods of using these compositions for the treatment of renal disease. More particularly, the invention discloses compositions of vitamins, minerals, amino acids, and / or proteins in amounts that can be used to supplement the nutritional deficiencies observed in patients afflicted with renal disease, renal insufficiency, or end-stage renal disease. The compositions of the invention can also be used as nutritional supplements for patients undergoing dialysis therapy or for patients on a restricted diet. In addition, the compositions can be used in combination or alone as a method of treating or managing a subject in the various stages of chronic renal disease, or throughout disease progression.
Owner:BAUM SETH J
Phosphate binder with reduced pill burden
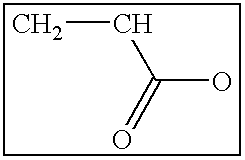
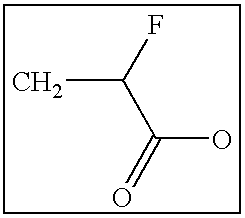
The present invention is generally directed to compositions and formulations that can be used for the treatment of diseases such as End Stage Renal Disease (“ESRD”) and Chronic Renal Insufficiency (“CRI”). Specifically, it is directed to lanthanum-based compounds that bind phosphate and that can be formulated to provide for a reduced pill burden relative to other phosphate binders. In a formulation aspect of the present invention, a formulation is provided the includes a lanthanum-based, phosphate binder. The formulation is typically characterized in that in may be swallowed without chewing. Formulations of the present invention, along with a lanthanum-based compound, may optionally include the following: mass diluting agents; binders; coatings; compression / encapsulation aids; disintegrants; lubricants; plasticizers; slip / anti-electrostatic agents; powder lubricants; and, sweeteners. Where the formulation is in the form of a tablet, it typically has a volume between 0.3 cm3 and 1.2 cm3, preferably between 0.35 cm3 and 0.50 cm3. Each tablet typically includes enough phosphate binder such that only 3 or less tablets need to be ingested each day for a patient suffering from ESRD.
Owner:SPECTRUM PHARMA INC
Methods of prognosis in chronic kidney disease
InactiveUS20120107420A1Reducing risk of deathAvoid deathBiocideDisease diagnosisNephrosisEnd stage renal disease
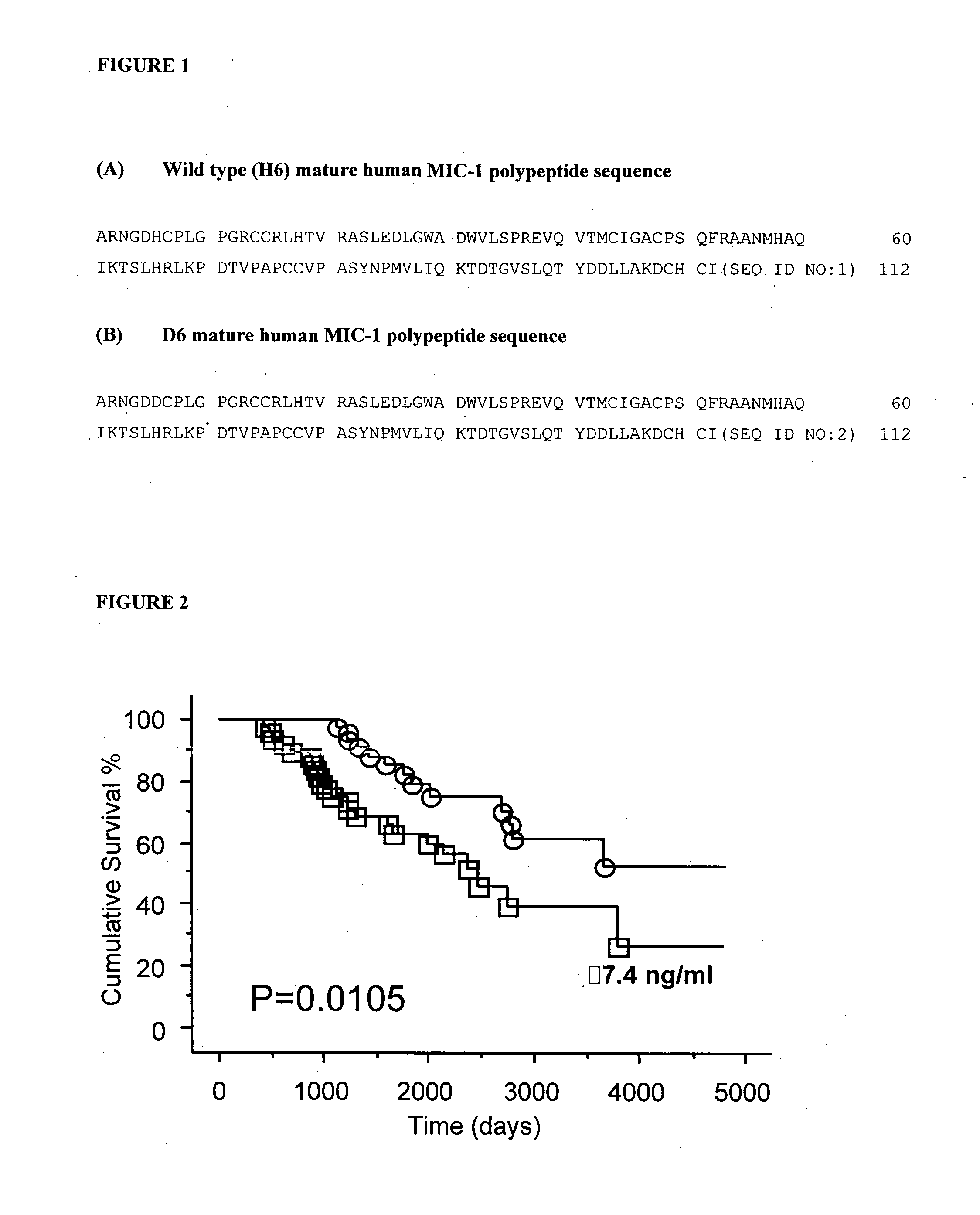
The present invention relates to methods of prognosing the survival of a diseased subject, particularly a subject with chronic kidney disease (CKD), as well as selecting an end-stage renal disease subject for a kidney transplant. The methods involve detecting an elevated amount of macrophage inhibitory cytokine-1 (MIC-1) in a test body sample from the diseased subject. A method of preventing or reducing the risk of death in a CKD subject which involves removing or inactivating MIC-1 present in the blood, plasma or serum of the subject, is also disclosed.
Owner:ST VINCENTS HOSPITAL SYDNEY
Implantable human kidney replacement unit
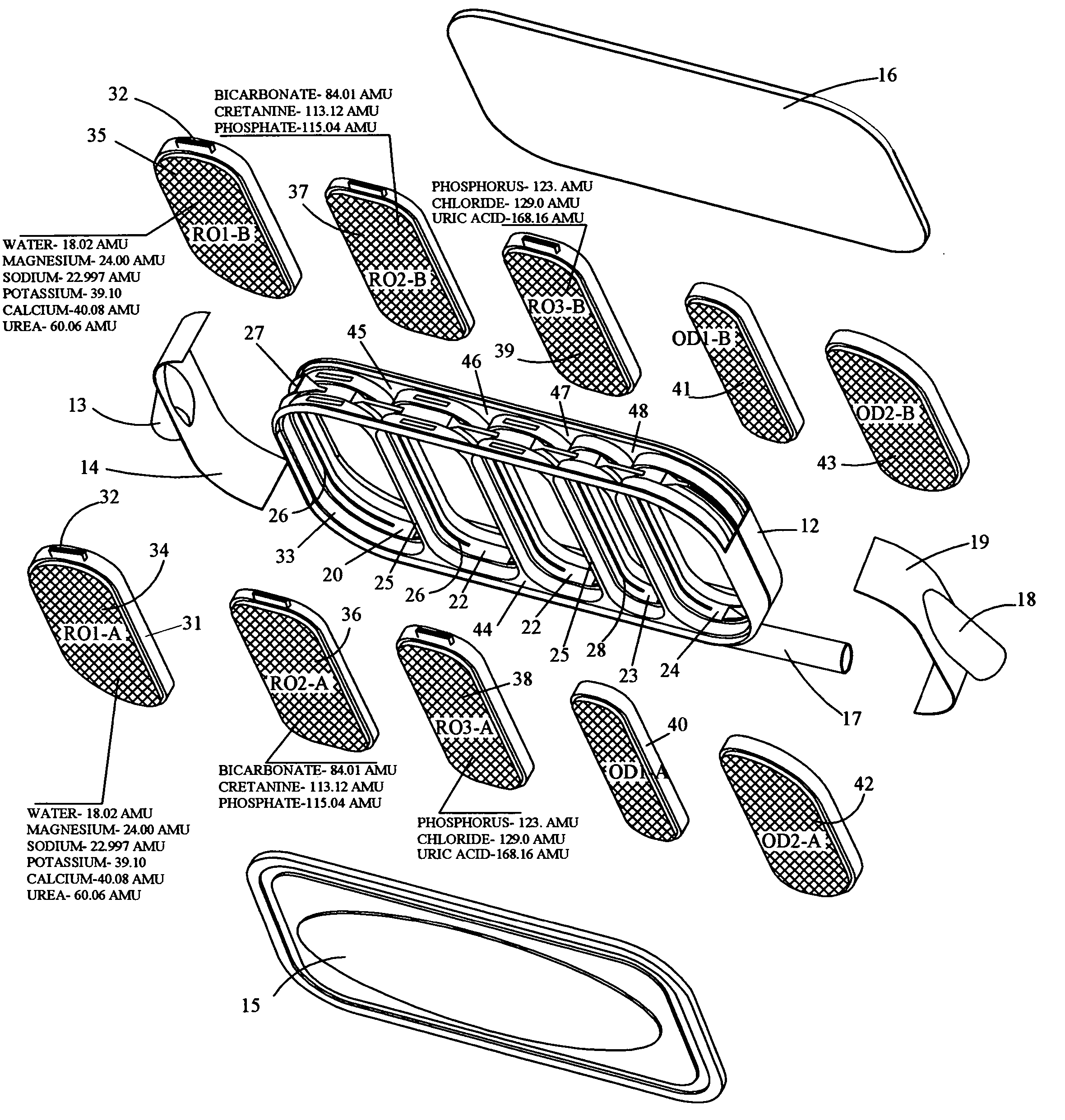
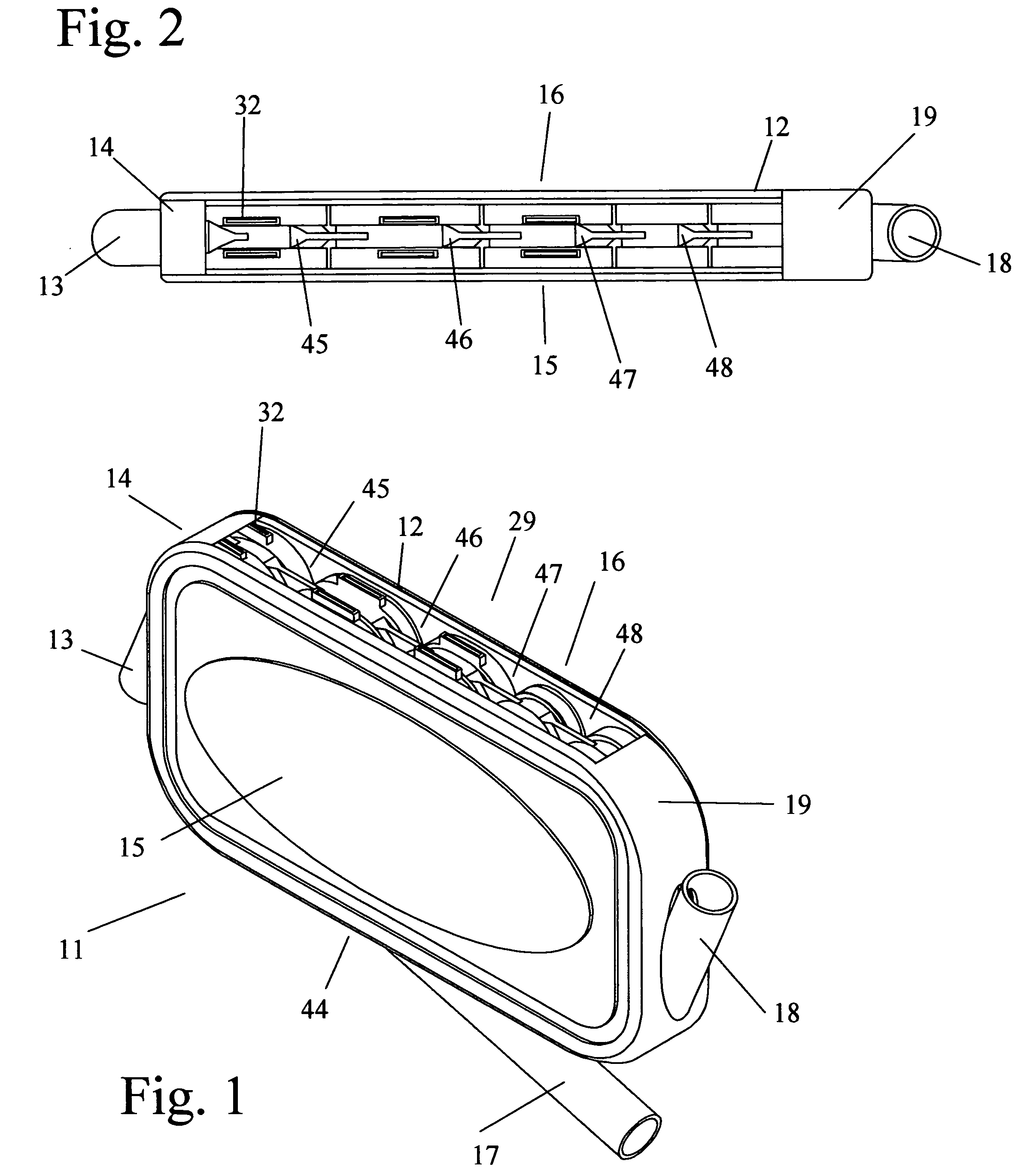
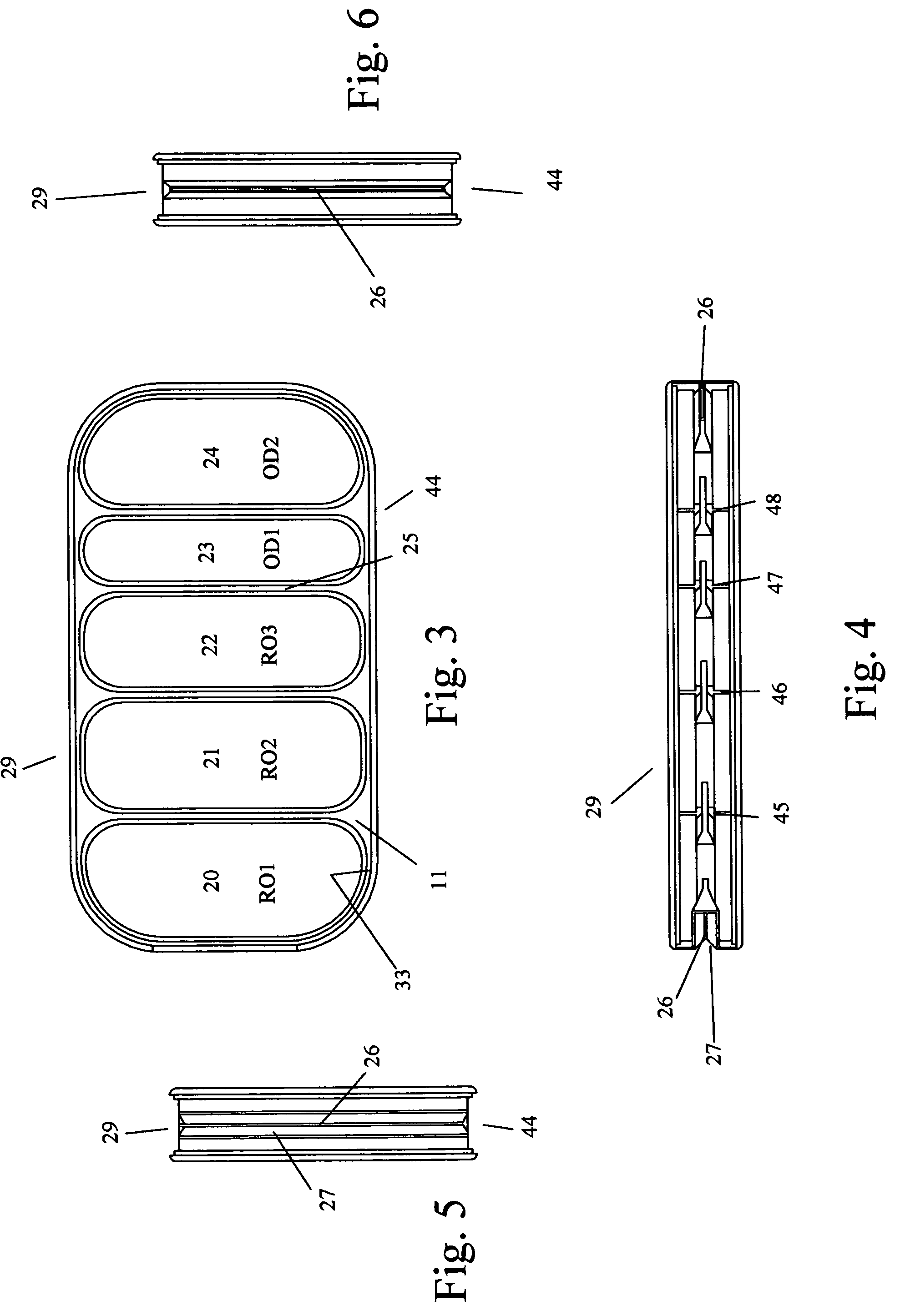
An implantable human kidney replacement unit. Fully functional self contained, providing patients with end stage renal disease the freedom of traveling and moving about normally. Replacing donor kidneys. Implanted in the flank with at least one inlet and outlet tube each, sutured to the iliac artery and vain, at least one urine tube to the ureter. The housing constructed of anti-coagulant bacteriostatic materials has a plurality of reverse-osmosis process chambers with semipemeable membranes through the unit, followed by osmosis-diffusion chambers and membranes. Blood from the artery enters the first of the chambers. Small molecules such as water, magnesium, sodium, potassium, calcium, urea etc. are extracted from the blood according to their weight in atomic mass units as blood wipes past the self-cleaning membrane cartridges in the chambers. Molecules are further separated and urea sent to the bladder with excess water and electrolytes. The remainder is channeled to at least one diffusion chamber and reabsorbed into the blood. The same process is repeated in the other chambers where selected larger molecules such as creatinine and phosphorus are excreted, and some diffused back into the blood.
Owner:LUDLOW ROLAND G
Compositions and methods for oxalate reduction
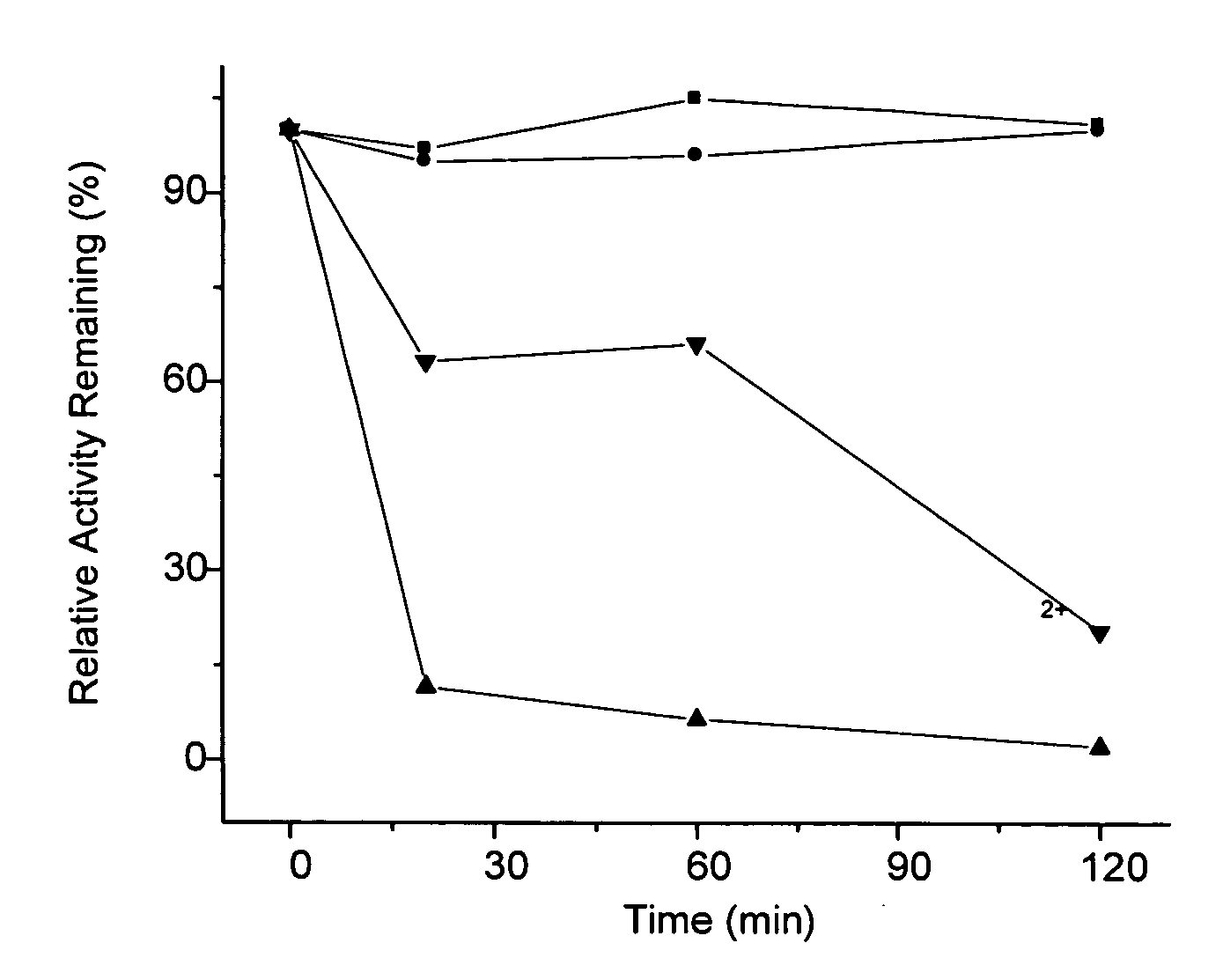
InactiveUS20070184118A1Reducing oxalateMaintain enzyme activityPowder deliveryPeptide/protein ingredientsUlcerative colitisEnd-stage kidney disease
The present invention comprises methods and compositions for the reduction of oxalate in humans. For example, the invention provides methods and compositions for the delivery of one or more oxalate-reducing enzymes embedded in particle compositions. The compositions of the present invention are suitable in methods of treatment or prevention of oxalate-related conditions including, but not limited to, hyperoxaluria, absorptive hyperoxaluria, enteric hyperoxaluria, primary hyperoxaluria, idiopathic calcium oxalate kidney stone disease (urolithiasis), vulvodynia, oxalosis associated with end-stage renal disease, cardiac conductance disorders, inflammatory bowel disease, Crohn's disease, ulcerative colitis, and patients who have undergone gastrointestinal surgery and bariatric surgery (surgery for obesity), and / or who have undergone antibiotic treatment.
Owner:OXTHERA INTPROP
Diagnostic polymorphisms for the ecnos promoter
InactiveUS20050084849A1Eliminate the effects ofDigital data processing detailsMicrobiological testing/measurementValvular diseaseProstate cancer
Disclosed are single nucleotide polymorphisms (SNIps) associated with breast cancer, lung cancer, prostate cancer, non-insulin dependent diabetes, end stage renal disease due to non-insulin dependent diabetes, hypertension, end stage renal disease F due to hypertension, myocardial infarction, colon cancer, hypertension, atherosclerotic peripheral vascular disease due to hypertension, cerebrovascular accident due to hypertension, cataracts due to hypertension, cardiomyopathy with hypertension, myocardial infarction due to hypertension, non-insulin dependent diabetes mellitus, atherosclerotic peripheral vascular disease due to non-insulin dependent diabetes mellitus, cerebrovascular accident due to non-insulin dependent diabetes mellitus, ischemic cardiomyopathy, ischemic cardiomyopathy with non-insulin dependent diabetes mellitus, myocardial infarction due to non-insulin dependent diabetes mellitus, atrial fibrillations without valvular disease, alcohol abuse, anxiety, asthma, chronic obstructive pulmonary disease. cholecystectomy, degenerative joint disease, end stage renal disease and frequent de-clots, end stage renal disease due to focal segmental glomerular sclerosis, end stage renal disease due to insulin dependent diabetes mellitus, or seizure disorder. Also disclosed are methods for using SNPs to determine susceptibility to these diseases; nucleotide sequences containing SNPs; kits for determining the presence of SNPs; and methods of treatment or prophylaxis based on the presence of SNPs.
Owner:VIRAL THERAPEUTICS
Combination drug therapy for reducing scar tissue formation
InactiveUS20080039362A1Reduce spreadPrevents a permanent catheter tip splayBiocidePeptide/protein ingredientsCombination drug therapySurgical site
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
Ion binding compositions
ActiveUS8192758B2Powder deliveryGranular deliveryEnd stage renal diseaseChronic venous insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR (INT) AG
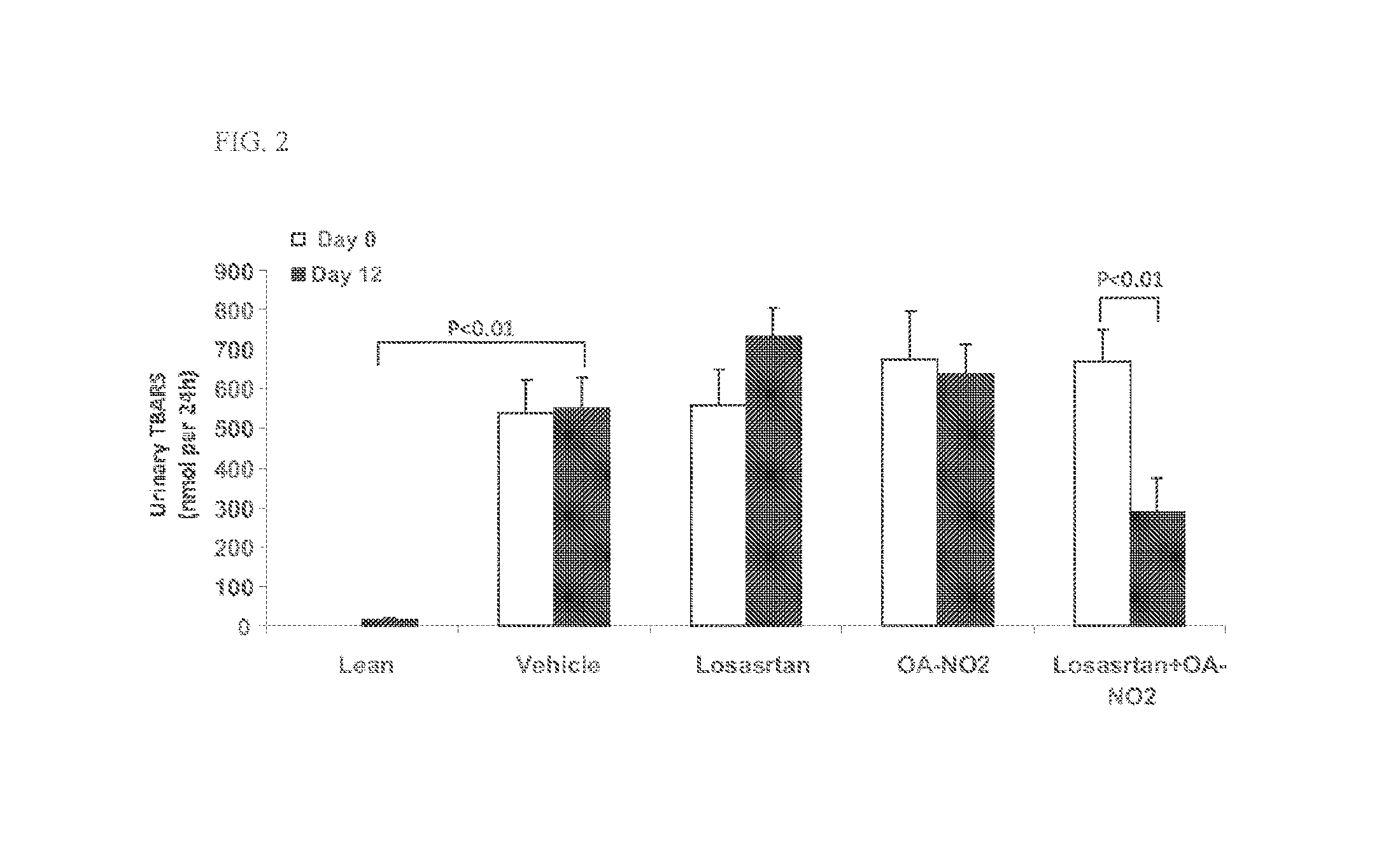
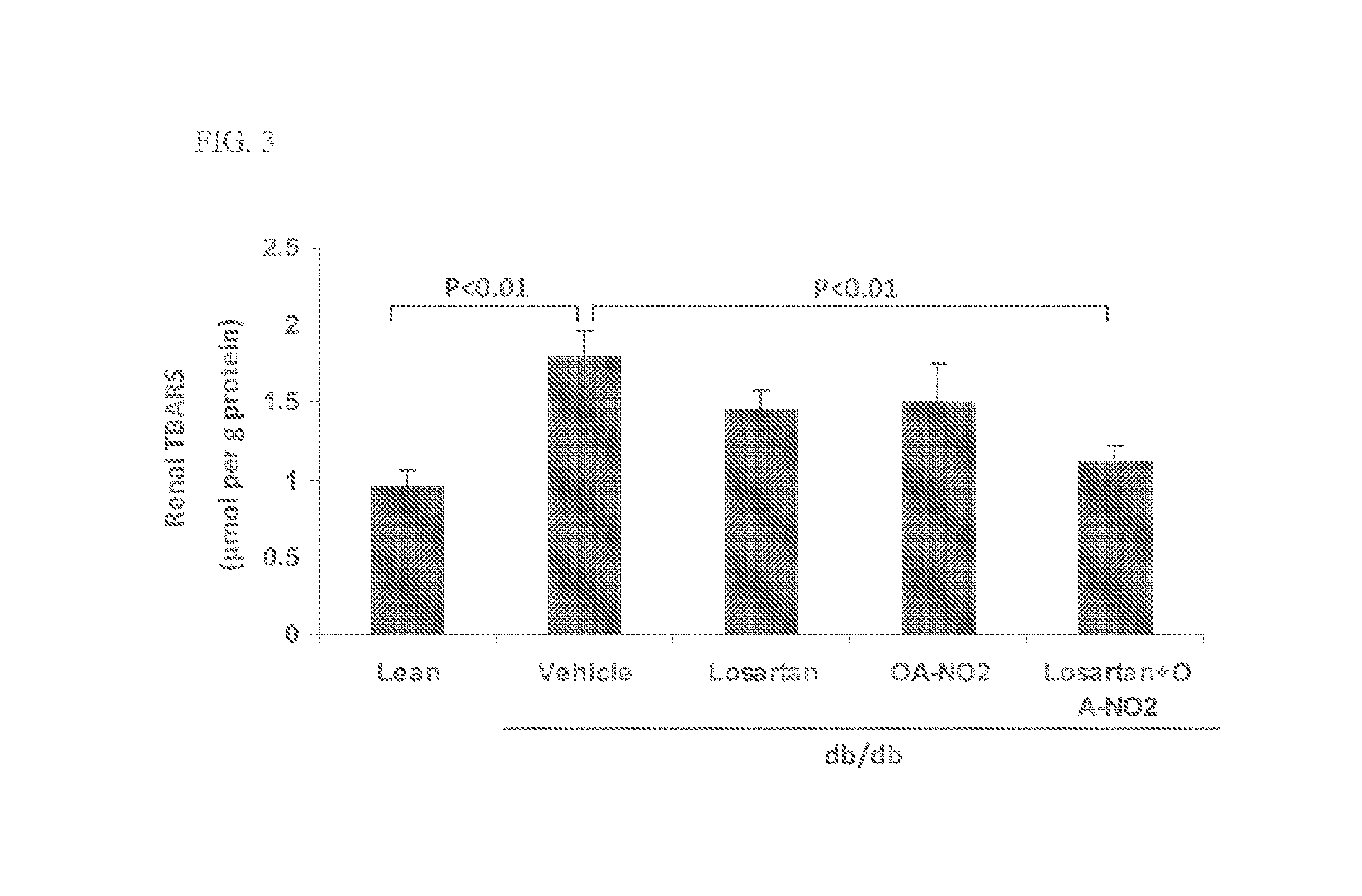
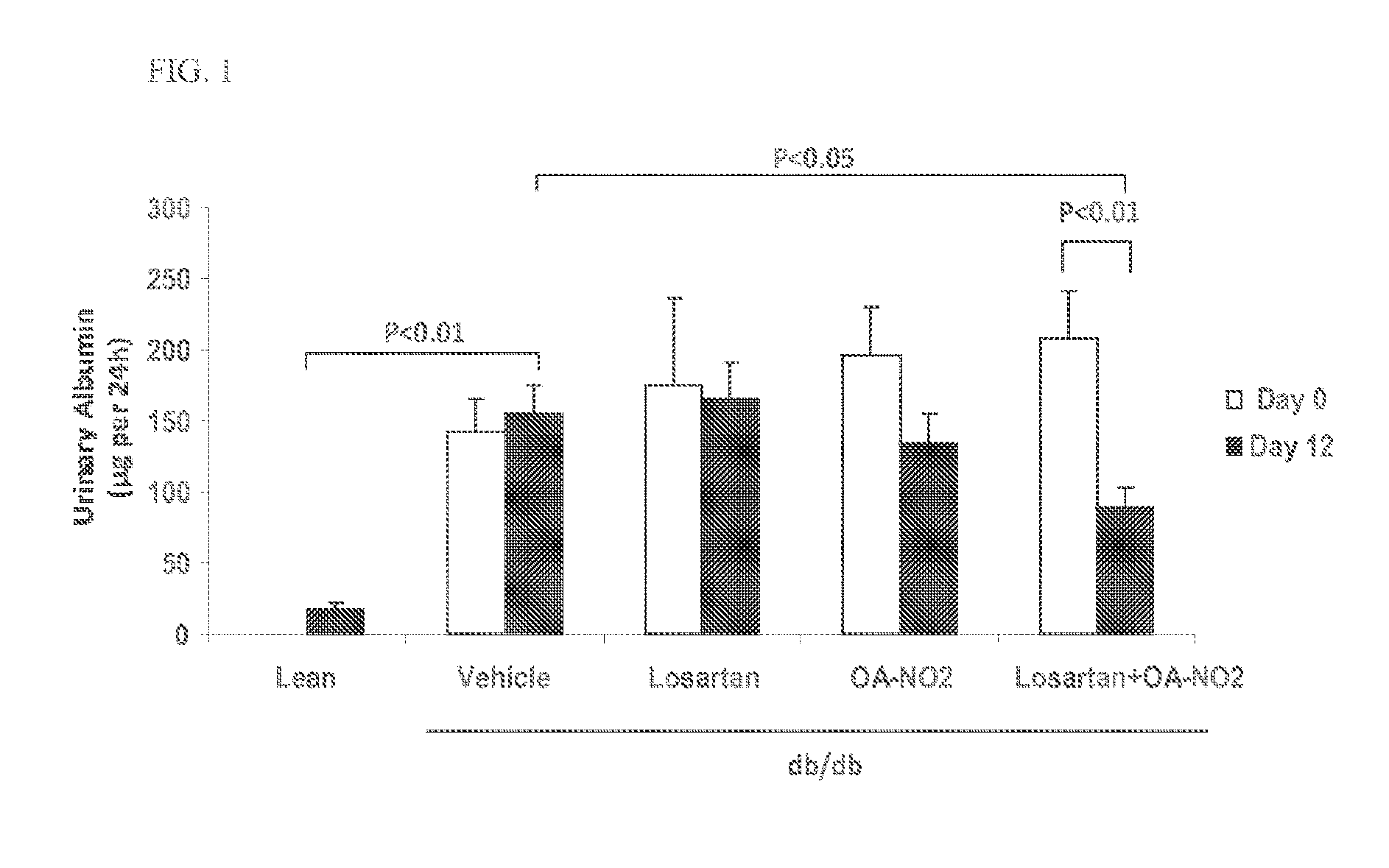
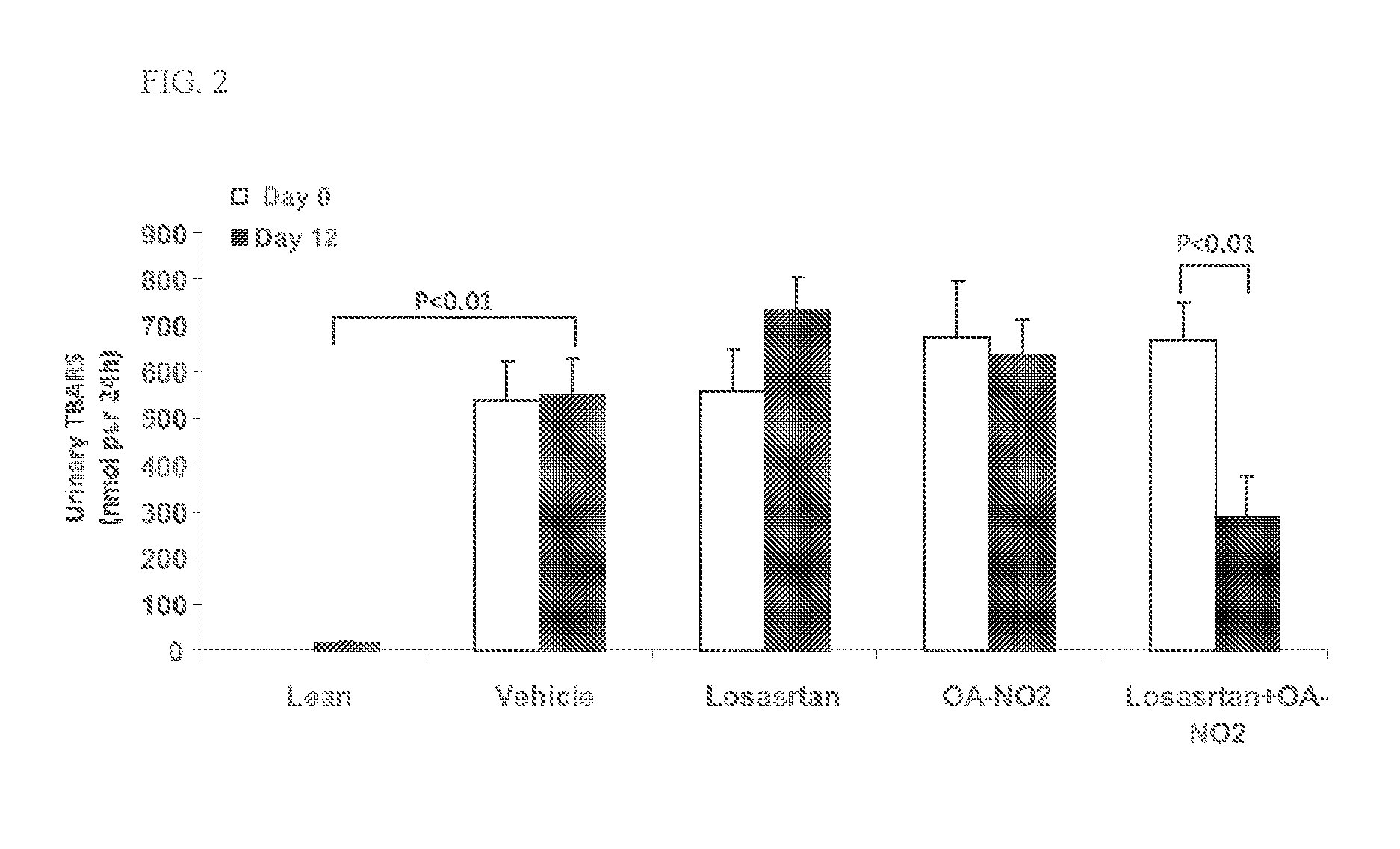
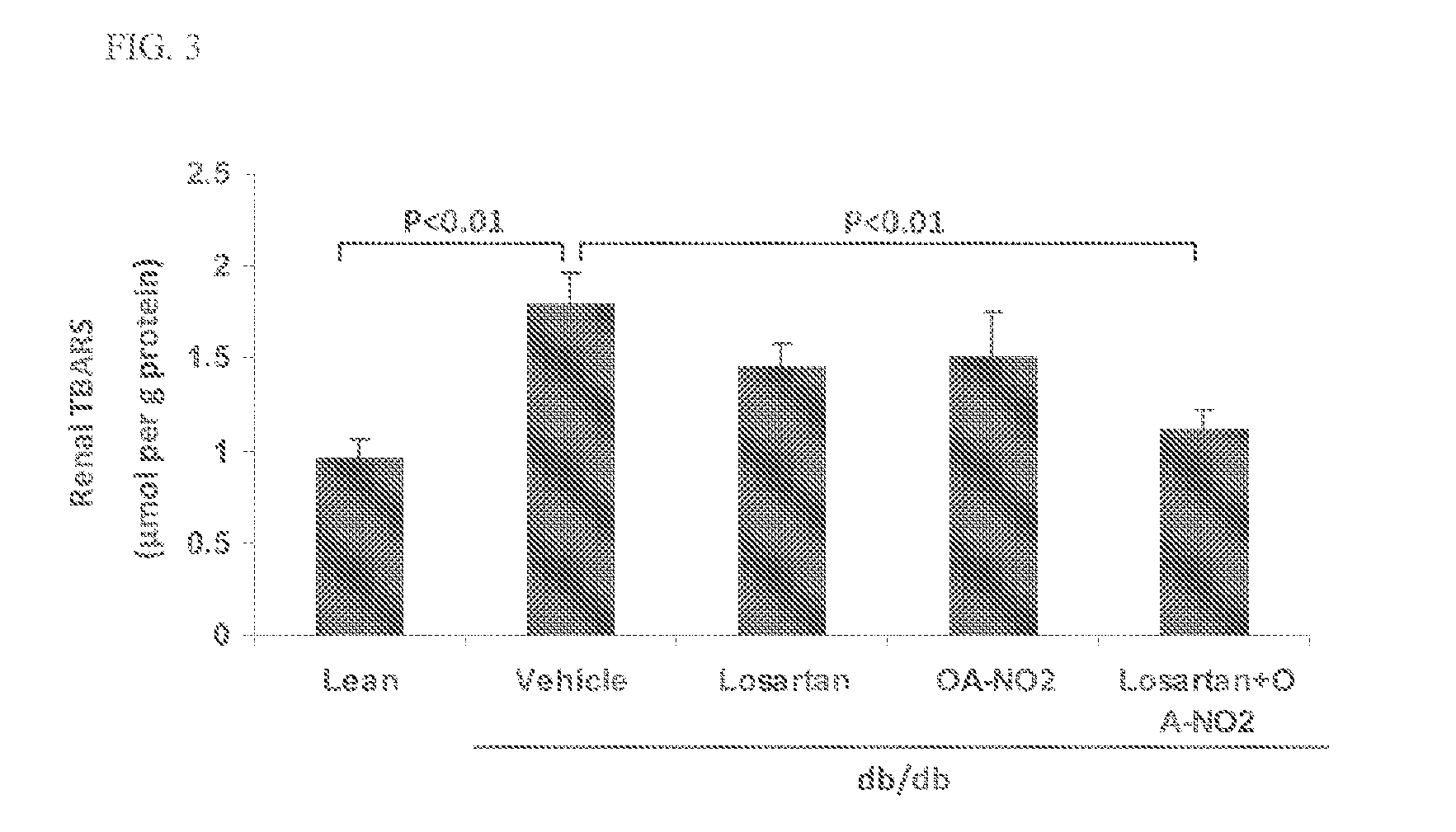
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS9192600B2Urinary disorderAnhydride/acid/halide active ingredientsNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Ion binding compositions
ActiveUS8282960B2Powder deliveryGranular deliveryEnd stage renal diseaseChronic venous insufficiency
Owner:VIFOR (INT) AG
Treating chronic uremic patients undergoing periodical dialysis
InactiveUS6914076B2Eliminate needWithout affecting maintenance and correctionBiocideAnimal repellantsNephrosisVein
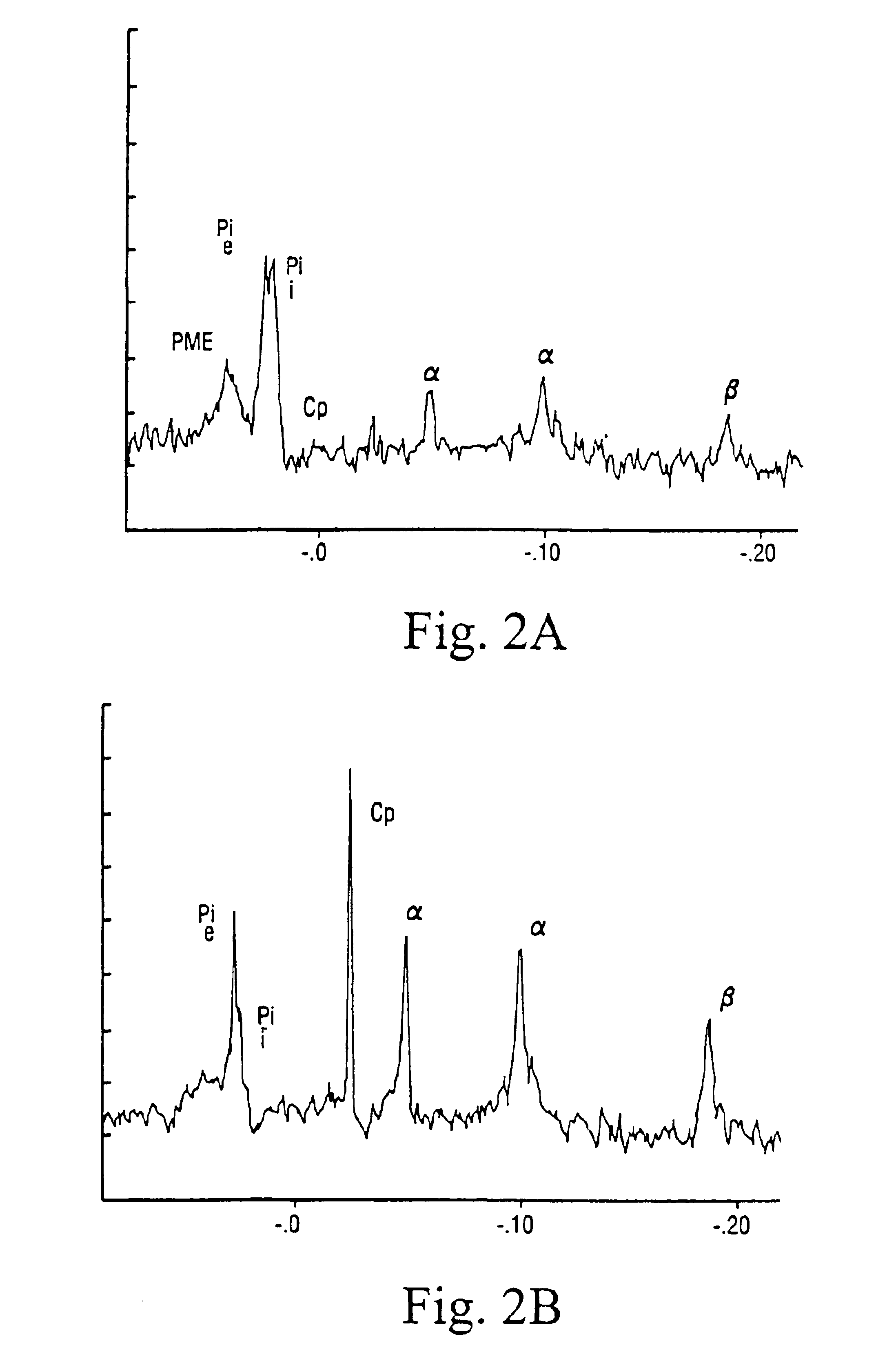
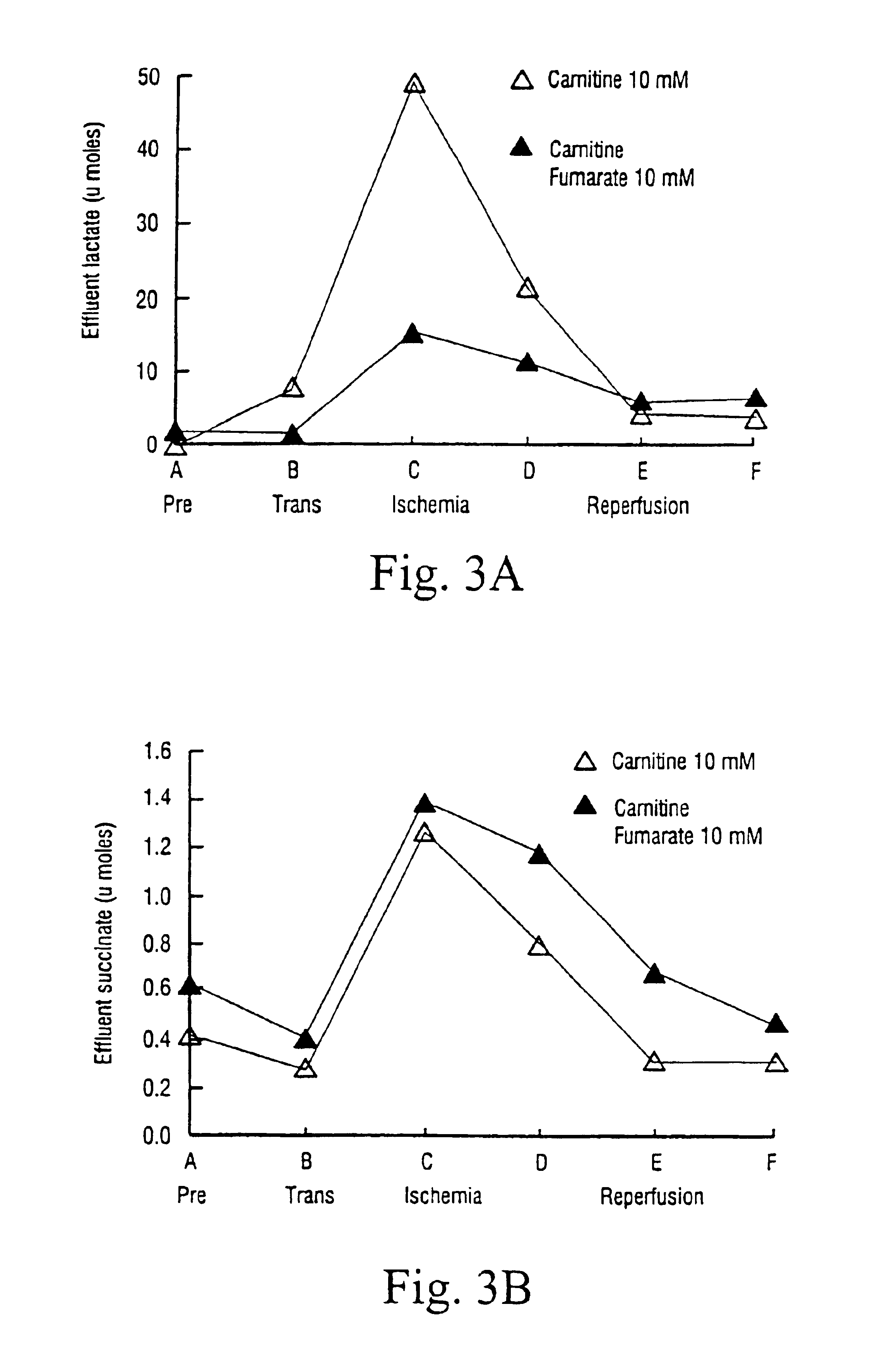
The method for the treatment of chronic uremic patients undergoing periodical dialysis is useful for preventing and / or treating carnitine deficiency in patients with end stage renal disease who are undergoing dialysis. The method according to the present invention comprises administering an effective dose of carnitine intravenously into the venous return line after each dialysis session.
Owner:ALFASIGMA SPA
Methods and compositions for treatment of ion imbalances
InactiveUS20060024265A1Powder deliverySynthetic polymeric active ingredientsEnd stage renal diseaseChronic renal insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides compositions comprising sodium-binding polymers and pharmaceutical compositions thereof. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
Ion binding compositions
ActiveUS7429394B2Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic venous insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR (INT) AG
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS20140243380A1Reducing mRNA expressionHigh expressionBiocideUrinary disorderNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Apheresis based treatment for kidney disease
InactiveUS20170035955A1Reduce formation rateAvoid performanceOther blood circulation devicesHaemofiltrationEnd stage renal diseaseFibrosis
A system and method for the treatment of kidney disease, either acute or chronic, in a mammal is disclosed. The method addresses the needs of a patient exhibiting fibrosis formation associated with levels of Gal-3 above normal. Removal of Gal-3 may make conventional treatments of kidney disease more effective, and help stabilize or improve the condition allowing avoidance of progression to end stage renal disease requiring hemodialysis or transplant. Depending on the condition of the patient, the treatment may help stabilize patients to avoid hemodialysis and allow the patient to wait until a transplant may be made available. A suitable patient is treated through apheresis where the patient's blood is withdrawn and ex vivo is treated to remove at least ten percent of Gal-3 from the withdrawn blood. Higher levels, and frequent repetition, may be needed to stabilize and / or improve kidney function in a patient sufficient for ongoing management.
Owner:ELIAZ THERAPEUTICS INC
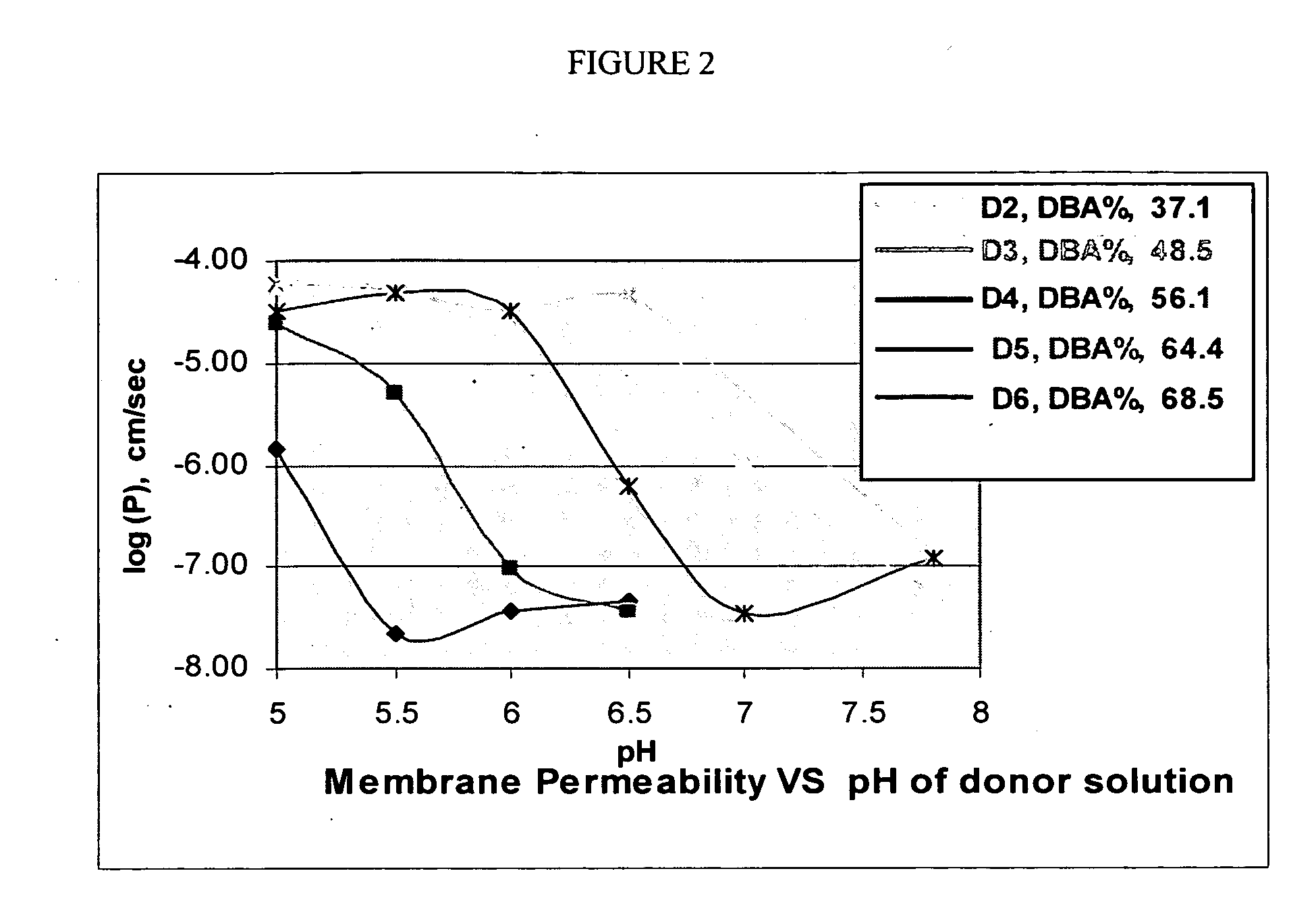
Coated Pharmaceutical Compositions
The present invention relates to polycarbophil coated crosslinked amine polymers and / or pharmaceutical compositions comprising polycarbophil coated crosslinked amine polymers. The polycarbophil coated crosslinked amine polymers have several therapeutic applications, including, but not limited to, hyperphosphatemia, chronic kidney disease and End-Stage Renal Disease.
Owner:GENZYME CORP
Methods and compositions for treatment of ion imbalances
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides compositions comprising sodium-binding polymers and pharmaceutical compositions thereof. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
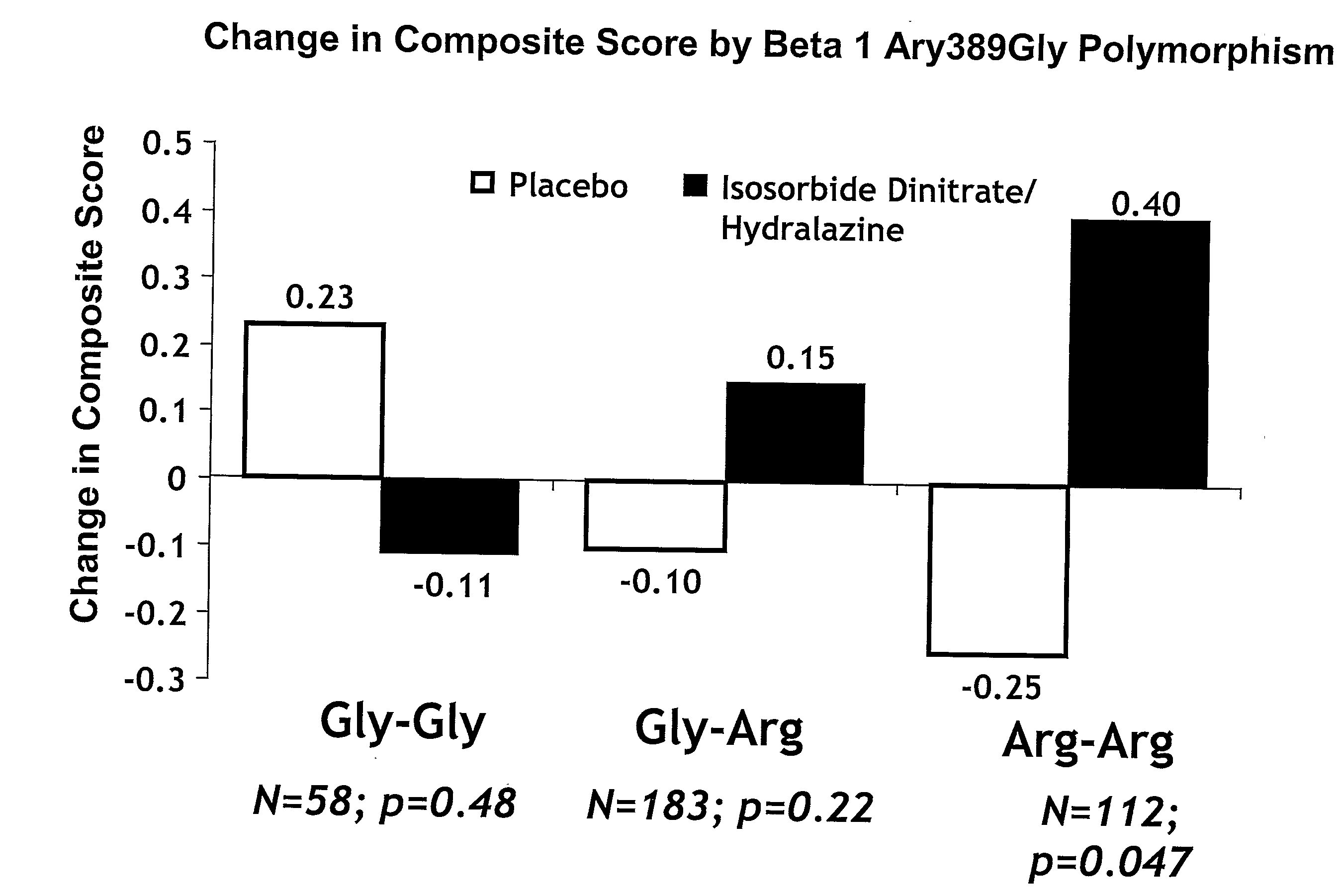
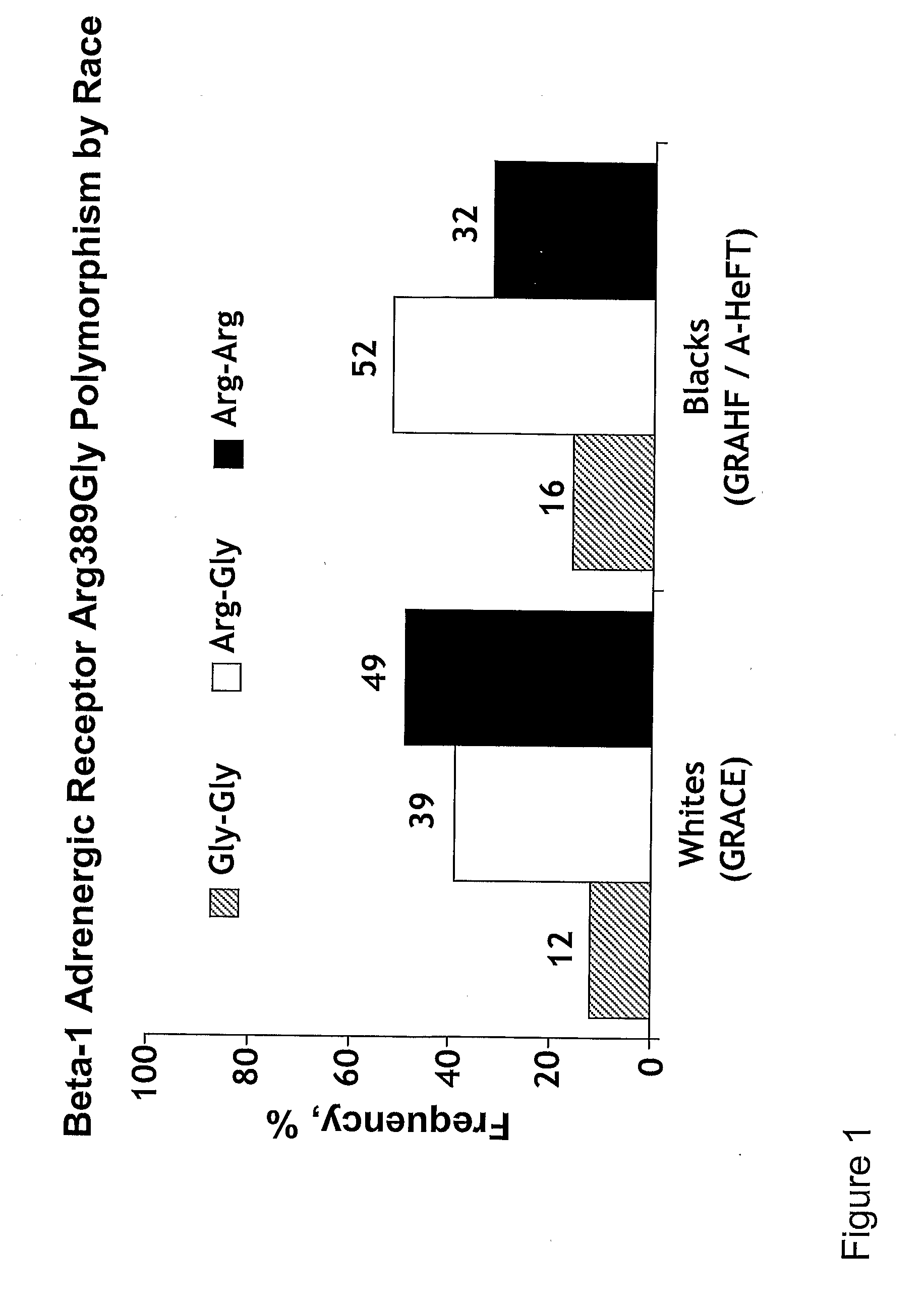
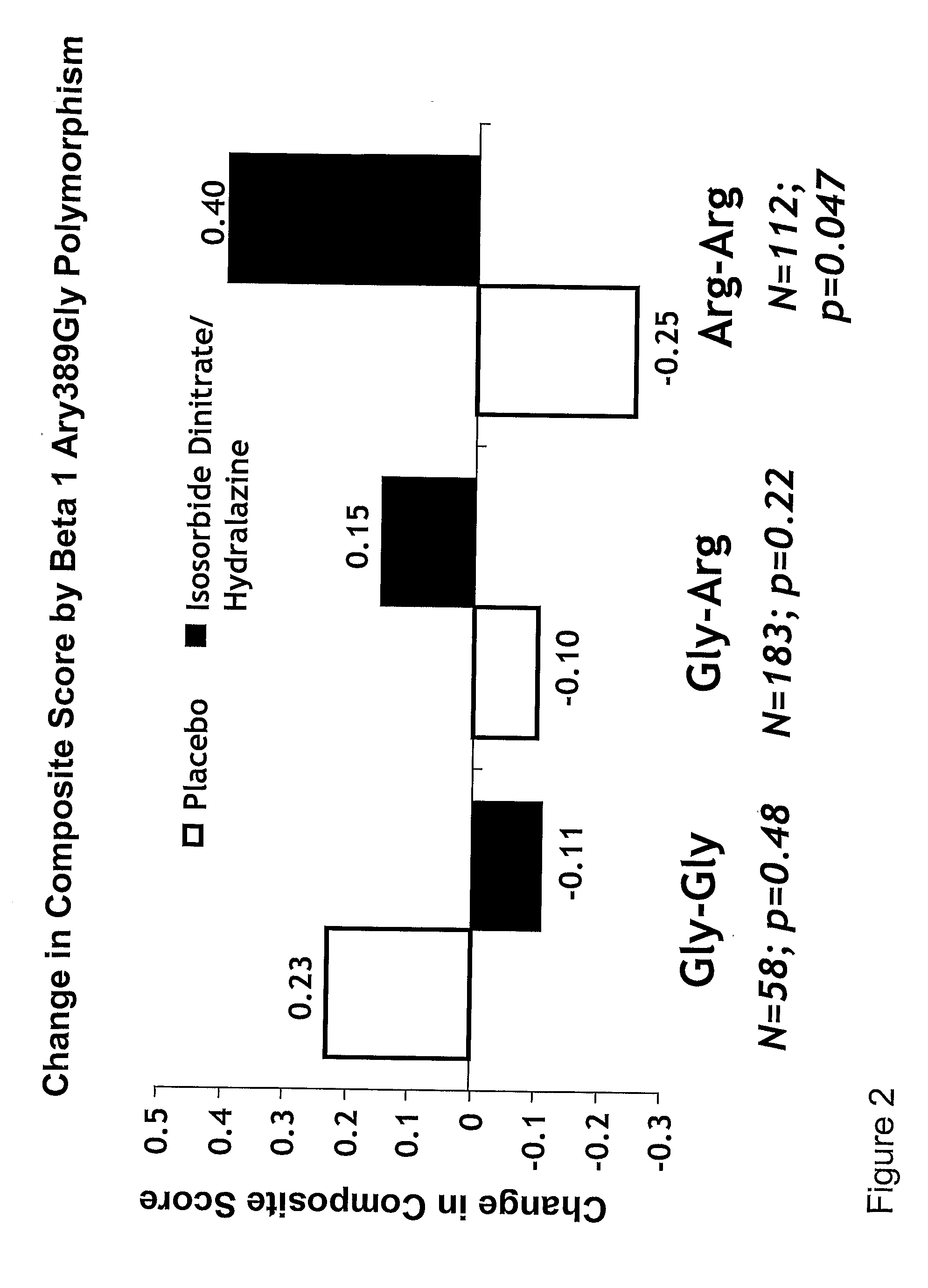
Genetic risk assessment in heart failure: impact of genetic variation of beta 1 adrenergic receptor gly389arg polymorphism
InactiveUS20090192128A1Reduce mortalityIncreased oxygen consumptionBiocideMicrobiological testing/measurementAntioxidantLeft ventricular size
The invention provides methods for (a) reducing mortality associated with heart failure; (b) improving oxygen consumption; (c) treating heart failure; (d) treating hypertension; (e) improving the quality of life in a heart failure patient; (f) inhibiting left ventricular remodeling; (g) reducing hospitalizations related to heart failure; (h) improving exercise tolerance; (j) increasing left ventricular ejection fraction; (k) decreasing levels of B-type natriuretic protein; (l) treating renovascular diseases; (m) treating end-stage renal diseases; (n) reducing cardiomegaly; (o) treating diseases resulting from oxidative stress; (p) treating endothelial dysfunctions; (q) treating diseases caused by endothelial dysfunctions; or (r) treating cardiovascular diseases; in a patient in need thereof, wherein the patient has a Arg389Arg polymorphism and / or a Gly389Gly polymorphism in the beta 1 adrenergic receptor gene, comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
Methods of treating erythropoietin-resistance
InactiveUS20060128805A1Halt erythropoietin-resistanceEffective manner to treatBiocidePeptide/protein ingredientsEnd stage renal diseaseIron Chelator
A human having an erythropoietin resistant condition is treated by the administration of an iron chelator. The human can have end-stage renal disease, chronic renal disease, cancer or anemia. Administration of the iron chelator can essentially halt or diminish an erythropoietin resistant condition in a human.
Owner:SHIVA BIOMEDICAL
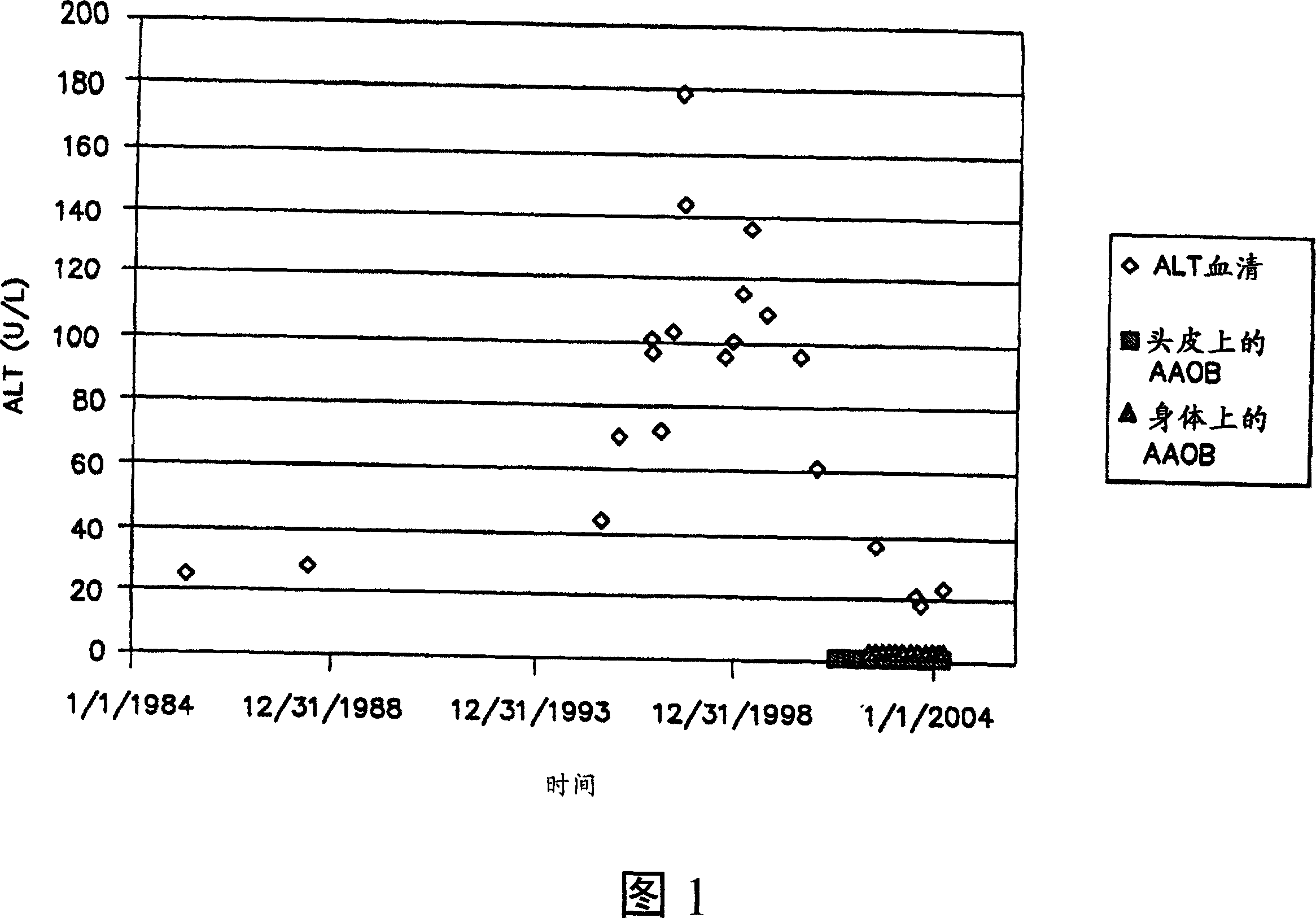
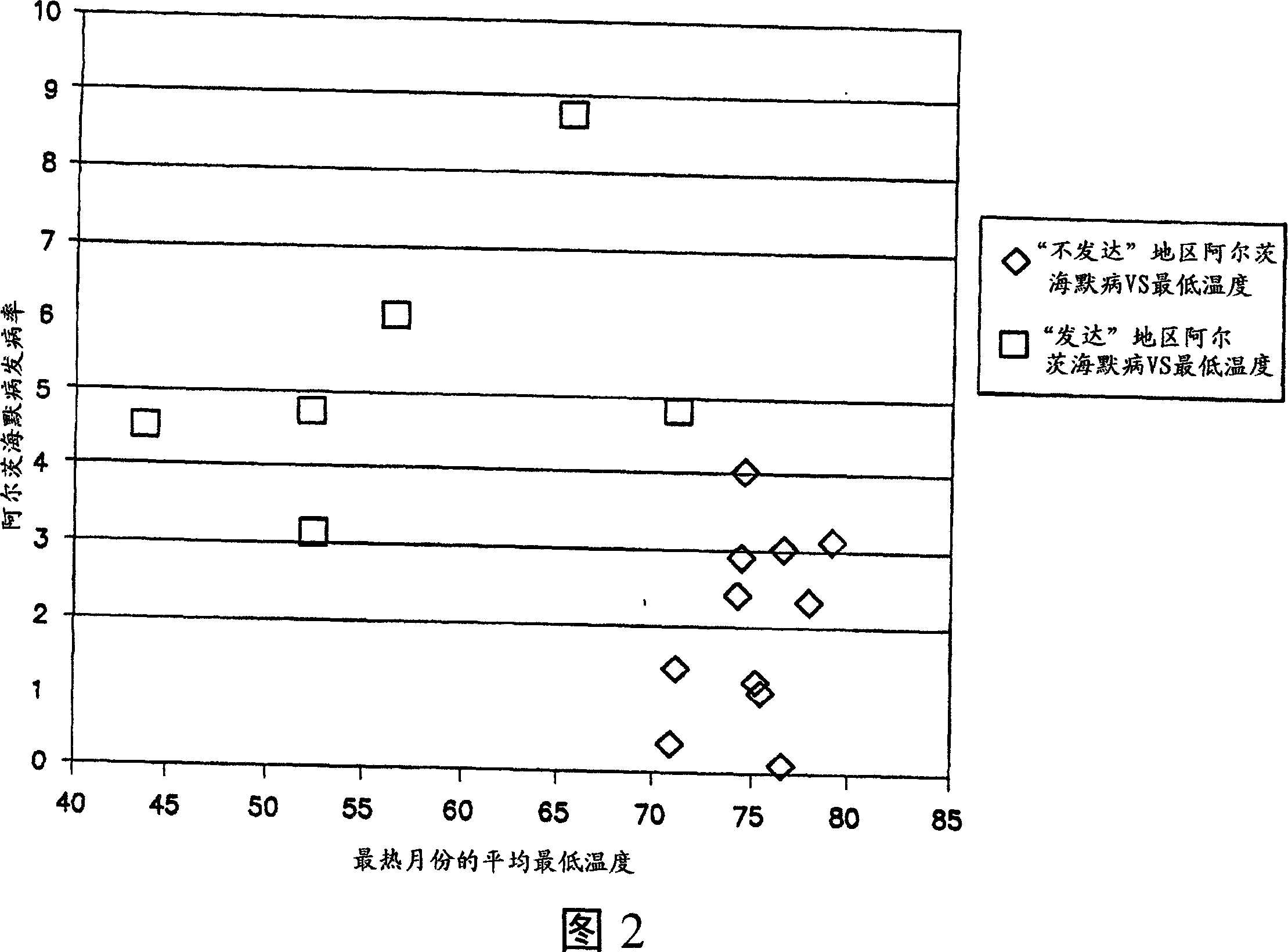
Methods of using ammonia oxidizing bacteria
A use of ammonia oxidizing bacteria in the manufacture of a medicament and a method for treating a subject who has developed or is at risk of developing at least one of hypertension, hypertrophic organ degeneration, Raynaud's phenomena, fibrotic organ degeneration, allergies, autoimmune sensitization, end stage renal disease, obesity, diabetes type 1, osteoporosis, impotence, hair loss, cancer, aging, autism, and an autism spectrum symptom comprising positioning ammonia oxidizing bacteria close proximity of a surface of the subject, of nitric oxide and nitric oxide precursors using ammonia oxidizing bacteria.
Owner:大卫·R·怀特洛克
Methods and devices for renal nerve blocking
Methods and apparatus are provided for renal neuromodulation using a pulsed electric field to effectuate electroporation or electrofusion. It is expected that renal neuromodulation (e.g., denervation) may, among other things, reduce expansion of an acute myocardial infarction, reduce or prevent the onset of morphological changes that are affiliated with congestive heart failure, and / or be efficacious in the treatment of end stage renal disease. Embodiments of the present invention are configured for extravascular delivery of pulsed electric fields to achieve such neuromodulation.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Floormat physiological sensor
InactiveUS20170188960A1Foster patient complianceFoster regular useElectrocardiographyEvaluation of blood vesselsEnd stage renal diseaseHemodynamics
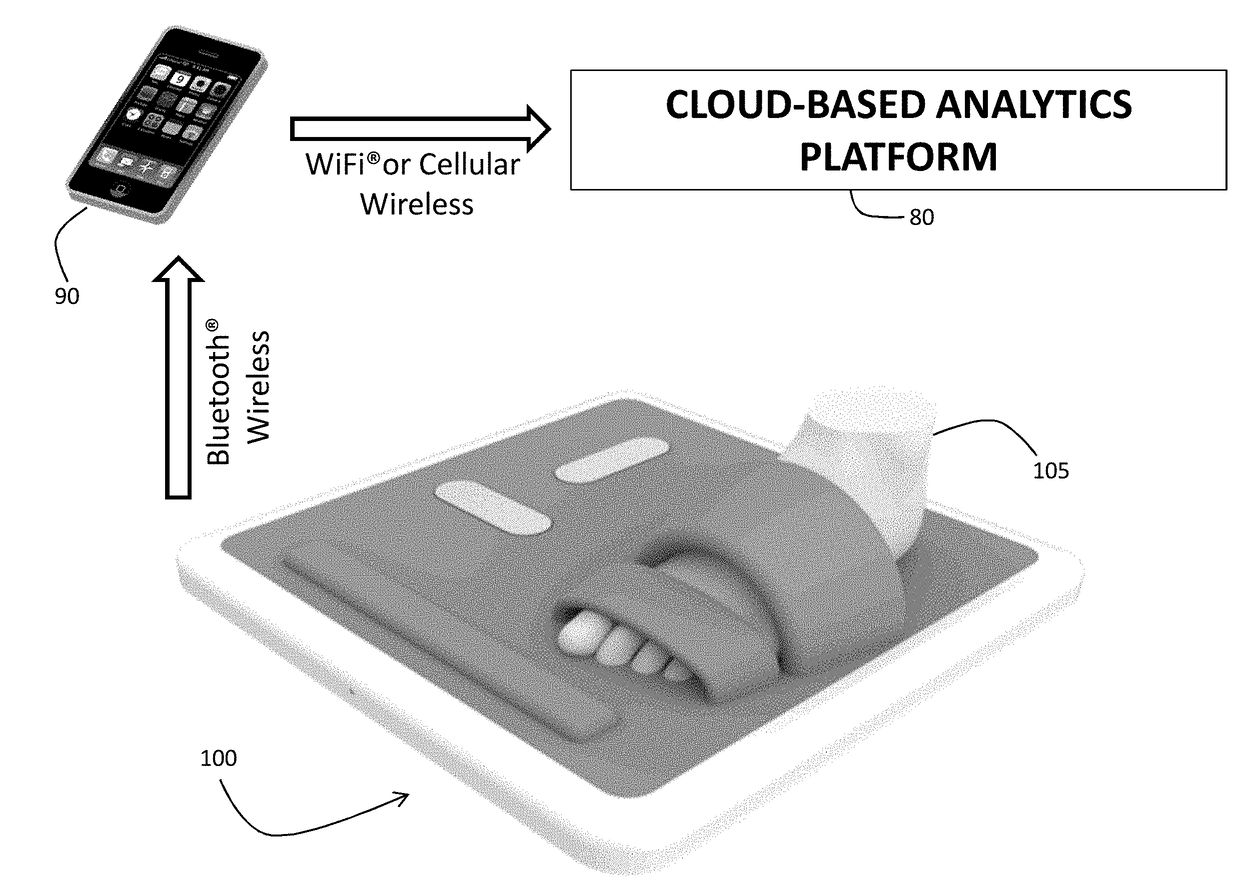
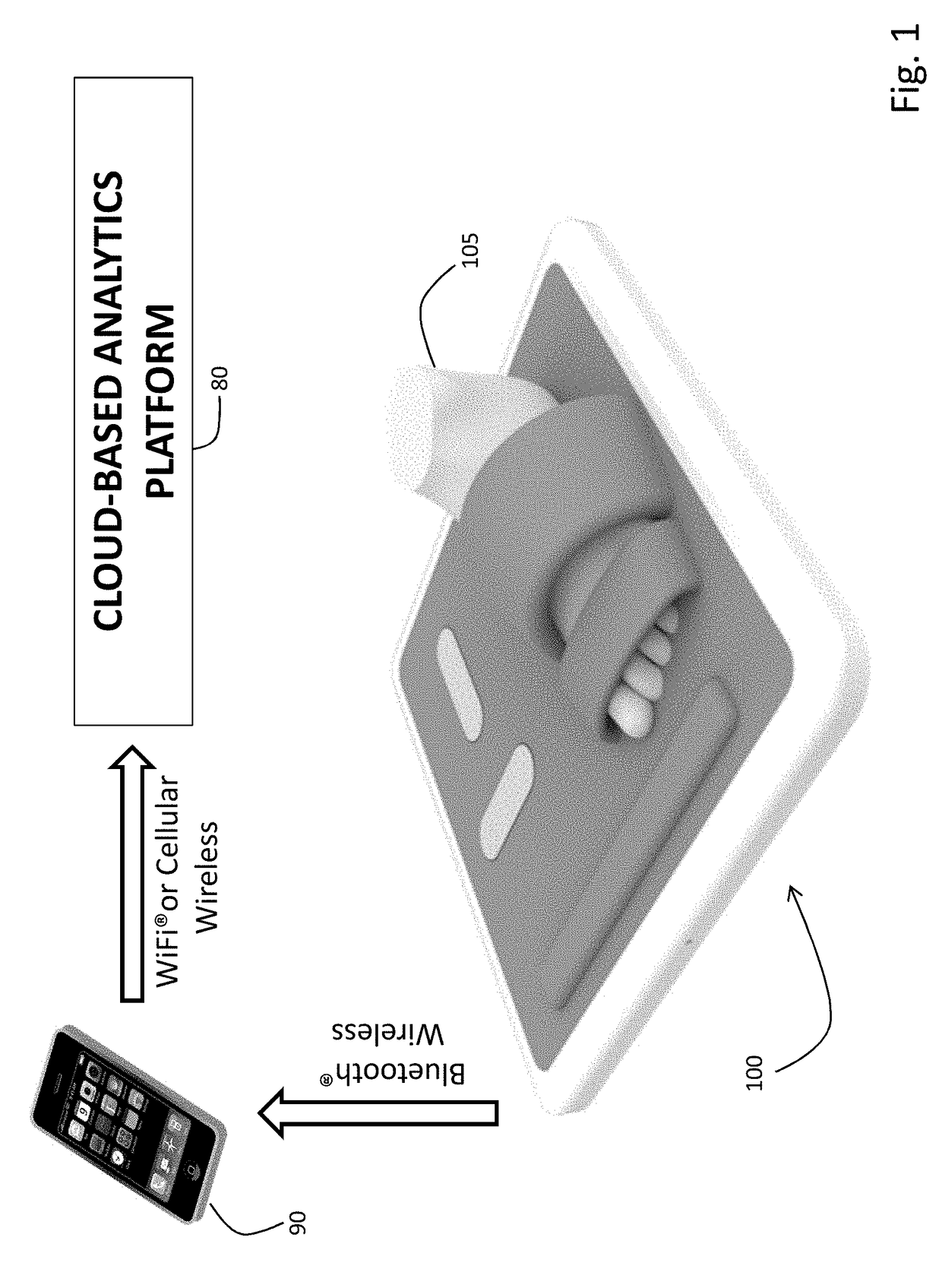
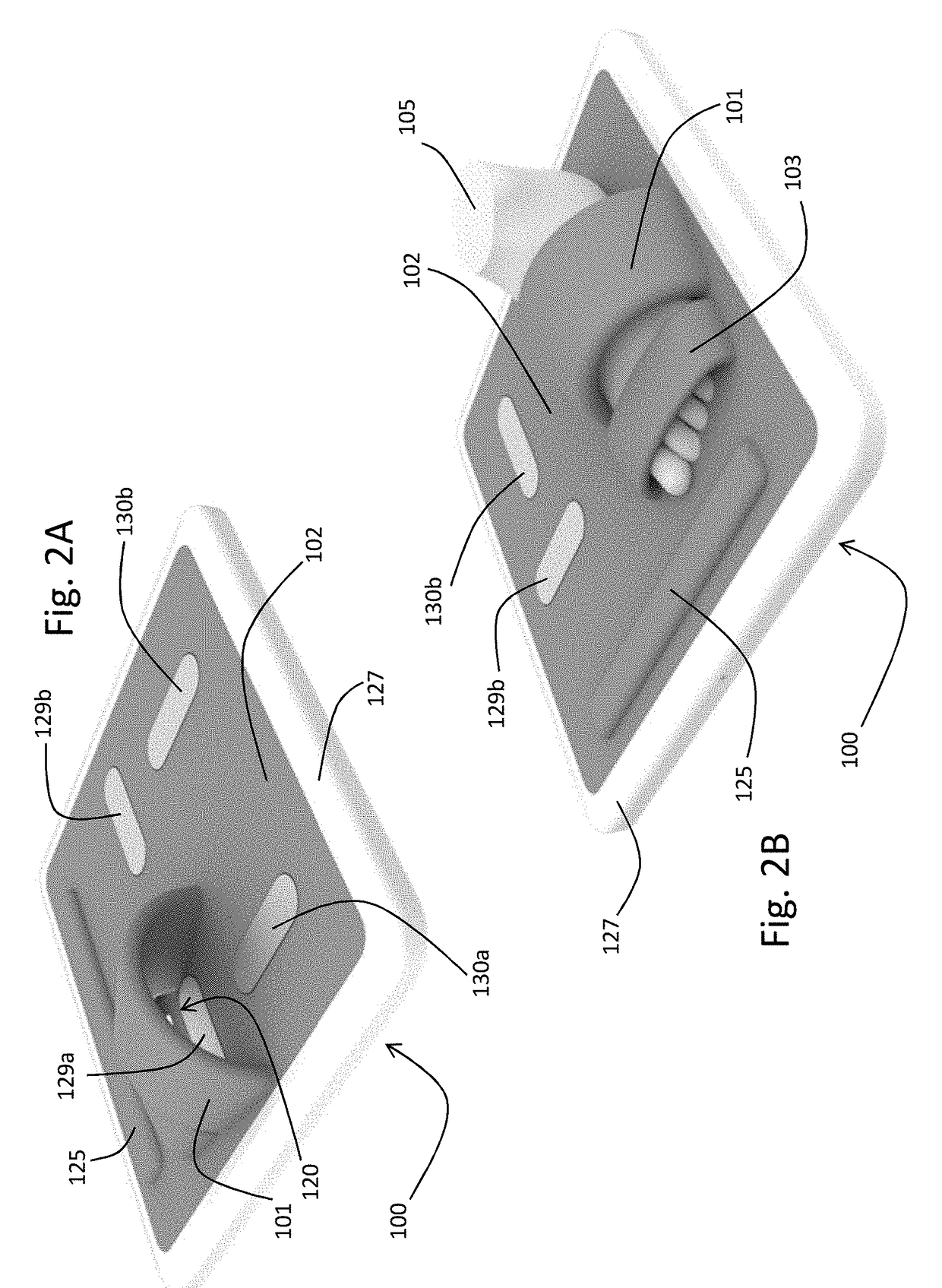
A stand-on physiological sensor (e.g. floormat) measures vital signs and various hemodynamic parameters, including blood pressure and ECG waveforms. The sensor is similar in configuration to a common bathroom scale and includes electrodes that take electrical measurements from a patient's feet to generate bioimpedance waveforms, which are analyzed digitally to extract various other parameters, as well as a cuff-type blood pressure system that takes physical blood pressure measurements at one of the patient's feet. Blood pressure can also be calculated / derived from the bioimpedance waveforms. Measured parameters are transmitted wirelessly to facilitate remote monitoring of the patient for heart failure, chronic heart failure, end-stage renal disease, cardiac arrhythmias, and other degenerative diseases.
Owner:TOSENSE
Peritoneal dialysates
InactiveUS20040096845A1Decrease in FIMicrobiological testing/measurementPharmaceutical delivery mechanismNitrogenous heterocyclic compoundTemperature and pressure
In peritoneal dialysis, a therapy for end stage renal disease patients, glucose has been used mainly as an osmotic agent in the dialysate. However due to the oxidation and decomposition of glucose, protein cross linking reaction progress to cause peritoneum sclerosis, resulting in cease of this therapy The present invention relates to the dialysate comprising protein cross linking suppressor(s) and / or protein cross linkage splitter(s) for the solution of the problem. Effective suppressors or splitters may be reductants, anti-oxidants, mercapto compounds, sulfide, hydrosulfide, salt of reductive sulfur oxyacid, thiourea and its derivatives, hydroxyl and / or carboxyl residue(s) containing cyclic compounds flavonoids, nitrogen containing heterocyclic compounds, hydrazyl compounds and uronic acid containing mucopoly-saccharides and the like. The dialysate is prepared by sterilizing the dialysate under high temperature and pressure separately from the suppressors and by adding the suppressors, that is sterilized separately, into the dialysate. By use of the dialysate in the present invention, peritoneal dialysis can keep on without suffering from problem,
Owner:JAPAN SCI & TECH CORP
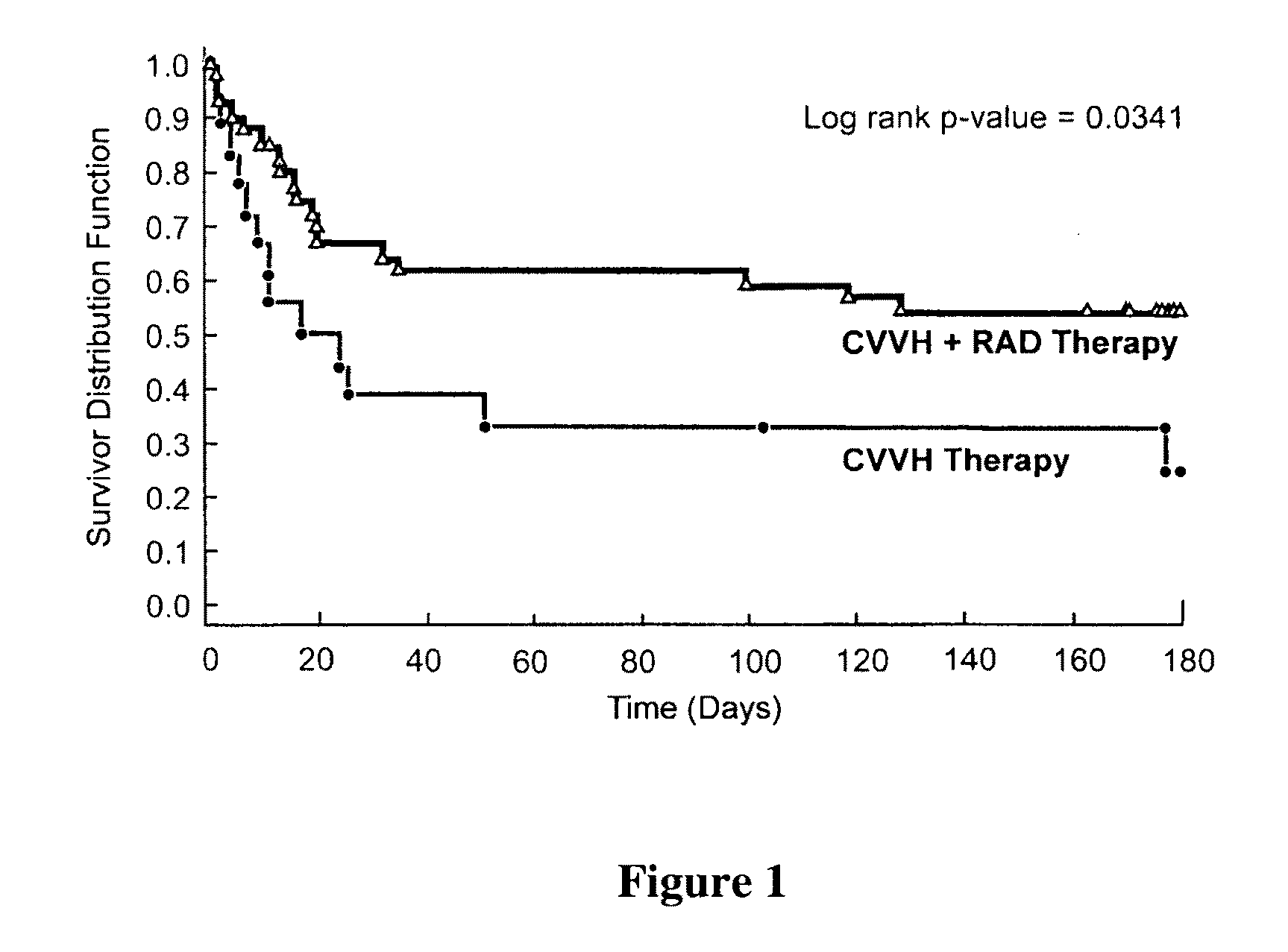
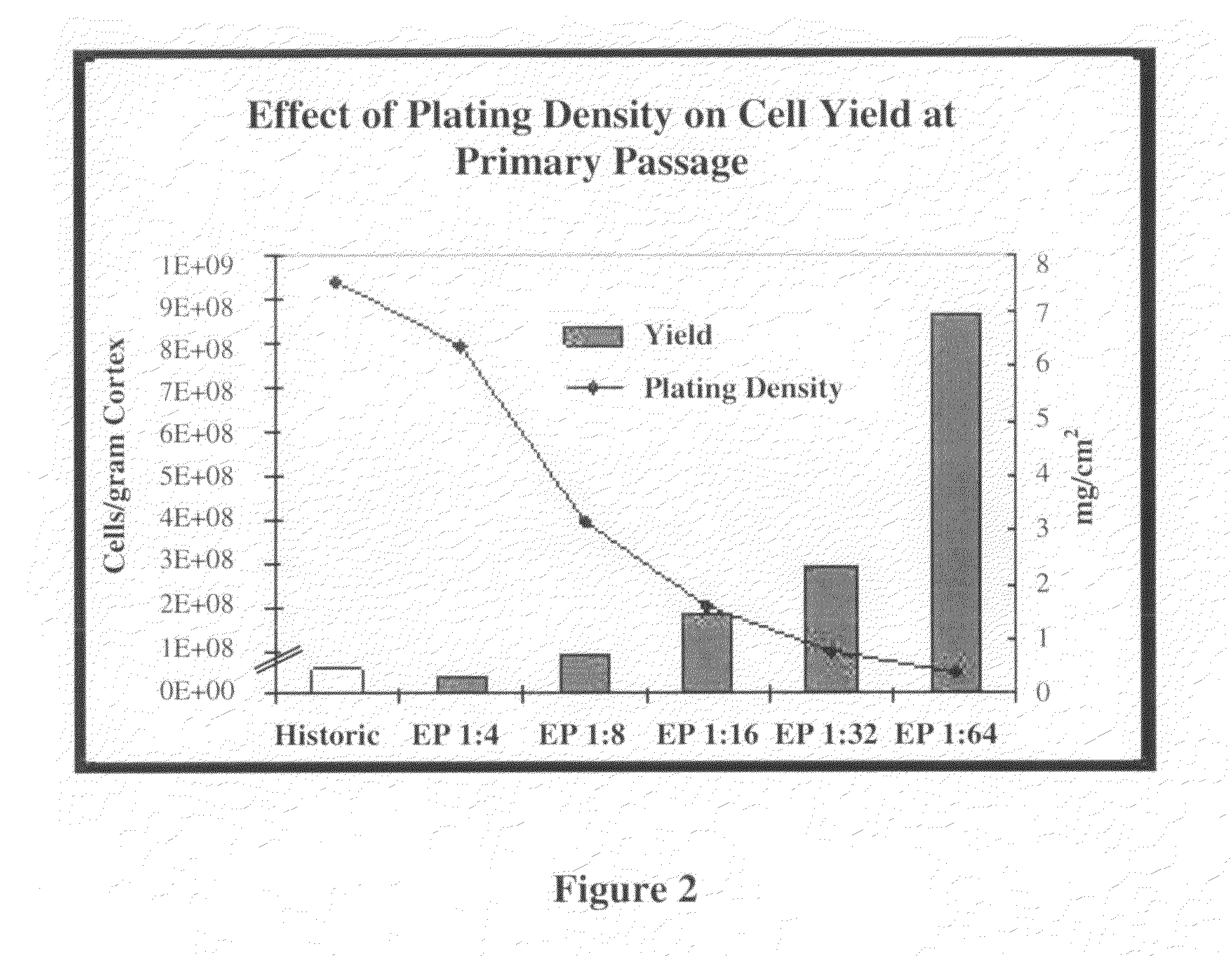
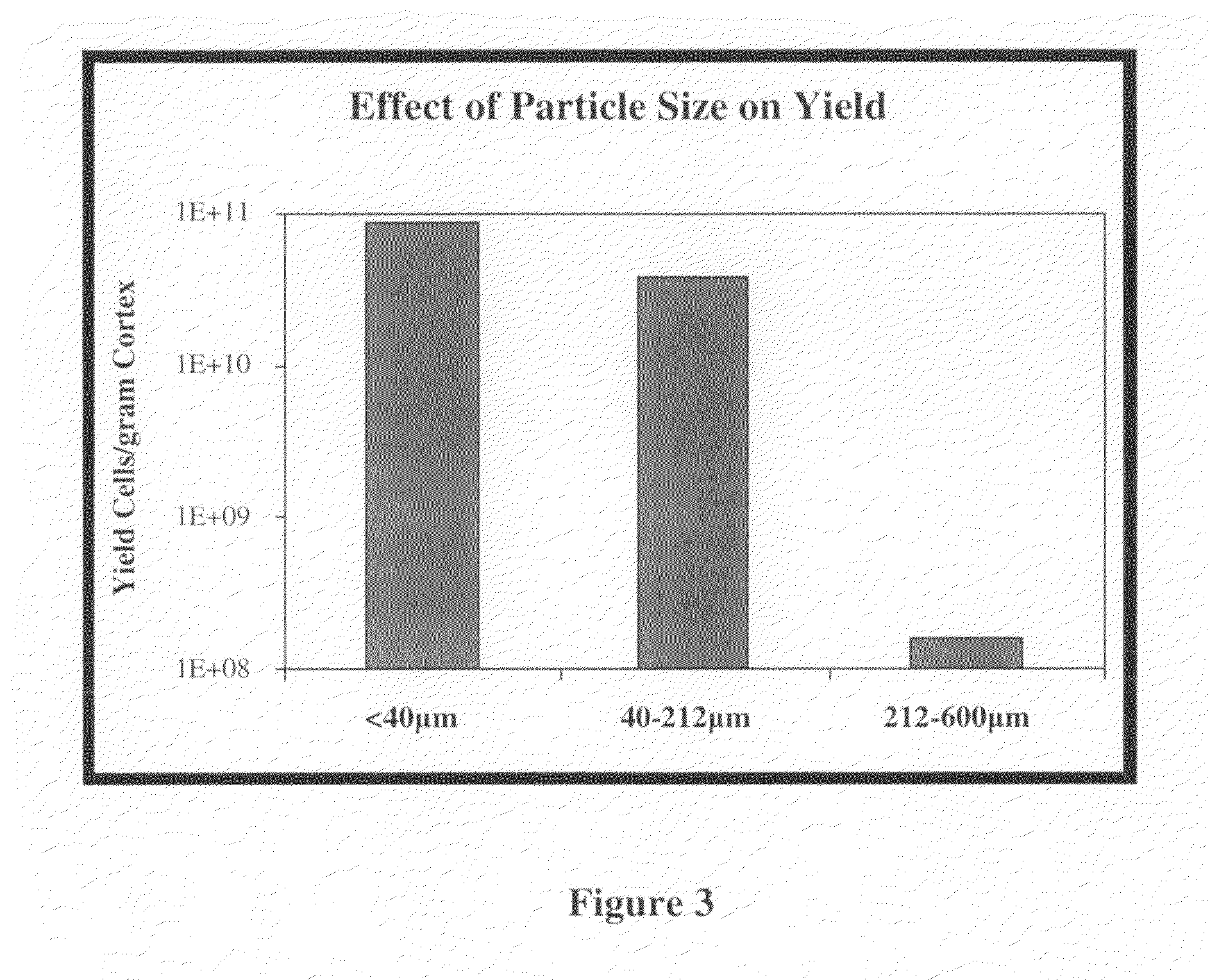
Methods for Enhanced Propagation of Cells
ActiveUS20100136687A1Enhancing kidney cell expansionQuality improvementCell culture active agentsUrinary disorderEnd stage renal diseaseMulti organ
The present invention relates generally to methods for the isolation and propagation of cells. For example, embodiments of the present invention relate to isolation and propagation methods for the manufacture of a large number of cells for use, for example, in biotherapeutic devices, such as devices for renal replacement therapy for the treatment of acute renal failure (ARF), acute tubular necrosis (ATN), multi-organ failure (MOF), sepsis, cardiorenal syndrome (CRS) and end-stage renal disease (ESRD).
Owner:SEASTAR MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com